Introduction
Glioblastoma (GBM) is the most common primary
malignancy in brain. The Central Brain Tumor Registry of the United
States reports the annual incidence of GBM at ~3.19/100,000 people
with a median survival of 15 months (1). The commonly used strategies for
treatment include surgery, radiation, and chemotherapy. Although
chemotherapy modestly increases survival in patients when surgery
and radiotherapy are unsuccessful, the prognosis of GBM is
primarily poor (2). Cancer
immunotherapy recognized as the fourth antitumor modality has
undergone a period of growth following encouraging data regarding
its clinical efficacy (3).
Immunotherapy approaches are under investigation for GBM, which
target the following aspects: enhancing immune response to tumors,
inhibiting or destroying molecular or cellular immunosuppressive
mediators induced by GBM cells, generating monoclonal antibodies
targeting the special tumor antigen in order to eliminate tumor
cells, and in vitro expanding tumor-specific lymphocytes
naturally arising or manually modified (4). The current overall therapeutic
outcomes of GBM are encouraging.
GBM is frequently associated with epidermal growth
factor receptor (EGFR) overexpression, and EGFR signaling is
important in various types of cancer, including GBM (5). Although noteworthy results of
antibody-based therapy in the treatment of melanoma, renal cell
carcinoma, and hematologic cancers have been observed, the
treatment has not directly benefitted GBM patients (6–8). The
antibody specific for EGFR, Erbitux®, showed little
effect on GBM patients (9). In
early-stage clinical trials, studies have shown promising results
for the use of bispecific antibody targeting CD3 and glioma antigen
(10–13). Of note, CD8+ T-cell
infiltrate is associated with prolonged survival of newly diagnosed
GBM patients (14). Additionally,
almost half of the T cells infiltrating GBM specimens were
CD56+ T cells (15), and
anti-CD3 x anti-GD2 bispecific antibody was able to redirect T-cell
cytolytic activity to a neuroblastoma target (16). The above-mentioned observations
suggest that adoptive immunotherapy for GBM mediated by bispecific
antibodies redirected effector lymphocyte is a promising
treatment.
To assess the efficiency of the antitumor effect of
the bispecific antibody, a reliable sensitive mouse model is
needed. The bioluminescence living imaging system as a novel image
measuring technology, has been used widely due to its high
sensitivity and accuracy in the detection of tumor growth (17). In the present study, we constructed
a U87MG-luc cell line that expressed luciferase stably, and a
linear correlation was identified between bioluminescent signal
intensity and the number of U87MG-luc cells in vitro and
in vivo. Clinically approved anti-CD3 antibody was then
chemically conjugated with Erbitux®. Considering
adjuvant immunotherapy with cytokine-induced killer (CIK) cells can
improve progression-free survival rates (18), combination treatments may further
improve the survival rates. Thus, the anti-CD3 x anti-EGFR
bispecific antibody (EGFRBi-Ab) was used to direct CIK cells to
kill the GBM target. Redirected with EGFRBi-Ab, CIK cells exhibited
enhanced specific cytotoxicity and cytokine production ability. The
efficacy of EGFRBi-Ab-armed CIK cells for the inhibition of
EGFR-positive GBM tumor was also investigated in a SCID-Beige mouse
model.
Materials and methods
Cell lines and vector construction
Human U87MG glioblastoma were obtained from the
American Type Culture Collection (ATCC; Rockville, MD, USA).
Colo205-luc, HT-29-luc, A549-luc, NCIH460-luc, BXPC-3-luc,
MDA-MB231-luc, HeLa-luc and PC-3M-luc cell lines were purchased
from Caliper Life Sciences (Hopkinton, MA, USA). Agents for cell
culture were from Gibco-Life Technologies (Carlsbad, CA, USA).
The 3.7ln-luc2 plasmid (previously constructed by
our laboratory) contained a BirA substrate peptide and c-myc tag
linked to a truncated membrane-anchored human low affinity nerve
growth factor receptor (ΔLNGFR). The 3.7BirA plasmid (also
previously constructed by our laboratory) contained an ER retention
signal and BirA enzyme as a reported gene. BirA substrate peptide
transported to the cell surface was biotinylated by BirA enzyme to
label the target cells (19).
Generation of the U87MG-luc reporter cell
line
The 3.7lnluc2 and 3.7BirA lentiviral transfer
vectors are self-inactivating lentivectors used for the separation
of the target luciferase reported cells. VSV-G pseudotyped
lentiviral vectors stocks were prepared by Lipofectamine 2000
transfection reagent-based transfection of 293T cells with 6
µg of packaging plasmids pLP1, pLP2 and pLP/VSVG
(Invitrogen, Carlsbad, CA, USA), respectively, and 3 µg of
transfer vector, respectively. The supernatant collected was
filtered through 0.45-µm filter and stored at −80°C. U87MG
(1×105) cells seeded in a 6-well tissue culture plate 1
day in advance were infected with the viruses at a MOI of 1 by spin
inoculation in a centrifuge at 1,800 x g for 90 min at 32°C with 8
µg/ml polybrene. Magnetic beads-based cell separation was
followed 72 h after post-infection according to the manufacturer's
instrucitons (19). After infection
and separation, target luciferase-labeled cells were cultured in a
black 96-well plate by limiting dilution. The transduction
efficiency and expressed protein tag were monitored by flow
cytometry. The bioluminescent imaging signals of selected
single-clone U87MG-luc reporter cell lines were measured using the
IVIS lumina system (Caliper Life Sciences) in a 96-well plate in
culture medium with D-luciferin substrate at a final concentration
of 0.15 mg/ml (Bc219-05; Synchem Chemie, Kassel, Germany).
