Introduction
Micro(nano)particles, also known as
micro(nano)vesicles (MV), have been the subject of extensive
studies as they play an important role in cell-to-cell
communication. In the present study, this traditional nomenclature
was used, although in fact, MV are differently classified, usually
according to size [exosomes, ectosomes, nanovesicles,
micro(nano)particles, and extracellular vesicles], or function
(oncosomes, argosomes, and tolerosomes) (1,2). The
name 'vesicles' seems to be a misnomer as MV have different shapes
and may be spherical, discoidal, elongated and cylindrical
(3,4). MV are released by various cells and
are present in body fluids, including blood, urine, pleural and
cerebrospinal fluids, and ascites (1,3,4). The
MV of tumor cells origin are classified as tumor-derived MV (TMV)
(5).
The increased number of circulating MV of poorly
defined origin was observed in patients with colorectal cancer
(CRC) (6,7), gastric (8), ovarian (9), and breast (10) cancer, and some were indicated as
possible predictors of progression. However, no evidence has been
provided concerning whether TMV are also involved. We have
previously found elevated numbers of HER-2/neu-positive MV in the
platelet-free plasma of gastric cancer patients that were
associated with advancement of the tumor, suggesting that some of
these MV are TMV (3). Detection of
TMV, among other MV, in blood may be of importance as they are
regarded as biomarkers of cancer progression and may offer new
diagnostic and therapeutic opportunities (1).
The present study was conducted to isolate MV from
the plasma of CRC patients, known to demonstrate an enhanced level
of MV (6) and healthy donors
(control) to define their properties. Large plasma pools obtained
from individual subjects were stepwise centrifuged at 15,000 x g
and 50,000 x g and size, structure, immunophenotype, including some
tumor markers, of MV obtained at each step were performed.
Interactions of MV with cancer cells and monocytes were also
studied. This study has shown that the circulating MV of CRC
patients are heterogeneous in all these aspects. As some MV
expressed tumor markers it seems to suggest that among them, TMV
are also present.
Materials and methods
Patients
Circulating MV were studied in 98 patients with CRC
(Duke's stages A-2, B-42, C-28, D-24 patients) and in control
healthy volunteers (n=89). Blood was obtained from patients before
any treatment and from control subjects by venous puncture and
collected to EDTA-containing tubes (Vacutainer system; BD
Biosciences, Franklin Lakes, NY, USA). To omit individual
variations, large pools of plasma were formed by mixing 1 ml of
plasma from individual subjects and were treated as representative
for patients and control populations and used for the isolation of
MV. These studies were approved by the Ethics Committee of the
Jagiellonian University Medical College.
Cancer cells
The SW480 colon cancer cell line (courtesy of
Professor Caroline Dive, Paterson Institute for Cancer Research,
University of Manchester, UK) was used as the TMV source and their
target. The cells were cultured in Dulbecco's modified Eagle's
medium (DMEM) with high glucose content (PAA Laboratories GmbH,
Pasching, Austria) supplemented with 10% of fetal bovine serum
(FBS; Biowest, Nuaille, France), previously centrifuged at 50,000 x
g for 1 h to remove bovine MV, at 37°C, in a 5% CO2
atmosphere and regularly tested for Mycoplasma sp. contamination
using a PCR-ELISA kit (Roche, Mannheim, Germany) and for endotoxin
contamination by the Limulus test (Charles River Laboratories,
Wilmington, MA, USA) according to the manufacturer's
instructions.
Isolation of MV
The MV were obtained by stepwise
ultracentrifugation. Blood samples were centrifuged at 3,000 x g
for 10 min to remove the cells. To omit individual variations, the
obtained plasma (~1 ml each sample) were pooled and centrifuged at
15,000 x g for 15 min to obtain platelet-free plasma. Following the
separation, the plasma contained ~2% while the pellet ~91% of
CD61+ particles (3). The
pellets obtained (called MV15) were suspended in
serum-free medium and washed three times at 15,000 x g. The
supernatants were then ultracentrifuged at 50,000 x g for 2 h. The
MV pellets obtained (known as MV50) were resuspended in
serum-free medium, washed three times at 50,000 x g and then
resuspended in 0.15% NaCl solution (measurements of electrokinetic
potential) or in the medium (other tests). The final suspensions
were ~40 times concentrated. In some experiments, the supernatants
from the SW480 cell line, were collected at confluency and
TMVSW480 (prepared in the same manner), were used.
Determination of MV size
distribution
The size of MV was determined in Zetasizer Nano ZS
apparatus (Malvern Instruments Ltd., Malvern, UK) equipped with a
laser of λ=633 nm, using the dynamic light scattering method (DLS),
with a nominal measuring range of 0.6–6,000 nm. Fluctuations of
light intensity due to Brownian's particle motions allowed
determination of their average speed, which in turn was
recalculated into effective particle size. The obtained values
represented the radius of spherical particles, which moved in
viscous media with the same velocity as the studied particles.
The ζ potential determination
Particle ζ potential was measured with the same
Zetasizer Nano ZS apparatus (Malvern Instruments) for samples
diluted 10 times in distilled water. Charged objects moving due to
applied electric field altered the frequency of scattered light
(Doppler effect), allowing determination of their electrophoretic
mobility, which was recalculated to the ζ potential using the
Smoluchowski equation.
Atomic force microscopy
Samples for atomic force microscopy (AFM) were
prepared from isolated MV50 by adsorbing onto modified
mica bases. The measurements were performed using an Ntegra Vita
microscope (NT-MDT Co., Zelenograd, Russia). The AFM images were
recorded using commercial silicon tip working in a tapping mode at
a resonance frequency within a range of 140–240 kHz with a
polysilicon cantilever (NT-MDT Co., Tempe, AZ, USA) of spring
constants k=3.4 N/m or k=5.8 N/m.
