Introduction
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL) is a transmembrane cytokine that is a promising
anticancer agent in cancer research (1). TRAIL has the ability to selectively
induce apoptosis in a wide range of tumor cells but does not induce
toxicity in most normal cells (2).
TRAIL also engages the extrinsic apoptotic pathway by binding to
the DR4 and DR5 death receptors, which triggers apoptotic signaling
(3). The activation of the death
receptor recruits Fas-associated death domain protein (FADD) and
eventually procaspase-8 to form the death-inducing signaling
complex (DISC), leading to the activation of the caspase cascade
(caspase-8, -9, -10 and -3) (4,5). It
has been reported that many tumor cells, including human lung
adenocarcinoma A549 cells, are resistant to the apoptotic effects
of the TRAIL signaling pathway (6).
Genistein, a major isoflavone compound of soybeans
and soy products, has been shown to have numerous beneficial
effects on diverse cell functions (7,8).
Previous studies indicate that genistein exerts anticancer
properties that include the inhibition of tumor cell proliferation,
enhanced tumor cell differentiation triggering cell cycle arrest,
and the induction of apoptosis in some cell types (9–11).
Genistein promotes the inhibition of protein tyrosine kinase by
competing with ATP for tyrosine kinase domain binding, resulting in
a decrease in cancer cell proliferation due to interruption of the
tyrosine kinase cascade stimulated by mitogens (12,13).
Genistein is also known as an estrogen receptor agonist that can
antagonize the multiplication of breast cancer cells due to
estradiol exposure (14).
Autophagy is a lysosomal-dependent degradation
process induced during starvation, hypoxic conditions, growth
factor deprivation, or endoplasmic reticulum stress in addition to
other stressors (15). However,
autophagy can also incite cell death due to mitophagy, the loss of
mitochondrial membrane potential, caspase cascade activation, and
finally lysosomal membrane permeabilization (16). Autophagic flux is the complete
mechanism of autophagy originating with the fusion of the
autophagosome with a lysosome, resulting in degradation and
recycling of the cargo (17).
Microtubule-associated protein 1 light chain 3 (LC3), which is a
ubiquitin-like protein necessary during proteolytic processing,
yields a 16-kDa LC3-I protein that conjugates with phosphatidyl
ethanolamine to yield a 14-kDa LC3-II form, where LC3-II is used as
a marker of complete autophagosome activation (18–20).
The p62 protein, also known as sequestosome 1 (SQSTM1), is a
ubiquitin-like protein involved in the lysosome-dependent
degradation system that directly interacts with LC3-II and
degradation in the autophagy process, and inhibition of autophagy
leads to increased p62 protein levels (18,19).
The role of autophagy in cancer is like a double-edged sword;
during tumorigenesis or oncogenesis processes it functions as a
mechanism for tumor suppression (21–23)
whereas when a tumor is formed, autophagy provides a cell survival
advantage to resist cell death induced by cancer therapy (24). Recent studies suggest that the
pharmacological or genetic inhibition of autophagy increases the
conventional effects of chemotherapy (25–27),
suggesting that the inhibition of autophagy may be an appropriate
and encouraging strategy for cancer treatment. Antimalarial drugs
such as chloroquine, which act as autophagy inhibitors, prevent
acidification of the lysosome and subsequent autophagosome-lysosome
fusion, inducing apoptosis (28–31).
In the present study, we demonstrated that
inhibition of autophagic flux by genistein enhanced TRAIL-induced
A549 cell death. A549 cells are TRAIL resistant and therefore a
single treatment with either genistein or TRAIL did not influence
cell death; therefore, we examined whether co-treatment of
genistein with TRAIL had a greater effect on A549 lung
adenocarcinoma cells. The enhancing effect of genistein on
TRAIL-induced cell death in A549 lung adenocarcinoma cells was
mediated by inhibition of autophagic flux.
Materials and methods
Cell culture
Cancer cells originating from A549 lung tumors were
obtained from the American Type Culture Collection (Global
Bioresource Center, Manassas, VA, USA). The cells were cultured in
RPMI-1640 (Gibco-BRL, Grand Island, NY, USA) medium supplemented
with 10% (v/v) fetal bovine serum and antibiotics (100 µg/ml
penicillin-streptomycin). The cell cultures were incubated in an
atmosphere containing 5% CO2 at 37°C.
Reagents
Recombinant genistein and chloroquine (20 µM)
were purchased from Sigma-Aldrich (St. Louis, MO, USA). Recombinant
TRAIL (100 ng/ml) was purchased from AbFrontier (Geumcheon-gu,
Seoul, Korea).
Cell viability tests
A549 cells were plated at 1.0×104 cells
in 12-well plates and incubated at 37°C for 24 h. The A549 cells
were pretreated with genistein in a dose-dependent manner (0, 10,
20 and 40 µM). After the 12-h genistein pretreatment,
recombinant TRAIL (100 ng/ml) protein was added and co-incubated
for 2 h. Additional cells were also pretreated with chloroquine (20
µM) for 1 h followed by genistein treatment. Cell morphology
was assessed by images taken under an inverted microscope (Nikon,
Japan), and cell viability was determined using the crystal violet
staining method. The cells were stained with a staining solution
(0.5% crystal violet in 30% ethanol and 3% formaldehyde) for 10 min
at room temperature, washed four times with phosphate-buffered
saline (PBS) and dried. Then, the cells were lysed with a 1% SDS
solution and measured at an absorbance of 550 nm. Cell viability
was calculated from the relative dye intensity compared with a
control.
