Introduction
Head and neck squamous carcinoma (HNSCC) is a common
cancer associated with high mortality, accounting for ~3–5% of all
human malignancies. Owing to the increasing consumption of alcohol
(1,2), tobacco (2,3), and
betel nut chewing (4) and increased
rates of opportunistic infection with human papilloma virus
(5), ~640,000 cases of HNSCC are
reported worldwide each year (6,7).
Despite improvements in clinical interventions, including surgery,
radiotherapy, chemotherapy, and chemoradiotherapy, the 5-year
survival rate in patients with HNSCC has not improved within the
last several decades (8).
Additionally, survival and morbidity rates in patients with HNSCC
have not improved significantly in the last 30 years. While
chemotherapy is thought to be one of the primary clinical
management strategies for HNSCC, the efficacy and safety of
clinical chemotherapeutic agents are not sufficient. Therefore,
recent strategies for developing chemotherapeutic agents have
focused on achieving cancer cell-specific apoptosis without
affecting normal cells (9).
Apoptosis, a process involving programmed cell
death, is an essential physiological process required for normal
tissue development and homeostasis (10). Recently, induction of apoptosis
through induction of DNA damage in cancer cells has been considered
as an effective anticancer strategy (11). Furthermore, based on our
understanding of apoptotic signaling pathways associated with
cancer cell-specific death, molecules targeting apoptotic pathways
have been tested in preclinical and clinical studies (11). However, currently available
chemotherapeutic agents that target molecules associated with
apoptosis have several clinical limitations, such as low efficacy
in cancer cells, high cytotoxicity in normal cells, and induction
of chemoresistance (12).
Therefore, recent studies associated with the development of
chemotherapeutic agents for cancer therapy have explored the
efficacy and safety of natural compounds from herbal plants.
Berberine
(C20H18NO4) is a type of
isoquinoline alkaloid purified from Rhizoma coptidis and
Cortex phellodendri. Recent pharmacological studies of
berberine have demonstrated that this molecule alleviates diabetic
nephropathy (13), rheumatoid
arthritis (14), and inflammation
(15). Moreover, berberine has
antioxidant effects (16,17), prevents obesity (18), and has anticancer activities in
breast (19), prostate (20), cervical (21), gastric (22), oral (23,24),
and ovarian cancers (25). Although
these anticancer effects of berberine and associated cell signaling
pathways have been reported in various cancers, the effects of
berberine on apoptosis have not been reported in HNSCC.
Therefore, the aim of this study is to determine
whether berberine could function as a chemotherapeutic agent in
HNSCC. Furthermore, we evaluated the effects of berberine on
apoptotic signaling pathway in HNSCC.
Materials and methods
Cell culture
FaDu cells, a human pharyngeal squamous carcinoma
cell line, were obtained from the American Type Culture Collection
(ATCC) and cultured according to the instructions provided by the
ATCC. Briefly, FaDu cells were maintained in minimum essential
medium (Life Technologies, Grand Island, NY, USA) containing 10%
fetal bovine serum (FBS). Cells were grown in a humidified
incubator at 37°C in an atmosphere containing 5%
CO2.
Cell cytotoxicity
The cells were cultured at a density of
1×105 cells/ml in 96-well culture plates for 24 h in a
humidified incubator at 37°C in an atmosphere containing 5%
CO2. Cultured FaDu cells were treated with 12.5 or 25
µM berberine for 24 h. Thereafter, 20 µl of 5 mg/ml
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was added to the cultured FaDu cells in the presence of berberine.
After 4 h, the supernatant was removed, and the formazan crystals
formed from reaction with MTT were dissolved in dimethyl sulfoxide
(200 µl/well). Finally, the optical density was measured at
570 nm using a spectrometer. Experiments were performed at least
three times.
Cell survival assay
Cell survival was measured as previously described
(26), using Green calcein-AM and
ethidium homodimer-1 (Life Technologies) to stain viable and dead
cells, respectively. To evaluate cell survival, FaDu cells were
plated on chamber slides and treated with 12.5 or 25 µM
berberine for 24 h. Thereafter, cell survival assays were performed
according to the manufacturer's protocol. Cells were then examined
and imaged using a fluorescence microscope (Eclipse TE200; Nikon
Instruments, Melville, NY, USA).
4′,6-Diamidino-2-phenylindole (DAPI)
staining
To observe nuclear condensation associated with
apoptosis, FaDu cells treated with 12.5 or 25 µM berberine
for 24 h were fixed with 4% paraformaldehyde prior to washing with
phosphate-buffered saline (PBS). The cells were then stained with 1
mg/ml DAPI (Life Technologies) for 20 min in the dark. Nuclear
condensation was observed by fluorescence microscopy (Eclipse
TE200; Nikon Instruments).
Quantification of apoptosis using flow
cytometric analysis
Flow cytometric analysis was performed in cells
co-stained with Annexin V and propidium iodide (PI; Cell Signaling
Technology, Danvers, MA, USA) to detect apoptosis. First, FaDu
cells were plated at 5×105 cells/ml in 6-well plates and
incubated for 24 h in a humidified incubator at 37°C with 5%
CO2. Thereafter, the cells were treated with 25
µM berberine for 24 h. Both floating and attached cells were
then collected, washed twice with ice-cold PBS, and resuspended in
500 µl of 1X binding buffer (BD Biosciences, San Diego, CA,
USA). Annexin V and PI were added to the cells, and cells were
incubated for 15 min at 37°C in the dark. The population of Annexin
V-positive cells and the cell cycle distribution were analyzed
using a BD CellQuest version 3.3 instrument (Becton-Dickinson, San
Jose, CA, USA) and WinMDI version 2.9 software (The Scripps
Research Institute, San Diego, CA, USA).
Western blot analysis
FaDu cells were plated at a density of
5×106 cells/ml in culture dishes and incubated for 24 h
in a humidified incubator at 37°C with 5% CO2. Cultured
FaDu cells were treated with 12.5 or 25 µM berberine for 24
h. Thereafter, cells were harvested, lysed using cell lysis buffer
(Cell Signaling Technology) containing protease and phosphatase
inhibitor cocktails, and incubated for 1 h at 4°C. Lysates were
centrifuged at 14,000 × g for 10 min at 4°C. The supernatant was
used as the cytosolic fraction. Total protein concentrations of the
cell lysates were determined by bicinchoninic acid protein assays
(Thermo Scientific, Rockford, IL, USA). Next, 5X loading buffer was
added to equal amounts of protein, and the mixtures were boiled at
90°C for 10 min. Total proteins were separated using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to nitrocellulose membranes. After blocking for 2 h
with 5% bovine serum albumin in Tris-buffered saline containing
Tween-20 at room temperature, membranes were incubated with primary
antibodies at 4°C overnight and then incubated with horseradish
peroxidase-conjugated secondary antibodies. The following
antibodies (purchased from Cell Signaling Technology) were used to
verify the apoptotic signaling pathways: anti-Fas ligand (FasL; 48
kDa), antitumor necrosis factor-related apoptosis-inducing ligand
(TRAIL; 28 kDa), anti-cleaved caspase-3 (17 and 19 kDa),
anti-cleaved caspase-7 (18 kDa), anti-cleaved caspase-8 (18 kDa),
anti-cleaved caspase-9 (37 kDa), anti-poly(ADP ribose) polymerase
(PARP; full-length form, 116 kDa, cleaved form, 85 kDa), anti-p53
(53 kDa), anti-B-cell lymphoma 2 (Bcl-2; 26 kDa), anti-Bcl
extra-large (Bcl-xL; 26 kDa), anti-Bcl-2-associated X protein (Bax;
21 kDa), anti-Bcl-2-associated death promoter (Bad; 23 kDa), and
anti-apoptotic protease-activating factor 1 (Apaf-1; 13 kDa).
