Introduction
Glioblastoma is the most aggressive primary brain
malignancy with a median survival rate of 14.6 months from
diagnosis in unselected patients, even following maximal, feasible
surgical resection, radiotherapy and standard adjuvant temozolomide
(TMZ) therapy (1). Only 0.4–0.5% of
all GBM patients with extracranial metastasis has been reported,
which may be attributable to the extremely shortened survival of
these patients (2). Combining
radiotherapy and TMZ provides better survival outcomes of
glioblastoma patients than radiotherapy alone (3). Survival and recurrence are
significantly associated with the extent of resection and residual
volume (4). Gross total resection
associated with survival improvement is not always possible as the
preservation of neurological functions is necessary. The efficacy
of current multimodality treatments including surgery,
radiotherapy, chemotherapy for this tumor remains
unsatisfactory.
Phenethyl isothiocyanate (PEITC) is one of the most
extensively studied isothiocyanates (5). PEITC can induce cell cycle arrest and
apoptotic cell death in various tumor types (6–12). In
our previous study, PEITC induced apoptosis through the extrinsic
(death receptor) and intrinsic (mitochondrial) pathways,
dysfunction of mitochondria and ROS-induced ER stress in GBM 8401
cells (13). PEITC displayed
anti-metastatic effects in vivo in a novel breast tumor
metastasis model (14), and
inhibited tumor migration and invasion via suppression of multiple
signal transduction pathways in human colon cancer HT29 cells
(15). Yet, there is no available
literature concerning how PEITC affects the migration and invasion
of human brain glioblastoma cells.
In the present study, we investigated the effects of
PEITC on human brain glioblastoma cells in regards to migration and
invasion through the signaling transduction pathways in GBM 8401
cells.
Materials and methods
Chemicals and reagents
PEITC, dimethyl sulfoxide (DMSO), propidium iodide
(PI), RNase, Tris-HCl, Triton X-100 and trypan blue were obtained
from Sigma Chemical Co. (St. Louis, MO, USA). RPMI-1640, fetal
bovine serum (FBS), L-glutamine, penicillin-streptomycin and
trypsin-EDTA were purchased from Gibco-BRL/Invitrogen (Carlsbad,
CA, USA). Matrigel invasion chambers were obtained from BD
Biosciences (San Jose, CA, USA).
Cell culture
The GBM 8401 cell line was purchased from the Food
Industry Research and Development Institute (Hsinchu, Taiwan).
Cells were plated onto 75-cm2 tissue culture flasks in
RPMI-1640 medium supplemented with 10% FBS, 100 U/ml penicillin and
100 μg/ml streptomycin, 2 mM L-glutamine and grown at 37°C
under a humidified 5% CO2 and 95% air at one atmosphere.
The cells were subcultured with a solution of 0.25% trypsin and
0.02% EDTA. The medium was changed every 2 days (16).
Cell morphological changes and
viability
GBM 8401 cells (1.6×105 cells/well) on a
12-well plate were treated with 0, 0.5, 1, 2 and 4 μM PEITC,
or 0 and 500 μM TMZ, and incubated for 0, 24 and 48 h. Cells
in each well were examined, and representative images were captured
at ×200 magnification using a Nikon TE2000-U inverted microscope
for morphological change examinations. After cells from each well
were trypsinized and collected by centrifugation at 1500 rpm for 5
min, and washed twice with PBS, 5 μg/ml PI in PBS was added
to determine the percentage of viable cells. Non-viable cells were
stained by PI dye exclusion (indicative of an intact membrane) and
displayed brighter fluorescence than the unstained (viable) cells.
Cells were counted by flow cytometric analysis with FACS Calibur
utilizing CellQuest software (Becton-Dickinson, San Jose, CA, USA)
(17).
Scratch wound healing assay
GBM 8401 cells (1×105 cells/well) were
placed for 24 h in 6-well plates, and a wound at confluence was
made with a pipette tip followed by washing with serum-free medium
to remove cell debris. The cells were photographed under phase
contrast microscopy (time=0) and then incubated in media with PEITC
(0, 2 and 4 μM), or with TMZ (500 μM) at 37°C in 5%
CO2 and allowed to migrate into the wound area for up to
48 h. Cells were gently washed with phosphate-buffered saline
(PBS). Images of the scratch wounds were quantified by ImageJ
software. The migration inhibition rate = (original scratch width -
new scratch width)/original scratch width × 100% (18).
Migration assay
GBM 8401 cells were cultured in serum-free RPMI-1640
medium containing 1% charcoal-stripped FBS for 48 h. The lower
chamber of the Transwell filter was coated with 10 μg type
IV collagen, and the lower chamber of each well was filled with
RPMI-1640 supplemented with 1% charcoal-stripped FBS. The filter in
the 6.5-mm Transwell was inserted in the 24-well plates, and the
GBM 8401 cells (~3.2×104 cells/filter) were placed on
the filter. The cells were treated with 0, 2 and 4 μM PEITC
and 500 μM TMZ for 48 h. Migrated cells were stained with 2%
crystal violet and were then examined and photographed under a
microscope (16,19).
Invasion assay
The same protocol was carried out as described in
the migration assay except that cells were placed on a
Matrigel-coated Transwell filter (Matrigel invasion chamber; BD
Biosciences) and were then examined and photographed under a
microscope (16,19).