Trypan blue exclusion assays
The number of viable cells was determined by trypan
blue dye exclusion (Sigma-Aldrich) using microscopy and a
hemocytometer. The results are expressed as a percentage relative
to the vehicle-treated controls.
Western blot assays
A549 cell lysates were prepared by harvesting cells,
washing in cold PBS, and resuspending in a lysis buffer [25 mM
HEPES (pH 7.4), 100 mM EDTA, 5 mM MgCl2, 0.1 mM DTT and
a protease inhibitor mixture] followed by sonication. Proteins (35
µg) were separated on a 10–15% SDS gel and transferred to a
nitrocellulose membrane. After a 1-h incubation with a 1:1,000
primary antibody dilution buffer (1% milk with PBS-Tween), the
membranes were developed by enhanced chemiluminescence using a
secondary antibody. The antibodies used for immunoblotting were LC3
(Novus Biologicals, Littleton, CO, USA), anti-p62 (Millipore,
Milford, MA, USA), cleaved caspase-3 (Cell Signaling Technology,
Inc., Danvers, MA, USA), cleaved caspase-8 (BD Pharmingen, San
Diego, CA, USA) and β-actin (Sigma-Aldrich). Images were examined
using a Fusion-FX7 imaging system (Vilber Lourmat, Marne-la-Vallée,
France).
Immunocytochemistry
The A549 cell line was cultured on glass coverslips
and treated with genistein, chloroquine, and/or TRAIL, washed with
PBS, and fixed with 3–4% paraformal-dehyde in PBS for 15 min at
room temperature. Cells were then washed twice with ice cold PBS,
incubated for 10 min in PBS containing 0.25% Triton X-100, and
washed in PBS an additional three times for 5 min. The cells were
blocked with 1% bovine serum albumin (BSA) in PBST for 30 min, and
then incubated with the primary antibody (anti-p62, diluted in 1%
BSA in PBST) in a humidified chamber for 1 h at room temperature or
overnight at 4°C. After the primary antibody treatment, the
solution was decanted and the cells were washed three times with
PBS for 5 min. The cells were then incubated with the secondary
antibody in 1% BSA for 1 h at room temperature in the dark, which