Furthermore, antibodies associated with mitogen-activated protein
kinase (MAPK) signaling (Cell Signaling Technology) were used as
follows: anti-phospho-extracellular signal-regulated kinase
(ERK)1/2 (42 and 44 kDa), anti-ERK1/2 (42 and 44 kDa),
anti-phospho-p38 (38 kDa), anti-p38 (38 kDa), anti-phospho-c-Jun N
terminal kinase (JNK; 46 and 54 kDa), anti-JNK (46 and 54 kDa),
anti-phospho-Akt (60 kDa), anti-Akt (60 kDa), and anti-β-actin (42
kDa). Antibodies against matrix metalloproteinase (MMP)-2 (63 kDa),
MMP-9 (92 kDa), and vascular endothelial growth factor (VEGF; 21
kDa), from Santa Cruz Biotechnology (Dallas, TX, USA), were used to
evaluate the suppression of FaDu cell migration in the presence of
berberine. The immunoreactive bands were visualized using the ECL
system (Amersham Biosciences, Piscataway, NJ, USA) and exposure to
radiographic film.
Caspase-3/-7 activity assay
The apoptotic activities of executioner caspases-3
and -7 were determined using the cell-permeable fluorogenic
substrate PhiPhiLux-G1D2 (OncoImmunin Inc.,
Gaithersburg, MD, USA), according to the manufacturer's
instructions.
Gelatin zymography
Equal volumes of cell culture supernatants were
mixed with non-reducing sample buffer [4% SDS, 0.15 M Tris (pH
6.8), and 20% glycerol containing 0.05% bromophenol blue] and
resolved on 10% polyacrylamide gels containing copolymerized 0.2%
(1 mg/ml) swine skin gelatin (Sigma-Aldrich, St. Louis, MO, USA).
After electrophoresis of the conditioned medium supernatant
samples, gels were washed twice for 15 min each with 2.5% Triton
X-100. Digestion was carried out by incubating the gel in
gelatinase buffer [50 mM Tris-HCl (pH 7.6), 10 mM CaCl2,
50 mM NaCl, and 0.05% Brij-35] at 37°C for 24 h. The gel was
stained with 0.1% Coomassie Brilliant Blue R-250 (GE Healthcare,
Piscataway, NJ, USA), and the locations of gelatinolytic activity
were revealed as clear bands on a background of uniform light blue
staining.
Migration assay
To perform migration assays, FaDu cells were
cultured on culture inserts (2×0.22 cm2; Ibidi,
Regensburg, Germany) at 1×104 cells/well. Wounds were
introduced by removing the culture inserts after 24 h of
incubation. The width of the wound was measured using images
obtained with an inverted microscope.
Quantitative polymerase chain reaction
(qPCR)
FaDu cells were plated at a density of
5×106 cells/ml on culture dishes and incubated for 24 h
at 37°C in an atmosphere containing 5% CO2. Cultured
FaDu cells were treated with 12.5 or 25 µM berberine for 24
h. Thereafter, total RNA was isolated using TRIzol reagent (Life
Technologies) according to the manufacturer's instructions. Total
RNA (1 µg) was reverse transcribed into first-strand cDNA
using the ThermoScript RT-PCR system (Life Technologies). For qPCR,
cDNA was amplified using a SureCycler 8800 (Agilent Technologies,
Santa Clara, CA, USA) and 2X TOPsimple DyeMIX-nTaq (Enzynomics,
Seoul, Korea), according to the instructions of the manufacturers.
Gene expression was determined by agarose gel electrophoresis.
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the
internal control for normalization. The following primers were used
for qPCR: MMP-2 forward, 5′-GGACAAGAACCAGATCACATACA-3′ and
reverse, 5′-CATACTTCACACGGACCACTT-3′ (human MMP-2; PCR product
size: 320 bp; NCBI accession no. NM_001302510.1); MMP-9
forward, 5′-GAGAACCAATCTCACCGACAG-3′ and reverse,
5′-GACACCAAACTGGATGACGA-3′ (human MMP-9; PCR product size, 412 bp;
NCBI accession no. NM_004994.2); and GAPDH forward,
5′-CTTTGGTATCGTG GAAGGACTC-3′ and reverse, 5′-CCTGCTTCACCACCTT
CTT-3′ (human GAPDH; PCR product size, 293 bp; NCBI accession no.
NM_001289746.1). After PCR, amplified PCR products were visualized
by DNA agarose gel electrophoresis. The differences in expression
levels are presented as histograms after densitometry analysis
using a VersaDoc imaging system (Bio-Rad, Hercules, CA, USA).
Caspase-dependent cell survival
assay
The cells were plated at a density of
1×105 cells/ml in 96-well plates and allowed to attach
overnight. After incubation, cultured cells were treated with 12.5
or 25 µM berberine in the presence or absence of 50
µM Z-VAD-fmk [N-benzyloxycarbonyl-Val-Ala-Asp (O-Me)
fluoromethyl ketone], a caspase-3 inhibitor (Sigma-Aldrich) and
were incubated for 24 h at 37°C. The cytotoxicity of berberine was
then measured by MTT assay.
Statistical analysis
Data are reported as the mean ± SD of three
individual experiments performed in triplicate. Statistical
analysis was carried out using Student's t-tests, and differences
with p-values of <0.05 were considered statistically
significant.
