Introduction
Oral squamous cell carcinoma (OSCC) commonly
develops on the surface of the tongue (1), larynx (2) or lip (3), the carcinogenesis of which involves
multistep malignant transformation of the surface squamous
epithelium (4). In accordance with
previous studies (5), an earlier
epidemiological analysis for patients with oral cancers in China
has shown that over a half of the examined oral malignancies are
SCC (6), suggesting SCC as the
predominate oral malignancy. Despite recent advances in novel
therapies (7), the prognosis for
patients with OSCC remains poor (8). Thus, it is imperative to identify a
novel therapeutic target in OSCC.
Frizzled2 (Fzd2) is a wingless-type MMTV integration
site family member (Wnt) receptor that was first cloned and
characterized by Sagara et al (9). As is the case for other Fzd isoforms,
Fzd2 has seven transmembrane domains, a cysteine-rich domain in the
N-terminal extracellular region and a C-terminal Ser/Thr-Xxx-Val
motif (9). Fzds and their ligands
Wnts were initially observed to play important roles in regulating
embryonic development (10).
Inappropriate activation of the Wnt/Fzd pathway has been reported
in numerous types of human cancer (11), such as hepatocellular carcinoma
(12) and colorectal cancer
(13). Targeting of frizzled
receptors decreases the growth and tumorigenicity of human
pancreatic, breast and non-small cell lung tumors (14). Despite these previous studies
suggesting an oncogenic property of Fzd2, Ding et al
contrarily identified Fzd2 as a tumor suppressor in salivary
adenoid cystic carcinomas in vitro (15). Since Fzd2 is frequently
overexpressed in head and neck SCC cancer cell lines as compared
with normal bronchial epithelial cells or primary oral squamous
epithelial cells (16), it is
likely that the aberrant overexpression of Fzd2 contributes to OSCC
carcinogenesis. Notably, Prgomet et al have found that the
ectopic expression of Wnt5a, a ligand for Fzd2, promotes OSCC cell
migration and invasion (17).
However their study did not elucidate whether Fzd2 also
participated in OSCC aggressiveness.
Therefore, the present study was conducted to
investigate whether and how Fzd2 affected OSCC cell migration and
invasion in a tongue SCC cell line and TSCCa cells. Stable
overexpression and knockdown of Fzd2 were performed in TSCCa cells.
In addition, the underlying mechanisms were examined.
Materials and methods
Cell culture
Human tongue SCC cancer cells (TSCCa cells) were
purchased from the Cell Bank of Wuhan University (Wuhan, China) and
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand
Island, NY, USA) supplemented with 10% fetal bovine serum (FBS;
HyClone, Logan, UT, USA), 100 U/ml penicillin and 100 µg/ml
streptomycin at 37°C in a humidified atmosphere of 5%
CO2.
Plasmid construction, stable and
transient transfection
Overexpression of Fzd2 in TSCCa cells was performed
using pcDNA3.1+ vector (Clontech Laboratories, Inc., Mountain View,
CA, USA). Briefly, a pair of primers was designed as forward,
5′-ctacaagcttagcatgcggccccgcag-3′ and
reverse, 5′-aatctcgagcgtccctcacacggtggtctca-3′
and synthesized to amplify the complete coding sequence (CDS) area
of Fzd2 gene (NM_001466.3) via reverse transcription PCR
(RT-PCR). The obtained fragments were first subcloned into the
UltraPower pUM-T simple vector (BioTeke, Beijing, China) between
HindIII and XhoI restriction enzyme, sites sequenced,
and then inserted into the pcDNA3.1+ vector (pcDNA3.1-Fzd2). The
empty pcDNA3.1+ vector served as a control (pcDNA3.1-NC). For
knockdown of Fzd2 in cancer cells, pRNA-H1.1 vector (GenScript
Biotechnology Co., Ltd., Nanjing, China) was utilized to generate
shRNA plasmids targeting Fzd2 mRNA in the present study. Briefly, a
pair of oligonucleotides encoding shRNA targeting Fzd2 mRNA (target
sequence, 5′-gaggccaactctcagtact-3′; 1,341–1,359 bp) was
synthesized, annealed and inserted into the pRNA-H1.1 vector
(pRNA-H1.1-Fzd2 shRNA). Scrambled shRNA was also obtained and
inserted into the pRNA-H1.1 plasmid as the negative control
(pRNA-H1.1-NC).