was followed by decanting the secondary antibody solution and
washing three times with PBS for 5 min. The cells were then
incubated with DAPI for 1 min and rinsed with PBS. Finally, the
cells were mounted with fluorescent mounting medium and visualized
via fluorescence microscopy.
Statistical analysis
The unpaired t-test or Welch's correction was used
for comparison between the two groups. The one-way analysis of
variance (ANOVA) method followed by the Tukey-Kramer test was used
for multiple comparisons. All statistical analyses were performed
using GraphPad Prism software. Results were considered significant
for values p<0.05, p<0.01 and p<0.001.
Results
Genistein enhances TRAIL-induced
apoptosis in A549 cells
To investigate the effects of genistein co-treatment
on TRAIL-induced apoptosis in A549 lung adenocarcinoma cells, the
cells were pretreated with varying genistein concentrations for 12
h and followed by treatment with TRAIL protein for an additional 2
h. We photographed the cells under light microscopy to investigate
cell morphological changes, and cell viability was analyzed using
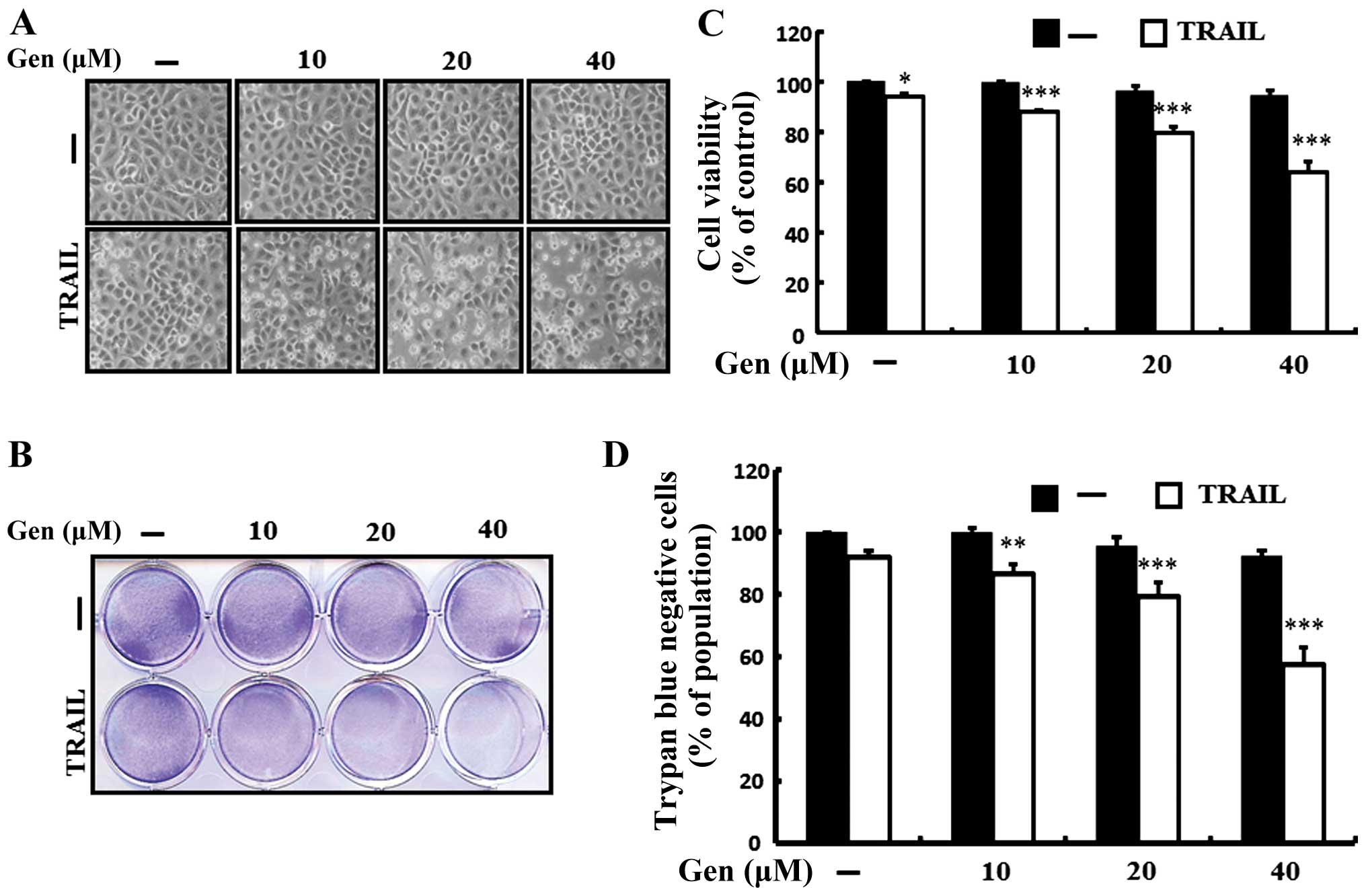
crystal violet and trypan blue exclusion assays. As shown in
Fig. 1, a single treatment of
genistein and TRAIL did not or only slightly influenced cell
viability with no morphological changes compared to the control
A549 cells; however, combination treatment of TRAIL and the
indicated doses of genistein significantly decreased cell viability
compared to genistein and TRAIL treatment alone. Cell morphology
also supported this enhanced effect of genistein, showing that the
combination of TRAIL and genistein treatment enhanced apoptotic
cell death compared with that of genistein or TRAIL treatment alone
(Fig. 1A). However, TRAIL and
genistein co-treatment reduced cell viability and significantly
increased apoptotic cell death in the A549 cells (Fig. 1B–D). These results indicate that