Gelatin zymography assay
GBM 8401 cells (1.6×105 cells/well) were
plated on 12-well tissue culture plates and incubated with 0, 2 and
4 μM PEITC or 500 μM TMZ for 24 and 48 h. The
conditioned medium was collected and separated by electrophoresis
on 10% SDS-PAGE with 0.2% gelatin (Sigma-Aldrich Corp.). The gels
were soaked in 2.5% Triton X-100 in dH2O twice for a
total of 60 min at 25°C at the end of the electrophoresis, and they
were incubated in substrate buffer (50 mM Tris HCl, 5 mM
CaCl2, 0.02% NaN3 and 1% Triton X-100, pH
8.0) at 37°C for 18 h. Bands related to the enzyme activity of
MMP-2 were visualized by negative staining using 0.2% Coomassie
blue in 50% methanol and 10% acetic acid (20). The bands were evaluated by Image J
software.
Western blot assay
GBM 8401 cells (2.4×106 cells/dish) were
placed in a 10-cm dish, and 0, 2 and 4 μM PEITC or 500
μM TMZ were added to the cells. The cells were incubated for
48 h. The cells were collected and lysed in lysate buffer composed
of 50 μM Tris (pH 8.0), 150 μM NaCl, 5 μM
ethylenediaminetetraacetic acid and 0.5% NP-40 with protease
inhibitor solution (Roche, Mannheim, Germany). The protein
concentration from each treatment was determined using the Bio-Rad
protein assay kit. Approximately 30 μg of protein from each
sample was separated on a 10% sodium dodecyl sulfate-polyacrylamide
electrophoretic gel (SDS-PAGE) and transferred to nitrocellulose
membranes (GE Healthcare, Piscataway NJ, USA). The blot was soaked
with blocking buffer, 5% non-fat dry milk in Tris-buffered saline
containing Tween-20 (TBS-T) for 1 h at 25°C. They were incubated
with the specific primary antibodies for matrix metalloproteinase
(MMP)-2, MMP-9, Ras, urokinase-type plasminogen activator (uPA),
Ras homolog gene family, member A (RhoA), growth factor
receptor-bound protein 2 (GRB2), p-p38, phospho-Jun NH2-terminal
kinase (p-JNK), p-extracellular-signal-regulated kinases (p-ERK),
p65, Son of sevenless homolog 1 (SOS1), rho-associated
coiled-coil-containing protein kinase 1 (Rock1) and MMP-13 (Santa
Cruz Biotechnology, Santa Cruz, CA, USA) in blocking buffer at 4°C
overnight. Immunoreactive proteins were detected with horseradish
peroxidase-conjugated secondary antibodies and detected by
chemiluminescence (GE Healthcare) and autoradiography using BioMax
LightFilm (Eastman Kodak, New Heaven, CT, USA) (21). The relative protein amounts from
each treatment were assessed by densitometry scanning of the X-ray
film, and analyzed by Eagle Eye Image system (Stratagene, La Jolla,
CA, USA).
Real-time polymerase chain reaction
(RT-PCR)
GBM 8401 cells (2.4×106 cells/dish) on
10-cm dish were treated with 0, 2 and 4 μM PEITC, or 500
μM TMZ, and incubated for 24 and 48 h. The cells from each
sample were collected, and the total RNA was extracted using the
Qiagen RNeasy Mini kit as previously described (16,22).
According to the standard protocol of the supplier (Applied
Biosystems), all RNA samples were reverse-transcribed for 30 min at
42°C with High Capacity cDNA reverse transcription kit.
Quantitative PCR conditions were: 2 min at 50°C, 10 min at 95°C,
and 40 cycles of 15 sec at 95°C, 1 min at 60°C using 1 μl of
the cDNA reverse-transcribed as described above, 2X SYBR-Green PCR
Master Mix (Applied Biosystems) and 200 nM of the forward and
reverse primers as shown in Table
I. Each assay was processed using the Applied Biosystems 7300
Real-Time PCR system in triplicate, and fold-changes in expression
were measured using the comparative CT method. The ratios of gene
expression to that of GAPDH are presented.
 | Table IPrimer sequence used for real-time
PCR. |
Table I
Primer sequence used for real-time
PCR.
| Primer name | Primer
sequence |
|---|
| MMP-2 | F:
CCCCAGACAGGTGATCTTGAC |
| R:
GCTTGCGAGGGAAGAAGTTG |
| MMP-7 | F:
GGATGGTAGCAGTCTAGGGATTAACT |
| R:
AGGTTGGATACATCACTGCATTAGG |
| MMP-9 | F:
CGCTGGGCTTAGATCATTCC |
| R:
AGGTTGGATACATCACTGCATTAGG |
| RhoA | F:
TCAAGCCGGAGGTCAACAAC |
| R:
ACGAGCTGCCCATAGCAGAA |
| GAPDH | F:
ACACCCACTCCTCCACCTTT |
| R:
TAGCCAAATTCGTTGTCATAC |
Statistical analysis
Results are expressed as mean ± SD of 3 experiments.
Differences between the PEITC-treated (experimental group) or the
TMZ-treated (positive control group), and the vehicle control group
were evaluated using the Student's t-test. A P-value <0.05 was
considered to indicate a statistically significant difference.