Immunophenotyping of MV
To characterise the MV phenotype the following
murine monoclonal antibodies (mAbs) were used: fluorescein
isothiocyanate (FITC)-conjugated anti-CD44, -CD61, phycoerythrin
(PE)-labelled anti-CD41, -CCR6 (chemokine receptor 6), -EGFR
(epidermal growth factor receptor) (all from BD Pharmingen, San
Diego, CA, USA) and FITC anti-CD44v6 (Bender MedSystems, Vienna,
Austria), PE anti-MUC1 (Mucin1) (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) and anti-HER-2/neu Alexa Fluor 488-conjugated
(BioLegend, San Diego, CA, USA). In parallel, staining with
appropriate isotype-matched mouse IgG (BD Pharmingen, Bender
MedSystems, Santa Cruz Biotechnology, Inc. or Biolegend) were used
as negative controls. MV samples were incubated with mAbs for 30
min at 4°C, resuspended in 0.3 ml of PBS containing 0.1% sodium
azide and analyzed by flow cytometry (FACS Canto), using FACS DiVa
v. 5.1 software. After gating on FSC and SSC log scale, 50,000
events were acquired and statistical analysis was performed
according to FITC, PE and Alexa Fluor 488 fluorescence of MV
stained with isotype controls.
Western blotting
MV were lysed in M-PER lysing buffer (Pierce,
Rockford, IL, USA) containing protease inhibitor cocktail (Roche).
Isolated protein (20 µm) was mixed with NuPAGE LDS Sample
Buffer (4X concentration) and NuPAGE Sample Reducing Agent (10X
concentration) (both from Life Technologies, Carlsbad, CA, USA).
Samples were heated (70°C, 10 min) and electrophoresed in 14%
polyacryl-amide gel containing SDS. The samples were then
transferred onto polyvinylidene fluoride membranes (Bio-Rad,
Hercules, CA, USA). After blocking for 1 h at room temperature in
Tris-buffered saline (TBS) with 0.1% Tween-20 and 1% bovine serum
albumin (BSA) (both from Sigma-Aldrich, St. Louis, MO, USA) the
membranes were incubated overnight at 4°C with murine mAbs against:
EGFR (clone R-1), HER-2/neu (clone F-11), and rabbit anti-MUC1
(clone H-295) (all from Santa Cruz Biotechnology, Inc.) diluted
1:1,000. As a loading control, mouse anti-actin (clone C-2; Santa
Cruz Biotechnology, Inc.) was used. After incubation, membranes
were washed in TBS supplemented with BSA and Tween-20 and incubated
for 1 h at room temperature with secondary goat anti-rabbit and
anti-mouse antibodies (dilution at 1:4,000) conjugated with
horseradish peroxidase (Santa Cruz Biotechnology, Inc.). The
protein bands were visualized with the SuperSignal West Pico
Chemiluminescence Substrate kit (Pierce) according to the
manufacturer's instructions and analyzed with Kodak Gel Logic 1500
Digital Imaging system (Kodak, Rochester, NY, USA).
Binding and engulfment of TMV
To test the ability of TMV to bind to cancer cells
and monocytes, TMV50 were stained with red PKH26
fluorescent dye (Sigma-Aldrich) for 5 min, according to the
manufacturer's instructions and then incubated with SW480 cells and
blood monocytes, isolated as previously described (11) (~2 TMV particles/cell, previously
titrated as detectable by FACS), for 2 and 24 h. To discriminate
between the cells which already engulfed TMV (intracellular
localization) from those with TMV only attached to their surface
(extracellular) a quenching assay with crystal violet (1 mg/ml
final concentration; Sigma-Aldrich), added to the cell suspension
(12), was introduced. In this
assay the extracellular fluorescence was quenched by crystal violet
(5). The samples were then analyzed
by FACS. Gate P1 was set according to the autofluorescence of cells
incubated in the medium without TMV.
Elimination of TMV from circulation
Severe combined immunodeficient (NOD SCID; Charles
River Laboratories, Sulzfeld, Germany) mice (2/group) were injected
intravenously into retroorbital veins with PKH26-labelled
TMVSW480 (5×107 in 100 µl PBS/mouse).
Handling and all the procedures were conductaed under laminar flow
conditions. Blood was drawn from tail vein into EDTA-containing
tubes at 5, 15, 30, 60 and 120 min after injection and then the
mice were euthanized. Spleen, liver, lung and kidney were removed
for further examination. Blood was centrifuged at 3,000 x g for 10
min and plasma and cells from the bottom were removed separately.
Cells from the pellet were washed. Plasma was further centrifuged
at 50,000 x g for 1 h and the pellet and plasma were analyzed for
the presence of PKH26-labelled TMV by FACS. Organ samples were
sectioned using Leica CM1850 cryostat (Leica Microsystems Nussloch
GmbH, Nussloch, Germany). The presence of TMVSW480 was
examined under the Olympus BX60 fluorescent microscope (Olympus
Corp., Tokyo, Japan).