genistein significantly increased TRAIL-induced apoptotic cell
death in the TRAIL-resistant A549 cells.
Genistein inhibits autophagic flux in
lung adenocarcinoma cells
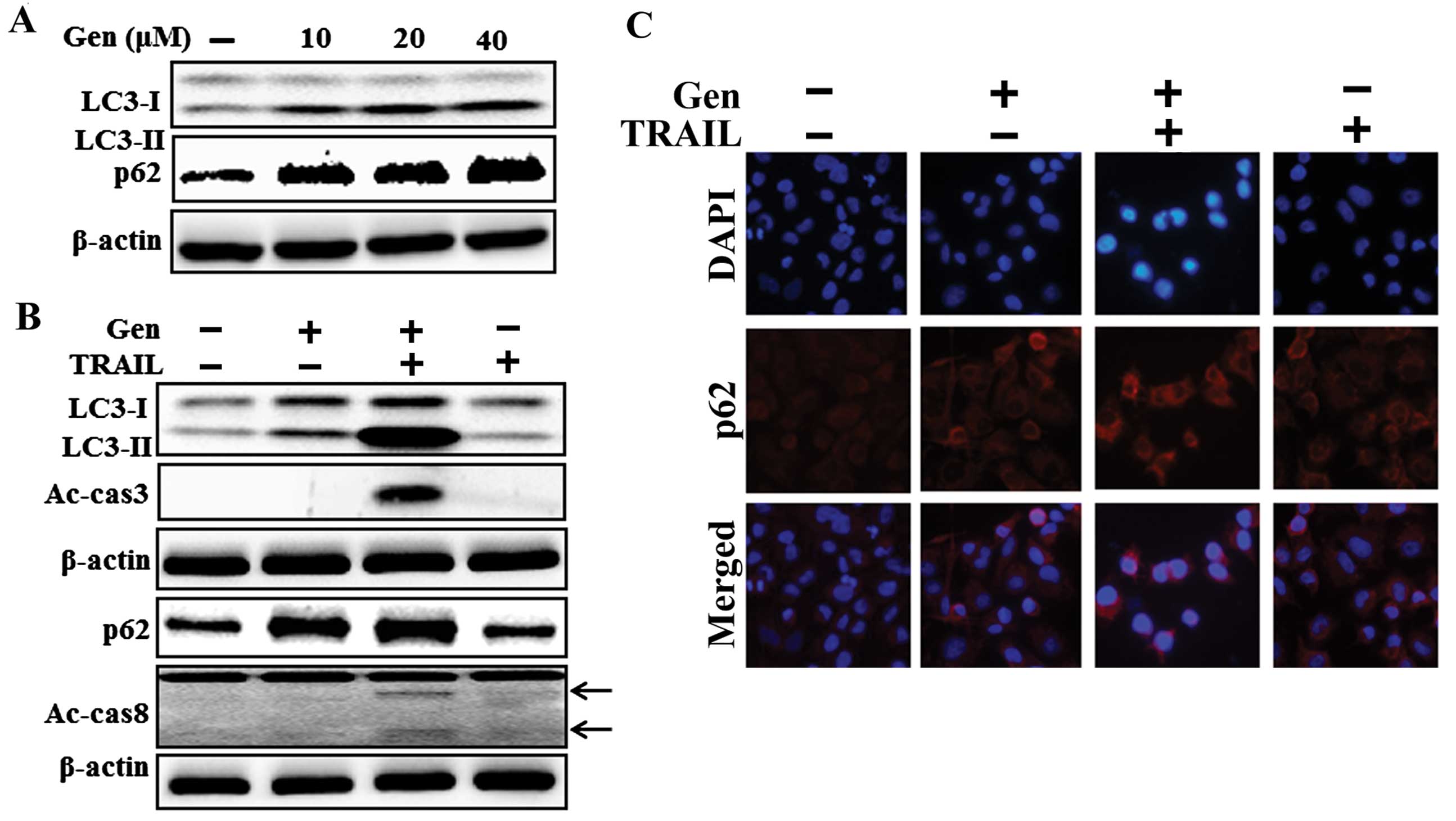
To investigate the effect of genistein on autophagic
flux in A549 cells, the cells were pretreated with varying
genistein concentrations for 12 h followed by treatment with TRAIL
protein for an additional 2 h. Whole cell lysates were subjected to
western blot analysis to determine changes in LC3-II, p62,
activated caspase-3, and activated caspase-8. The production level
of p62 and the conversion of LC3-I to LC3-II were increased after
genistein treatment in a dose-dependent manner (Fig. 2A). The combined TRAIL and genistein
treatment enhanced LC3-II and p62 protein levels compared with
those of genistein or TRAIL treatment alone. These results were
also confirmed by the presence of the intracellular apop-tosis
indicators activated caspase-3 and activated caspase-8 (Fig. 2B). The expression levels of TRAIL
receptors such as DR4 and DR5 were not changed by treatment with
genistein alone or by a combination of genistein and TRAIL in the
A549 cells (data not shown). The immunocytochemistry results showed
that co-treatment of genistein with TRAIL enhanced p62 protein
levels compared to those of genistein or TRAIL treatment alone
(Fig. 2C). These results indicate
that genistein alone or combined treatment with TRAIL and genistein
inhibits autophagy flux in A549 cells.
Genistein enhances TRAIL-induced lung
cancer cell death by inhibition of autophagic flux
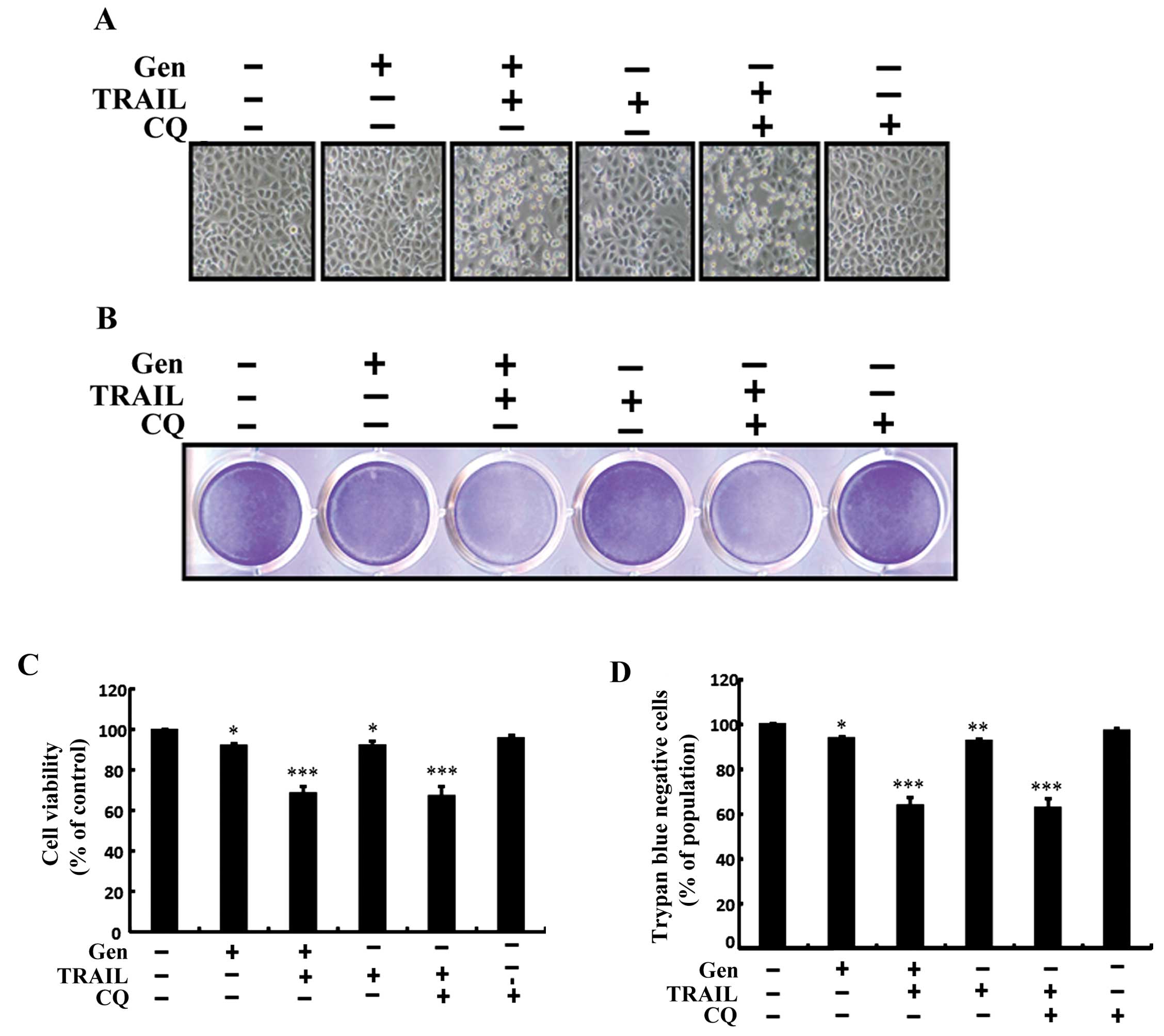
We used chloroquine, which acts as an autophagy
inhibitor by preventing acidification of the lysosome, to
investigate the effect of genistein-mediated enhancement of
TRAIL-induced cell death in A549 lung adenocarcinoma cells. The
cells were pretreated with the indicated genistein doses for 12 h
and then treated with TRAIL protein for an additional 2 h.