Results
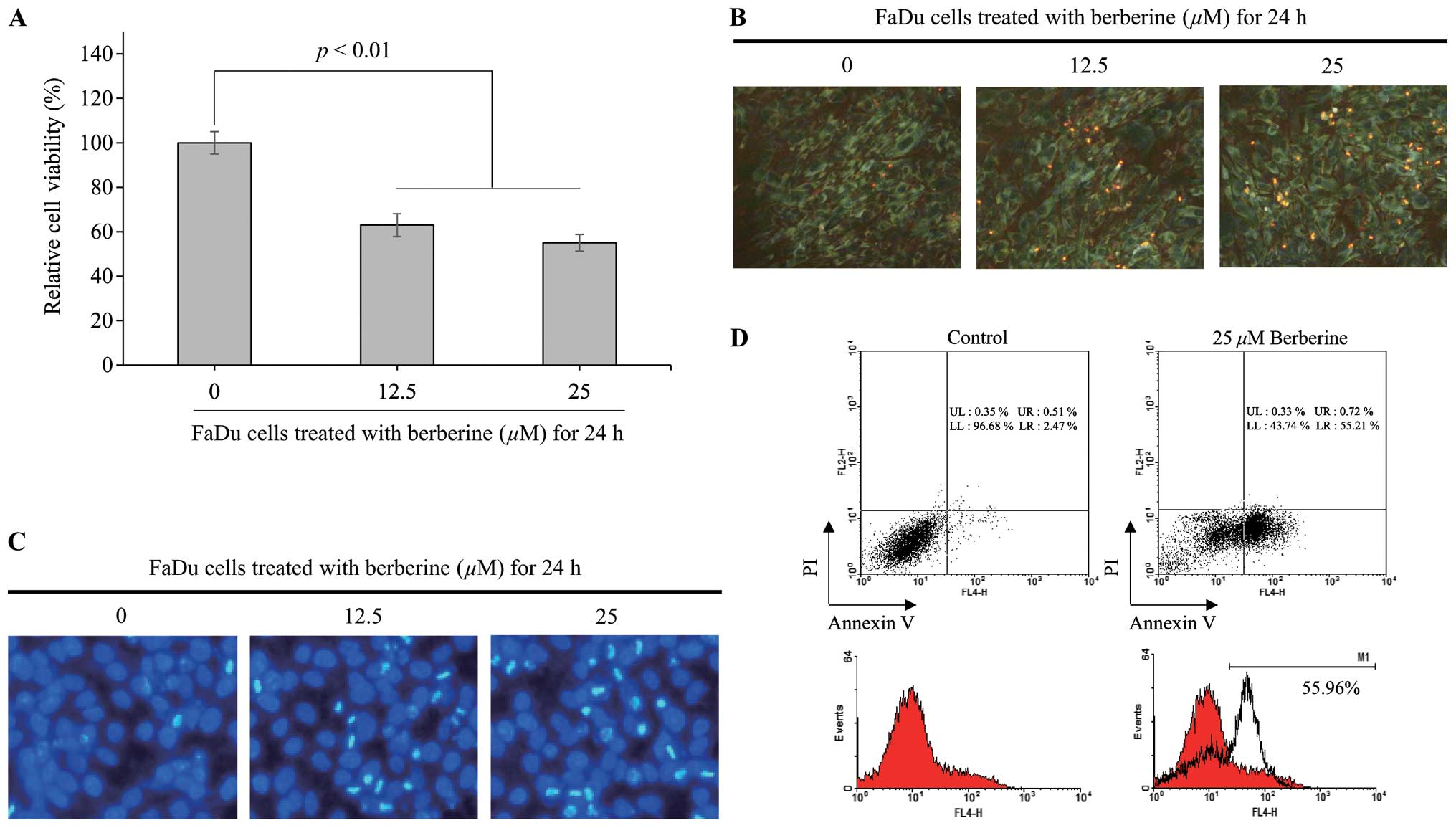
Berberine exhibited cytotoxicity in FaDu
cells
To determine whether berberine was cytotoxic to
HNSCC cells, the viability of FaDu cells was measured by MTT assay
at 24 h after treatment with 12.5 or 25 µM berberine. As
shown in Fig. 1A, the viability of
FaDu cells was decreased by ~37% (p<0.01) and 45% (p<0.01) by
12.5 and 25 µM berberine, respectively, compared with that
in untreated control cells. Furthermore, to confirm the viability
of FaDu cells in presence of 12.5 or 25 µM berberine, we
performed assays for viable and dead using cell-permeable Green
calcein-AM to stain live cells (green fluorescence) and ethidium
homodimer-1 to stain dead cells (red fluorescence). As shown in
Fig. 1B, the density of FaDu cells
treated with 12.5 or 25 µM berberine for 24 h was reduced
compare with that in untreated control cells. Moreover, the number
of dead cells stained with ethidium homodimer-1 was increased in
FaDu cells treated with berberine. Therefore, these data suggested
that berberine was cytotoxic to FaDu cells in a
concentration-dependent manner.
Berberine-induced FaDu cell death was
associated with apoptosis
To determine whether the apoptotic pathway was
involved in the observed berberine-induced FaDu cell death, FaDu
cells were treated with 12.5 or 25 µM berberine for 24 h,
and nuclear staining with DAPI was performed to visualize nucleus
condensation, a typical feature of cells undergoing apoptosis. As
shown in Fig. 1C, berberine
treatment caused a decrease in the density of FaDu cells compared
with that in untreated control cells, consistent with the results
of the viable and dead cell assay. Furthermore, the number of FaDu
cells with condensed nuclei was increased by berberine treatment in
a concentration-dependent manner. Next, to further verify the
effects of berberine on apoptosis in FaDu cells, we performed FACS
analysis using PI and Annexin V staining. As shown in Fig. 1D, the population of apoptotic cells
was significantly increased by ~54.29% in FaDu cells treated with
25 µM berberine for 24 h compared with that in untreated
control cells. Taken together, these data further supported that
berberine induced cytotoxic effects through an apoptotic pathway in
FaDu cells.
Both death receptor-dependent extrinsic
and mitochon-dria-dependent intrinsic apoptotic signaling pathways
were involved in berberine-induced apoptosis in FaDu cells
Next, to elucidate the apoptotic signaling pathways
involved in berberine-induced apoptosis in FaDu cells, we measured
the expression of death receptor ligands, such as FasL and TRAIL,
by western blotting in FaDu cells treated with 12.5 or 25 µM
berberine for 24 h. As shown in Fig.
2A, berberine significantly increased the expression of FasL
and TRAIL in FaDu cells. Subsequently, both cleaved caspase-8 and
cleaved caspase-7 were significantly increased in FaDu cells
treated with berberine in a concentration-dependent manner. These
data clearly suggested that the death receptor-dependent extrinsic
apoptotic signaling pathway was involved in berberine-induced
apoptosis in FaDu cells. Furthermore, the expression levels of
anti-apoptotic factors Bcl-2 and Bcl-xL were significantly
decreased in FaDu cells treated with berberine in a
concentration-dependent manner, as shown in Fig. 2B. Conversely, the expression levels
of pro-apoptotic factors Bax, Bad, Apaf-1, and cleaved caspase-9
were significantly increased by berberine in FaDu cells. These data
indicated that the mitochondria-dependent intrinsic apoptotic
signaling pathway was triggered by cleaved caspase-8 and was
involved in berberine-induced apoptosis in FaDu cells. However,
caspase-3 is a target molecule of both death receptor-dependent
extrinsic and mitochondria-dependent intrinsic apoptosis.
Therefore, we determined the expression of cleaved caspase-3 and
its downstream target molecule PARP. As shown in Fig. 2C, cleaved caspase-3 was
significantly upregu-lated in FaDu cells treated with berberine.
Subsequently, levels of both the pro-form and cleaved form of PARP,
which functions downstream of caspase-3, were significantly
increased by berberine treatment in FaDu cells. To further confirm
the activation of caspase-3 in FaDu cells treated with berberine,
we performed PhiphiLux-caspase-3/-7 assays. As shown in Fig. 2D, the population of FaDu cells
stained with green, representing cleaved caspase-3/-7, was
increased following berberine treatment in a
concentration-dependent manner. Taken together, these data
indicated that berberine-induced apoptosis in FaDu cells was
mediated by the activation of caspase-3 through both the death
receptor-dependent extrinsic pathway and the mitochondria-dependent
intrinsic apoptotic signaling pathway.