P-values are indicated in the figure legends
Results
Effect of PEITC on cell morphological
changes and the viability of GBM 8401 cells
GBM 8401 cells were treated with 0, 0.5, 1, 2 and 4
μM PEITC or 500 μM TMZ for 24 and 48 h to determine
the cytotoxic effects of PEITC. No marked morphological change in
the GBM 8401 cells was induced by PEITC (Fig. 1A). Total percentages of viable cells
were measured by flow cytometric assay. PEITC or TMZ did not
decrease the percentage of viable GBM 8401 cells in a dose- and
time-dependent manner (Fig. 1B).
The total number of viable cells was not significantly decreased in
the GBM 8401 cells following exposure to concentrations as high as
4 μM PEITC or 500 μM TMZ after a 24- and 48-h
treatment. Consequently, concentrations of ≤4 μM PEITC or
500 μM TMZ were selected for use in subsequent
experiments.
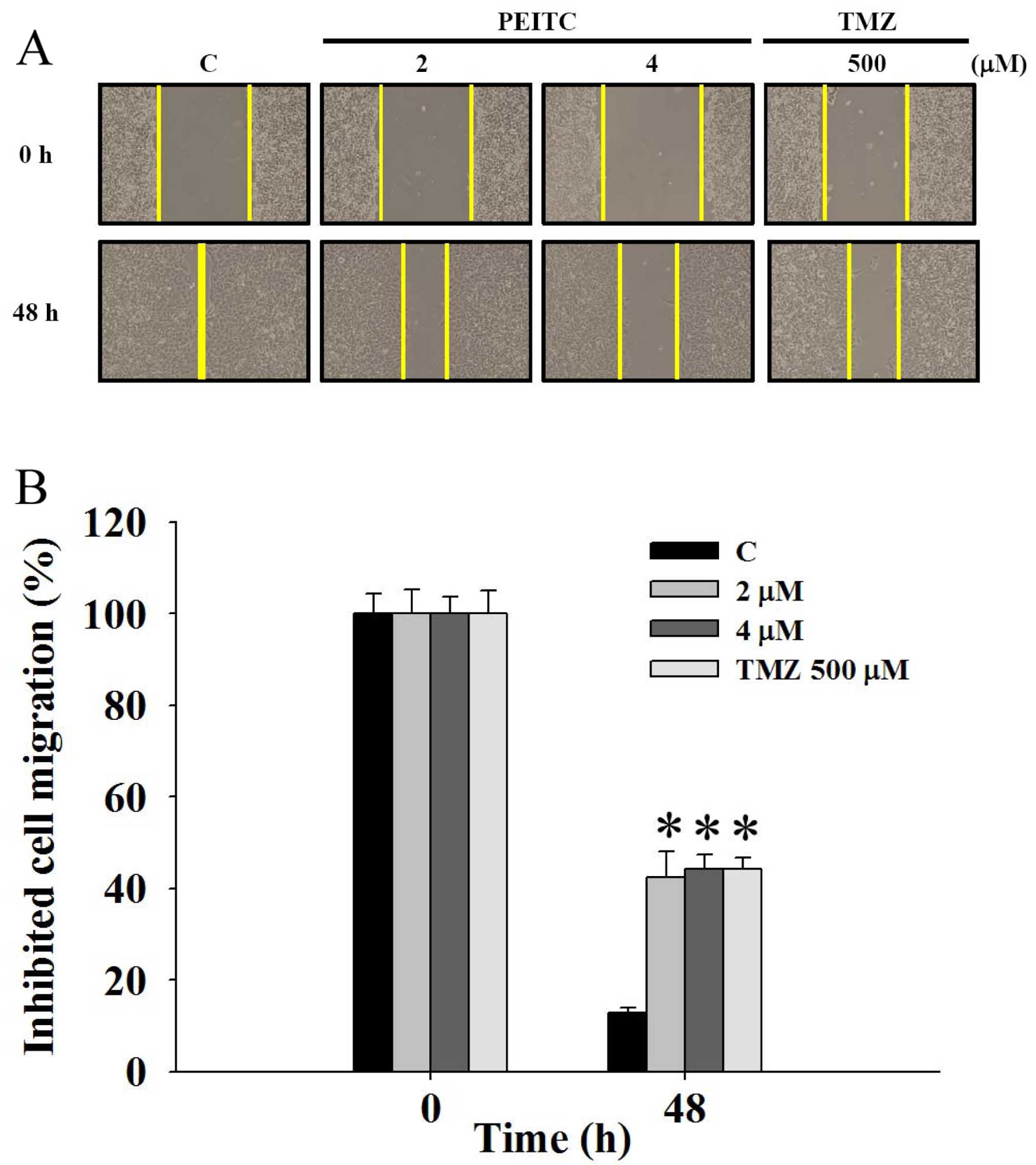
PEITC inhibits the migration of GBM 8401
cells
GBM 8401 cells were incubated with different
concentrations of PEITC and 500 μM TMZ for 48 h to determine
the effects of PEITC on cell migration. The scratch wound healing
assay was performed, and the results are shown in Fig. 2. An apparent and gradual increase in
cells in the wounded zone at different concentrations of PEITC was
observed with light microscopy. The migration inhibition rates were
12.7, 42.4, 44.3 and 44.2% after cells were treated with 0, 2 and 4
μM PEITC and 500 μM TMZ for 48 h, respectively
(Fig. 2B). The effects of PEITC on
the migration of GBM 8401 cells as determined from the scratch
wound healing assay were dose-dependent.