In vitro cell proliferation
The U87MG and U87MG-luc cells were seeded in a
96-well plate and incubated at 37°C overnight. One hundred
microliters of fresh medium containing 10 µl Cell Counting
Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan) was added to
each well and incubated for an additional 1 h. The absorbency of
U87MG and U87MG-luc cells was measured at 450 nm by a 96-well plate
reader (DG5032; Huadong, Nanjing, China) after incubation.
According to the manufacturer's instructions, the proliferation of
U87MG and U87MG-luc cells was assessed by the absorbance
values.
FlowJo cytometric analysis
Anti-CD3-FITC, anti-CD56-APC, anti-human B7-H1-PE
and anti-mouse IgG2a-FITC secondary antibodies were purchased from
eBioscience (San Diego, CA, USA). Anti-human B7-H3-PE was purchased
from R&D System (Minneapolis, MN, USA), anti-human GD2 was
purchased from Millipore (Billerica, MA, USA) and anti-human IgG
Fc-PE secondary antibody was purchased from BioLegend (San Diego,
CA, USA). The cells were assayed with a Guava flow cytometer
EasyCyte (Guava Technologies, Hayward, CA, USA) and the data
analysis was carried out with the FlowJo software version 7.6.1
(Tree Star Inc., Ashland, OR, USA).
Preparation of CIK cells from peripheral
blood lymphocytes (PBMCs)
Peripheral mononuclear blood cells (PBMCs) were
separated by Ficoll density gradient centrifugation. Blood was
obtained from healthy donors as supplied by the Beijing Blood Bank.
CIK cells were expanded 15 days from PBMCs as previously described
(20). Briefly, the CIK cells were
stimulated by combination of IFN-γ, interleukin-1α, interleukin-2
(Peprotech, Rocky Hill, NJ, USA) and anti-CD3 mAb (OKT3;
eBioscience). Fresh medium containing fresh interleukin-2 was added
every 2 or 3 days and the cells were cultured for 15 days prior to
being cryopreserved. The study was performed according to the
protocols approved by the Biomedical Research Ethics Committee of
CAS Key Laboratory of Pathogenic Microbiology and Immunology.
Synthesis of anti-CD3 x anti-EGFR
bispecific antibody (EGFRBi-Ab) and arming of CIK cells
Anti-EGFR (Erbitux®; Merck Serono,
Darmstadt, Germany) or anti-HER2 (Herceptin®; Roche,
Indianapolis, IN, USA) was reacted with sulfo-SMCC and anti-CD3
(OKT3) was reacted with Traut's reagents as previously described
(21,22). Cryopreserved CIK cells were thawed,
and armed with EGFRBi-Ab at a concentration of 50 ng/106
cells at room temperature for 30 min followed by washing the cells
to eliminate unbound antibodies. The combination of OKT3 (50
ng/106 cells) and Erbitux® (50
ng/106 cells) pre-incubated CIK cells were used as
unarmed control CIK cells.
In vitro cytotoxicity assay
Cytotoxicity was measured with a luciferase
quantitative assay (21–23). Target cells were seeded in
triplicate in 96-well microplates at 1×104/well prior to
the addition of EGFRBi-armed, Her2Bi-armed, or unarmed CIK cells at
various effector-to-target (E/T) ratios. Effector and tumor cells
were allowed to interact at 37°C for 18 h. A final concentration of
0.15 mg/ml D-luciferin was added to each well. The IVIS lumina
system was used to measure the bioluminescent image signal.
ELISA assay
U87MG-luc cells were seeded (1×104/well)
in 96-well microplates in triplicate overnight. The medium was
removed, and fresh medium or medium containing EGFRBi-armed CIK or
control CIK cells was added to wells at an E/T of 20:1. The CIK and
target U87MG-luc cells were incubated at 37°C for 18 h. The
supernatants were collected and production of IFN-γ, TNF-α, and
IL-2 was quantified by the human cytokine ELISA kit (eBioscience)
according to the manufacturer's instructions.
In vivo antitumor effect of
EGFR-BiAb-armed CIK cells
Female eight-week-old SCID-Beige mice were purchased
from the Peking University Health Science Center (Beijing, China).
The U87MG-luc cells (5×106) were injected subcutaneously
into the dorsal region of female SCID-Beige mice. For the tumor
growth inhibition studies, on the following day, EGFRBiAb-armed CIK
cells (5×107/mouse) or control CIK cells were injected
intravenously. Tumor growth was monitored on the indicated day by
the IVIS lumina imaging system with Living Image software. The
signal intensity of tumor burdens was expressed as total
photons/second/cm2 (p/s/cm2/sr).
Statistical analysis and
reproducibility
Experiments were repeated at least three times. Data
were analyzed using Graphpad Prism 5 software and were presented as
the means ± SDs. Unpaired Student's t-test (two-tailed) or the
Mann-Whitney test was used for comparison of two groups where
appropriate. One-way analysis of variance (ANOVA) followed by
Dunnett's post hoc were used for multiple comparisons. Pearson's
correlation coefficient (R) was analyzed. P<0.05 was considered
statistically significant. The number with a significant difference
from a control is denoted by an asterisk in the figures.
Results
Establishment of human U87MG-luc
glioblastoma cell line stably expressing luciferase
To establish U87MG-luc cells, 3.7ln-luc2 and 3.7BirA
lentivirus plasmids (Fig. 1A) were
constructed and used to produce pseudotyped lentiviruses. The U87MG
cells infected with the lentivirus were able to express luciferase
protein and process the transmembrane protein on the surface of
cells. After adding the substrate d-biotin, BirA-tag was
biotinylated, attached to streptavidin beads, and positive cells
were subsequently separated (Fig.