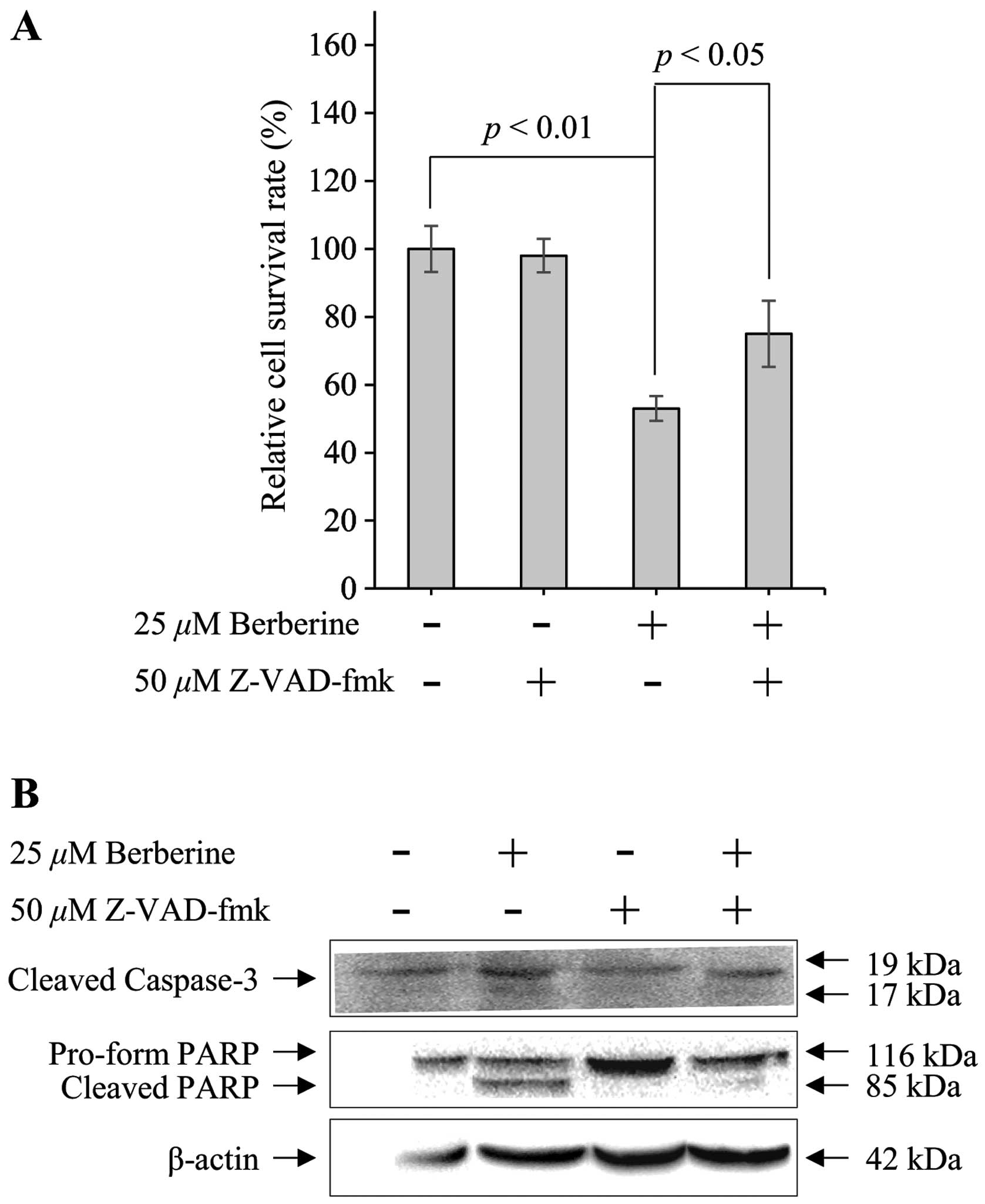
Berberine-induced apoptosis in FaDu cells
was regulated by caspase activation
Apoptotic signaling pathways are mediated by the
activation of the caspase cascade, a hallmark of apoptosis.
Therefore, to determine whether berberine-induced apoptosis in FaDu
cells was dependent on the activation of caspases, FaDu cells were
pretreated for 30 min with 50 µM Z-VAD-fmk, a pan-caspase
inhibitor, prior to treatment with 25 µM berberine. MTT
assays were then performed to measure cell viability, and western
blotting was used to observe changes in the expression of caspase-3
and PARP. As shown in Fig. 3A, 25
µM berberine decreased the viability of FaDu cells by ~45%
compared with that in untreated control cells. However, the
viability of FaDu cells was partially recovered (by ~74%) in the
presence of Z-VAD-fmk and berberine. Furthermore, the
berberine-induced upregulation of cleaved caspase-3 was decreased
by Z-VAD-fmk in FaDu cells. Subsequently, Z-VAD-fmk significantly
suppressed berberine-induced cleavage of the pro-form of PARP in
FaDu cells (Fig. 3B). Therefore,
these data indicated that berberine-induced apop-tosis in FaDu
cells was regulated by the activation of caspases.
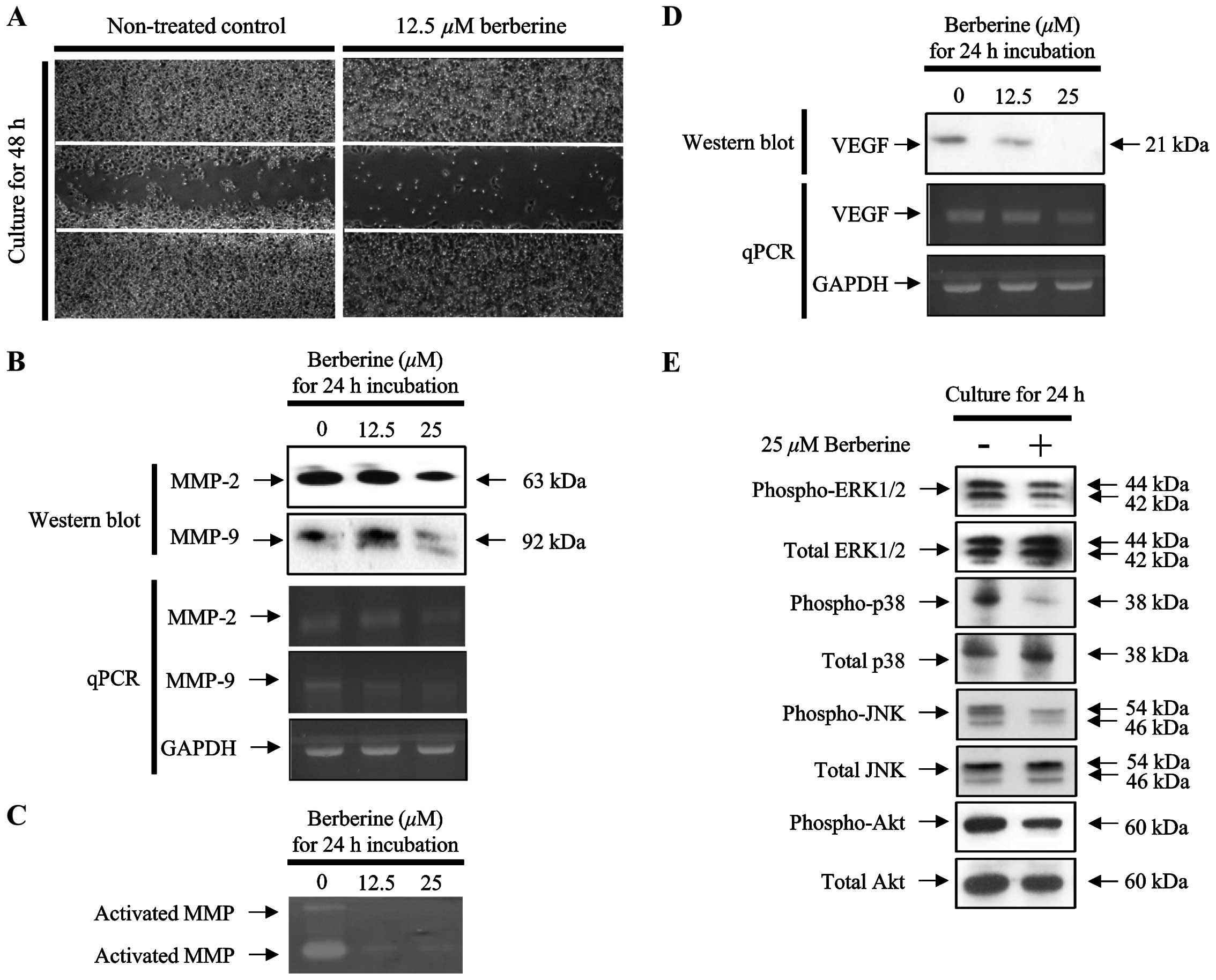
Berberine suppressed the migration of
FaDu cells through the expression and activation of MMP-2 and
MMP-9
Suppression of metastasis is a critical anticancer
effect that should be considered during the development of
chemotherapeutic agents. Therefore, we measured the migration of
FaDu cells following treatment with berberine for 24 and 48 h. As
shown in Fig. 4A, migration was
significantly inhibited in FaDu cells treated with 12.5 µM
berberine compared with that in untreated control cells.