For establishment of oral cancer cells with
increased or decreased Fzd2 expression, G418 (Invitrogen Life
Technologies, Carlsbad, CA, USA) screen was performed. TSCCa cells
(3x105) were first seeded in 6-well plates and allowed
to grow for 24 h. The cells were transfected with pcDNA3.1-Fzd2,
pRNA-H1.1-Fzd2 shRNA or negative control plasmids (2 µg for
each) using Lipofectamine 2000 (Invitrogen Life Technologies)
according to the manufacturer's intstructions. At 24 h-post
transfection, G418 (200 µg/ml) was added into the cell
culture to screen the cells for 1–2 weeks. Cell clones that
resisted G418 were selected and assayed for Fzd2 mRNA and protein
expression.
To examine whether the signal transducer and
activator of transcription-3 (STAT3) signaling pathway was involved
in Fzd2-mediated TSCCa cell metastasis, shRNA targeting STAT3 mRNA
(NM_139276.2; target sequence, 5′-ggtgtctccactggtctat-3′;
2,238–2,256 bp) was obtained and used to transiently transfect
TSCCa cells using Lipofectamine 2000. After 24 h the cells
transfected with pRNA-H1.1-STAT3 shRNA plasmid were subjected to
further analysis.
Quantitative PCR
Total RNAs were isolated from cell lysates using an
RNA Simple Total RNA kit (Tiangen, Beijing, China), and then
processed for cDNA synthesis with a Super M-MLV Reverse
Transcriptase kit (BioTeke Corp., Beijing, China). Fzd2 mRNA levels
were determined using SYBR-Green (Solarbio, Beijing, China), and
normalized to β-actin via a comparative threshold cycle (CT) method
(2−ΔΔCt) (18). Primers
used for determining Fzd2 mRNA expression were as follows: forward,
5′-cacggacatcgcctacaacc-3′ and reverse,
5′-gcaccttcaccagcggatag-3′.
Western blot analysis
Total proteins were extracted from cells with NP-40
lysis buffer (Beyotime, Shanghai, China), fractionated on sodium
dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE),
transferred onto polyvinylidene fluoride (PVDF) membranes
(Millipore, Bedford, MA, USA) and blocked with 5% (w/v) skim milk.
The membranes were incubated with goat polyclonal antibodies
against Fzd2 (1:200 dilution) and phosphorylated p-STAT3Tyr
705 (1:200 diluted) (both from Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), and rabbit polyclonal antibodies
against epithelialcadherin (E-cadherin; 1:400 dilution; Boster,
Wuhan, China), vimentin (1:500 dilution), Snail (1:500 dilution),
Slug (1:500 dilution) (all from Bioss, Beijing, China), matrix
metalloproteinase (MMP)-2 (1:400 dilution), MMP-9 (1:400 dilution)
(both from Boster), MMP-13 (1:500 dilution; Bioss), a-disintegrin
and metalloproteinase with thrombospondin motifs-5 (ADAMTS5; 1:250
dilution; Abcam, Cambridge, UK) and total STAT-3 (1:200 dilution;
Santa Cruz Biotechnology, Inc.) at 4°C overnight. HRP-conjugated
secondary antibodies were used to incubate the membranes for 45 min
at 37°C. The protein blots were visualized with an enhanced
chemiluminescence (ECL) kit (Seven Seas Pharmtech Co., Ltd.,
Shanghai, China), and normalized to a protein density of endogenous
β-actin.
Assessment of cell migration and
invasion
Transwell inserts (8-µm pores; Corning
Incorporated Life Sciences, Tewksbury, MA, USA) coated with or
without Matrigel (BD Biosciences, San Jose, CA, USA) were used to
detect cell invasion and migration, respectively. For the invasion
assay, the upper surface of the filter was first coated with
Matrigel. Cancer cells (2x104) in 200 µl
serum-free DMEM medium were placed onto the upper chambers, and the
lower chambers were filled with complete media. After 24 h, the
inner surface of the upper chambers was wiped with cotton swabs,
and the invasive cells were fixed with paraformaldehyde (Sinopharm,
Shanghai, China) for 20 min, and then stained with hematoxylin for
5 min. The stained cells were then counted in five random areas in
triplicate wells. A cell migration assay was performed using the
Transwell inserts without Matrigel.
Immunofluorescent staining
TSCCa cells were first grown to subconfluence on
coverslips, fixed in paraformaldehyde and then permeabilized with
0.1% Triton X-100 (Amresco China, Shanghai, China). E-cadherin
protein was immunodetected with a rabbit polyclonal antibody
against E-cadherin (1:200 dilution; Boster) and Cy3-conjugated
secondary antibody, and the nuclei were visualized with
4′,6-diamidino-2-phenylindole (DAPI; Biosharp, Hefei, China).