Results
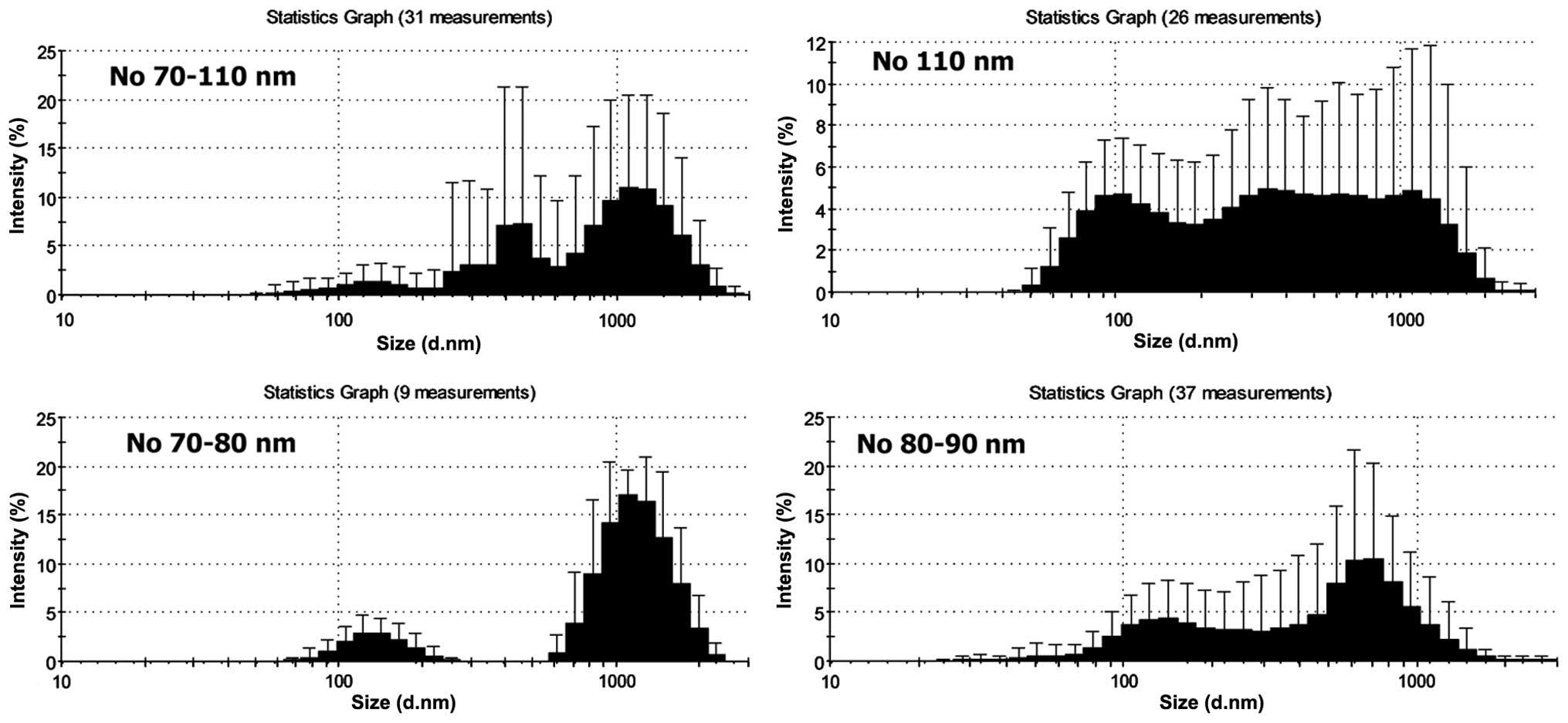
Size distribution
The size of isolated MV15 and
MV50, as defined by DLS, revealed heterogeneity and
relatively small differences between them (Fig. 1). Broad distribution of sizes and
rather high SD indicated polydispersity of samples. The size ranges
were 70(80)–1,300(1,400) nm in MV15 and MV50
and no differences were observed between patients and controls. The
most frequent number of particles had sizes 105 nm in
MV15 and 70–90 nm in MV50, both in patients
and control samples (differences were not significant). The
negative ζ potential of MV15 from patients (−17.6±1.52
mV) and controls (−15.8±1.76 mV) did not differ, while the
MV50 of patients had a significantly higher (P≥0.05) ζ
potential (−16.8±0.90 mV) than that from the controls (−10.7±1.64
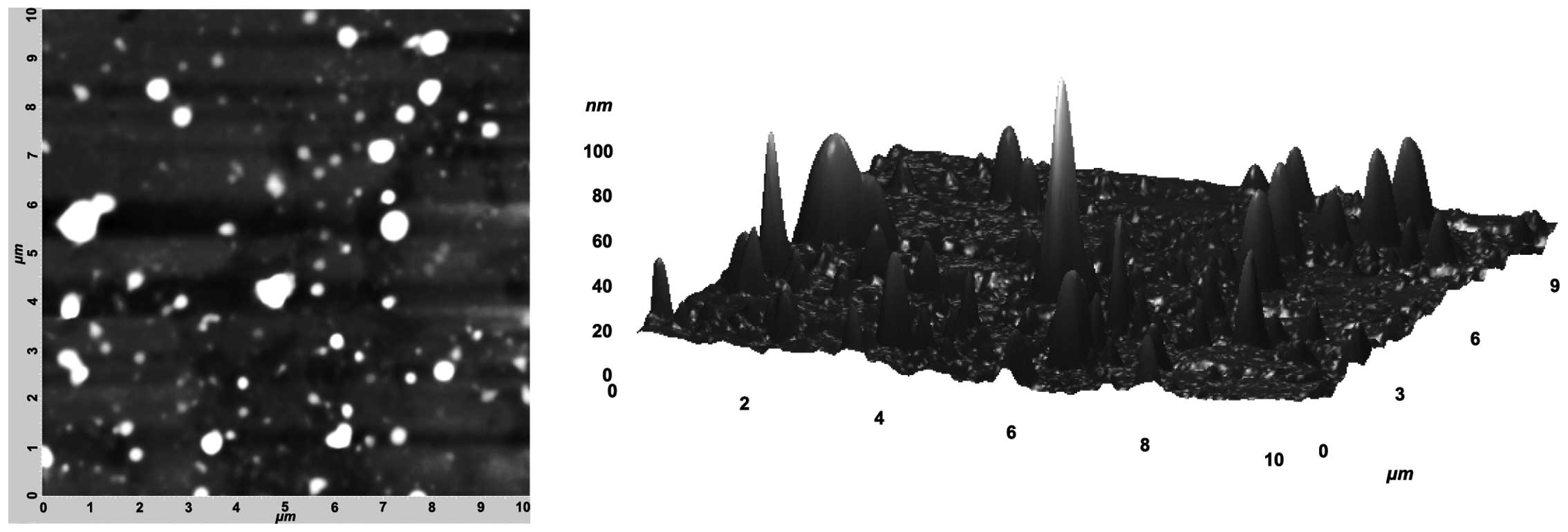
mV). AFM was used to investigate the structure of MV. It was not
possible to analyze MV15 because of the presence of
large homogenous clusters, which disturb the measurements. The
MV50 isolated from the plasma of patients showed highly
heterogeneous MV differing both in shapes and sizes (Fig. 2A and B). A comparison of sizes
obtained from AFM and DLS showed their high compatibility.
Immunophenotype was determined by flow cytometry and western
blotting.
