Introduction
Endometrial adenocarcinoma (EC) is a major cause of
morbidity and mortality for women worldwide. According to
epidemiological data it is the fourth most common malignancy among
women in Poland. The mortality ratio resulting from this cancer led
to the twelfth place in terms of the causes of cancer deaths in
Poland (1). The exact molecular
mechanism of the ethiopatology of EC is still a matter of
continuous interest. However, it is known that the most important
risk factors for the development of this kind of cancer are
unopposed estrogen exposure, genetic mutations and obesity
(2). Endometrial cancers have been
assigned, based on histological and molecular pathology
observations, as two major types (3). Most common type I estrogen-dependent
adenocarcinoma with endometrioid morphology (EC1). Type II cancers
include more aggressive histological variants such as clear-cell
and serous carcinomas and uterine carcinosarcomas. The risk of EC1
is reported to be linked with unopposed estrogen exposure and
action (4–6). The above-mentioned hormonal changes
correlated with the increased estrogen receptor (ER) β transcript
abundance leading to increased proliferation of endometrial cells
with an increasing frequency of mutations in the cells (7).
The biological effect of lysophosphatidic acid (LPA)
in the human uterus is mediated through four major, G
protein-coupled transmembrane receptors: LPA receptor (LPAR)1-LPAR4
(8). In the human body, two general
pathways of LPA production have been demonstrated. In each pathway,
at least two major phospholipase activities are required:
phospholipase A2 (PLA2) and phospholipase D [(PLD), also called
autotaxin (ATX)] (9,10). There are some studies in the
literature that LPA signaling may play a role in pathogenesis of
both benign and malignant endometrial tumors. Billon-Denis et
al (11) presented LPA
influence on the growth of leiomyomas or fibroids. Treatment of
leiomyoma tumor-derived cell line with LPA-entailed DNA synthesis
through ERK activation (11). The
authors also proposed that LPA produced in leiomyomas in
vivo, may be involved in tumor cell proliferation (11). There are also studies indicating
that LPA promoted endometrial cancer invasion via the induction of
matrix metalloproteinase-7 (MMP-7) (12,13).
However, the number of studies on the significance of LPA-dependent
signaling in endometrial tumor cells is still limited.
Additionally, there are certain conflicting data on the usefulness
of the LPAR status, ATX or PLA2 expressions as the independent
prognostic factors in endometrial cancer patients as well as on the
possibility of LPA-dependent targeted molecular therapy in
endometrial cancer.
The aim of our study was to investigate LPARs, ATX
and PLA2 expression in type 1 endometrial cancer and normal
endometrium with correlation to clinicopathological features.
Materials and methods
Patients and samples
The study was approved by the Local Ethics Committee
of the Faculty of Medical Sciences, University of Warmia and
Masuria in Olsztyn.
Tissue samples were obtained from 37 postmenopausal
women who underwent total abdominal hysterectomy because of EC.
Standard histopathological parameters were determined by the
pathologist. In each case, age, the presence of hypertension,
obesity and type 2 diabetes were determined. The age of patients
ranged from 46 to 82 years (mean, 64 years). Tumor stage, age range
and body mass index (BMI) of endometrial cancer patients are
presented in Table I. For the
control samples, normal endometrium explants of middle-to-late
proliferative phase of menstrual cycle were obtained during
hysterectomies due to uterine leiomyomas from 10 premenopausal
women (age range, 33–56 years). The explants for gene and protein
expression analyses were frozen in liquid nitrogen and kept at
−80°C until molecular studies were performed.
 | Table IRepresentative clinicopathological
characteristics of 37 endometrial cancers. |
Table I
Representative clinicopathological
characteristics of 37 endometrial cancers.
| Characteristics | No. of cases |
|---|
| Tumor stage and
grade | |
| Stage IA grade
1 | 6 |
| Stage IA grade
2 | 7 |
| Stage IA grade
3 | 1 |
| Stage IB grade
2 | 7 |
| Stage IB grade
3 | 3 |
| Stage II | 10 |
| Stage III | 3 |
| Age (years) | |
| ≤60 | 15 |
| >60 | 22 |
| Body mass index
(BMI) | |
| 18–24.9 | 6 |
| 25–30 | 9 |
| >30 | 22 |
Total RNA extraction and reverse
transcription (RT)
Total RNA was extracted from tissue explants using
TRIzol according to the manufacturer's instructions. RNA samples
were stored at −80°C. Before use, RNA content and quality was
evaluated by spectrophotometric measurement and agarose gel
electrophoresis. One microgram of each sample of total RNA was
reverse transcribed using a QuantiTect Reverse Transcription kit
(#205311; Qiagen). The RT reaction was performed in a total
reaction volume of 20 µl, following the manufacturer's
instructions and products stored at −20°C until real-time PCR
amplification.
Quantitative real-time PCR
The quantification of mRNA for the studied genes was
conducted by real-time PCR using specific primers for LPAR1,
LPAR2, LPAR3, LPAR4, ATX and
PLA2. The results of mRNA expression were normalized to
glyceraldehyde-3-phosphate dehydrogenase (GAPDH, an internal
control) mRNA expression and were expressed as arbitrary units.
This housekeeping gene was chosen using NormFinder software,
comparing three candidate genes: GAPDH, β-actin and
H2A.1. The primers were designed using an online software
package (http://bioinfo.ut.ee/primer3/). Primer sequences and
the sizes of the amplified fragments of all transcripts are shown
in Table II. Real-time PCR was
performed with an ABI Prism 7900 (Applied Biosystems Life
Technologies, Foster City, CA, USA) sequence detection system using
Maxima® SYBR-Green/ROX qPCR Master Mix (#K0222;
Fermentas, Thermo Scientific, USA). The PCR reactions were
performed in 384-well plates. Each PCR reaction well (10 µl)
contained 3 µl of RT product, 5 µM each of forward
and reverse primers and 5 µl SYBR-Green PCR Master Mix.