Thereafter, the coverslips were mounted onto glass slides and
viewed under a microscope (Olympus, Tokyo, Japan).
Statistical analysis
Data are presented as the mean ± standard deviation
(SD), and were assessed by one-way analysis of variance followed by
the Bonferroni post hoc test. P<0.05 was considered to indicate
a statistically significant result.
Results
Stable overexpression and silencing of
Fzd2 in OSCC cells
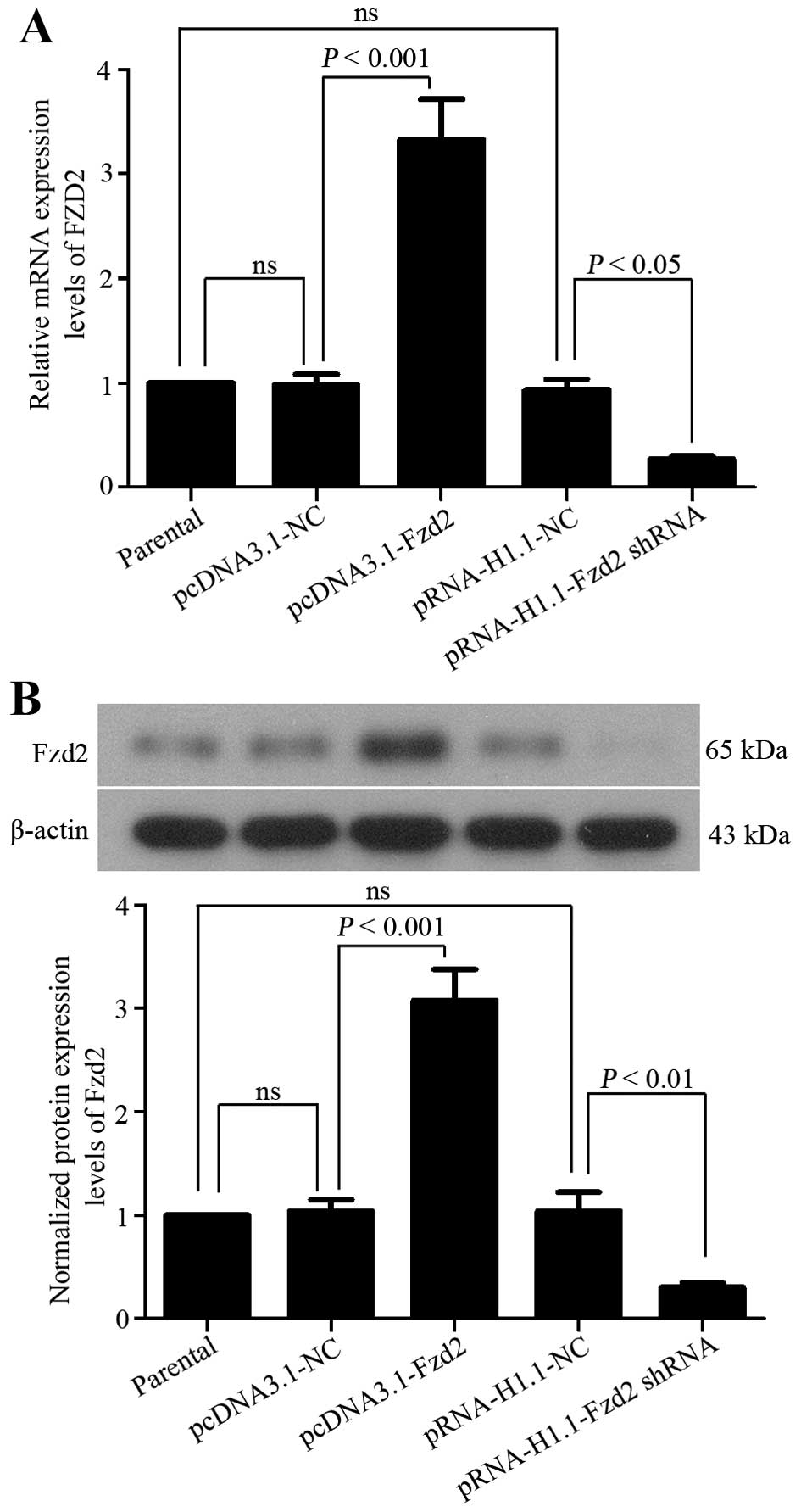
To up- or downregulate Fzd2 expression in TSCCa
cells that express a moderate level of Fzd2, pcDNA3.1-Fzd2,
pRNA-H1.1-Fzd2 shRNA plasmids or their corresponding negative
control plasmids were constructed to transfect the TSCCa cells. The
mRNA and protein expression levels of Fzd2 was then determined in
cells resisting G418 with quantitative PCR and western blot
analysis, respectively. The pcDNA3.1-Fzd2 plasmids led to a
significant upregulation in Fzd2 expression in TSCCa cells
(Fig. 1). In addition, Fzd2
expression in the TSCCa cells transfected with the Fzd2 shRNA
showed efficient depletion (Fig.