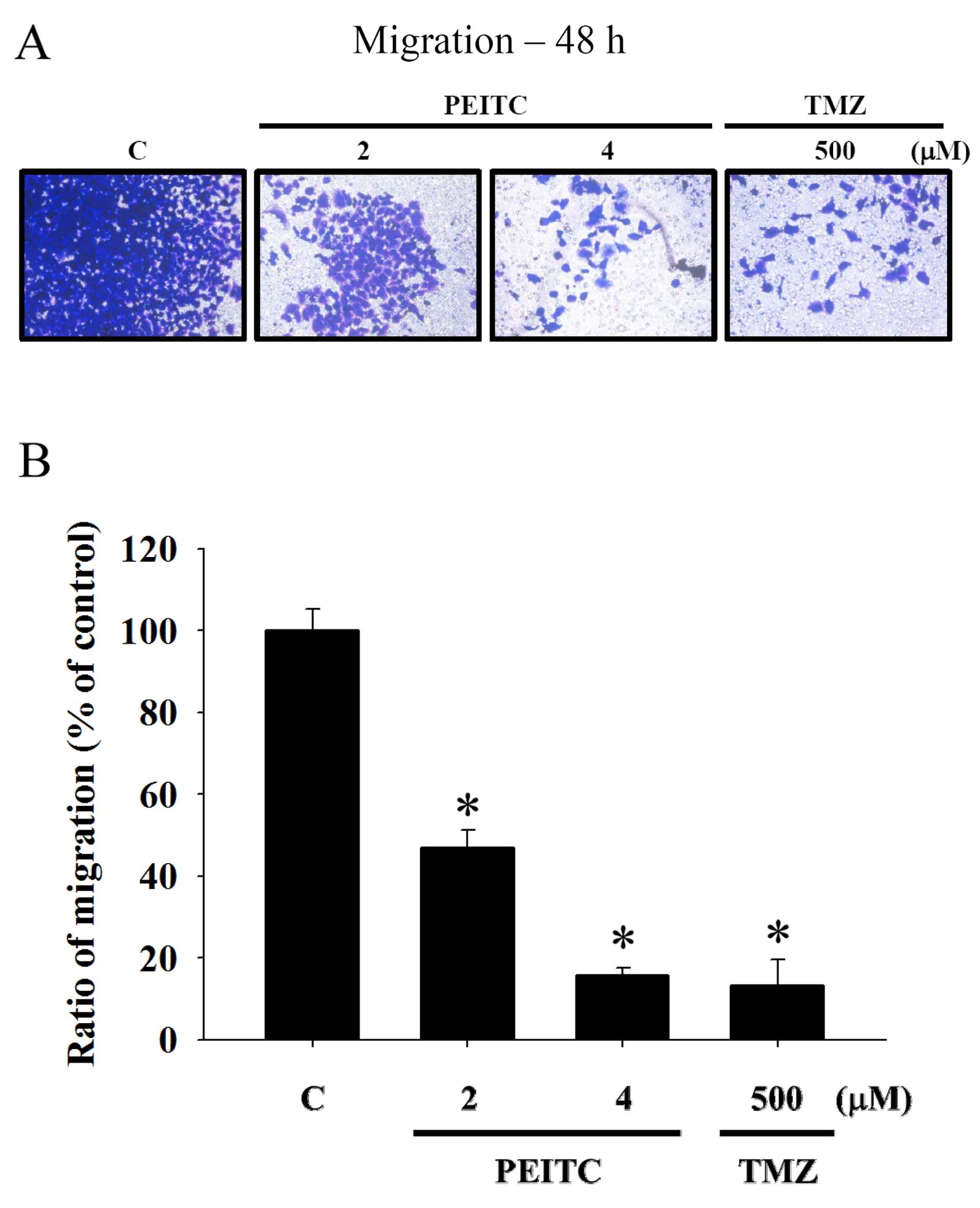
Results from the Transwell migration assay indicated
that PEITC significantly inhibited the migration of GBM 8401 cells
at concentrations between 2 and 4 μM (Fig. 3), and the percentage of inhibition
ranged from 46.89 to 15.75% when cells were incubated with PEITC
for 48 h (Fig. 3B). These effects
of PEITC on the migration of GBM 8401 cells as determined by the
Transwell migration assay were also dose-dependent. TMZ also had an
inhibitory effect on GBM 8401 cell migration at the concentration
of 500 μM.
PEITC inhibits the invasion of GBM 8401
cells
GBM 8401 cells were able to invade through a filter
coated with Matrigel from the upper to the lower chamber in the
control (Fig. 4), while penetration
of the filter by GBM 8401 cells was inhibited by PEITC at
concentrations between 2 and 4 μM. The percentage of
inhibition ranged from 27.80 to 7.31% after a 48-h treatment
(Fig. 4B). The effects of PEITC on
invasion were in a dose-dependent manner. The invasion of GBM 8401
cells was also inhibited by 500 μM TMZ.