Real-time PCR was performed under the following conditions: 95°C
for 10 min, followed by 40 cycles of 94°C for 15 sec and 60°C for
60 sec. Subsequently, in each PCR reaction melting curves were
obtained to ensure single product amplification. In order to
exclude the possibility of genomic DNA contamination in the RNA
samples, the reactions were also performed either with blank-only
buffer samples or in the absence of the reverse transcriptase
enzyme. The specificity of PCR products for all examined genes was
confirmed by gel electrophoresis and sequencing. The efficiency
range for the target and the internal control amplifications was
between 95 and 100%. For relative quantification of mRNA expression
levels, the previously reported real-time PCR algorithm was used
(14).
 | Table IIPrimers used for real-time PCR. |
Table II
Primers used for real-time PCR.
| Gene | Primer
sequence | Fragment size
(bp) | GenBank accession
no. |
|---|
| LPAR1 |
5′-GGCTATGTTCGCCAGAGGACTAT-3′ | | |
|
5′-TCCAGGAGTCCAGCAGATGATAA-3′ | 135 | NM_001401.3 |
| LPAR2 |
5′-GCTCTGTCGAGCCTGCTTGTCTTC-3′ | | |
|
5′-ACAGTCTTGACCAGGCTGAGCGTG-3′ | 149 | NM_004720.5 |
| LPAR3 |
5′-AAACTTTCCTTTGGCTCTGGAC-3′ | | |
|
5′-ATTCCAGCGAAGAAATCGGC-3′ | 458 | NM_012152.2 |
| LPAR4 |
5′-GGGTGACAGAAGATTCATTGACTTCC-3′ | | |
|
5′-GGCCAGGAAACGATCCACACTA-3′ | 415 | NM_001278000.1 |
| ATX |
5′-CGTGAAGGCAAAGAGAACACG-3′ | | |
|
5′-AAAAGTGGCATCAAATACAGG-3′ | 776 | NM_006209.4 |
| PLA2 |
5′-ACATCTGCAAAAGCGCAAGG-3′ | | |
|
5′-CCTGCTGTCAGGGGTTGTAG-3′ | 374 | NM_024420.2 |
| GAPDH |
5′-CTGCACCACCAACTGCTTAG-3′ | | |
|
5′-GGGCCATCCACAGTCTTCT-3′ | 120 | NM_002046.5 |
Western blot analysis
For immunoblotting, protein fractions were obtained
from the tissue samples and total protein from the cells. Briefly,
luteal tissues were homogenized on ice in RIPA buffer containing
150 mM NaCl, 50 nM Tris Base, pH 7.2, 0.1% SDS, 1% Triton X-100,
0.5% sodium deoxycholate and 5 mM EDTA in the presence of the
protease inhibitor cocktail (#11697498001; Roche). Lysates were
then sonicated and centrifuged at 10,000 x g for 15 min at 4°C. The
protein samples were stored at −70°C for further analysis. The
protein concentration was determined according to Bradford
(15). Equal amounts (50 µg)
of membrane fraction were dissolved in SDS gel-loading buffer (50
mM Tris-HCl, pH 6.8, 4% SDS, 20% glycerol and 2%
β-mercaptoethanol), heated to 95°C for 5 min and separated by 12%
SDS-PAGE. Separated proteins were electroblotted using a semidry
transfer method onto polyvinylidene difluoride membranes
(Immobilon-P Transfer Membrane, #IPVH00010; Millipore) in transfer
buffer (0.3 mM Tris buffer, pH 10.4, 10% methanol, 25 mM Tris
buffer, pH 10.4, 10% methanol, 25 mM Tris buffer, pH 9.4, 10%
methanol, 40 mM glycine). After blocking in 5% non-fat dry milk in
TBS-T buffer (Tris-buffered saline with 0.1% Tween-20) for 1.5 h at
25.6°C, the membranes were incubated overnight with rabbit
polyclonal anti-LPAR2, ATX and cPLA2 antibodies (concentration
1:100, #sc-25490, #sc-66813 and #sc-438, respectively; Santa Cruz
Biotechnology), rabbit polyclonal anti-LPAR1 and LPAR3 antibodies
(concentration 4 µg/ml or 1:200, #10005280 and #10004840,
respectively; Cayman Chemicals), goat polyclonal anti-LPAR4
(concentration 1:100; Santa Cruz Biotechnology #sc-46021) and
monoclonal anti-GAPDH antibody produced in the mouse (concentration
0.05 µg/ml, #G8795; Sigma) at 4°C. Subsequently, the
proteins were detected by incubating the membranes with an
anti-rabbit IgG-alkaline phosphatase antibody produced in the goat
(concentration 1:20,000 for LPAR1, LPAR2, LPAR3, ATX and PLA2,
#A3687; Sigma), donkey anti-goat IgG-alkaline phosphatase antibody
(concentration 1:20,000 for LPAR4, #A4187; Sigma) or anti-mouse
IgG-alkaline phosphatase antibody produced in the goat
(concentration for all antibodies, 1:20,000 for GAPDH, #A3562;
Sigma) for 1.5 h at 25.6°C. After washing again in TBS-T buffer,
the immune complexes were visualized using an alkaline phosphatase
visualization procedure. The specific bands were quantified using
Kodak 1D software (Eastman Kodak, Rochester, NY, USA). GAPDH was
used as an internal control for protein loading.