Additional cells were also pretreated with chloroquine for 1 h
followed by genistein treatment. We photographed the cells under
light microscopy to investigate morphological changes, and cell
viability was analyzed using crystal violet and trypan blue
exclusion assays. As shown in Fig.
3, treatment of A549 cells with either TRAIL or genistein alone
did not or only slightly influenced cell death and no morphological
changes were identified compared to the control. After combined
treatment with TRAIL and chloroquine, cell death was strongly
enhanced. Cell morphology results also supported this enhanced cell
death effect by TRAIL and chloroquine compared to that of genistein
or TRAIL treatment alone (Fig. 3A).
The combined treatment of chloroquine and TRAIL reduced cell
viability and significantly increased cell death in the A549 lung
cancer cells (Fig. 3B–D). These
results indicate that genistein-mediated enhanced TRAIL-induced
cell death was due to the inhibition of autophagic flux.
Genistein-mediated enhancement of the
TRAIL-induced apoptotic pathway by inhibition of autophagic
flux
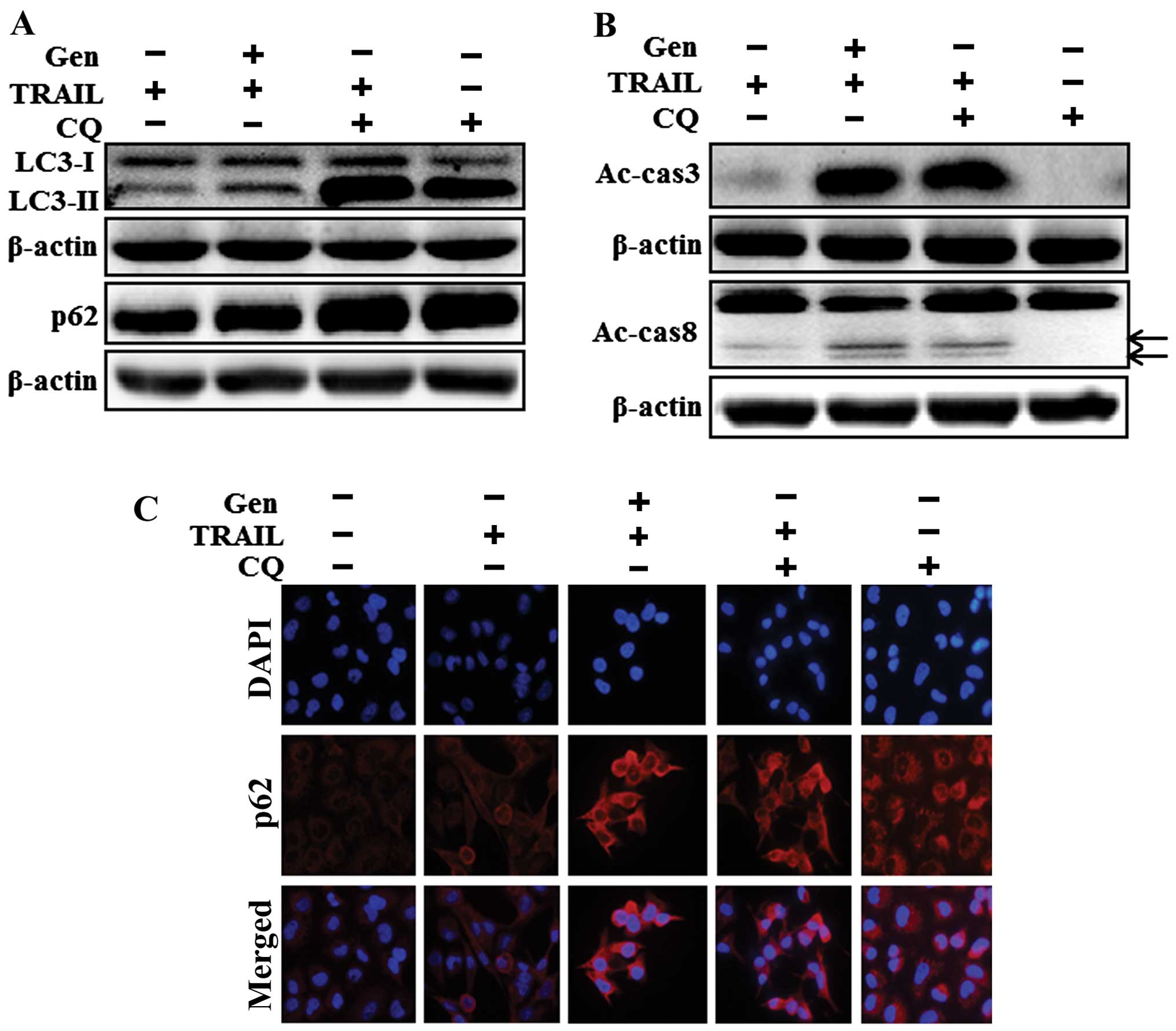
We investigated the effect of genistein-mediated
enhancement of the TRAIL-induced apoptotic pathway by inhibiting
autophagic flux with chloroquine, which acts as an autophagy
inhibitor. The cells were pretreated with the indicated doses of
genistein for 12 h followed by treatment with TRAIL protein for an
additional 2 h. Additional cells were also pretreated with
chloroquine (20 µM) for 1 h followed by genistein treatment.