PEITC decreases the enzyme activity of
MMP-2 in GBM 8401 cells
Gelatin zymography assay indicated that the enzyme
activity of MMP-2 was reduced in a dose-dependent manner after GBM
8401 cells were treated with 2 and 4 μM PEITC for 24 and 48
h (Fig. 5). The enzyme activity of
MMP-2 were also decreased after cells were treated with 500
μM TMZ for 24 and 48 h (Fig.
5).
PEITC inhibits the levels of proteins
associated with migration and invasion in GBM 8401 cells
Western blot assay was applied to determine the
effects of PEITC and TMZ on the levels of proteins associated with
the migration and invasion of GBM 8401 cells. PEITC decreased the
protein levels of Ras, uPA, RhoA, GRB2 (Fig. 6A), p-p38, p-JNK, p-ERK, p65
(Fig. 6B), SOS1, MMP-2, MM-9 and
MMP-13 (Fig. 6C) in a
dose-dependent manner after cells were treated with 2 and 4
μM PEITC for 48 h. TMZ reduced the protein levels of Ras,
uPA, RhoA, GRB2, p-p38, p-JNK, p-ERK, p65, SOS1, MMP-2, MMP-9 and
MMP-13 (Fig. 6).
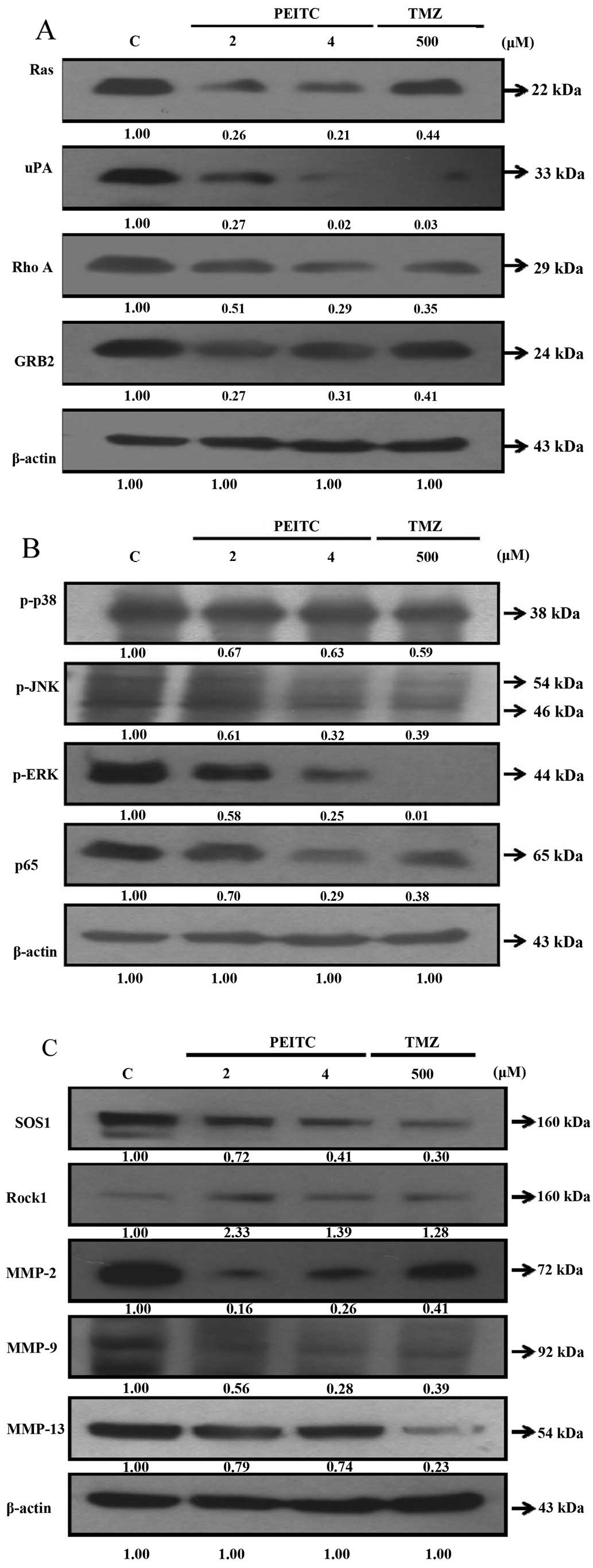 | Figure 6Effects of PEITC on the levels of
proteins associated with migration and invasion in GBM 8401 cells.
Cells were treated with 0, 2 and 4 μM PEITC or 500 μM
TMZ for 48 h. The proteins levels from each sample were determined
by SDS-PAGE and western blotting. (A) Ras, uPA, Rho A, GRB2; (B)
p-p38, p-JNK, p-ERK, p65 and (C) SOS1, Rock1, MMP-2, MMP-9 and
MMP-13. |
PEITC inhibits mRNA expression levels in
GBM 8401 cells
To investigate the effects of PEITC on the
expression of migration- and invasion-associated genes in GBM 8401
cells, the cells were treated with 2 and 4 μM PEITC for 24
and 48 h. Real-time PCR analyses were applied to assess the mRNA
expression levels of these genes. PEITC inhibited the mRNA levels
of MMP-2 (Fig. 7A), MMP-7 (Fig. 7B), MMP-9 (Fig. 7C) and RhoA (Fig. 7D) in a dose- and time-dependent
manner.