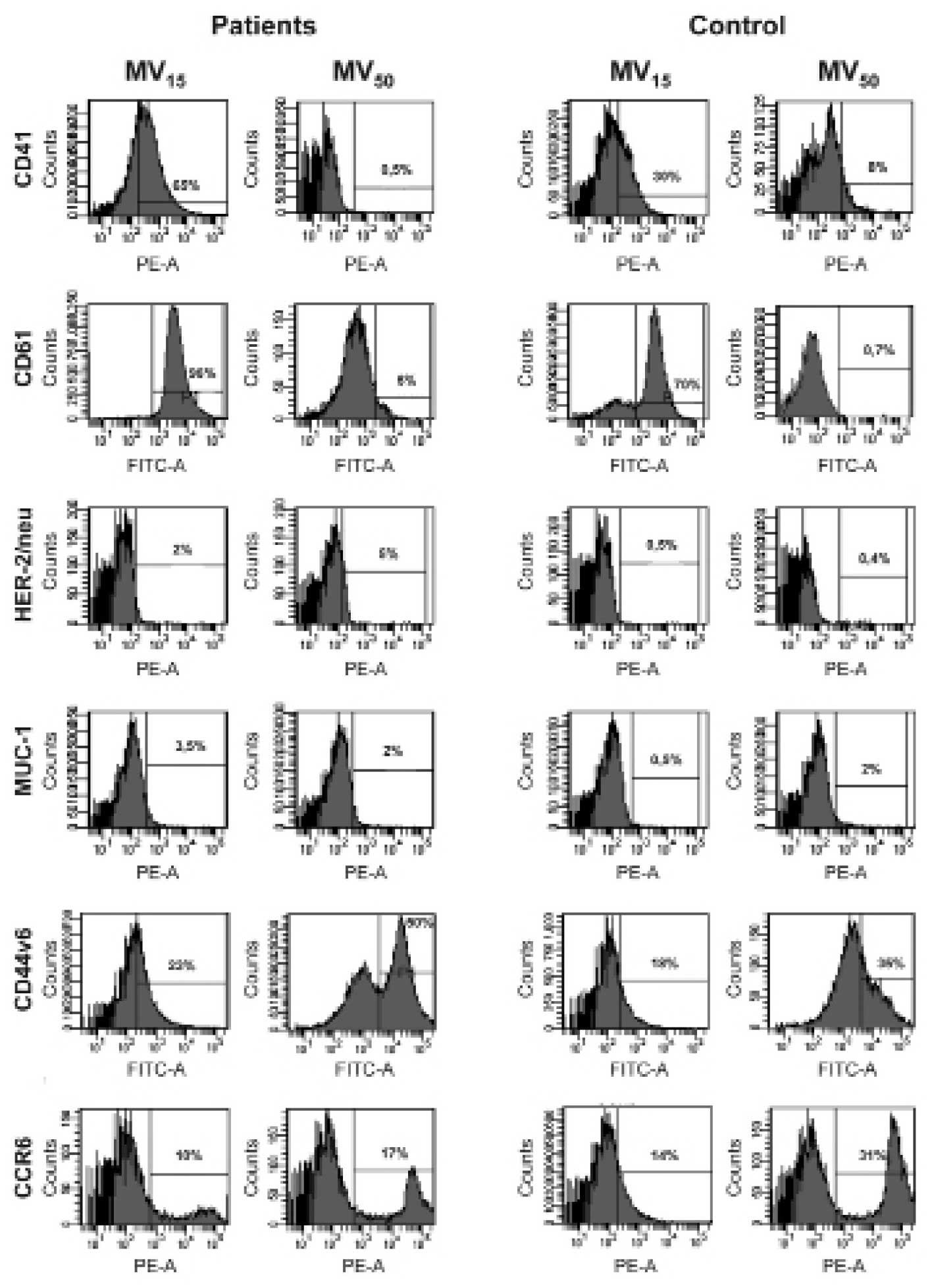
Expression of determinants and tumor
markers
Among several determinants tested on MV by FACS, we
concentrated on these of platelet origin (CD41, CD61), those
involved in cancer cell interactions with other cells (CD44v6,
CCR6) and tumor-associated (HER-2/neu, EGFR and MUC1). Expression
of platelet markers (CD41, CD61) was significantly higher on
MV15 than MV50, for patients and donors
(Fig. 3) among which there was no
difference. HER-2/neu-positive particles were more apparent in
patients than donors, and in the former their proportion was higher
in MV50 than MV15. Expression of MUC1 on
MV15 was higher in patients but no difference between
them was observed on MV50 protein content. The
proportion of CD44v6 and CCR6 was higher on MV50 than
MV15 but patients and controls showed no differences in
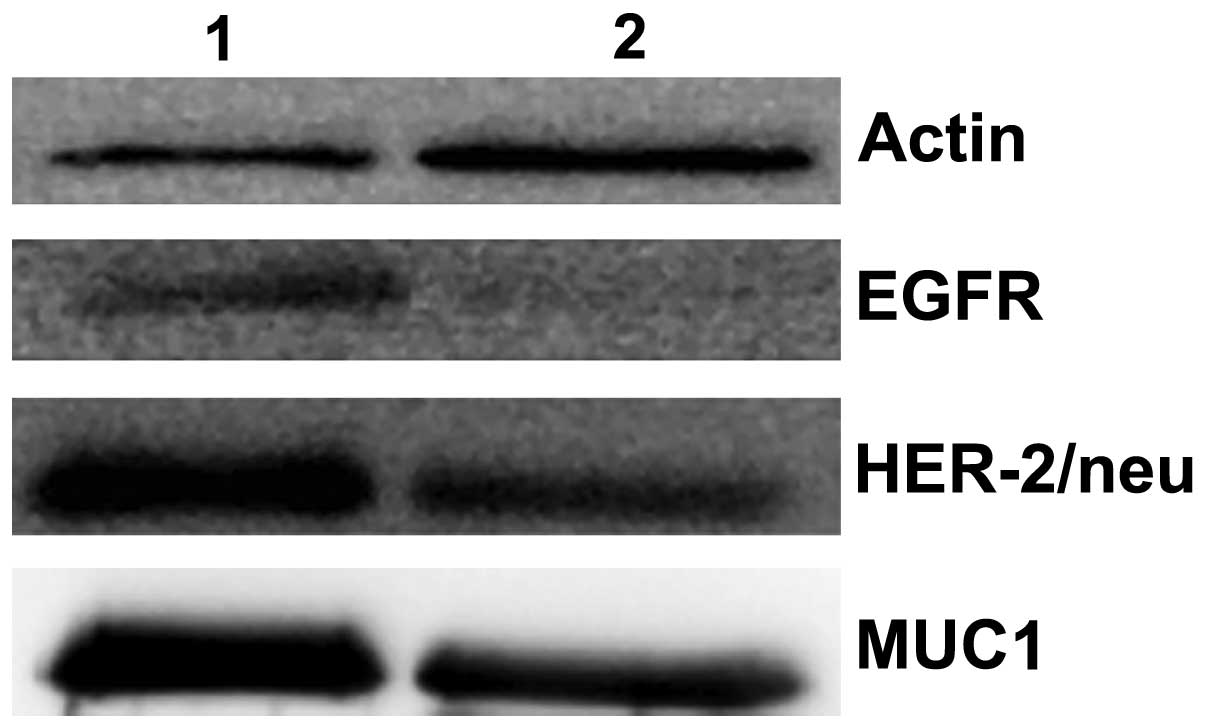
their expression in MV15 and MV50. Western
blot analysis of MV50 from the patients showed stronger
bands of EGFR (HER-1/Erb B1), HER-2/neu and MUC1 as compared to the
controls (Fig. 4).