1). These results indicated that TSCCa cells with a higher or
lower expression of Fzd2 were successfully established in the
present study.
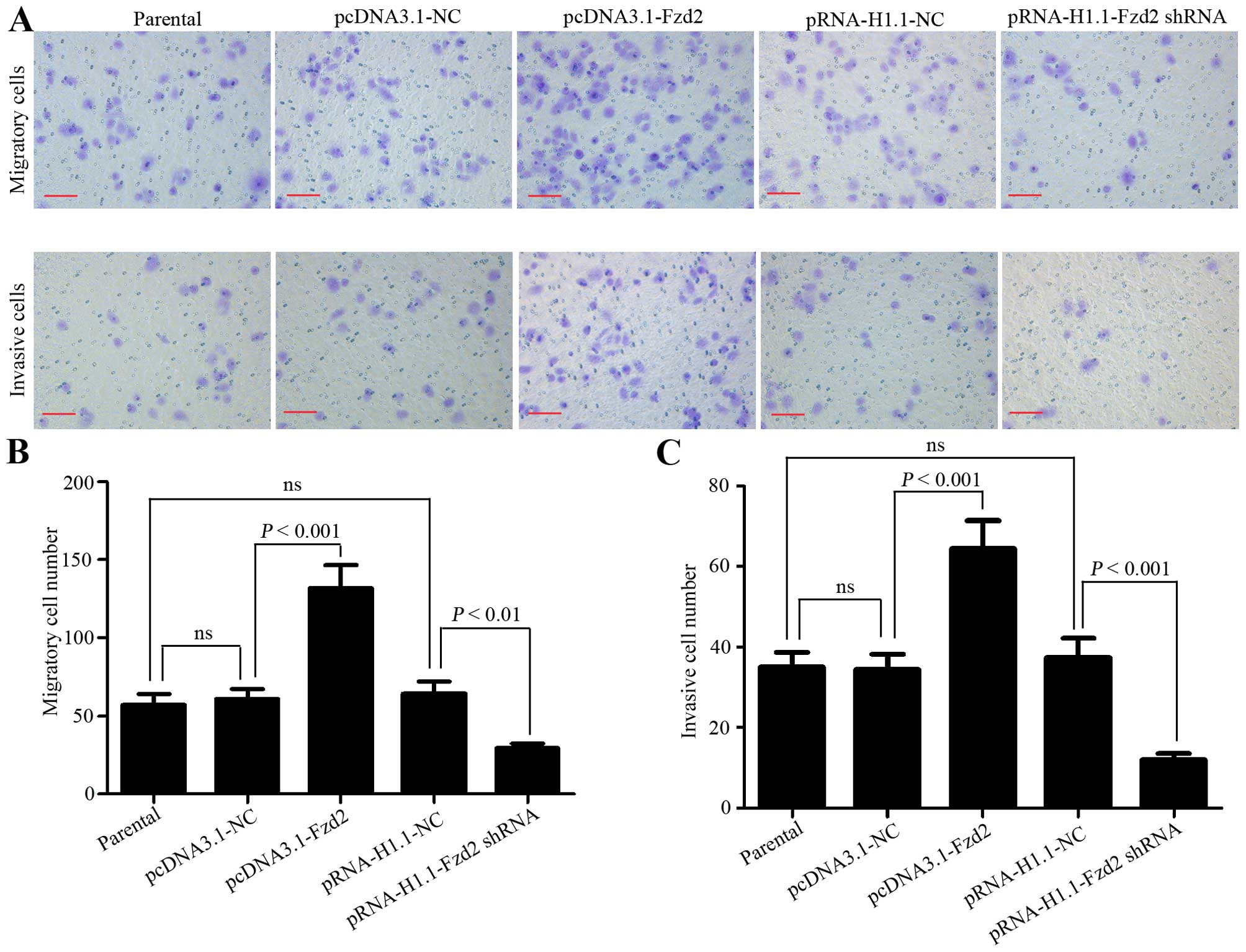
Fzd2 mediates OSCC cell migration and
invasion in vitro
Transwell inserts coated with or without Matrigel
were used to investigate the impact of Fzd2 on TSCCa cell invasion
and migration, respectively. The results showed that the migratory
cell number was increased from 60.8±6.3 to 131.6±15 when Fzd2 was
overexpressed, but decreased from 64±8.2 to 29.2±3.3 when Fzd2 was
underexpressed (Fig. 2A and B).