Furthermore, the protein and mRNA expression levels of MMP-2 and
MMP-9, which are known to be associated with cancer cell migration,
were decreased in FaDu cells treated with berberine in a
concentration-dependent manner (Fig.
4B). Therefore, to verify the activation of MMPs secreted from
berberine-treated FaDu cells into the conditioned medium, we
performed the gelatin zymography assays. As shown in Fig. 4C, the clear bands formed by the
digestion of gelatin, which is a substrate of MMP-2 and MMP-9, were
less visible in FaDu cells treated with berberine in a
concentration-dependent manner. Interestingly, the mRNA and protein
expression levels of VEGF were significantly downregulated by
berberine treatment in FaDu cells (Fig.
4D).
Berberine suppressed the phosphorylation
of MAPK in FaDu cells
The expression of MMPs is closely associated with
the activation of the MAPK signaling pathway in various types of
cancer cells. Therefore, we investigated whether the activation of
the MAPK signaling pathway was altered in FaDu cells treated with
berberine. As shown in Fig. 4E, the
phosphorylation of ERK1/2, p38, and JNK was significantly reduced
in FaDu cells treated with 25 µM berberine for 24 h.
Discussion
Recent studies have shown that cancers of the oral
cavity and pharynx have collectively become the six most common
type of cancer worldwide, representing a global public health
problem (27). Although clinical
interventions for the management of oral cancer have improved, the
5-year survival rate for patients with oral cancer is only ~50%
(28).
Surgery is used to treat oral cancer and usually
involves the removal of malignant and adjacent normal tissue in the
oral cavity. However, such surgery may have various clinical side
effects, including changes in the appearance and function of the
oral cavity; this may in turn cause more severe emotional
disturbance than that observed with other types of cancer (29). To reduce the clinical side effects
caused by surgical approaches, chemotherapeutic approaches have
become increasingly important in oral cancer. However, such
chemotherapeutic approaches may have low efficacy and may cause
additional adverse side effects, thereby restricting the use of
many potential chemotherapeutic agents. Recent studies have focused
on biologically active materials purified from medicinal herbs that
are traditionally used in folk medicine and are considered
biologically safe in order to develop chemotherapeutic agents with
high efficacy and fewer side effects. According to the previously
described prerequisites for chemotherapeutic agents, we reported
that berberine did not affect the viability of normal human primary
oral keratinocytes (23).
Furthermore, berberine exhibited cytotoxic effects in KB oral
cancer cells derived from an epidermal carcinoma of the oral cavity
and caused significant cytotoxicity in FaDu cells, as shown in
Fig. 1A and B. These data suggested
that berberine may have fewer side effects by causing cell death
specifically in cancer cells without affecting normal cells.
However, the IC50 value of berberine in FaDu cells was
~25 µM, which was 8-fold higher than that in KB cells, U937
human leukemic monocyte lymphoma cells, and B16 mouse melanoma
cells (23,30). Moreover, the IC50 value
of berberine in SNU-5 human gastric carcinoma cells is 48
µM, which is ~2-fold higher than that in FaDu cells.
Therefore, the IC50 value of berberine may exhibit
tissue-type specificity.
Chromatin condensation caused by DNA fragmentation
is a typical feature of apoptotic cells (31). In the present study, although we did
not observe DNA fragmentation in FaDu cells treated with berberine,
chromatin condensation was observed, as shown in Fig. 1C. Furthermore, FACS analysis using
Annexin V and PI showed that the population of Annexin V-positive
FaDu cells was significantly upregulated following treatment with
berberine. Annexin V, a Ca2+-dependent
phospholipid-binding protein, has high affinity for membrane
phosphatidylserine translocated to the cell surface from the inner
plasma membrane at early stage of apoptosis. However, apoptosis or
necrosis occurring at the late stage of cell death is caused by the
translocation of membrane phosphatidylserine, which precedes the
loss of membrane integrity. Therefore, PI staining was performed to
stain the membranes of dead and damaged cells to identify apoptotic
cells in early and late stages. As shown in Fig. 1D, the apoptotic population was
significantly increased by 55.21% in FaDu cells treated with 25
µM berberine compared with that in untreated control cells.
Interestingly, in FaDu cells treated with berberine for 24 h,
early-stage apoptotic cells accounted for ~54.49% of the
population, while the percentage of late-stage apoptotic cells was
much lower. Taken together, these data showed that apoptotic cell
death was involved in berberine-induced FaDu cell death.
However, apoptosis (programmed cell death), is
tightly regulated by the death receptor-mediated extrinsic pathway
and the mitochondria-dependent intrinsic pathway (32). Furthermore, the selective modulation
of both apoptotic signaling pathways could provide important
insights into the targeting of apoptosis during the development of
novel chemotherapeutic agents (11). Therefore, to examine the involvement
of berberine-induced apoptotic signaling pathways in FaDu cells, we
observed changes in the expression and activation of pro- and
anti-apoptotic factors associated with death receptor-dependent
extrinsic and mitochondria-dependent intrinsic apoptotic signaling
pathways. In the death receptor-mediated extrinsic pathway, death
receptor-specific ligands, such as FasL and TRAIL, trigger the
sequential activation of pro-apoptotic factors, such as caspase-8,
caspase-3, and PARP, thereby inducing cell death (33). Furthermore, activated caspase-8
induces cleavage of cytosolic BH3 interacting-domain death agonist
(BID) to truncated BID (tBID), resulting in loss of mitochondrial
transmembrane potential through the insertion of Bax into the outer
mitochondrial membrane; this triggers the mitochondria-dependent
intrinsic apoptotic signaling pathway (34). Sequentially, the upregulation of Bad
expression and the activation of caspase-9 activate downstream
pro-apoptotic factors, such as caspase-3 and PARP, thereby inducing
cell death. Moreover, the downregulation of anti-apoptotic factors,
such as Bcl-2 and Bcl-xL, accelerate mitochondria-dependent
intrinsic apoptotic cell death (35,36).