1B). A single U87MG-luc clone was obtained by serial limiting
dilutions in 96-well plates and the luciferase expression was
detected and analyzed by the IVIS lumina imaging system with Living
Image software. We seeded a number of U87MG-luc cells in 96-well
plates ranging from 1,562 to 100,000 (1,562, 3,125, 6,250, 12,500,
25,000, 50,000 and 100,000) per well. The results showed that there
was a good correlation between the level of luciferase activity and
the number of U87MG-luc cells (Fig.
1C). The linear correlation between the luciferase
activity-related bioluminescent signal and the number of cells is
shown in Fig. 1C
(R2=0.99). Moreover, the expression of myc-BirA-tag was
detected by FACS staining with anti-myc antibody (Fig. 1D).
To investigate whether U87MG-luc cells could be used
to establish a subcutaneous tumor model in SCID-Beige mice,
5×106 U87MG-luc cells were injected subcutaneously in
the dorsal thigh of female mice (n=6), and the bioluminescent
signal reflecting tumor growth was monitored and analyzed using the
IVIS lumina imaging system with Living Image software at the
indicated days (Fig. 1E). Mean
tumor luminescence was also calculated (Fig. 1F). Tumor size was measured and a
linear correlation between mean luminescence and mean tumor size
was observed (R2=0.99, data not shown).
Luciferase gene has no effect on cell
proliferation and surface molecular expression of U87MG cells
To investigate whether the luciferase gene itself
had a negative effect on U87MG cell growth in vitro,
proliferation assays were performed (Fig. 2A). To measure the proliferation of
cells stably expressing luciferase, the proliferation rates of
U87MG and U87MG-luc cells were detected by a CCK-8 assay as
described in Materials and methods. The results showed that there
was no significant difference in proliferation between the U87MG
and U87MG-luc cells.
We detected the expression of EGFR, Her2, B7-H3,
B7-H1 and GD2, the attractive molecules used as the targets for
glioblastoma, with the specific antibody on the surface of U87MG
and U87MG-luc, respectively. As shown in Fig. 2B, a high expression of EGFR, B7-H3
and GD2 and a low expression of B7-H1 was detected on U87MG and
U87MG-luc cells. By contrast, Her2 was not detected on the cells.
Moreover, the expression level of these molecules was similar on
the surface of U87MG and U87MG-luc cells. Taken together, these
results indicated that the luciferase gene did not affect the
proliferation or target the molecular expression of U87MG
cells.
Therefore in the subsequent experiment, we selected
one of the molecules with a high expression on U87MG-luc and EGFR,
as the target for arming CIK cells.
Preparation and characterization of
EGFRBi-Ab and CIK cells