Binding and ingestion by SW480 cells and
monocytes of MV
Since MV are present at the tumor site (1), it was of interest to determine whether
MV circulating in plasma are able to interact with cancer cells,
i.e., whether MV are taken up by the cells. For this purpose, the
cells from a colon cancer cell line (SW480) were exposed to
PKH26-labelled MV50 (as enriched in MV of tumor cells
origin) isolated from the plasma of patients and controls and
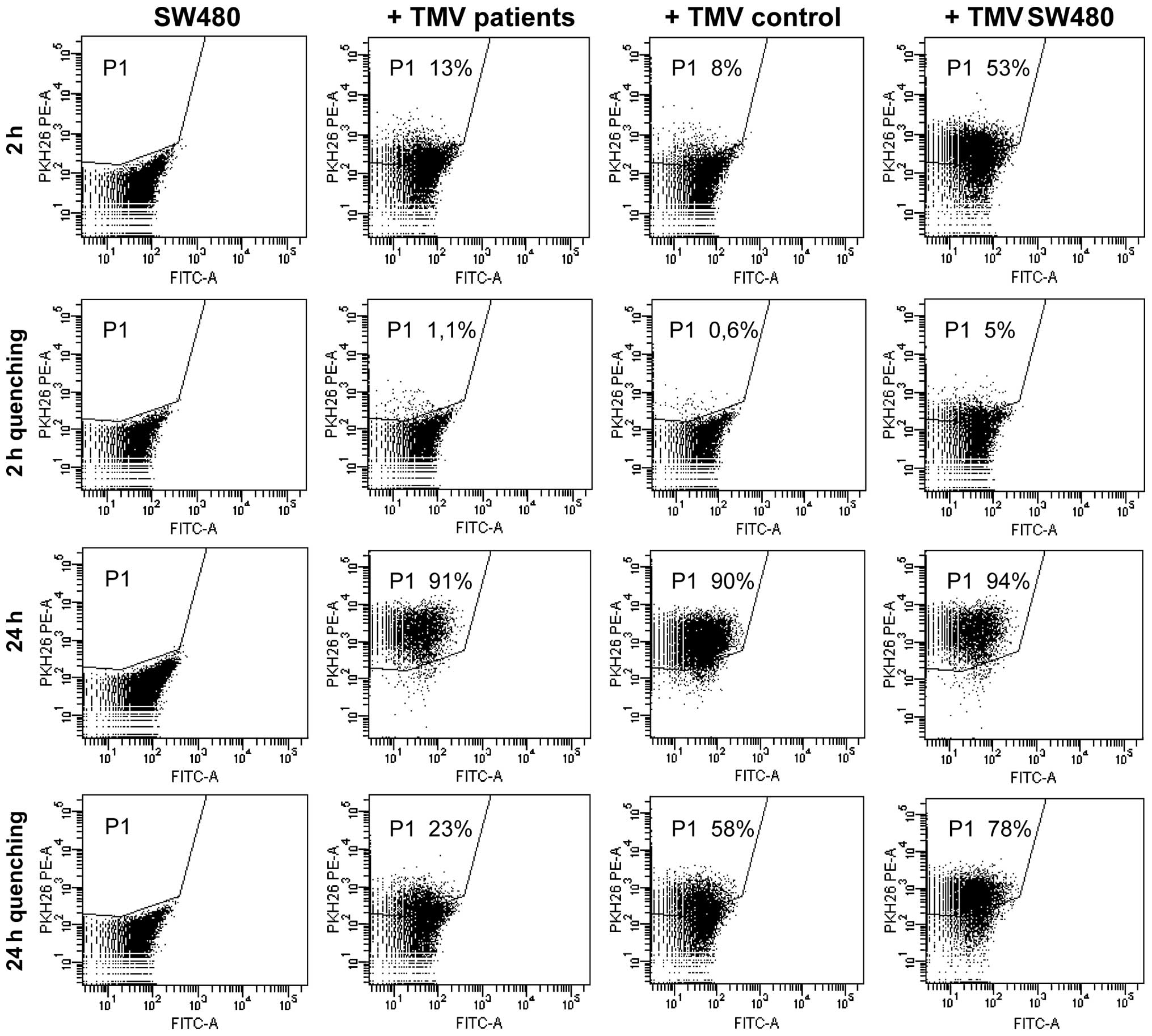
TMVSW480 ('pure' TMV) for 2 and 24 h, followed by FACS
analysis. After incubation for 2 h only a small proportion of
MV50 was seen mostly extracellularly as judged by almost
total quenching with crystal violet (Fig. 5). No difference was observed for
MV50 between patients and donors, but
TMVSW480 attachment was significantly higher (53%).