Therefore, we measured changes in the expression
levels of death receptor ligands, such as FasL and TRAIL, which
initiate not only the death receptor-dependent extrinsic apoptotic
signaling pathway, but also the mitochondria-dependent intrinsic
apoptotic signaling pathway through the activation of its
downstream target caspase-8. As shown in Fig. 2A, the expression of death receptor
ligands FasL and TRAIL was significantly upregulated in FaDu cells
treated with berberine in a concentration-dependent manner.
Subsequently, the upregulated death receptor ligands (i.e., FasL
and TRAIL) initiated activation of the caspase cascade through the
caspase-8/PARP axis, defined as the death receptor-dependent
extrinsic apoptotic signaling pathway. Moreover, the activated
caspase-8 triggered the mitochondria-dependent intrinsic apoptotic
signaling pathway, which involves upregulation or activation of
pro-apoptotic factors, such as Bax, Bad, Apaf-1, and caspase-9, and
downregulation of anti-apoptotic factors, such as Bcl-2 and Bcl-xL,
as shown in Fig. 2B. Taken
together, these data clearly demonstrated that berberine-induced
apoptosis in FaDu cells was mediated by both the death
receptor-dependent extrinsic and mitochondria-dependent intrinsic
apoptotic signaling pathways. Similar to the our results in present
study, Kim et al (24)
reported that the berberine-induced apoptosis in KB oral cancer
cells was mediated by both death receptor-dependent extrinsic and
mitochondria-dependent intrinsic apoptotic signaling pathways
triggered by the upregulation of the death ligand FasL.
However, the end stage of both apoptotic signaling
pathways is mediated by activation of caspase-3 and PARP (Fig. 2C). Consistent with this, we observed
activation of caspase-3 using the cell-permeable fluorogenic
caspase substrate PhiPhiLux caspase-3/-7 in FaDu cells treated with
berberine, as shown in Fig. 2D.
These data suggested that berberine-induced apoptosis in FaDu cells
may depend on activation of the caspase cascade, a critical early
step in apoptosis. As shown in Fig.
3A, the cytotoxic effects of berberine in FaDu cells were
partially decreased in the presence of Z-VAD-fmk, a pan-caspase
inhibitor. Furthermore, the activation of caspase-9 and its
downstream target PARP was significantly decreased in FaDu cells
co-treated with berberine and Z-VAD-fmk (Fig. 3B). These data further supported that
berberine-induced apoptosis in FaDu cells was dependent on
activation of the caspase cascade.
Interestingly, the expression of the tumor
suppressor p53 was increased in berberine-treated FaDu cells in a
concentration-dependent manner, as shown in Fig. 2B. Upregulation and activation of p53
lead to G1 phase cell cycle arrest (37) and cause initiation of apoptosis
through induction of Bax (38) and
p53 upregulated modulator of apoptosis (PUMA) expression in the
context of DNA damage (39). Recent
studies have reported that berberine-induced upregulation of the
tumor suppressor p53 promotes cell cycle arrest in hepatocellular
carcinoma cells (40) and prostate
cancer cells (41). Therefore,
although we did not observe berberine-induced cell cycle arrest in
FaDu cells in the present study, cell cycle arrest may be induced
in FaDu cells treated with berberine. Further studies are required
to examine these additional potential anticancer effects of
berberine.
In addition, recent studies have suggested that p53
may contribute to the regulation of cell invasion and migration
(42); indeed, p53 mutations cause
loss of cell growth suppression and increase cell migration and
invasion (43–45). Therefore, we measured the migration
of FaDu cells in the presence of berberine. As shown in Fig. 4A, berberine significantly suppressed
the migration of FaDu cells compared with that in untreated
control. Furthermore, the expression and activation of MMPs, such
as MMP-2 and MMP-9, which are associated with cancer cell
migration, were significantly suppressed in FaDu cells treated with
berberine in a concentration-dependent manner, as shown in Fig. 4B and C. Signals from tumor cells can
promote the expression and activation of MMPs, which degrade the
extracellular membrane, thereby initiating cancer cell migration.
In particular, the expression levels of MMP-2 and MMP-9 are closely
associated with the development and progression of cancer (46). Therefore, MMP expression has
interesting implications in the potential use of MMP inhibitors as
chemotherapeutic agents. In the present study, our results clearly
suggested that berberine inhibited the migration of FaDu cells
through suppression of MMP expression and activation.
In the present study, we showed the berberine
reduced the mRNA and protein levels of VEGF, a representative
growth factor associated with angiogenesis, as shown in Fig. 4D. Angiogenesis plays a crucial role
in the development of many types of cancer (47). In particular, the upregulation of
VEGF not only promotes endothelial cell proliferation and
migration, but also increases tumor size by promoting the formation
of new blood vessels and increasing the permeability of existing
blood vessels (47). Therefore,
anti-angiogenesis therapy may be a promising strategy in the
development of chemotherapeutic agents. Moreover, although we did
not observe the biological relationship between p53 and the
expression of VEGF in the present study, others have reported that
p53 indirectly downregulates VEGF expression (48,49).
Previously, we showed that berberine induced upregulation of p53 in
FaDu cells, as shown in Fig. 2B.
Therefore, berberine-induced p53 expression may be associated with
changes in the expression of VEGF in FaDu cells, representing one
of the anticancer effects of berberine.
Next, to verify the cellular signaling pathways
associated with the anticancer activities of berberine, we measured
changes in the expression and activation of MAPK and PI3K/Akt
signaling pathway components in FaDu cells treated with berberine.
Phorbol 12-myristate (PMA) has been shown to induce MMP expression
(50) through activation of the
PI3K/Akt and MAPK signaling pathways (51). Recently, Im et al reported
that inhibition of MAPK activation suppresses PMA-induced MMP-9
expression in MCF-7 human breast cancer cells (53). Furthermore, Lin et al showed
that resveratrol suppresses 12-O-tetradecanoylphorbol-13-acetate
(TPA)-induced MMP-9 expression through inhibition of the MAPK
pathway in oral cancer cells (54).
Although the signaling pathways that lead to upregulation of MMP
expression are still incompletely understood, recent studies have
suggested that the MAPK signaling pathway may regulate the
expression of MMPs associated with cancer cell migration. Thus, the
regulation of MMP expression using specific chemical inhibitors or
natural compounds targeting cellular signaling pathways, such as
the PI3K/Akt and MAPK pathways, could be used as potential
chemotherapeutic agents. In the present study, we showed that the
phosphorylation of MAPKs, such as ERK1/2 and p38, as well as that
of PI3K/Akt was significantly suppressed in FaDu cells treated with
berberine for 24 h; interestingly, the phosphorylation of JNK was
not inhibited, as shown in Fig. 4E.