Similar change patterns of the invasive cell number were obtained
from the invasion assay (pcDNA3.1-NC vs. pcDNA3.1-Fzd2=34.4±3.9 vs.
64.6±6.9; pRNA-H1.1-NC vs. pRNA-H1.1-Fzd2 shRNA=37.4±4.9 vs.
12±1.6; Fig. 2A and C). These
results revealed that the migratory and invasive capabilities of
TSCCa cells were positively correlated with Fzd2 expression levels,
suggesting that Fzd2 mediated the aggressive metastasis of OSCC
cells in vitro.
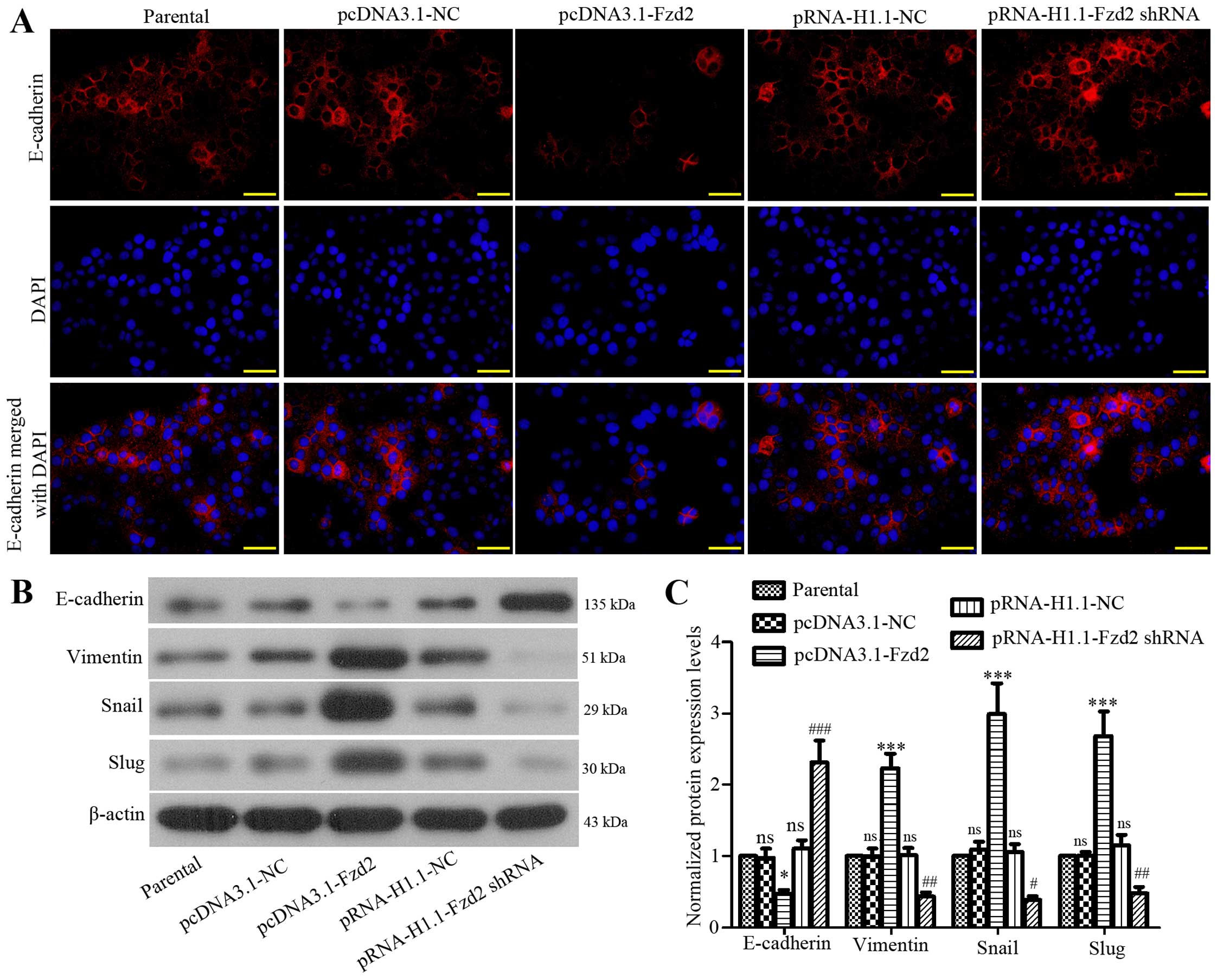
Fzd2 promotes the epithelial-mesenchymal
transition (EMT) in OSCC cells
Tumor cells usually undergo partial or full EMT, and
are converted to migratory and invasive cells (19). We here determined whether Fzd2
played a role in the EMT process of TSCCa cells. The expression of
E-cadherin (an epithelial marker) was significantly increased in
TSCCa cells when Fzd2 was inhibited, but decreased when Fzd2 was
overexpressed as determined by immunofluorescent staining (Fig. 3A). The subsequent western blot
analysis confirmed the immunofluorescent staining results of
E-cadherin (Fig. 3B and C).