Statistical analysis
Mean values ± standard deviation (SD) and median
were calculated. The Student's t-test was used to compare normally
distributed continuous variables and Mann-Whitney-U test for
abnormal distribution. The Spearman's and Pearson's correlation
coefficients were estimated. Linear regression analysis was also
used. The analysis were performed using Statistica and GraphPad
Prism software, accepting P<0.05 as significant.
Results
Expression profile of LPAR1, LPAR2,
LPAR3, LPAR4, ATX and PLA2 in EC and normal endometrium
All the studied tumors as well as normal endometria
expressed LPAR1, LPAR2, LPAR3, LPAR4, ATX and PLA2 mRNAs and
protein levels. All the examined LPARs (except for LPAR3 protein)
and enzymes responsible for LPA synthesis showed significantly
higher mRNA and protein expression in cancerous than healthy
endometrium (P<0.05). The cancer samples showed the highest
LPAR2 and LPAR1 transcript and protein expression ranging from
0.001115 to 0.1907, mean 0.03125±SD 0.051 for LPAR2 mRNA and from
1.5 to 6.9, mean 4.1±SD 1.6 for LPAR2 protein in cancer tissue
comparing to normal endometria ranging from 0.001 to 0.01, mean
0.0063±SD 0.0033 for LPAR2 mRNA and from 0.9 to 4.1, mean 2.6±SD
1.1 for LPAR2 protein (P<0.05, Table III and Fig. 1 c and d). LPAR1 mRNA expression in
the cancerous tissue ranged from 0.001 to 0.19, mean 0.03±SD 0.05
and LPAR1 protein level from 1.1 to 6.9, mean 2.5±SD 1.5, whereas
LPAR1 mRNA expression in normal endometria ranged from 0.0009 to
0.009, mean 0.005±SD 0.002 and LPAR1 protein level in healthy
tissue ranged from 0.9 to 2.9, mean 1.7±SD 0.7 (P<0.05, Table III and Fig. 1 a and b). We also found
significantly higher LPAR4, ATX and PLA2 transcript and protein