Therefore, these data suggested that berberine suppressed the
expression of MMPs through inhibition of MAPK and PI3K/Akt
signaling pathways in FaDu cells.
In addition, recent studies have reported the
suppression of angiogenesis by targeting VEGF through the PI3K/Akt
and MAPK signaling pathways in various types of cancer (54,55).
Therefore, the PI3K/Akt and MAPK signaling pathways are closely
associated with the regulation of VEGF expression in cancer.
Furthermore, the inhibition of PI3K/Akt and MAPK signaling pathways
may suppress tumor growth by inhibition of angiogenesis through the
downregulation of VEGF expression (56).
In conclusion, we demonstrated the anticancer
effects of berberine in FaDu cells. In particular, berberine
induced apoptosis, suppressed migration through the downregulation
of MMP expression, and exerted anti-angiogenic effects through the
downregulation of VEGF expression. Taken together, these findings
suggested that berberine may be a promising candidate
chemotherapeutic agent for the treatment of HNSCC.
Acknowledgments
This study was supported by a research fund from the
Chosun University Dental Hospital, 2015.
References
|
1
|
Sturgis EM and Miller RH: Second primary
malignancies in the head and neck cancer patient. Ann Otol Rhinol
Laryngol. 104:946–954. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rothman KJ: The effect of alcohol
consumption on risk of cancer of the head and neck. Laryngoscope.
88(Suppl 8): 51–55. 1978.PubMed/NCBI
|
|
3
|
Maier H, Dietz A, Gewelke U, Heller WD and
Weidauer H: Tobacco and alcohol and the risk of head and neck
cancer. Clin Investig. 70:320–327. 1992.PubMed/NCBI
|
|
4
|
Rothman KJ: Epidemiology of head and neck
cancer. Laryngoscope. 88:435–438. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin YS, Jen YM, Wang BB, Lee JC and Kang
BH: Epidemiology of oral cavity cancer in Taiwan with emphasis on
the role of betel nut chewing. ORL J Otorhinolaryngol Relat Spec.
67:230–236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mannarini L, Kratochvil V, Calabrese L,
Gomes Silva L, Morbini P, Betka J and Benazzo M: Human papilloma
virus (HPV) in head and neck region: Review of literature. Acta
Otorhinolaryngol Ital. 29:119–126. 2009.
|
|
7
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lacko M, Braakhuis BJ, Sturgis EM,
Boedeker CC, Suárez C, Rinaldo A, Ferlito A and Takes RP: Genetic
susceptibility to head and neck squamous cell carcinoma. Int J
Radiat Oncol Biol Phys. 89:38–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park MR, Kim SG, Cho IA, Oh D, Kang KR,
Lee SY, Moon SM, Cho SS, Yoon G, Kim CS, et al: Licochalcone-A
induces intrinsic and extrinsic apoptosis via ERK1/2 and p38
phosphorylation-mediated TRAIL expression in head and neck squamous
carcinoma FaDu cells. Food Chem Toxicol. 77:34–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fesik SW: Promoting apoptosis as a
strategy for cancer drug discovery. Nat Rev Cancer. 5:876–885.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Renehan AG, Booth C and Potten CS: What is
apoptosis, and why is it important? BMJ. 322:1536–1538. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khan KH, Blanco-Codesido M and Molife LR:
Cancer therapeutics: Targeting the apoptotic pathway. Crit Rev
Oncol Hematol. 90:200–219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luqmani YA: Mechanisms of drug resistance
in cancer chemotherapy. Med Princ Pract. 14(Suppl 1): 35–48. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ni WJ, Ding HH and Tang LQ: Berberine as a
promising anti-diabetic nephropathy drug: An analysis of its
effects and mechanisms. Eur J Pharmacol. 760:103–112. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu Z, Jiao Q, Ding J, Liu F, Liu R, Shan
L, Zeng H, Zhang J and Zhang W: Berberine induces dendritic cell
apoptosis and has therapeutic potential for rheumatoid arthritis.
Arthritis Rheum. 63:949–959. 2011. View Article : Google Scholar
|
|
16
|
Xiao HB, Sun ZL, Zhang HB and Zhang DS:
Berberine inhibits dyslipidemia in C57BL/6 mice with
lipopolysaccharide induced inflammation. Pharmacol Rep. 64:889–895.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Z, Geng YN, Jiang JD and Kong WJ:
Antioxidant and anti-in flammatory activities of berberine in the
treatment of diabetes mellitus. Evid Based Complement Alternat Med.
2014:2892642014. View Article : Google Scholar
|
|
18
|
Tan Y, Tang Q, Hu BR and Xiang JZ:
Antioxidant properties of berberine on cultured rabbit corpus
cavernosum smooth muscle cells injured by hydrogen peroxide. Acta
Pharmacol Sin. 28:1914–1918. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Zhao Y, Zhang M, Pang X, Xu J,
Kang C, Li M, Zhang C, Zhang Z, Zhang Y, et al: Structural changes
of gut microbiota during berberine-mediated prevention of obesity
and insulin resistance in high-fat diet-fed rats. PLoS One.
7:e425292012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim S, Choi JH, Kim JB, Nam SJ, Yang JH,
Kim JH and Lee JE: Berberine suppresses TNF-alpha-induced MMP-9 and
cell invasion through inhibition of AP-1 activity in MDA-MB-231
human breast cancer cells. Molecules. 13:2975–2985. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Cao B, Liu X, Fu X, Xiong Z, Chen L,
Sartor O, Dong Y and Zhang H: Berberine suppresses androgen
receptor signaling in prostate cancer. Mol Cancer Ther.
10:1346–1356. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin JP, Yang JS, Chang NW, Chiu TH, Su CC,
Lu KW, Ho YT, Yeh CC, Mei-Dueyang, Lin HJ, et al: GADD153 mediates
berberine-induced apoptosis in human cervical cancer Ca ski cells.
Anticancer Res. 27:3379–3386. 2007.PubMed/NCBI
|
|
23
|
Lin JP, Yang JS, Wu CC, Lin SS, Hsieh WT,
Lin ML, Yu FS, Yu CS, Chen GW, Chang YH, et al: Berberine induced
down-regulation of matrix metalloproteinase-1, -2 and -9 in human
gastric cancer cells (SNU-5) in vitro. In Vivo. 22:223–230.
2008.PubMed/NCBI
|
|
24
|
Kim JS, Oh D, Yim MJ, Park JJ, Kang KR,
Cho IA, Moon SM, Oh JS, You JS, Kim CS, et al: Berberine induces
FasL-related apoptosis through p38 activation in KB human oral
cancer cells. Oncol Rep. 33:1775–1782. 2015.PubMed/NCBI
|
|
25
|
Kuo CL, Chi CW and Liu TY: Modulation of
apoptosis by berberine through inhibition of cyclooxygenase-2 and
Mcl-1 expression in oral cancer cells. In Vivo. 19:247–252.
2005.PubMed/NCBI
|
|
26
|
Jin P, Zhang C and Li N: Berberine
exhibits antitumor effects in human ovarian cancer cells.