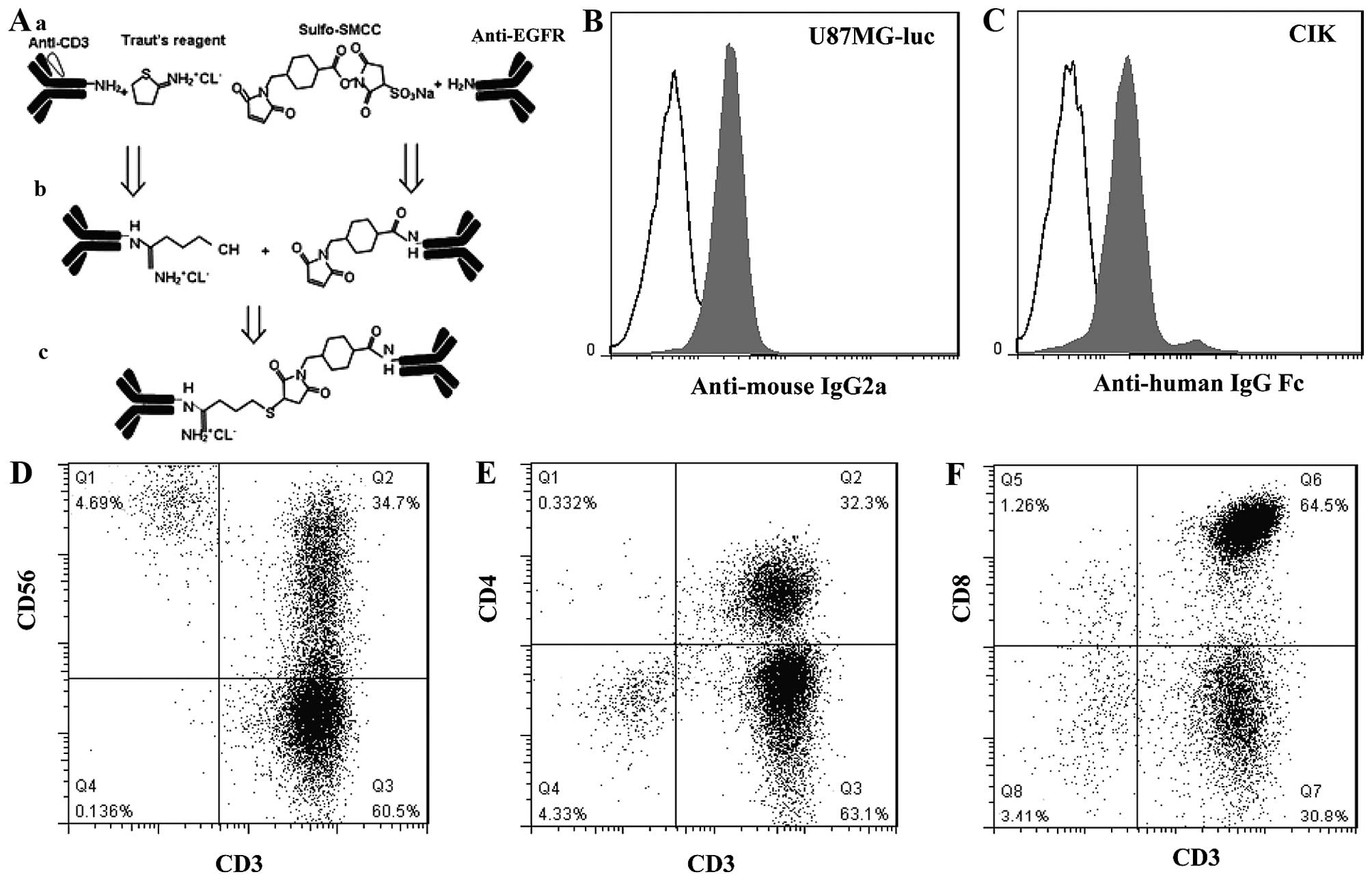
Bispecific antibody EGFRBiAb was prepared as
described in Materials and methods (Fig. 3A). Firstly, the binding specificity
of EGFRBi against EGFR was assessed. U87MG-luc cells were stained
with EGFRBi-Ab or a combination of OKT3 and Erbitux®
(used as the unarmed control for EGFRBi-Ab). Anti-mouse IgG2a-FITC
was then added to detect the CD3 moiety of EGFRBi-Ab. Only
functionally bispecific EGFRBi antibody bound to U87MG-luc cells by
EGFR-recognized Erbitux® and were detected through mouse
origin OKT3 by anti-mouse IgG2a secondary antibody. As shown in
Fig. 3B, positively stained cells
were detected in 87% of the U87MG-luc population with a mean
fluorescent intensity (MFI) of 24. On the other hand, the bound CD3
moiety of EGFRBi-Ab to CIK cells was assessed by staining with
PE-labeled anti-human IgG Fc to detect the EGFR moiety of BiAb.
Positively stained cells were detected in 88% of the CIK cell
population with an MFI of 33 (Fig.
3C).
To produce a sufficient number of effector cells,
PBMCs from buffy coat were stimulated by the combination of IFN-γ,
IL-1α, IL-2 with OKT3 for 15 days as described in Methods and
materials. The CIK cells were then quantitatively analyzed by FACS.
As shown in Fig. 3D-F, the CIK
cells contained almost 96.74±1.49% of CD3+ cells, i.e.,
20.02±9.4% of CD3+CD56+ cells (Fig. 3D), 36.1±8.37% of
CD3+CD4+ (Fig.
3E), and 61.46±7.43% of CD3+CD8+
(Fig. 3F) approximately. For the
CD3− population, the majority of cells were
CD56-positive, 2.73±1.43% (Fig.
3D). Overall, these data suggested that the CIK cells mainly
comprised T cells and NK T cells with a small population of NK
cells.
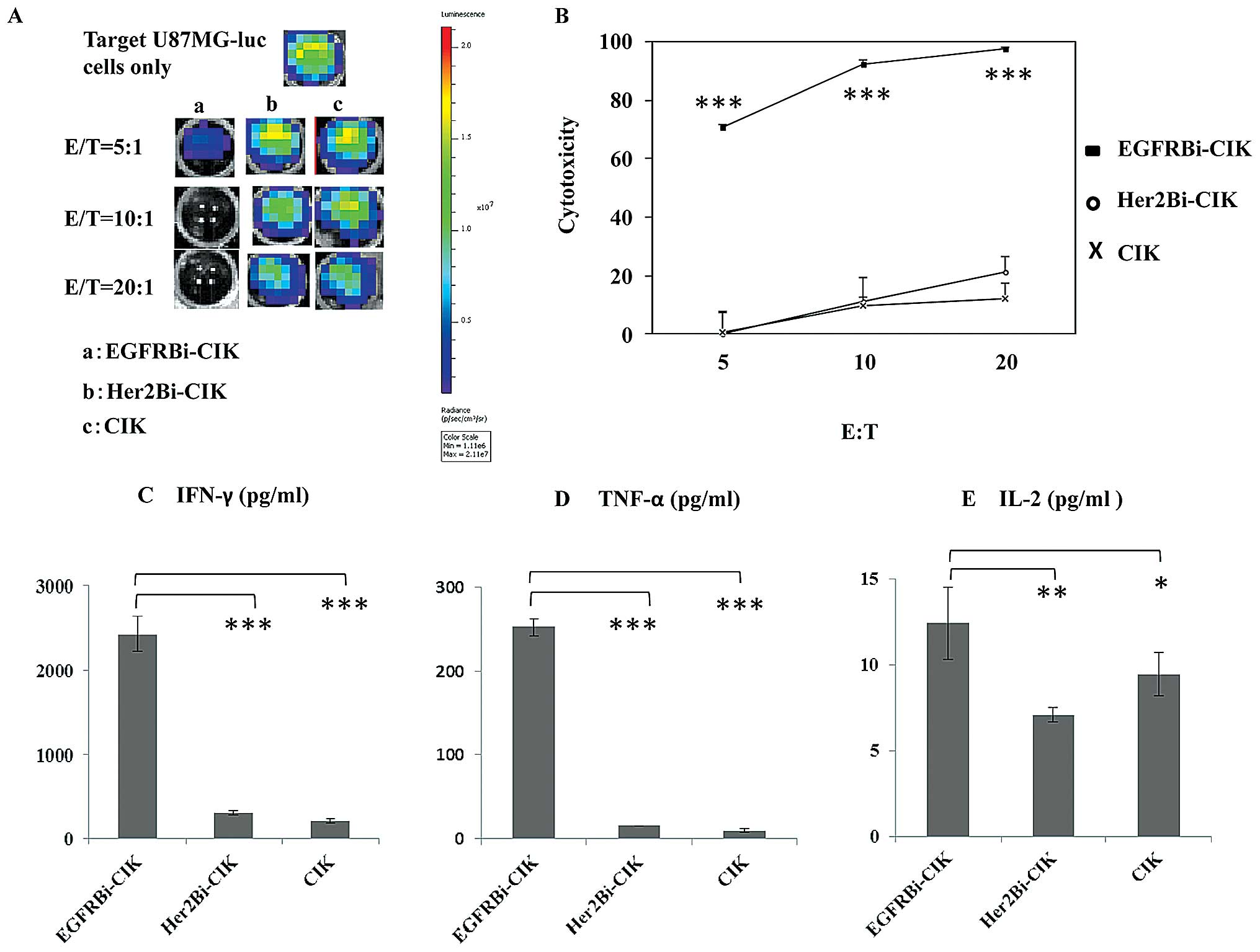
Cytotoxity effects of EGFRBi-armed CIK
cells with IFN-γ TNF-α and IL-2 production on glioblastoma
cells
The amount of EGFRBi-Ab required to arm CIK cells
ranged from 5 to 500 ng/106 cells, with 50 ng and 500
ng/106 cells showing a similar cytotoxicity. Therefore,
we selected 50 ng/106 cells as the concentration of
EGFRBi-Ab for all the subsequent experiments, and CIK cells mixed
with individual OKT3 and Erbitux® were considered
unarmed CIK controls. Cytotoxicity assays were performed at E/T
ratios of 5:1, 10:1 and 20:1 for 18 h. The bioluminescent images
correlated with the number of living U87MG-luc cells (Fig. 4A). After 18-h incubation with
EGFRBi-armed CIK or unarmed CIK or Her2Bi-armed CIK cells,
bioluminescent image signal expressed in photons per second was
converted into a living cell number and the cytotoxicity assays
were calculated at the indicated E/T ratios. As shown in Fig. 4B, an increasing E/T ratio was
correlated directly with the percentage of cytotoxicity in all the