Incubation for 24 h resulted in a significantly higher proportion
of positive cells with or without quenching, indicating that
approximately half of MV50 were localized
intracellularly. This provides clear evidence for the attachment
and engulfment of MV50 by cancer cells and indicated the
lack of uptake specificity. The other noteworthy issue was the
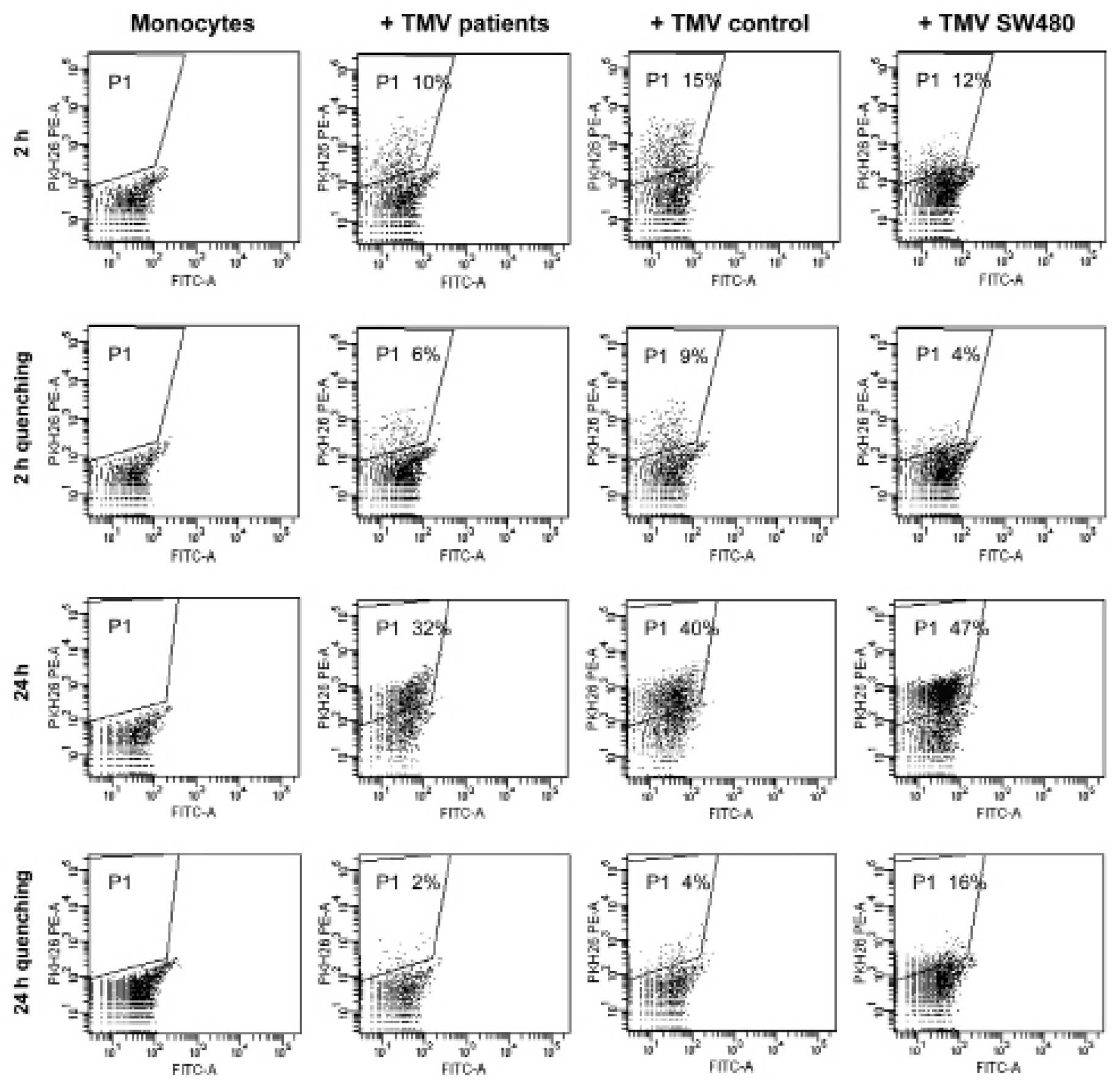
interaction of MV50 with monocytes. Monocytes were
selected as the most common cell-infiltrating tumors
(tumor-infiltrating monocytes, TIM). Monocytes were exposed to
PKH26-labelled MV50, similarly for 2 and 24 h. Fig. 6 shows that uptake of MV50
from patients and control by monocytes occurred, and the proportion
of positive cells was similar as observed in the case of cancer
cells.
As the plasma level of circulating MV is relatively
constant, the issue was whether it was due to elimination/delivery
of MV. As the number of MV50 from patients and controls
was extremely low to reach their level observed in human plasma,
TMVSW480 were used to determine the elimination rate
from blood and specific organ localization. Trace levels of
PKH26-labelled TMVSW480 were detected in blood cells and
plasma (~1.0% of injected) only at 5 min after intravenous
injection into NOD SCID mice. At this time no labelled TMV in the
liver, lung, spleen and kidney were detected. At 15 min after
injection labelled TMVSW480 were observed in the liver
and lung tissue sections, but not kidney and spleen. The highest
fluorescence intensity was observed in the former. No TMV were
detected later on in any of the examined organs. The results
indicated that TMV were rapidly eliminated from blood and that the
liver was mainly responsible for their 'trapping' and
elimination.
Discussion
Many micro(nano)particles, also known as
microvesicles (MV), are released by tumor cells (TMV). TMV play a
pivotal role in cancer and may serve as a putative diagnostic tool
and therapeutic target (1).
However, MV are also shed by many cells of the body (e.g.,
platelets, hepatocytes, endothelium), including those present in
tumor stroma (13). MV circulating
in blood are of different cell origin. Their level is increased in
many types of cancer (3,8–10).
Detection of circulating TMV (oncosomes) in blood is limited by the
fact that their estimated frequency among host MV is approximately
1% (1). Since most common
platelet-derived MV (CD41+, CD61+) attenuate
TMV, the studies were undertaken to remove them from the plasma of
CRC patients. Our previous observation in gastric cancer patients
indicated that plasma centrifugation at 15,000 x g led to the
substantial depletion of MV of platelet origin (3). In the present study, stepwise
centrifugation of plasma at 15,000 x g and 50,000 x g was used to
characterize more precise fractions obtained at these gravities,
known as MV15 and MV50, respectively. Using
these fractions we characterized MV size, electrokinetic potential,
morphology, expression of tumor markers, and MV50
interactions in vitro with cancer cells and monocytes.