expression in cancerous tissue (mean 0.0011±SD 0.009 for LPAR4
mRNA, mean 0.76±SD 0.52 for LPAR4 protein and mean 0.005± SD 0.004
for ATX mRNA, mean 1.3±SD 0.6 for ATX protein and mean 0.004±SD
0.004 for PLA2 mRNA, mean 1.05±SD 0.4 for PLA2 protein) comparing
to normal endometria (mean 0.000074±SD 0.00008 for LPAR4 mRNA, mean
0.32±SD 0.38 for LPAR4 protein and mean 0.0014±SD 0.0012 for ATX
mRNA, mean 0.5±SD 0.2 for ATX protein and mean 0.0014±SD 0.002 for
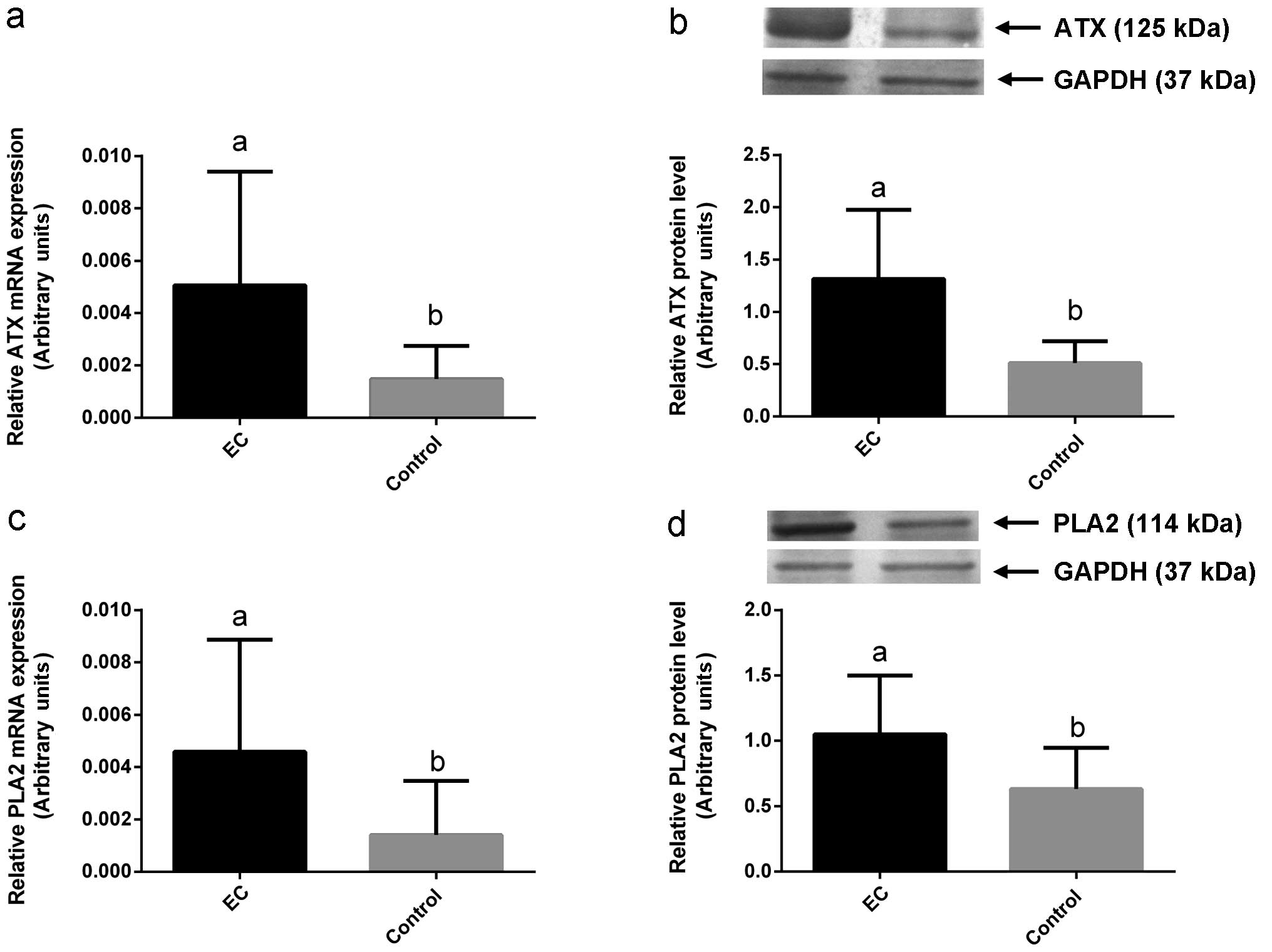
PLA2 mRNA, mean 0.6±SD 0.3 for PLA2 protein) (P<0.05, Table III, Fig. 1 g and h and Fig. 2). We found significantly higher
LPAR3 transcript expression in cancer tissue (mean 0.002±SD 0.001
for LPAR3 mRNA) comparing to normal endometria (mean 0.0009±SD
0.0007 for LPAR3 mRNA) (P<0.05, Table III and Fig. 1e). We did not find any difference in
LPAR3 protein level between cancerous and normal tissues
(P>0.05, Table III and
Fig. 1f).
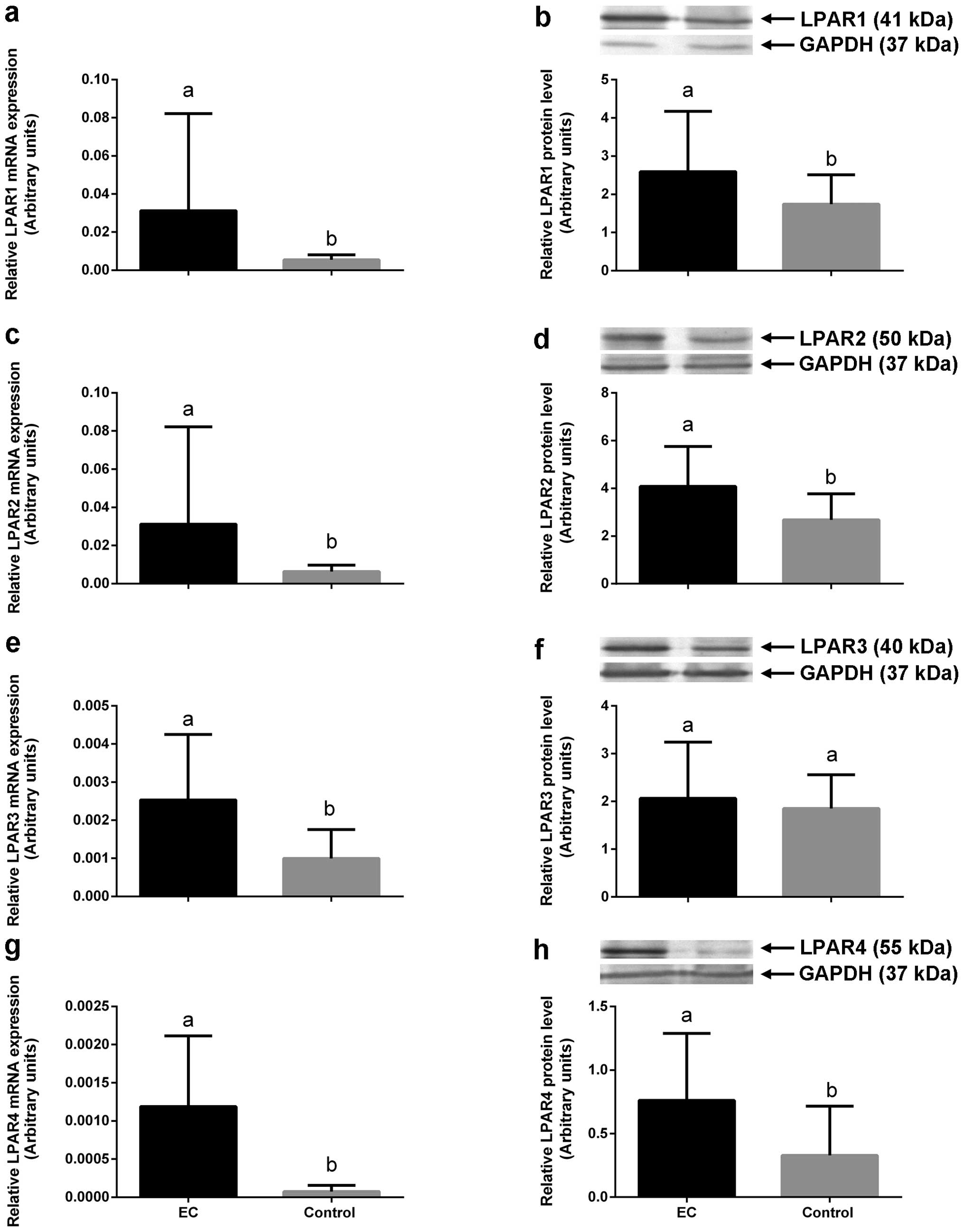 | Figure 1The expression of mRNAs (a, c, e and
g) and proteins (b, d, f and h) for LPAR1, LPAR2, LPAR3 and LPAR4,
respectively, in EC tissue (black bars) and normal endometrium
(control, grey bars). All values are expressed as the mean ± SEM of
LPAR1, LPAR2, LPAR3 and LPAR4 expression. Different letters
indicate significant differences (P<0.05). LPAR,
lysophosphatidic acid receptor; EC, endometrial cancer. |
 | Table IIIComparison of mRNA and protein levels
of LPARs, ATX and PLA2 between studied endometrial cancers (EC) and
normal endometrium tissues (control) using qRT-PCR and western
blotting studies. |
Table III
Comparison of mRNA and protein levels
of LPARs, ATX and PLA2 between studied endometrial cancers (EC) and
normal endometrium tissues (control) using qRT-PCR and western
blotting studies.