tested CIK effectors. The percentage of cytotoxicity of
EGFRBi-armed CIK cells was significantly greater than that of the
other groups at each E/T ratio.
To analyze the cytokines along with the
cytotoxicity, supernatants of cell cultures were analyzed for
cytokine production at an E/T of 20:1. As shown in Fig. 4C-E, significant increase was
observed for IFN-γ, TNF-α and IL-2 secretion in EGFRBi-armed CIK
cells over their unarmed CIK counterparts or Her2Bi-armed CIK cells
when co-cultured with U87MG-luc cells.
Cytotoxity effects of EGFRBi-armed CIK
cells on different tumor cell lines
We assessed the ability of EGFRBi-armed CIK cells to
respond to a wide range of human EGFR-positive carcinoma, including
colorectal (Colo205-luc and HT-29-luc), pancreatic (BXPC3-luc),
lung (A549-luc and NCIH460-luc), breast (MDA-MB231-luc), cervical
(HeLa-luc), and prostate (PC-3M-luc) cancer. After 18-h incubation
with EGFRBi-armed CIK or unarmed CIK cells (Fig. 5), the percentage of cytotoxicity
with EGFRBi-armed CIK cells was significantly greater than that
with unarmed control effectors at E/T ratios of 5:1 and 10:1 in the
EGFR-positive cancer cells.
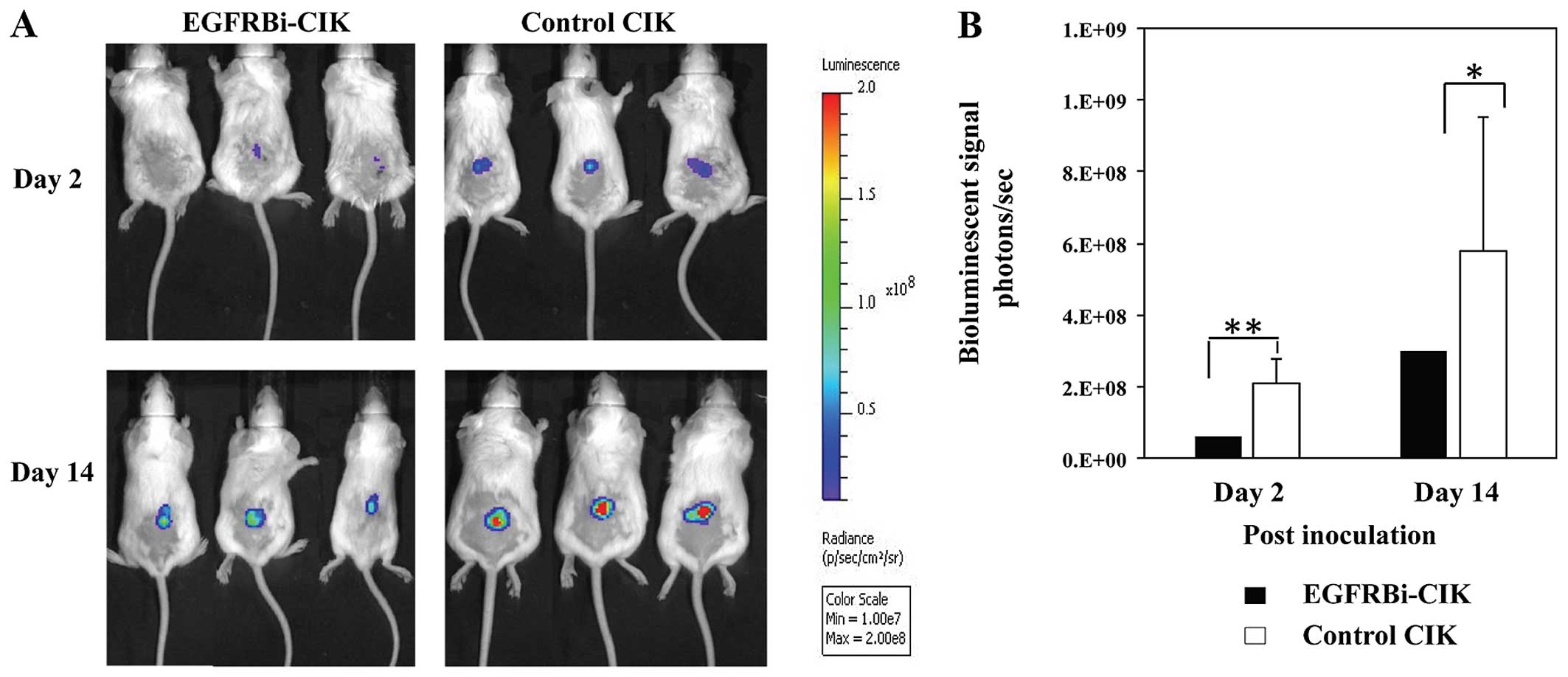
EGFRBi-armed CIK cells inhibit U87MG
tumor growth in SCID-Beige mice
To determine whether EGFRBi-armed CIK cells
suppressed tumor growth in vivo, SCID-Beige mice were
engrafted subcutaneously with U87MG-luc cells. On the following
day, the mice were treated with unarmed control CIK cells or
EGFRBi-CIK cells, respectively. After injection, the mice were
given no further treatment but were monitored with bioluminescent
imaging on the indicated day. This bioluminescent imaging model
allows the monitoring of tumor cell fate as early as the first few
days after inoculation, when tumor formation cannot be detected by
palpation, and three representative mice of each group were shown
(Fig. 6A). The mice treated with
unarmed CIK cells showed stronger luminescence than the
EGFRBi-armed CIK cells. Furthermore, after comparing the mean
luminescence of the two groups, significant differences in
inhibition of tumor growth were observed between them (Fig. 6B). Therefore, compared with the
unarmed control CIK cells, EGFRBi-armed CIK cells inhibited tumor
growth in vivo.
Discussion
Although the therapeutical antibody for EGFR, such
as Erbitux®, significantly improved survival rates in
patients with metastatic colorectal cancer, these results could not
been duplicated in GBM (9).
Intratumor heterogeneity and EGFR pathway redundancy limited the
clinical utility of the antibody-based therapy in GBM (24). Bispecific antibody comprises an
immune effector cell-specific antibody and its hetero-conjugated
mAb specific to a selected tumor-associated antigen (TAA). Such a