Different methods are used for determination of MV
size, e.g., electron microscopy, DLS (3) or AFM, alone or combined with
microfluidics (14,15). We used the DLS method and found an
almost identical range of sizes (70–1,400 nm) among MV15
and MV50 fractions, both in patients and controls. From
the size distribution of nanoparticles it appears that
MV15, from patients and control were more heterogeneous
(trimodal pattern) while MV50 showed a bimodal size
distribution. The most common frequency of particle size in
MV15 was approximately 105 nm, while in MV50
80–90 nm. This size suggesta that most MV are equivalent to
exosomes (16). DLS results are in
concordance with data from the AFM analysis, which indicated a
similar mean range of sizes, and different shapes of
MV50. There was no difference between patients and
control samples (not shown). In comparison to MV15 the
MV50 of CRC patients exhibited an increased expression
of some tumor markers, mainly HER-2/neu, MUC1 as defined by flow
cytometry and EGFR (HER-1/Erb B1), HER-2/neu and MUC1 as determined
by western blotting. The level of CD44v6 and CCR6 was also higher
in MV50 than MV15 but no differences between
patients and control were identified. The general conclusion was
that MV50 from patients contained more TMV, i.e.,
expressing tumor markers as compared to the controls and
MV15. This finding can be extrapolated to suggest that
in patients, tumor marker positive circulating MV50 are
present in elevated numbers. Since the two markers are
overexpressed in colon cancer cells (17,18),
it appears to indicate that CRC tumors are the major source of such
MV, i.e., TMV. The presence of HER-2/neu and MUC1 in healthy
individuals is not noteworthy as normal colon epithelial cells also
express at least HER-2/neu (19),
while MUC1 is not a truly specific tumor marker (20). It is notable that EGFR on
MV50 was detected by western blotting but not by FACS.
The likely explanation may be that it is not expressed on the
MV50 surface, but present inside only. This is in
agreement with the observation that SW480 cells are EGFR-positive,
while EGFR is not detected on TMVSW480 (unpublished
observation). The higher ζ potential of patients' MV50,
may also be associated with the presence of MV of tumor origin.
As MV of tumor and tumor-infiltrating cells origin
are observed in the tumor bed (21–23),
we determined whether MV50 present in plasma may
interact with cancer cells. Colon cancer cells (SW480) were used as
a target for MV50 from patients and controls. SW480
cells were exposed to PKH-26-labelled MV50 from plasma
and TMVSW480 as controls. At 2 h the MV attached to the
cells, while at 24 h engulfment occurred. There was no significant
difference in the magnitude of the MV uptake, thus indicating a
lack of specificity in interactions of MV with cancer cells. Uptake
of tumor-derived exosomes i.e., with particles sizes <100 nm has
already been described (24,25).
However, these small MV were isolated from supernatants of culture
cell lines (24–26). Thus, to the best of our knowledge,
the present results are the first to demonstrate that circulating
('native') MV can also interact with cancer cells. Although some
specificity of tumor-derived exosomes towards cancer cells over
'immortalized' cells was suggested, such exosomes are not
preferentially associated with their parental cell lines (16). The lack of specificity may be due to
various endocytic pathways of uptake, not only exosomes, but also
'native MV' (25,26). Uptake of plasma MV by cancer cells
indicate that such MV, such as TMV, may deliver their cargo to
target cells (5). Since TMV are
considered a potential drug delivery means (1), the lack of specificity in uptake of
MV50 may be advantageous as isolation of circulating MV
from healthy subjects is relatively simple, as shown in the present
study. Additionally, it is unlikely that MV of tumor origin may
deliver 'unwanted' cargo. Uptake of plasma MV by monocytes may also
be involved in their delivery to the tumor site, as monocytes are
prominent participants of the tumor infiltrate (27,28).
It was noteworthy to identify where TMV are
accumulated in the body and the expeditious manner in which they
are eliminated. These studies were undertaken in NOD SCID mice
which were infused with 'pure' TMVSW480 labelled with
fluorescent dye PKH-26 in numbers required to reach the level of
total MV observed in the blood of patients. No presence of these
TMV in blood cells was observed 5 min after injection, suggesting
their rapid elimination. At 15–30 min, their accumulation was
observed mostly in the liver, and lungs. The finding suggests that
TMV are taken up by tissue macrophages (abundantly present in these
organs), which is supported by observations that TMV were attached
to human blood monocytes in vitro within 2 h and then
engulfed within 24 h (unpublished). However, we were unable to
establish the sequence of subsequent intracellular events, as at
latter times TMV were not identified in these organs, e.g.,
possible degradation by enzymes for which there is an abundance in
macrophages (29). However, these
findings suggest that continuous delivery of MV is necessary in the
body because their continuous level was detected.
In conclusion, this study provides evidence for the
heterogeneity in many respects of circulating MV in CRC patients,
some of which are likely to be of tumor origin. These MV may affect
the tumor and may be carried by monocytes. However, their precise
role in this type of cancer remains unclear and requires additional
studies.
Acknowledgments
We would like to thank Ms. I Ruggiero for skillful
technical assistance. Grant sponsors included the National Science
Centre (grant no. N N401 290239). Publication of this study was
supported by the Leading National Research Center (KNOW), Faculty
of Medicine, Jagiellonian University Medical College.
References
|
1
|
Rak J: Extracellular vesicles - biomarkers
and effectors of the cellular interactome in cancer. Front
Pharmacol. 4:212013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choi DS, Kim DK, Kim YK and Gho YS:
Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass
Spectrom Rev. 34:474–490. 2015. View Article : Google Scholar
|
|
3
|
Baran J, Baj-Krzyworzeka M, Węglarczyk K,
Szatanek R and Zembala M, Barbasz J, Czupryna A, Szczepanik A and
Zembala M: Circulating tumour-derived microvesicles in plasma of
gastric cancer patients. Cancer Immunol Immunother. 59:841–850.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mrvar-Brecko A, Sustar V, Jansa V, Stukelj
R, Jansa R, Mujagić E, Kruljc P, Iglic A, Hägerstrand H and
Kralj-Iglic V: Isolated microvesicles from peripheral blood and
body fluids as observed by scanning electron microscope. Blood
Cells Mol Dis. 44:307–312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baj-Krzyworzeka M, Szatanek R, Węglarczyk
K, Baran J, Urbanowicz B, Brański P, Ratajczak MZ and Zembala M:
Tumour-derived microvesicles carry several surface determinants and
mRNA of tumour cells and transfer some of these determinants to
monocytes. Cancer Immunol Immunother. 55:808–818. 2006. View Article : Google Scholar
|
|
6
|
Silva J, Garcia V, Rodriguez M, Compte M,
Cisneros E, Veguillas P, Garcia JM, Dominguez G, Campos-Martin Y,
Cuevas J, et al: Analysis of exosome release and its prognostic
value in human colorectal cancer. Genes Chromosomes Cancer.