| Group | Number | qRT-PCR study
| Western blotting
study
|
|---|
| Mean | SD | P-value | Mean | SD | P-value |
|---|
| LPAR1 | EC | 37 | 0.031 | 0.05 | 0.0001 | 2.5 | 1.5 | 0.05 |
| Control | 10 | 0.005 | 0.002 | | 1.7 | 0.7 | |
| LPAR2 | EC | 37 | 0.03 | 0.05 | 0.0008 | 4.1 | 1.6 | 0.002 |
| Control | 10 | 0.006 | 0.003 | | 2.7 | 1.1 | |
| LPAR3 | EC | 37 | 0.002 | 0.001 | 0.008 | 2.1 | 1.1 | ns |
| Control | 10 | 0.0009 | 0.0007 | | 1.8 | 0.7 | |
| LPAR4 | EC | 37 | 0.001 | 0.0009 | <0.0001 | 0.8 | 0.5 | 0.004 |
| Control | 10 | 0.00007 | 0.0008 | | 0.32 | 0.38 | |
| ATX | EC | 37 | 0.005 | 0.004 | 0.0006 | 1.3 | 0.6 | 0.0002 |
| Control | 10 | 0.0014 | 0.0012 | | 0.5 | 0.2 | |
| PLA2 | EC | 37 | 0.004 | 0.004 | 0.0008 | 1.1 | 0.4 | 0.009 |
| Control | 10 | 0.0014 | 0.0021 | | 0.6 | 0.3 | |
Correlations between LPARs, ATX and PLA2
expression with the selected clinical, pathological and metabolic
features
Statistically positive correlations were found
between depth of myoinvasion-pT category (where T1A-tumor limited
to the endometrium or invades less than one half of the myometrium;
T1B-tumor invades one half or more of the myometrium; T2-tumor
invades stromal connective tissue of the cervix but does not extend
beyond the uterus; and T3-tumor involves the uterine serosa,
parametrium, vagina or adnexa) and levels of LPAR1, LPAR2 and PLA2
transcripts and proteins. In detail: LPAR1 was positively
correlated with the depth of myoinvasion (P=0.00012, r=0.58 for
mRNA and P=0.006, r=0.43 for protein, respectively), LPAR2 was
positively correlated with the depth of myoinvasion (P=0.00012,
r=0.58 for mRNA and P=0.00022, r=0.57 for protein, respectively),
PLA2 was positively correlated with the depth of myoinvasion
(P=0.0059, r=0.44 for mRNA and P=0.01, r=0.4 for protein,
respectively). Additionally, we found positive correlations between
LPAR3 and LPAR4 transcripts with the depth of myoinvasion
(P=0.0003, r=0.56 for LPAR3 mRNA and P=0.0035, r=0.46 for LPAR4
mRNA, respectively). Interestingly, we also found positive
correlations between LPAR1, LPAR2, LPAR4 and PLA2 mRNA and protein
expression with the International Federation of Gynecology and
Obstetrics (FIGO) stage. In detail: LPAR1 was positively correlated
with FIGO stage (P=0.0022, r=0.48 for mRNA and P=0.000091, r=0.59
for protein, respectively), LPAR2 was positively correlated with
FIGO stage (P=0.002, r=0.48 for mRNA and P=0.000015, r=0.64 for
protein, respectively), LPAR4 was positively correlated with FIGO
stage (P=0.001, r=0.51 for mRNA and P=0.017, r=0.38 for protein,
respectively) and PLA2 was positively correlated with FIGO stage
(P=0.0018, r=0.49 for mRNA and P=0.0008, r=0.6 for protein,
respectively). Additionally, we found positive correlations between
LPAR3 mRNA and ATX protein with FIGO stage (P=0.0001, r=0.59 for
LPAR3 mRNA and P=0.000001, r=0.7 for ATX protein,
respectively).
We also found that the expression of LPAR1, LPAR2
and PLA2 at mRNA and protein level was positively associated with
the age of patients (P=0.01, r=0.38 for LPAR1 mRNA; P=0.0056,
r=0.44 for LPAR1 protein; P=0.019, r=0.38 for LPAR2 mRNA; P=0.0009,
r=0.52 for LPAR2 protein; P=0.015, r=0.39 for PLA2 mRNA and
P=0.005, r=0.44 for PLA2 protein, respectively). The expression of
LPAR3 mRNA as well as LPAR4 and ATX protein levels were positively
correlated with the age of the examined women (P=0.0038, r=0.46 for
LPAR3 mRNA; P=0.027, r=0.36 for LPAR4 protein and P=0.003, r=0.47
for ATX protein, respectively). We found positive correlation
between the expression of LPAR1 mRNA, LPAR2 mRNA and protein and
LPAR3 mRNA with the BMI of the examined patients (P=0.047, r=0.32
for LPAR1 mRNA; P=0.047, r=0.32 for LPAR2 mRNA; P=0.03, r=0.36 for
LPAR2 protein and P=0.02, r=0.38 for LPAR3 mRNA, respectively). We
found no association between the expression levels of the studied
factors and diabetes or hypertension amongst the examined patients
(P>0.05).