bispecific antibody may redirect immune-potent effector cells to
target tumor cells. EGFR is an ideal candidate used as a target in
various types of tumor imaging and antibody-based therapeutic
approaches. Preclinical findings have shown that arming activated T
cells with bispecific EGFR antibody can target EGFR+
cancers (25). In addition,
adjuvant immunotherapy with CIK cells may prevent recurrence, and
improve progression-free survival rates, and the quality of life
(18). The combination of CIK cell
therapy with conventional adjuvant or palliative therapies was
superior to the standard therapy alone, indicating the benefit of
CIK cell therapy for cancer patients (26). In our study, the CIK cells comprised
T cells and NK T cells, as well as a small population of NK
cells.
In the present study, we armed CIK cells with
bispecific Ab and tested whether EGFR is a useful target for GBM.
The results showed that, EGFRBi-armed CIK cells exhibited
significant cytotoxic activity against human GBM U87MG cells in
vitro. We also validated the specific lysis of a wide range of
EGFR-positive human tumor cells, including lung, colorectal,
pancreatic, breast, cervical, and prostate cancer by EGFRBi-Ab
redirected CIK cells in vitro. Additionally, EGFRBi-armed
CIK cells secreted a higher level of IFN-γ, TNF-α, and IL-2 than
unarmed CIK cells. It is conceivable that arming leads to binding
specifically to tumor cells and the triggering of CIK cell
activation and cytokine secretion. The increase in tumoricidal
cytokines suggested that armed CIK cell infusions may vaccinate
patients against their own tumors. Infusion of EGFRBi-armed CIK
cells also markedly inhibited the growth of GBM cells in the
xenograft mouse model.
The in vivo bioluminescence imaging (BLI)
system has developed rapidly in recent years. With the sensitive,
noninvasive, and quantitative system of BLI technology, it is
possible to localize and monitor the orthotopic and metastatic
growth of tumor in vivo (27). Specifically, BLI is widely used in
cancer research and therapy (28).
In the present study, we initially constructed a stable human
U87MG-luc GBM cell line that expressed a high level of luciferase.
As our data has shown, there was a good correlation between the
luciferase activity and the number of cells. Furthermore, similar
to the study by Tiffen et al (29), the luciferase gene did not affect
the surface expression of EGFR, Her2, B7-H3, B7-H1, GD2 and the
proliferation of GBM U87MG cells. We also established a xenograft
subcutaneous human GBM tumor model in SCID-Beige mice with
U87MG-luc cells and supplied a sensitive model for investigation of
the pathogenesis of GBM, and assessment of the efficiency of
immunotherapies.