Whole cell lysates were subjected to western blot analysis to
determine changes in LC3-II, p62, activated caspase-3 and activated
caspase-8 protein levels. The genistein and TRAIL co-treatment, and
the combined treatment of TRAIL and chloroquine, resulted in
increased LC3-II and p62 protein levels relative to that of
chloroquine or TRAIL treatment alone, confirming that genistein
inhibited autophagic flux in the A549 cells (Fig. 4A). Chloroquine and TRAIL
co-treatment inhibited cell viability and significantly enhanced
apoptotic cell death in the A549 cells. These results were
confirmed by the presence of intracellular apoptosis indicator
activated caspase-3 (Fig. 4B) and
there was no clear difference in activated caspase-8 expression
since only TRAIL treatment slightly induced activated caspase-8
expression compared to co-treatment of TRAIL and chloroquine but
was not statistically significant. The immunocytochemistry results
showed that the TRAIL and chloroquine combined treatment increased
p62 levels compared to those of chloroquine or TRAIL treatment
alone (Fig. 4C). These results
indicate that the genistein-enhanced TRAIL-induced apoptotic
pathway was due to inhibition of autophagic flux.
Discussion
The purpose of the present study was to investigate
the function of genistein and co-treatment of genistein and TRAIL
on A549 human adenocarcinoma cells. The results suggest that
genistein enhanced TRAIL-induced tumor cell death in A549 cells by
inhibition of autophagic flux.
TRAIL, also known as Apo2L, may be a safe and potent
biological agent that can be applied for cancer therapy in humans.
It has gained huge interest in medical science as it can
specifically induce cancer cells, transformed cells, and
virus-infected cells to undergo apoptosis without displaying any
toxicity in normal cells (32–36).
However, the efficacy and mode of action for this effective
therapeutic agent are still being investigated. Genistein
(4′,5,7-trihydroxyisoflavone), commonly found in soy products, has
been used as an alternative hormone replacement therapy and has a
lower risk of cancer and neurodegenerative diseases (37). It has been reported that genistein
enhances the induction of apoptosis by chemotherapeutic agents in
several types of cancer cells (38,39).
Autophagic flux is the complete mechanism of autophagy by which
cytoplasmic components are recruited to lysosomes for degradation
(40,41). Anti-rheumatoid arthritis drugs, such
as chloroquine, have been shown to disrupt autophagy in clinical
trials of cancer therapy (42).
Most primary tumor cell lines and a range of
patient-created cancer cell lines ultimately become resistant to
the apoptotic effects of TRAIL (43). Jin et al (6) showed that lung adenocarcinoma A549
cells are resistant to cell death by TRAIL. We also observed in our
present study that a single treatment of genistein or TRAIL alone
did not or only slightly induced cell death in A549 adenocarcinoma
cells. However, the combined treatment of genistein and TRAIL
strongly induced cell death in the A549 cells (Fig. 1). This evidence suggests that
genistein, which acts as an anticancer agent in combination with
TRAIL, can be used to sensitize TRAIL-mediated apoptosis in
TRAIL-resistant A549 cells. Some reports have shown that genistein
induces autophagy and also initiates apoptosis in ovarian cancer
cells (44). However, our results
indicate that treatment with varying concentrations of genistein
alone resulted in a dose-dependent increase in LC3-II and p62
levels in A549 adenocarcinoma cells. The combined TRAIL and
genistein treatment enhanced LC3-II, p62, activated caspase-3, and
activated caspase-8 protein production levels compared to the
control by inhibiting autophagic flux (Fig. 2). Recently, researchers have shown
that co-treatment of genistein and TRAIL inhibited pancreatic
cancer cell growth (45) and
enhanced TRAIL-mediated apoptosis in lung A549 cells through
regulation of the AKT pathway (46). Pharmacological or genetic inhibition
of autophagy induces cell death in various types of cancer cells,
and we investigated the sensitivity of A549 cell proliferation to
pharmacological inhibition of autophagy with chloroquine.
Co-treatment with genistein and TRAIL increased LC3-II, p62,
activated caspase-3 and activated caspase-8 production levels.
Furthermore, TRAIL and chloroquine combined treatment increased
LC3-II and p62 levels compared to those of chloroquine or TRAIL
alone. These results were confirmed by increased levels of the
intracellular apoptosis indicators activated caspase-3 and
activated caspase-8 (Figs. 3 and
4).