Discussion
Several studies have investigated the effects of
PEITC on human glioma cells (23,24).
In our previous study, PEITC was found to induce the apoptosis of
human brain glioblastoma cells (13). In the present study, the cell
morphology of GBM 8401 cells was not significantly altered
(Fig. 1A), and the cell viability
was not significantly decreased following exposure to PEITC at a
concentration as high as 4 μM, or 500 μM TMZ after a
24- and 48-h treatment (Fig. 1B).
Thus, the concentrations of PEITC and TMZ for cell migration and
invasion studies were determined. Based on the Transwell migration
assay, PEITC significantly inhibited the migration of GBM 8401
cells at concentrations between 2 and 4 μM in a
dose-dependent manner (Fig. 3), and
the percentage of inhibition ranged from 46.89 to 15.75% when cells
were incubated with PEITC for 48 h (Fig. 3B). Based on the invasion assay,
PEITC also significantly inhibited the invasion of GBM 8401 cells
at concentrations between 2 and 4 μM in a dose-dependent
manner (Fig. 4), and the percentage
of inhibition ranged from 27.80 to 7.31% after a 48-h treatment
(Fig. 4B). The current standard
chemotherapy, TMZ, also had inhibitory effects on the migration and
invasion of GBM 8401 cells at the concentration of 500
μM.
MMPs, a family of zinc-dependent endopeptidases,
play roles in brain development, synaptic plasticity and repair
after injury to the pathogenesis of various brain disorders
(25). MMP-mediated extracellular
matrix (ECM) degradation promotes tumor invasion, progression and
is involved in angiogenesis and metastasis. MMPs are able to
degrade almost all known ECM components and play important roles in
mediating glioblastoma tumor cell invasion (26). The levels of MMP-2, MMP-9 and
membrane type 1 (MT1)-MMP expression in gliomas are higher than
those in normal brain tissue. MMP-13 enzymatic activity was found
to be critical to the highly invasive potential of cancer stem
cells of human glioblastoma cell line U251 (27). The levels of MMP-7 expression are
correlated with tumor aggressiveness and poor prognosis in solid
tumors, but they are highly variable in patients with glioblastoma
(27). Cross-talk between the tumor
and the surrounding stroma to regulate MMP-7 exists; the expression
of MMP-7 in human U87 glioma cells is low in culture, but higher
when the cells are implanted within the brain. In the present
study, PEITC reduced the enzyme activity of MMP-2 in a dose- and
time-dependent manner after GBM 8401 cells were treated with 2 and
4 μM PEITC for 24 and 48 h (Fig.
5). PEITC also decreased the protein levels of MMP-2, MMP-9 and
MMP-13 (Fig. 6C) in a
dose-dependent manner after cells were treated with 2 and 4
μM PEITC for 48 h. PEITC inhibited the mRNA levels of MMP-2
(Fig. 7A), MMP-7 (Fig. 7B), MMP-9 (Fig. 7C) in a dose- and time-dependent
manner. Taken together, PEITC may inhibit the migration and
invasion of GBM 8401 cells through reduction in the enzyme activity
of MMP-2, the protein levels of MMP-2, MMP-9 and MMP-13, and the
mRNA levels of MMP-2, MMP-7 and MMP-9.
uPA converts plasminogen to plasmin-activating MMPs,
and GBM cell invasion may be enhanced by uPA-mediated direct
activation of MMP-9 (28). The
enhanced invasive capacity of peritumoral cells in GBM requires
simultaneous Rac and RhoA activation (29). Knockdown of GRB2, mediating receptor
tyrosine kinase-induced activation of RAS and downstream signaling,
can reduce invasive activity of breast cancer (30). Epidermal growth factor receptor
(EGFR) vIII-mediated migration and transformation of U87MG
(PTEN-mutant) glioblastoma cells was found to be downregulated by
the effects of signal regulatory protein α1 (SIRPα1) on the
activation loop of SHP-2/FAK/GRB2/SOS-1/MAPK (31). Knockdown of RhoA inhibited the
expression of p-JNK and phospho-c-Jun (p-c-Jun), reduced MMP-2
activity and cell invasion in human glioma U251 cells under hypoxic
conditions (32). The
ROCK-dependent signaling pathway is involved in glioma migration,
and antidromic effects on glioma migration are executed by
selective knockdown of either ROCK1 or ROCK2 (33). ROCK1 knockdown inhibits cell
proliferation, while ROCK2 knockdown promotes it. In the present
study, PEITC inhibited the protein levels of Ras, uPA, RhoA, GRB2
(Fig. 6A), p-p38, p-JNK, p-ERK, p65
(Fig. 6B) and SOS1 (Fig. 6C) in a dose-dependent manner after
cells were treated with 2 and 4 μM PEITC for 48 h. PEITC
decreased the mRNA levels of RhoA (Fig.
7D) in a dose- and time-dependent manner. Taken together, PEITC
may inhibit the migration and invasion of GBM 8401 cells through
reduction in the protein levels of Ras, uPA, RhoA, GRB2, p-p38,
p-JNK, p-ERK, p65, SOS1, Rock1 and the mRNA levels of RhoA.