Anticancer Agents Med Chem. 15:511–516. 2015. View Article : Google Scholar
|
|
27
|
Kim JS, Ellman MB, An HS, Yan D, van
Wijnen AJ, Murphy G, Hoskin DW and Im HJ: Lactoferricin mediates
anabolic and anti-catabolic effects in the intervertebral disc. J
Cell Physiol. 227:1512–1520. 2012. View Article : Google Scholar
|
|
28
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar
|
|
29
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Silverman S Jr: Oral cancer: Complications
of therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
88:122–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Letasiová S, Jantová S, Cipák L and
Múcková M: Berberine-anti-proliferative activity in vitro and
induction of apoptosis/necrosis of the U937 and B16 cells. Cancer
Lett. 239:254–262. 2006. View Article : Google Scholar
|
|
32
|
Oberhammer FA, Hochegger K, Fröschl G,
Tiefenbacher R and Pavelka M: Chromatin condensation during
apoptosis is accompanied by degradation of lamin A+B, without
enhanced activation of cdc2 kinase. J Cell Biol. 126:827–837. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hensley P, Mishra M and Kyprianou N:
Targeting caspases in cancer therapeutics. Biol Chem. 394:831–843.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ikner A and Ashkenazi A: TWEAK induces
apoptosis through a death-signaling complex comprising
receptor-interacting protein 1 (RIP1), Fas-associated death domain
(FADD), and caspase-8. J Biol Chem. 286:21546–21554. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li H, Zhu H, Xu CJ and Yuan J: Cleavage of
BID by caspase 8 mediates the mitochondrial damage in the Fas
pathway of apoptosis. Cell. 94:491–501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fischer B, Coelho D, Dufour P, Bergerat
JP, Denis JM, Gueulette J and Bischoff P: Caspase 8-mediated
cleavage of the pro-apoptotic BCL-2 family member BID in
p53-dependent apoptosis. Biochem Biophys Res Commun. 306:516–522.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhu J, Xiong L, Yu B and Wu J: Apoptosis
induced by a new member of saponin family is mediated through
caspase-8-dependent cleavage of Bcl-2. Mol Pharmacol. 68:1831–1838.
2005.PubMed/NCBI
|
|
38
|
Harper JW, Adami GR, Wei N, Keyomarsi K
and Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent
inhibitor of G1 cyclin-dependent kinases. Cell. 75:805–816. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chipuk JE, Kuwana T, Bouchier-Hayes L,
Droin NM, Newmeyer DD, Schuler M and Green DR: Direct activation of
Bax by p53 mediates mitochondrial membrane permeabilization and
apoptosis. Science. 303:1010–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang P, Yu J and Zhang L: The nuclear
function of p53 is required for PUMA-mediated apoptosis induced by
DNA damage. Proc Natl Acad Sci USA. 104:4054–4059. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang N, Zhu M, Wang X, Tan HY, Tsao SW and
Feng Y: Berberine-induced tumor suppressor p53 up-regulation gets
involved in the regulatory network of miR-23a in hepatocellular
carcinoma. Biochim Biophys Acta. 1839:849–857. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang Y, Liu Q, Liu Z, Li B, Sun Z, Zhou H,
Zhang X, Gong Y and Shao C: Berberine, a genotoxic alkaloid,
induces ATM-Chk1 mediated G2 arrest in prostate cancer cells. Mutat
Res. 734:20–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu JX, Zhang DG, Zheng JN and Pei DS:
Rap2a is a novel target gene of p53 and regulates cancer cell
migration and invasion. Cell Signal. 27:1198–1207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Boudreau HE, Casterline BW, Burke DJ and
Leto TL: Wild-type and mutant p53 differentially regulate NADPH
oxidase 4 in TGF-β-mediated migration of human lung and breast
epithelial cells. Br J Cancer. 110:2569–2582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Momota H, Narita Y, Matsushita Y, Miyakita
Y and Shibui S: p53 abnormality and tumor invasion in patients with
malignant astrocytoma. Brain Tumor Pathol. 27:95–101. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Muller PA, Caswell PT, Doyle B, Iwanicki
MP, Tan EH, Karim S, Lukashchuk N, Gillespie DA, Ludwig RL,
Gosselin P, et al: Mutant p53 drives invasion by promoting integrin
recycling. Cell. 139:1327–1341. 2009. View Article : Google Scholar
|
|
47
|
Chambers AF and Matrisian LM: Changing
views of the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tomao F, Papa A, Rossi L, Zaccarelli E,
Caruso D, Zoratto F, Benedetti Panici P and Tomao S: Angiogenesis
and anti-angiogenic agents in cervical cancer. Onco Targets Ther.
7:2237–2248. 2014. View Article : Google Scholar
|
|
49
|
Mukhopadhyay D, Tsiokas L and Sukhatme VP:
Wild-type p53 and v-Src exert opposing influences on human vascular
endothelial growth factor gene expression. Cancer Res.
55:6161–6165. 1995.PubMed/NCBI
|
|
50
|
Qin G, Kishore R, Dolan CM, Silver M,
Wecker A, Luedemann CN, Thorne T, Hanley A, Curry C, Heyd L, et al:
Cell cycle regulator E2F1 modulates angiogenesis via p53-dependent
transcriptional control of VEGF. Proc Natl Acad Sci USA.
103:11015–11020. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ehrenfeld P, Conejeros I, Pavicic MF,
Matus CE, Gonzalez CB, Quest AF, Bhoola KD, Poblete MT, Burgos RA
and Figueroa CD: Activation of kinin B1 receptor increases the
release of metalloproteases-2 and -9 from both estrogen-sensitive
and -insensitive breast cancer cells. Cancer Lett. 301:106–118.
2011. View Article : Google Scholar
|
|
52
|
Eberhardt W, Huwiler A, Beck KF, Walpen S
and Pfeilschifter J: Amplification of IL-1 beta-induced matrix
metalloproteinase-9 expression by superoxide in rat glomerular
mesangial cells is mediated by increased activities of NF-kappa B
and activating protein-1 and involves activation of the
mitogen-activated protein kinase pathways. J Immunol.
165:5788–5797. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Im NK, Jang WJ, Jeong CH and Jeong GS:
Delphinidin suppresses PMA-induced MMP-9 expression by blocking the
NF-κB activation through MAPK signaling pathways in MCF-7 human
breast carcinoma cells. J Med Food. 17:855–861. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lin FY, Hsieh YH, Yang SF, Chen CT, Tang
CH, Chou MY, Chuang YT, Lin CW and Chen MK: Resveratrol suppresses
TPA-induced matrix metalloproteinase-9 expression through the
inhibition of MAPK pathways in oral cancer cells. J Oral Pathol
Med. Nov 17–2014.Epub ahead of print. PubMed/NCBI
|
|
55
|
Tong Q, Qing Y, Wu Y, Hu X, Jiang L and Wu
X: Dioscin inhibits colon tumor growth and tumor angiogenesis
through regulating VEGFR2 and AKT/MAPK signaling pathways. Toxicol
Appl Pharmacol. 281:166–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang W, Ren F, Wu Q, Jiang D, Li H and Shi
H: MicroRNA-497 suppresses angiogenesis by targeting vascular
endothelial growth factor A through the PI3K/AKT and MAPK/ERK
pathways in ovarian cancer. Oncol Rep. 32:2127–2133.
2014.PubMed/NCBI
|