51:409–418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi DS, Yang JS, Choi EJ, Jang SC, Park
S, Kim OY, Hwang D, Kim KP, Kim YK, Kim S, et al: The protein
interaction network of extracellular vesicles derived from human
colorectal cancer cells. J Proteome Res. 11:1144–1151. 2012.
View Article : Google Scholar
|
|
8
|
Kim HK, Song KS, Park YS, Kang YH, Lee YJ,
Lee KR, Kim HK, Ryu KW, Bae JM and Kim S: Elevated levels of
circulating platelet microparticles, VEGF, IL-6 and RANTES in
patients with gastric cancer: Possible role of a metastasis
predictor. Eur J Cancer. 39:184–191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Giusti I, D'Ascenzo S and Dolo V:
Microvesicles as potential ovarian cancer biomarkers. Biomed Res
Int. 2013:7030482013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Galindo-Hernandez O, Villegas-Comonfort S,
Candanedo F, González-Vázquez MC, Chavez-Ocaña S,
Jimenez-Villanueva X, Sierra-Martinez M and Salazar EP: Elevated
concentration of microvesicles isolated from peripheral blood in
breast cancer patients. Arch Med Res. 44:208–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mytar B, Baran J, Gawlicka M, Ruggiero I
and Zembala M: Immunophenotypic changes and induction of apoptosis
of monocytes and tumour cells during their interactions in vitro.
Anticancer Res. 22:2789–2796. 2002.
|
|
12
|
Van Amersfoort ES and Van Strijp JA:
Evaluation of a flow cytometric fluorescence quenching assay of
phagocytosis of sensitized sheep erythrocytes by polymorphonuclear
leukocytes. Cytometry. 17:294–301. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luga V and Wrana JL: Tumor-stroma
interaction: Revealing fibroblast-secreted exosomes as potent
regulators of Wnt-planar cell polarity signaling in cancer
metastasis. Cancer Res. 73:6843–6847. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ashcroft BA, de Sonneville J, Yuana Y,
Osanto S, Bertina R, Kuil ME and Oosterkamp TH: Determination of
the size distribution of blood microparticles directly in plasma
using atomic force microscopy and microfluidics. Biomed
Microdevices. 14:641–649. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuana Y, Oosterkamp TH, Bahatyrova S,
Ashcroft B, Garcia Rodriguez P, Bertina RM and Osanto S: Atomic
force microscopy: A novel approach to the detection of nanosized
blood microparticles. J Thromb Haemost. 8:315–323. 2010. View Article : Google Scholar
|
|
16
|
Smyth TJ, Redzic JS, Graner MW and
Anchordoquy TJ: Examination of the specificity of tumor cell
derived exosomes with tumor cells in vitro. Biochim Biophys Acta.
1838:2954–2965. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang JL, Hanley JR, Yu Y, Berney CR,
Russell PJ and Crowe PJ: In vivo overexpression of c-erbB-2
oncoprotein in xenografts of mice implanted with human colon cancer
lines. Anticancer Res. 17:3463–3468. 1997.PubMed/NCBI
|
|
18
|
Yu Z, Cui B, Jin Y, Chen H and Wang X:
Novel irreversible EGFR tyrosine kinase inhibitor 324674 sensitizes
human colon carcinoma HT29 and SW480 cells to apoptosis by blocking
the EGFR pathway. Biochem Biophys Res Commun. 411:751–756. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cohen JA, Weiner DB, More KF, Kokai Y,
Williams WV, Maguire HC Jr, LiVolsi VA and Greene MI: Expression
pattern of the neu (NGL) gene-encoded growth factor receptor
protein (p185neu) in normal and transformed epithelial tissues of
the digestive tract. Oncogene. 4:81–88. 1989.PubMed/NCBI
|
|
20
|
Labouvie C, Machado J, Carneiro F, Seitz G
and Blin N: Expression pattern of gastrointestinal markers in
native colorectal epithelium, lesions, and carcinomas. Oncol Rep.
4:1367–1371. 1997.PubMed/NCBI
|
|
21
|
Gromov P, Gromova I, Olsen CJ,
Timmermans-Wielenga V, Talman ML, Serizawa RR and Moreira JM: Tumor
interstitial fluid - a treasure trove of cancer biomarkers. Biochim
Biophys Acta. 1834:2259–2270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang M, Chen J, Su F, Yu B, Su F, Lin L,
Liu Y, Huang JD and Song E: Microvesicles secreted by macrophages
shuttle invasion-potentiating microRNAs into breast cancer cells.
Mol Cancer. 10:1172011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dayan D, Salo T, Salo S, Nyberg P,
Nurmenniemi S, Costea DE and Vered M: Molecular crosstalk between
cancer cells and tumor microenvironment components suggests
potential targets for new therapeutic approaches in mobile tongue
cancer. Cancer Med. 1:128–140. 2012. View
Article : Google Scholar
|
|
24
|
Tian T, Wang Y, Wang H, Zhu Z and Xiao Z:
Visualizing of the cellular uptake and intracellular trafficking of
exosomes by live-cell microscopy. J Cell Biochem. 111:488–496.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Escrevente C, Keller S, Altevogt P and
Costa J: Interaction and uptake of exosomes by ovarian cancer
cells. BMC Cancer. 11:1082011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ,
Chang LF, Zhou Q and Sui SF: Cellular internalization of exosomes
occurs through phagocytosis. Traffic. 11:675–687. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leek RD, Lewis CE, Whitehouse R, Greenall
M, Clarke J and Harris AL: Association of macrophage infiltration
with angiogenesis and prognosis in invasive breast carcinoma.
Cancer Res. 56:4625–4629. 1996.PubMed/NCBI
|
|
28
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: Tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zembala M and Buckle AM: Monocytes in
malignant disease. Human Monocytes. Zembala M and Asherson GL:
Academic Press; London: pp. 513–528. 1989
|