In conclusion, our experiments indicated that PEITC
has potent anticancer activities through the inhibition of the
migration and invasion of GBM 8401 cells. PEITC decreased the
expression levels of MMP-2, MMP-7, MMP-9, MMP-13, Ras, uPA, RhoA,
GRB2, p-p38, p-JNK, p-ERK, p65 and SOS1 in GBM 8401 cells in
vitro (Fig. 8). PEITC may have
therapeutic potential, and our findings have elucidated the
possible molecular mechanisms and signaling pathways of the
anticancer properties of PEITC in regards to human brain
glioblastoma cells.
Acknowledgments
The present study was supported by grant
TCVGH-1044903B from the Taichung Veterans General Hospital,
Taichung, Taiwan.
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, et al
European Organisation for Research and Treatment of Cancer Brain
Tumor and Radiotherapy Groups; National Cancer Institute of Canada
Clinical Trials Group: Radiotherapy plus concomitant and adjuvant
temozolomide for glioblastoma. N Engl J Med. 352:987–996. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lun M, Lok E, Gautam S, Wu E and Wong ET:
The natural history of extracranial metastasis from glioblastoma
multiforme. J Neurooncol. 105:261–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang LJ, Zhou CF and Lin ZX: Temozolomide
and radiotherapy for newly diagnosed glioblastoma multiforme: A
systematic review. Cancer Invest. 32:31–36. 2014. View Article : Google Scholar
|
|
4
|
Chaichana KL, Jusue-Torres I,
Navarro-Ramirez R, Raza SM, Pascual-Gallego M, Ibrahim A,
Hernandez-Hermann M, Gomez L, Ye X, Weingart JD, et al:
Establishing percent resection and residual volume thresholds
affecting survival and recurrence for patients with newly diagnosed
intracranial glioblastoma. Neurooncol. 16:113–122. 2014.
|
|
5
|
Moon YJ, Brazeau DA and Morris ME: Dietary
phenethyl isothiocyanate alters gene expression in human breast
cancer cells. Evid Based Complement Alternat Med. 2011(462525)2011,
http://dx.doi.org/10.1155/2011/462525.
|
|
6
|
Antosiewicz J, Ziolkowski W, Kar S,
Powolny AA and Singh SV: Role of reactive oxygen intermediates in
cellular responses to dietary cancer chemopreventive agents. Planta
Med. 74:1570–1579. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen YR, Han J, Kori R, Kong AN and Tan
TH: Phenylethyl isothiocyanate induces apoptotic signaling via
suppressing phosphatase activity against c-Jun N-terminal kinase. J
Biol Chem. 277:39334–39342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu R, Kim BR, Chen C, Hebbar V and Kong
AN: The roles of JNK and apoptotic signaling pathways in
PEITC-mediated responses in human HT-29 colon adenocarcinoma cells.
Carcinogenesis. 24:1361–1367. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jakubikova J, Bao Y and Sedlak J:
Isothiocyanates induce cell cycle arrest, apoptosis and
mitochondrial potential depolarization in HL-60 and
multidrug-resistant cell lines. Anticancer Res. 25:3375–3386.
2005.PubMed/NCBI
|
|
10
|
Kang L and Wang ZY: Breast cancer cell
growth inhibition by phenethyl isothiocyanate is associated with
down-regulation of oestrogen receptor-alpha36. J Cell Mol Med.
14:1485–1493. 2010. View Article : Google Scholar :
|
|
11
|
Telang U, Brazeau DA and Morris ME:
Comparison of the effects of phenethyl isothiocyanate and
sulforaphane on gene expression in breast cancer and normal mammary
epithelial cells. Exp Biol Med (Maywood). 234:287–295. 2009.
View Article : Google Scholar
|
|
12
|
Tseng E, Scott-Ramsay EA and Morris ME:
Dietary organic isothiocyanates are cytotoxic in human breast
cancer MCF-7 and mammary epithelial MCF-12A cell lines. Exp Biol
Med (Maywood). 229:835–842. 2004.
|
|
13
|
Chou YC, Chang MY, Wang MJ, Harnod T, Hung
CH, Lee HT, Shen CC and Chung JG: PEITC induces apoptosis of human
brain glioblastoma GBM8401 cells through the extrinsic- and
intrinsic -signaling pathways. Neurochem Int. 81:32–40. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gupta P, Adkins C, Lockman P and
Srivastava SK: Metastasis of breast tumor cells to brain is
suppressed by phenethyl isothiocyanate in a novel in vivo
metastasis model. PLoS One. 8:e672782013. View Article : Google Scholar :
|
|
15
|
Lai KC, Hsu SC, Kuo CL, Ip SW, Yang JS,
Hsu YM, Huang HY, Wu SH and Chung JG: Phenethyl isothiocyanate
inhibited tumor migration and invasion via suppressing multiple
signal transduction pathways in human colon cancer HT29 cells. J
Agric Food Chem. 58:11148–11155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin CC, Chen JT, Yang JS, Lu HF, Hsu SC,
Tan TW, Lin YT, Ma YS, Ip SW, Wu JJ, et al: Danthron inhibits the
migration and invasion of human brain glioblastoma multiforme cells
through the inhibition of mRNA expression of focal adhesion kinase,
Rho kinases-1 and metalloproteinase-9. Oncol Rep. 22:1033–1037.