In conclusion, we report that genistein enhanced
apoptosis in A549 cells by inhibition of autophagic flux. In
addition, combination treatment with genistein and TRAIL strongly
enhanced apoptosis in TRAIL-resistant A549 cells, suggesting that
genistein enhances TRAIL-induced tumor cell death in
TRAIL-resistant A549 adenocarcinoma cells by inhibition of
autophagic flux.
Acknowledgments
This study was supported by a grant from the
National Research Foundation of Korea (NRF), funded by the Korean
Government (MISP) (no. 2013R1A4A1069486).
References
|
1
|
Ashkenazi A, Pai RC, Fong S, Leung S,
Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert
A, et al: Safety and antitumor activity of recombinant soluble Apo2
ligand. J Clin Invest. 104:155–162. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bellail AC, Qi L, Mulligan P, Chhabra V
and Hao C: TRAIL agonists on clinical trials for cancer therapy:
The promises and the challenges. Rev Recent Clin Trials. 4:34–41.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang S and El-Deiry WS: TRAIL and
apoptosis induction by TNF-family death receptors. Oncogene.
22:8628–8633. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gonzalvez F and Ashkenazi A: New insights
into apoptosis signaling by Apo2L/TRAIL. Oncogene. 29:4752–4765.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnstone RW, Frew AJ and Smyth MJ: The
TRAIL apoptotic pathway in cancer onset, progression and therapy.
Nat Rev Cancer. 8:782–798. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin CY, Park C, Hwang HJ, Kim GY, Choi BT,
Kim WJ and Choi YH: Naringenin up-regulates the expression of death
receptor 5 and enhances TRAIL-induced apoptosis in human lung
cancer A549 cells. Mol Nutr Food Res. 55:300–309. 2011. View Article : Google Scholar
|
|
7
|
Kang JL, Lee HW, Kim HJ, Lee HS,
Castranova V, Lim CM and Koh Y: Inhibition of SRC tyrosine kinases
suppresses activation of nuclear factor-kappaB, and serine and
tyrosine phosphorylation of IkappaB-alpha in
lipopolysaccharide-stimulated raw 264.7 macrophages. J Toxicol
Environ Health A. 68:1643–1662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ravindranath MH, Muthugounder S, Presser N
and Viswanathan S: Anticancer therapeutic potential of soy
isoflavone, genistein. Adv Exp Med Biol. 546:121–165. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi YH, Zhang L, Lee WH and Park KY:
Genistein-induced G2/M arrest is associated with the inhibition of
cyclin B1 and the induction of p21 in human breast carcinoma cells.
Int J Oncol. 13:391–396. 1998.PubMed/NCBI
|
|
10
|
Choi YH, Lee WH, Park KY and Zhang L:
p53-independent induction of p21 (WAF1/CIP1), reduction of cyclin
B1 and G2/M arrest by the isoflavone genistein in human prostate
carcinoma cells. Jpn J Cancer Res. 91:164–173. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sarkar FH, Adsule S, Padhye S, Kulkarni S
and Li Y: The role of genistein and synthetic derivatives of
isoflavone in cancer prevention and therapy. Mini Rev Med Chem.
6:401–407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shao ZM, Wu J, Shen ZZ and Barsky SH:
Genistein exerts multiple suppressive effects on human breast
carcinoma cells. Cancer Res. 58:4851–4857. 1998.PubMed/NCBI
|
|
13
|
Hoffman R: Potent inhibition of breast
cancer cell lines by the isoflavonoid kievitone: Comparison with
genistein. Biochem Biophys Res Commun. 211:600–606. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peterson G and Barnes S: Genistein
inhibits both estrogen and growth factor-stimulated proliferation
of human breast cancer cells. Cell Growth Differ. 7:1345–1351.
1996.PubMed/NCBI
|
|
15
|
Kourtis N and Tavernarakis N: Autophagy
and cell death in model organisms. Cell Death Differ. 16:21–30.
2009. View Article : Google Scholar
|
|
16
|
Kroemer G and Jäättelä M: Lysosomes and
autophagy in cell death control. Nat Rev Cancer. 5:886–897. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mizushima N: Autophagy: Process and
function. Genes Dev. 21:2861–2873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Klionsky DJ, Abeliovich H, Agostinis P,
Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA,
Ballabio A, et al: Guidelines for the use and interpretation of
assays for monitoring autophagy in higher eukaryotes. Autophagy.
4:151–175. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mizushima N and Yoshimori T: How to
interpret LC3 immuno-blotting. Autophagy. 3:542–545. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mizushima N, Yoshimori T and Levine B:
Methods in mammalian autophagy research. Cell. 140:313–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Degenhardt K, Mathew R, Beaudoin B, Bray
K, Anderson D, Chen G, Mukherjee C, Shi Y, Gélinas C, Fan Y, et al:
Autophagy promotes tumor cell survival and restricts necrosis,
inflammation, and tumorigenesis. Cancer Cell. 10:51–64. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karantza-Wadsworth V, Patel S, Kravchuk O,
Chen G, Mathew R, Jin S and White E: Autophagy mitigates metabolic
stress and genome damage in mammary tumorigenesis. Genes Dev.