Discussion
Cancer is a disease involving abnormal cell growth
with the potential to invade or spread to other parts of the body.
It is usually composed of cells of the impaired growth control
mechanisms (16). Although there is
a relatively high possibility for good prognosis for the early
diagnosed cases of EC, there are still over 20% of deaths due to
this carcinoma (17,18). This situation clearly reflects the
failure of the available diagnostic tools in EC, especially in
identifying its premalignant stages. Therefore there is still an
urgent need for developing efficient prognostic markers and
individual, targeted therapies for EC.
The results of many studies confirmed the important
role of the LPA signaling system in the development of the
reproductive organ related tumors, especially ovarian cancers. It
was documented that LPA was produced by ovarian cancer cells and
acted as the ovarian cancer activating factor (19–21).
Moreover, LPA levels in the serum samples from ovarian cancer
patients were much higher than in the serum samples from the group
of healthy patients (22).
Increased levels of LPA were also found in ascites of ovarian
cancer patients and in the corresponding plasma samples (19,23–25).
The in vivo performed studies using HEC1A, the EC cell line,
demonstrated that the physiological level of LPA stimulated the
invasion and proliferation of those cells (12,13).
Moreover, Wang et al (13)
reported LPA as a strong promoter of the urokinase plasminogen
activator, with elevated levels correlating with tumor malignancy.
Similarly, in our study all the examined enzymes responsible for
LPA synthesis showed significantly higher mRNA and protein
expression in cancerous than healthy endometrium. We found over 2
times higher ATX and PLA2 expression in cancerous tissue comparing
to normal endometria. The data confirm the possibility of higher
LPA synthesis and action in endometrial cancer compared to healthy
uterus.
There are continuous efforts to establish whether
different cellular effects of LPA on cell proliferation, motility
and invasion in cancer cells depend on the activation of the
certain type of LPARs. Of these, several studies documented the
overexpression of LPAR2 and LPAR3 in ovarian cancer cell lines in
comparison to normal ovarian epithelial cells (21,26,27).
The elevated expression of LPAR2 and LPAR3 stimulated the migration
and invasion of ovarian cancer cells (28). The data seem to be in agreement with
the results of our study, where all the examined LPARs showed
significantly higher mRNA and protein expression in cancer than
healthy endometrium. The studied cancerous samples showed the
highest LPAR2 and LPAR1 transcript and protein expression comparing
to normal endometria. Moreover, the transcript and protein
expression for LPAR4 was significantly higher in cancer tissue
comparing to normal endometria. In case of LPAR3, only mRNA
expression was significantly higher in cancer tissue comparing to
normal endometria. Our data suggest that LPAR1 and especially
LPAR2, with the highest expression in our study, may be mainly
involved in LPA-induced proliferation and angiogenesis in the
cancer tissue. Although, we did not examine that issues directly,
there are data in the literature that LPAR2 was directly involved
in the promotion of angiogenesis in ovarian tumors via the
stimulation of vascular endothelial growth factor (VEGF) expression
(26,29). Also, Fujita et al (30) documented the correlation between the
LPAR2 and LPAR3 expression levels and the induction of VEGF
expression in ovarian cancer cells. Moreover, the study of Yu et
al (28) proved that the
knockdown of LPAR2 and LPAR3 led to the suppression of the
production of VEGF in ovarian cancer cells. Although, there is some
information in the literature on the connection between LPA
signaling and tumorigenesis in ovaries, LPA involvement in the
ethiopathology of endometrial cancer is still not well examined.
Most of already published studies were performed in vivo
using the EC cell line, HEC1A. Hope et al (12) reported that among the 4 principle
LPARs (LPAR1, LPAR2, LPAR3 and LPAR4), LPAR2 was predominantly
expressed by HEC1A cells. This agrees with the data obtained in our
study that endometrial cancer tissue show the highest LPAR2
transcript and protein expression compared to normal endometria.
Wang et al (13) documented
that the knockdown of LPAR2 caused the supression of the
LPA-induced HEC1A invasion, but there were no significant changes
in the level of migration of HEC1A cells (13). Besides, the knockdown of LPAR2
blocked LPA-induced activation of MMP-7 which usually plays an
important regulatory role in cell surface proteolysis and is
capable of binding to a variety of cell surface proteins, such as
E-cadherin, β-integrin and tumor necrosis factor-α (13). In endometrial cancer, the
overexpression of MMP-7 initiates the activation of MMP-2 which
promotes cancer invasion (12). All
of the above data point to LPAR1 and LPAR2 as the main receptors
responsible for LPA action in the endometrial cancer tissue and at
the same time the most promising predictors of the endometrial
cancer progression.
To support the above-mentioned hypothesis, we found
positive correlations between depth of myoinvasion and levels of
LPAR1, LPAR2 and PLA2 transcripts and proteins. We also found
positive correlations between LPAR3 and LPAR4 transcripts and the
depth of myoinvasion. There were also positive correlations between
LPAR1, LPAR2, LPAR4 and PLA2 mRNA and protein expression with the
FIGO stage. Additionally, we found positive correlations between
LPAR3 mRNA and ATX protein with FIGO stage. The expression of
LPAR1, LPAR2 and PLA2 at mRNA and protein level and the expression
of LPAR3 mRNA as well as LPAR4 and ATX protein levels were also
positively associated with the age of patients. Moreover, we found
positive correlation between the expression of LPAR1 and LPAR3 mRNA
and LPAR2 mRNA and protein with the BMI of the examined patients.