2009.PubMed/NCBI
|
|
17
|
Lu HF, Lai TY, Hsia TC, Tang YJ, Yang JS,
Chiang JH, Lu CC, Liu CM, Wang HL and Chung JG: Danthron induces
DNA damage and inhibits DNA repair gene expressions in GBM 8401
human brain glioblastoma multiforms cells. Neurochem Res.
35:1105–1110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shang HS, Chang JB, Lin JH, Lin JP, Hsu
SC, Liu CM, Liu JY, Wu PP, Lu HF, Au MK, et al: Deguelin inhibits
the migration and invasion of U-2 OS human osteosarcoma cells via
the inhibition of matrix metalloproteinase-2/-9 in vitro.
Molecules. 19:16588–16608. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu KW, Chen JC, Lai TY, Yang JS, Weng SW,
Ma YS, Lu PJ, Weng JR, Chueh FS, Wood WG, et al: Gypenosides
inhibits migration and invasion of human oral cancer SAS cells
through the inhibition of matrix metalloproteinase-2 -9 and
urokinase-plasminogen by ERK1/2 and NF-kappa B signaling pathways.
Hum Exp Toxicol. 30:406–415. 2011. View Article : Google Scholar
|
|
20
|
Liao CL, Lai KC, Huang AC, Yang JS, Lin
JJ, Wu SH, Gibson Wood W, Lin JG and Chung JG: Gallic acid inhibits
migration and invasion in human osteosarcoma U-2 OS cells through
suppressing the matrix metalloproteinase-2/-9, protein kinase B
(PKB) and PKC signaling pathways. Food Chem Toxicol. 50:1734–1740.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ho CC, Huang AC, Yu CS, Lien JC, Wu SH,
Huang YP, Huang HY, Kuo JH, Liao WY, Yang JS, et al: Ellagic acid
induces apoptosis in TSGH8301 human bladder cancer cells through
the endoplasmic reticulum stress- and mitochondria-dependent
signaling pathways. Environ Toxicol. 29:1262–1274. 2014.
|
|
22
|
Lin HJ, Su CC, Lu HF, Yang JS, Hsu SC, Ip
SW, Wu JJ, Li YC, Ho CC, Wu CC, et al: Curcumin blocks migration
and invasion of mouse-rat hybrid retina ganglion cells (N18)
through the inhibition of MMP-2, -9, FAK, RhoA and Rock-1 gene
expression. Oncol Rep. 23:665–670. 2010.PubMed/NCBI
|
|
23
|
Gupta B, Chiang L, Chae K and Lee DH:
Phenethyl isothiocyanate inhibits hypoxia-induced accumulation of
HIF-1α and VEGF expression in human glioma cells. Food Chem.
141:1841–1846. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee DH, Kim DW, Lee HC, Lee JH and Lee TH:
Phenethyl isothiocyanate sensitizes glioma cells to TRAIL-induced
apoptosis. Biochem Biophys Res Commun. 446:815–821. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim YS and Joh TH: Matrix
metalloproteinases, new insights into the understanding of
neurodegenerative disorders. Biomol Ther (Seoul). 20:133–143. 2012.
View Article : Google Scholar
|
|
26
|
Chintala SK, Tonn JC and Rao JS: Matrix
metalloproteinases and their biological function in human gliomas.
Int J Dev Neurosci. 17:495–502. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Inoue A, Takahashi H, Harada H, Kohno S,
Ohue S, Kobayashi K, Yano H, Tanaka J and Ohnishi T: Cancer
stem-like cells of glioblastoma characteristically express MMP-13
and display highly invasive activity. Int J Oncol. 37:1121–1131.
2010.PubMed/NCBI
|
|
28
|
Zhao Y, Lyons CE Jr, Xiao A, Templeton DJ,
Sang QA, Brew K and Hussaini IM: Urokinase directly activates
matrix metallo-proteinases-9: A potential role in glioblastoma
invasion. Biochem Biophys Res Commun. 369:1215–1220. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ruiz-Ontañon P, Orgaz JL, Aldaz B,
Elosegui-Artola A, Martino J, Berciano MT, Montero JA, Grande L,
Nogueira L, Diaz-Moralli S, et al: Cellular plasticity confers
migratory and invasive advantages to a population of
glioblastoma-initiating cells that infiltrate peritumoral tissue.
Stem Cells. 31:1075–1085. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu Y, Zhang H, Lit LC, Grothey A,
Athanasiadou M, Kiritsi M, Lombardo Y, Frampton AE, Green AR, Ellis
IO, et al: The kinase LMTK3 promotes invasion in breast cancer
through GRB2-mediated induction of integrin β1. Sci Signal.
7:ra582014. View Article : Google Scholar
|
|
31
|
Kapoor GS and O'Rourke DM: SIRPalpha1
receptors interfere with the EGFRvIII signalosome to inhibit
glioblastoma cell transformation and migration. Oncogene.
29:4130–4144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tong JJ, Yan Z, Jian R, Tao H, Hui OT and
Jian C: RhoA regulates invasion of glioma cells via the c-Jun
NH2-terminal kinase pathway under hypoxia. Oncol Lett. 4:495–500.
2012.
|
|
33
|
Mertsch S and Thanos S: Opposing signaling
of ROCK1 and ROCK2 determines the switching of substrate
specificity and the mode of migration of glioblastoma cells. Mol
Neurobiol. 49:900–915. 2014. View Article : Google Scholar :
|