Moreover, the expression levels of the E-cadherin suppressors Snail
and Slug, and mesenchymal marker vimentin (20) were increased in TSCCa cells after
Fzd2 overexpression, and decreased following Fzd2 depletion
(Fig. 3B and C). The results showed
that Fzd2 contributed to OSCC cell EMT by mediating the above
mentioned EMT-related proteins.
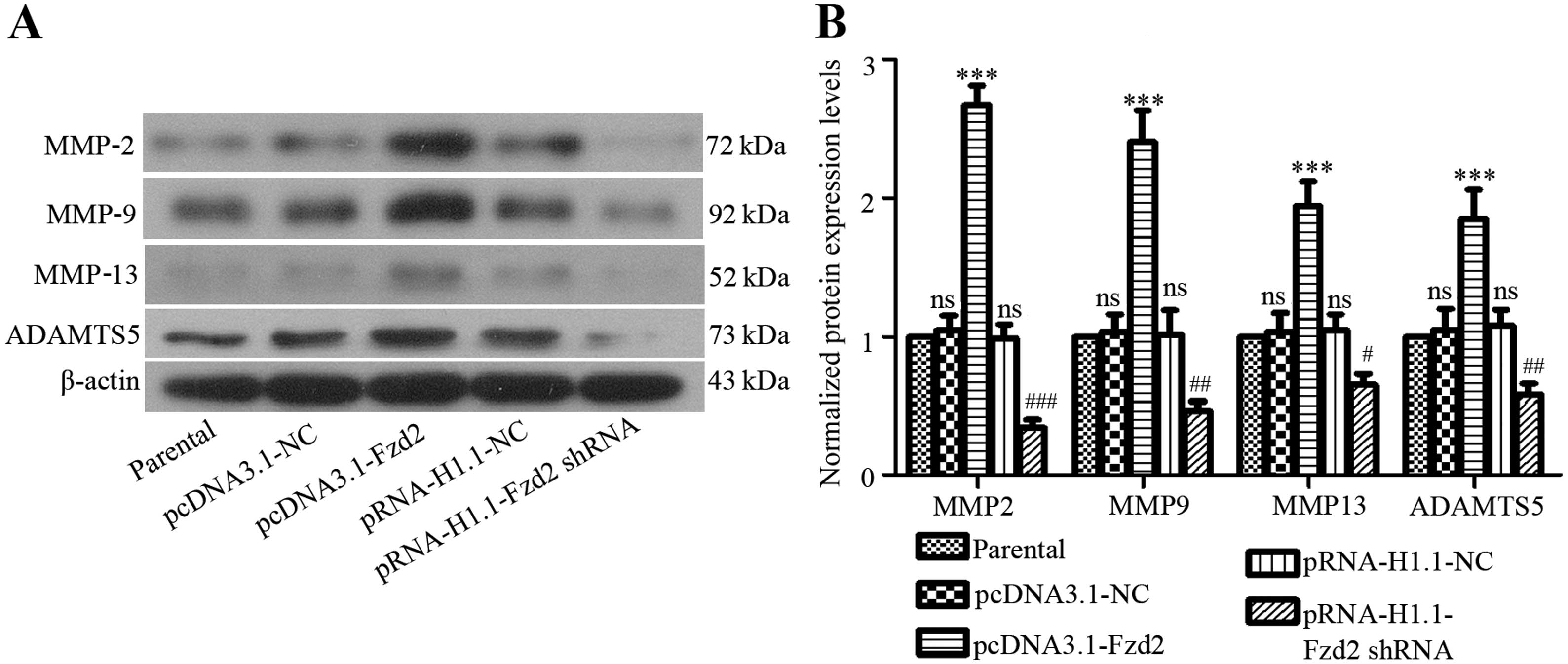
Fzd2 mediates the expression of multiple
matrix metalloproteinases in OSCC cells
Degradation of extracellular matrix (ECM) enabled
the invasive behavior of tumor cells (21). The involvement of zinc
metalloproteinases, including MMPs and ADAMTSs, which are
representative families, have been identified in oral cancer
development (22,23). We therefore examined whether the
expression levels of important MMPs in TSCCa cells were affected by
Fzd2 in the present study. As compared with the negative control
cells, the expression levels of MMP-2, -9 and -13 and ADAMTS5 were
upregulated in Fzd2-overexpressed TSSCa cells, but downregulated in
Fzd2-depleted TSSCa cells (Fig.