BMI is an unquestionable risk factor of endometrial cancer
(31,32). Therefore, it is not surprising that
LPA signaling connected with overexpression of the enzymes
responsible for LPA synthesis and their receptors is associated
with the excess of adipose tissue, as we have shown in the present
study. Some other studies, focused on the increased BMI and
treatment outcome in EC and demonstrated that elevated BMI was
rather a favorable prognosticator (33–35).
Although, there is often the association between BMI and
hypertension and prognosis for tumor malignancy (36,37),
in our study we found no association between the expression levels
of the studied factors and diabetes or hypertension amongst the
examined patients.
In summary, when we compared endometrial cancer
versus non-cancerous endometrial tissue, we were able to show
overexpression of all examined LPARs and enzymes responsible for
LPA synthesis in cancer tissue. Especially, owing to the highest
LPAR2 and LPAR1 transcript and protein expression in cancerous
tissue and positive correlations of both these receptors with the
depth of myoinvasion and the FIGO stage, LPAR2 and LPAR1 seem to be
the most promising predictors of the endometrial cancer progression
as well as the main receptors responsible for LPA action in the
endometrial cancer tissue.
Acknowledgments
All procedures performed in studies involving human
participants were in accordance with the Ethical Standards of the
Institutional and/or National Research Committee and with the 1964
Helsinki Declaration and its later amendments or comparable ethical
standards.
References
|
1
|
Didkowska J, Wojciechowska U, Tarkowski W
and Zatoński W: Cancer in Poland in 2009. Polish National Cancer
Registry. Department of Epidemiology and Cancer Prevention. The
Maria Skłodowska-Curie Memorial Cancer Center; Warsaw: 2011
|
|
2
|
Schouten LJ, Goldbohm RA and van den
Brandt PA: Anthropometry, physical activity, and endometrial cancer
risk: Results from the Netherlands Cohort Study. J Natl Cancer
Inst. 96:1635–1638. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bokhman JV: Two pathogenetic types of
endometrial carcinoma. Gynecol Oncol. 15:10–17. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parazzini F, La Vecchia C, Bocciolone L
and Franceschi S: The epidemiology of endometrial cancer. Gynecol
Oncol. 41:1–16. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Potischman N, Hoover RN, Brinton LA,
Siiteri P, Dorgan JF, Swanson CA, Berman ML, Mortel R, Twiggs LB,
Barrett RJ, et al: Case-control study of endogenous steroid
hormones and endometrial cancer. J Natl Cancer Inst. 88:1127–1135.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zeleniuch-Jacquotte A, Akhmedkhanov A,
Kato I, Koenig KL, Shore RE, Kim MY, Levitz M, Mittal KR, Raju U,
Banerjee S, et al: Postmenopausal endogenous oestrogens and risk of
endometrial cancer: Results of a prospective study. Br J Cancer.
84:975–981. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park CK, Apte S, Acs G and Harris EER:
Cancer endometrium. Abeloff's Clinical Oncology. Abeloff M,
Armitage J, Niederhuber J, Kastan M and McKenna W: 4th edition.
Elsevier; Philadelphia: 2008, View Article : Google Scholar
|
|
8
|
Ye X and Chun J: Lysophosphatidic acid
(LPA) signaling in vertebrate reproduction. Trends Endocrinol
Metab. 21:17–24. 2010. View Article : Google Scholar :
|
|
9
|
Aoki J: Mechanisms of lysophosphatidic
acid production. Semin Cell Dev Biol. 15:477–489. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okudaira S, Yukiura H and Aoki J:
Biological roles of lysophosphatidic acid signaling through its
production by autotaxin. Biochimie. 92:698–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Billon-Denis E, Tanfin Z and Robin P: Role
of lysophosphatidic acid in the regulation of uterine leiomyoma
cell proliferation by phospholipase D and autotaxin. J Lipid Res.
49:295–307. 2008. View Article : Google Scholar
|
|
12
|
Hope JM, Wang FQ, Whyte JS, Ariztia EV,
Abdalla W, Long K and Fishman DA: LPA receptor 2 mediates
LPA-induced endometrial cancer invasion. Gynecol Oncol.
112:215–223. 2009. View Article : Google Scholar
|
|
13
|
Wang FQ, Ariztia EV, Boyd LR, Horton FR,
Smicun Y, Hetherington JA, Smith PJ and Fishman DA:
Lysophosphatidic acid (LPA) effects on endometrial carcinoma in
vitro proliferation, invasion, and matrix metalloproteinase
activity. Gynecol Oncol. 117:88–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao S and Fernald RD: Comprehensive
algorithm for quantitative real-time polymerase chain reaction. J
Comput Biol. 12:1047–1064. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun X, Cheng G, Hao M, Zheng J, Zhou X,
Zhang J, Taichman RS, Pienta KJ and Wang J: CXCL12 / CXCR4 / CXCR7
chemokine axis and cancer progression. Cancer Metastasis Rev.
29:709–722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Llauradó M, Ruiz A, Majem B, Ertekin T,
Colás E, Pedrola N, Devis L, Rigau M, Sequeiros T, Montes M, et al:
Molecular bases of endometrial cancer: New roles for new actors in
the diagnosis and the therapy of the disease. Mol Cell Endocrinol.