In addition to adoptive cell therapy, the immune
checkpoint blockade in passive immunotherapy has been successful in
the treatment of various types of cancer, thereby encouraging a
resurgence of interest in GBM (30). A new approach being evaluated in
clinical trials involves the use of monoclonal antibodies to block
immunosuppressive molecules such as PD-1 expressed by T cells. The
monoclonal antibody specific for PD-1 is a promising treatment for
GBM due to its tumor-expressed ligand PD-L1 (B7-H1), for predicting
the efficacy of targeting the PD-1/PD-L1 pathway (31). In our study, administration of
EGFRBi-armed CIK cells suppressed established tumor growth in
vivo, although this did not completely eradicate the tumor
cells. This occurred due to the insufficient persistence of
armed-CIK cells. Function sustaining and trafficking of human CIK
cells in the xenograft tumor model was more difficult than that of
CIK cells in the immune system of the patient. In combination with
anti-PD-1/PD-L1 antibody, anti-GD2 antibody or anti-B7-H3 antibody
may further improve the in vivo efficacy of EGFRBi
antibody-armed CIK cells (31–34).
The combination of EGFRBi antibody- and GD2Bi antibody-armed CIK
cells, and/or B7-H3Bi antibody-armed CIK cells is also a promising
approach.
In summary, to the best of our knowledge, this study
has shown for the first time that EGFRBi-Ab is capable of enhancing
CIK cells ability to kill GBM and other EGFR-positive cancers. In
addition, a sensitive model for evaluating antitumor effect for GBM
has been generated. The in vitro and in vivo
antitumor effect of EGFRBi-armed CIK cells supports their further
clinical use for the treatment of GBM.
Acknowledgments
This present study is funded by the grants from the
Ministry of Science and Technology of China (S&T major program
no. 2012ZX1004701-001-002), the Basic Research Program of China
(973, no. 2013CB531502) and the National Nature Science Foundation
of China (no. 31400754, 31370889, 81273270, 81041110, 81471590 and
81402549).
Abbreviations:
|
ANOVA
|
one-way analysis of variance
|
|
GBM
|
glioblastoma
|
|
CIK
|
cytokine-induced killer
|
|
EGFR
|
epidermal growth factor receptor
|
|
EGFRBi-Ab
|
anti-CD3 x anti-EGFR bispecific
antibody
|
|
PBMCs
|
peripheral mononuclear blood cells
|
|
TAA
|
tumor- associated antigen
|
References
|
1
|
Thakkar JP, Dolecek TA, Horbinski C,
Ostrom QT, Lightner DD, Barnholtz-Sloan JS and Villano JL:
Epidemiologic and molecular prognostic review of glioblastoma.
Cancer Epidemiol Biomarkers Prev. 23:1985–1996. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stewart LA: Chemotherapy in adult
high-grade glioma: A systematic review and meta-analysis of
individual patient data from 12 randomised trials. Lancet.
359:1011–1018. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mellman I, Coukos G and Dranoff G: Cancer
immunotherapy comes of age. Nature. 480:480–489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Agrawal NS, Miller R Jr, Lal R, Mahanti H,
Dixon-Mah YN, DeCandio ML, Vandergrift WA III, Varma AK, Patel SJ,
Banik NL, et al: Current studies of immunotherapy on glioblastoma.
J Neurol Neurosurg. 1:210001042014.PubMed/NCBI
|
|
5
|
Bonavia R, Inda MM, Cavenee WK and Furnari
FB: Heterogeneity maintenance in glioblastoma: A social network.
Cancer Res. 71:4055–4060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gyorki DE, Spillane J, Speakman D,
Shackleton M and Henderson MA: Current management of advanced
melanoma: A transformed landscape. ANZ J Surg. 84:612–617. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Escudier B and Albiges L: Pazopanib for
the treatment of advanced renal cell cancer. Expert Opin Orphan
Drugs. 2:605–616. 2014. View Article : Google Scholar
|
|
8
|
Capietto AH, Keirallah S, Gross E, Dauguet
N, Laprévotte E, Jean C, Gertner-Dardenne J, Bezombes C,
Quillet-Mary A, Poupot M, et al: Emerging concepts for the
treatment of hematological malignancies with therapeutic monoclonal
antibodies. Curr Drug Targets. 11:790–800. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neyns B, Sadones J, Joosens E, Bouttens F,
Verbeke L, Baurain JF, D'Hondt L, Strauven T, Chaskis C, In't Veld
P, et al: Stratified phase II trial of cetuximab in patients with
recurrent high-grade glioma. Ann Oncol. 20:1596–1603. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hishii M, Nitta T, Ebato M, Okumura K and
Sato K: Targeting therapy for glioma by LAK cells coupled with
bispecific antibodies. J Clin Neurosci. 1:261–265. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jacobs SK, Wilson DJ, Melin G, Parham CW,
Holcomb B, Kornblith PL and Grimm EA: Interleukin-2 and lymphokine
activated killer (LAK) cells in the treatment of malignant glioma:
Clinical and experimental studies. Neurol Res. 8:81–87.