4).
STAT3 signaling pathway is involved in
Fzd2-mediated OSCC cell invasion
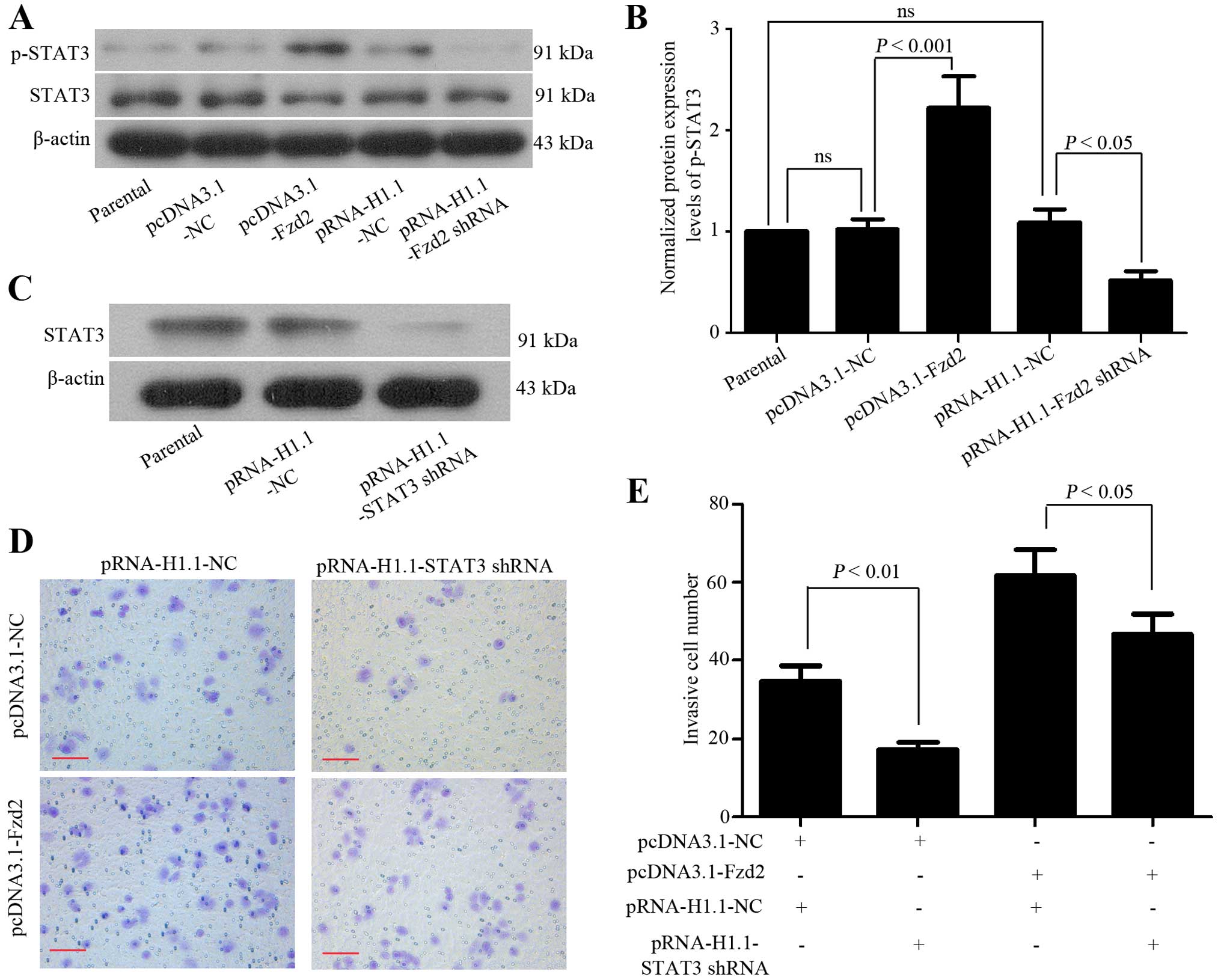
Since the aberrant activation of STAT3 signaling
transduction is involved in the invasive behaviors of cancer cells
(24), we investigated whether this
pathway was involved in Fzd2-mediated OSCC cell invasion. The
western blot analysis showed that the phosphorylation of STAT3 was
enhanced in TSCCa cells overexpressing Fzd2, but suppressed in
those depleting Fzd2 (Fig. 5A and
B). To examine whether the activated STAT3 signal contributed
to Fzd2-induced OSCC cell invasion, a specific shRNA targeting
STAT3 mRNA was used to transfect the TSCCa cells that overexpressed
Fzd2. STAT3 expression in the TSCCa cells transfected with the
STAT3 shRNA showed efficient depletion (Fig. 5C). The matrigel invasion assay
revealed that the Fzd2 overexpression induced the invasion of TSCCa
cells was attenuated when endogenous STAT3 was suppressed (Fig. 5D and E). These results suggested
that Fzd2 mediated OSCC cell invasion at least partly through
mediation of the STAT3 signaling pathway.