21:1621–1635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mathew R, Karantza-Wadsworth V and White
E: Role of autophagy in cancer. Nat Rev Cancer. 7:961–967. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen N and Debnath J: Autophagy and
tumorigenesis. FEBS Lett. 584:1427–1435. 2010. View Article : Google Scholar :
|
|
25
|
Apel A, Herr I, Schwarz H, Rodemann HP and
Mayer A: Blocked autophagy sensitizes resistant carcinoma cells to
radiation therapy. Cancer Res. 68:1485–1494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carew JS, Espitia CM, Esquivel JA II,
Mahalingam D, Kelly KR, Reddy G, Giles FJ and Nawrocki ST:
Lucanthone is a novel inhibitor of autophagy that induces cathepsin
D-mediated apoptosis. J Biol Chem. 286:6602–6613. 2011. View Article : Google Scholar :
|
|
27
|
Boya P, González-Polo RA, Casares N,
Perfettini JL, Dessen P, Larochette N, Métivier D, Meley D,
Souquere S, Yoshimori T, et al: Inhibition of macroautophagy
triggers apoptosis. Mol Cell Biol. 25:1025–1040. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Poole B and Ohkuma S: Effect of weak bases
on the intraly-sosomal pH in mouse peritoneal macrophages. J Cell
Biol. 90:665–669. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fan C, Wang W, Zhao B, Zhang S and Miao J:
Chloroquine inhibits cell growth and induces cell death in A549
lung cancer cells. Bioorg Med Chem. 14:3218–3222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang PD, Zhao YL, Deng XQ, Mao YQ, Shi W,
Tang QQ, Li ZG, Zheng YZ, Yang SY and Wei YQ: Antitumor and
antimetastatic activities of chloroquine diphosphate in a murine
model of breast cancer. Biomed Pharmacother. 64:609–614. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoon YH, Cho KS, Hwang JJ, Lee SJ, Choi JA
and Koh JY: Induction of lysosomal dilatation, arrested autophagy,
and cell death by chloroquine in cultured ARPE-19 cells. Invest
Ophthalmol Vis Sci. 51:6030–6037. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Van Geelen CM, de Vries EG and de Jong S:
Lessons from TRAIL-resistance mechanisms in colorectal cancer
cells: Paving the road to patient-tailored therapy. Drug Resist
Updat. 7:345–358. 2004. View Article : Google Scholar
|
|
33
|
Srivastava RK: TRAIL/Apo-2L: Mechanisms
and clinical applications in cancer. Neoplasia. 3:535–546. 2001.
View Article : Google Scholar
|
|
34
|
Shankar S and Srivastava RK: Enhancement
of therapeutic potential of TRAIL by cancer chemotherapy and
irradiation: Mechanisms and clinical implications. Drug Resist
Updat. 7:139–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
LeBlanc H, Lawrence D, Varfolomeev E,
Totpal K, Morlan J, Schow P, Fong S, Schwall R, Sinicropi D and
Ashkenazi A: Tumor-cell resistance to death receptor-induced
apoptosis through mutational inactivation of the proapoptotic Bcl-2
homolog Bax. Nat Med. 8:274–281. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim H, Kim EH, Eom YW, Kim WH, Kwon TK,
Lee SJ and Choi KS: Sulforaphane sensitizes tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL)-resistant hepatoma
cells to TRAIL-induced apoptosis through reactive oxygen
species-mediated up-regulation of DR5. Cancer Res. 66:1740–1750.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gencel VB, Benjamin MM, Bahou SN and
Khalil RA: Vascular effects of phytoestrogens and alternative
menopausal hormone therapy in cardiovascular disease. Mini Rev Med
Chem. 12:149–174. 2012. View Article : Google Scholar :
|
|
38
|
Banerjee S, Li Y, Wang Z and Sarkar FH:
Multi-targeted therapy of cancer by genistein. Cancer Lett.
269:226–242. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sarkar FH and Li Y: Using chemopreventive
agents to enhance the efficacy of cancer therapy. Cancer Res.
66:3347–3350. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Codogno P and Meijer AJ: Autophagy and
signaling: Their role in cell survival and cell death. Cell Death
Differ. 12(Suppl 2): 1509–1518. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Klionsky DJ and Emr SD: Autophagy as a
regulated pathway of cellular degradation. Science. 290:1717–1721.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sotelo J, Briceño E and López-González MA:
Adding chloroquine to conventional treatment for glioblastoma
multiforme: A randomized, double-blind, placebo-controlled trial.
Ann Intern Med. 144:337–343. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Stegehuis JH, de Wilt LH, de Vries EG,
Groen HJ, de Jong S and Kruyt FA: TRAIL receptor targeting
therapies for non-small cell lung cancer: Current status and
perspectives. Drug Resist Updat. 13:2–15. 2010. View Article : Google Scholar
|
|
44
|
Gossner G, Choi M, Tan L, Fogoros S,
Griffith KA, Kuenker M and Liu JR: Genistein-induced apoptosis and
autophagocytosis in ovarian cancer cells. Gynecol Oncol. 105:23–30.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nozawa F, Itami A, Saruc M, Kim M, Standop
J, Picha KS, Cowan KH and Pour PM: The combination of tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL/Apo2L) and
genistein is effective in inhibiting pancreatic cancer growth.
Pancreas. 29:45–52. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Park SY and Seol DW: Regulation of Akt by
EGF-R inhibitors, a possible mechanism of EGF-R inhibitor-enhanced
TRAIL-induced apoptosis. Biochem Biophys Res Commun. 295:515–518.
2002. View Article : Google Scholar : PubMed/NCBI
|