358:244–255. 2012. View Article : Google Scholar
|
|
18
|
Jereczek-Fossa B, Badzio A and Jassem J:
Surgery followed by radiotherapy in endometrial cancer: Analysis of
survival and patterns of failure. Int J Gynecol Cancer. 9:285–294.
1999. View Article : Google Scholar
|
|
19
|
Xu Y, Shen Z, Wiper DW, Wu M, Morton RE,
Elson P, Kennedy AW, Belinson J, Markman M and Casey G:
Lysophosphatidic acid as a potential biomarker for ovarian and
other gynecologic cancers. JAMA. 280:719–723. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen Z, Belinson J, Morton RE and Xu Y and
Xu Y: Phorbol 12-myristate 13-acetate stimulates lysophosphatidic
acid secretion from ovarian and cervical cancer cells but not from
breast or leukemia cells. Gynecol Oncol. 71:364–368. 1998.
View Article : Google Scholar
|
|
21
|
Fang X, Gaudette D, Furui T, Mao M,
Estrella V, Eder A, Pustilnik T, Sasagawa T, Lapushin R, Yu S, et
al: Lysophospholipid growth factors in the initiation, progression,
metastases, and management of ovarian cancer. Ann NY Acad Sci.
905:188–208. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choi JW, Herr DR, Noguchi K, Yung YC, Lee
CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, et al: LPA
receptors: Subtypes and biological actions. Annu Rev Pharmacol
Toxicol. 50:157–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiao Y, Chen Y, Kennedy AW, Belinson J and
Xu Y: Evaluation of plasma lysophospholipids for diagnostic
significance using electrospray ionization mass spectrometry
(ESI-MS) analyses. Ann NY Acad Sci. 905:242–259. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiao YJ, Schwartz B, Washington M, Kennedy
A, Webster K, Belinson J and Xu Y: Electrospray ionization mass
spectrometry analysis of lysophospholipids in human ascitic fluids:
Comparison of the lysophospholipid contents in malignant vs
nonmalignant ascitic fluids. Anal Biochem. 290:302–313. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoon HR, Kim H and Cho SH: Quantitative
analysis of acyl-lysophosphatidic acid in plasma using negative
ionization tandem mass spectrometry. J Chromatogr B Analyt Technol
Biomed Life Sci. 788:85–92. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goetzl EJ, Kong Y and Mei B:
Lysophosphatidic acid and sphingosine 1-phosphate protection of T
cells from apoptosis in association with suppression of Bax. J
Immunol. 162:2049–2056. 1999.PubMed/NCBI
|
|
27
|
Furui T, LaPushin R, Mao M, Khan H, Watt
SR, Watt MA, Lu Y, Fang X, Tsutsui S, Siddik ZH, et al:
Overexpression of edg-2/vzg-1 induces apoptosis and anoikis in
ovarian cancer cells in a lysophosphatidic acid-independent manner.
Clin Cancer Res. 5:4308–4318. 1999.
|
|
28
|
Yu S, Murph MM, Lu Y, Liu S, Hall HS, Liu
J, Stephens C, Fang X and Mills GB: Lysophosphatidic acid receptors
determine tumorigenicity and aggressiveness of ovarian cancer
cells. J Natl Cancer Inst. 100:1630–1642. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu YL, Tee MK, Goetzl EJ, Auersperg N,
Mills GB, Ferrara N and Jaffe RB: Lysophosphatidic acid induction
of vascular endothelial growth factor expression in human ovarian
cancer cells. J Natl Cancer Inst. 93:762–768. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fujita T, Miyamoto S, Onoyama I, Sonoda K,
Mekada E and Nakano H: Expression of lysophosphatidic acid
receptors and vascular endothelial growth factor mediating
lysophosphatidic acid in the development of human ovarian cancer.
Cancer Lett. 192:161–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Renehan AG, Tyson M, Egger M, Heller RF
and Zwahlen M: Body-mass index and incidence of cancer: A
systematic review and meta-analysis of prospective observational
studies. Lancet. 371:569–578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Crosbie EJ, Zwahlen M, Kitchener HC, Egger
M and Renehan AG: Body mass index, hormone replacement therapy, and
endometrial cancer risk: A meta-analysis. Cancer Epidemiol
Biomarkers Prev. 19:3119–3130. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Everett E, Tamimi H, Greer B, Swisher E,
Paley P, Mandel L and Goff B: The effect of body mass index on
clinical/pathologic features, surgical morbidity, and outcome in
patients with endometrial cancer. Gynecol Oncol. 90:150–157. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Martra F, Kunos C, Gibbons H, Zola P,
Galletto L, DeBernardo R and von Gruenigen V: Adjuvant treatment
and survival in obese women with endometrial cancer: An
international collaborative study. Am J Obstet Gynecol.
198:89.e1–89.e8. 2008. View Article : Google Scholar
|
|
35
|
Münstedt K, Wagner M, Kullmer U, Hackethal
A and Franke FE: Influence of body mass index on prognosis in
gynecological malignancies. Cancer Causes Control. 19:909–916.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Litta P, Di Giuseppe J, Moriconi L, Delli
Carpini G, Piermartiri MG and Ciavattini A: Predictors of
malignancy in endometrial polyps: A multi-institutional cohort
study. Eur J Gynaecol Oncol. 35:382–386. 2014.PubMed/NCBI
|
|
37
|
Nevadunsky NS, Van Arsdale A, Strickler
HD, Moadel A, Kaur G, Levitt J, Girda E, Goldfinger M, Goldberg GL
and Einstein MH: Obesity and age at diagnosis of endometrial
cancer. Obstet Gynecol. 124:300–306. 2014. View Article : Google Scholar : PubMed/NCBI
|