1986.PubMed/NCBI
|
|
12
|
Dillman RO, Duma CM, Schiltz PM, DePriest
C, Ellis RA, Okamoto K, Beutel LD, De Leon C and Chico S:
Intracavitary placement of autologous lymphokine-activated killer
(LAK) cells after resection of recurrent glioblastoma. J
Immunother. 27:398–404. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pfosser A, Brandl M, Salih H,
Grosse-Hovest L and Jung G: Role of target antigen in
bispecific-antibody-mediated killing of human glioblastoma cells: A
pre-clinical study. Int J Cancer. 80:612–616. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang I, Tihan T, Han SJ, Wrensch MR,
Wiencke J, Sughrue ME and Parsa AT: CD8+ T-cell
infiltrate in newly diagnosed glioblastoma is associated with
long-term survival. J Clin Neurosci. 17:1381–1385. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Waziri A, Killory B, Ogden AT III, Canoll
P, Anderson RC, Kent SC, Anderson DE and Bruce JN: Preferential in
situ CD4+CD56+ T cell activation and
expansion within human glioblastoma. J Immunol. 180:7673–7680.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yankelevich M, Kondadasula SV, Thakur A,
Buck S, Cheung NK and Lum LG: Anti-CD3 x anti-GD2 bispecific
antibody redirects T-cell cytolytic activity to neuroblastoma
targets. Pediatr Blood Cancer. 59:1198–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Henriquez NV, van Overveld PG, Que I,
Buijs JT, Bachelier R, Kaijzel EL, Löwik CW, Clezardin P and van
der Pluijm G: Advances in optical imaging and novel model systems
for cancer metastasis research. Clin Exp Metastasis. 24:699–705.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hontscha C, Borck Y, Zhou H, Messmer D and
Schmidt-Wolf IG: Clinical trials on CIK cells: First report of the
international registry on CIK cells (IRCC). J Cancer Res Clin
Oncol. 137:305–310. 2011. View Article : Google Scholar
|
|
19
|
Han H, Liu Q, He W, Ong K, Liu X and Gao
B: An efficient vector system to modify cells genetically. PLoS
One. 6:e263802011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume
KG and Weissman IL: Use of a SCID mouse/human lymphoma model to
evaluate cytokine-induced killer cells with potent antitumor cell
activity. J Exp Med. 174:139–149. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han H, Ma J, Zhang K, Li W, Liu C, Zhang
Y, Zhang G, Ma P, Wang L, Zhang G, et al: Bispecific
anti-CD3xanti-HER2 antibody mediates T cell cytolytic activity to
HER2-positive colorectal cancer in vitro and in vivo. Int J Oncol.
45:2446–2454. 2014.PubMed/NCBI
|
|
22
|
Ma J, Han H, Liu D, Li W, Feng H, Xue X,
Wu X, Niu G, Zhang G, Zhao Y, et al: HER2 as a promising target for
cytotoxicity T cells in human melanoma therapy. PLoS One.
8:e732612013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu X, Tao L, Rivera A, Williamson S, Song
XT, Ahmed N and Zhang X: A simple and sensitive method for
measuring tumor-specific T cell cytotoxicity. PLoS One.
5:e118672010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Padfield E, Ellis HP and Kurian KM:
Current therapeutic advances targeting EGFR and EGFRvIII in
glioblastoma. Front Oncol. 5:52015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reusch U, Sundaram M, Davol PA, Olson SD,
Davis JB, Demel K, Nissim J, Rathore R, Liu PY and Lum LG: Anti-CD3
x anti-epidermal growth factor receptor (EGFR) bispecific antibody
redirects T-cell cytolytic activity to EGFR-positive cancers in
vitro and in an animal model. Clin Cancer Res. 12:183–190. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jäkel CE, Vogt A, Gonzalez-Carmona MA and
Schmidt-Wolf IG: Clinical studies applying cytokine-induced killer
cells for the treatment of gastrointestinal tumors. J Immunol Res.
2014:8972142014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Badr CE and Tannous BA: Bioluminescence
imaging: Progress and applications. Trends Biotechnol. 29:624–633.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nogawa M, Yuasa T, Kimura S, Kuroda J,
Sato K, Segawa H, Yokota A and Maekawa T: Monitoring
luciferase-labeled cancer cell growth and metastasis in different
in vivo models. Cancer Lett. 217:243–253. 2005. View Article : Google Scholar
|
|
29
|
Tiffen JC, Bailey CG, Ng C, Rasko JE and
Holst J: Luciferase expression and bioluminescence does not affect
tumor cell growth in vitro or in vivo. Mol Cancer. 9:2992010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patel MA, Kim JE, Ruzevick J, Li G and Lim
M: The future of glioblastoma therapy: Synergism of standard of
care and immunotherapy. Cancers (Basel). 6:1953–1985. 2014.
View Article : Google Scholar
|
|
31
|
Keeren K, Friedrich M, Gebuhr I, Philipp
S, Sabat R, Sterry W, Brandt C, Meisel C, Grütz G, Volk HD, et al:
Expression of tolerance associated gene-1, a mitochondrial protein
inhibiting T cell activation, can be used to predict response to
immune modulating therapies. J Immunol. 183:4077–4087. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Swaika A, Hammond WA and Joseph RW:
Current state of anti-PD-L1 and anti-PD-1 agents in cancer therapy.
Mol Immunol. Mar 4–2015.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Suzuki M and Cheung NK: Disialoganglioside
GD2 as a therapeutic target for human diseases. Expert Opin Ther
Targets. 19:349–362. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou Z, Luther N, Ibrahim GM, Hawkins C,
Vibhakar R, Handler MH and Souweidane MM: B7-H3, a potential
therapeutic target, is expressed in diffuse intrinsic pontine
glioma. J Neurooncol. 111:257–264. 2013. View Article : Google Scholar
|