Discussion
Findings of a recent study have shown that the
ectopic expression of Wnt5a (a Fzd2 ligand) promotes OSCC cell
migration and invasion in vitro (17), suggesting that the Wnt5a-Fzd2
pathway may be involved in OSCC metastasis. Although the
overexpression of Fzd2 has been identified in a variety of OSCC
cell lines (25), its functional
role in OSCC development and progression remains elusive. The
present study was thus conducted to examine the potential effects
of Fzd2 on OSCC cell migration and invasion through loss- or
gain-of-function experiments.
The most common location for OSCC is tongue
(26), and therefore the TSCCa cell
line established from tongue SCC (27) was used in the present study. Loss-
or gain-of-function of Fzd2 was performed in TSCCa cells,
respectively. The results of the present study showed that the
migratory and invasive abilities of TSCCa cells were enhanced when
the endogenous Fzd2 was upregulated, but suppressed when Fzd2 was
downregulated. These results suggested that Fzd2 played an
oncogenic role in OSCC cells in vitro.
The metastatic process requires cancer cells to exit
the primary tumor sites and to acquire migratory and invasive
capabilities, in which EMT plays a critical role (20). EMT initiation leads to the
destabilization of epithelial cell-cell junctions, which is
accompanied by the degradation of multiple junction proteins
(28). Since the downregulation of
E-cadherin is the hallmark of EMT as previously described (19), we determined whether Fzd2 affected
its expression in OSCC cells. We found that while Fzd2
overexpression induced a marked reduction in E-cadherin expression
in TSCCa cells, Fzd2 knockdown induced an increase in E-cadherin.
Opposite expression alterations in E-cadherin transcriptional
suppressors, Snail and Slug (20),
were observed in these cells. It has been reported that changes in
the intermediate filament are also associated with cell motility
and invasive abilities (20).
Vimentin filaments usually replace intermediate cytokeratin
filaments in mesenchymal-like cells that potentially favor local
invasion and metastasis (29). Our
results showed that TSCCa cells with enhanced migratory and
invasive properties had a higher expression of vimentin, and that
vimentin expression was positively regulated by Fzd2. In accordance
with a comprehensive study conducted in liver, lung, colon and
breast cancer cell lines (30), the
present study demonstrated that Fzd2 played a role in the EMT
process of OSCC cells at least partly through regulation of
EMT-related factors.
ECM is a highly dynamic structure and its
dysregulation has been recognized to contribute to numerous
pathological conditions, including invasive cancers (22). Cancer cells use multiple modes to
invade through ECM (31), and this
process is mediated by specific enzymes that are responsible for
ECM degradation such as metalloproteinases (22). MMP-2, -9 and -13 are major
metalloproteinases, the increased expression levels of which are
frequently observed in OSCCs (32–34).
The present results showing that the expression levels of these
MMPs in TSCCa cells were positively regulated by Fzd2 suggest that
the MMPs were involved in Fzd2-mediated OSCC cell migration and
invasion. ADAM proteins share a metalloproteinase domain with MMPs,
and have been classified as membrane-anchored ADAMs and secreted
ADAMs with thrombospondin motifs, referred to as ADAMTSs (23). ADAMTS5 is a representative
metalloproteinase in the ADAM family, and accumulated lines of
evidence have shown that these matrix-degrading proteases are
expressed in malignant tumors and participate in tumorigenesis
(23). Notably, ADAMTS5 is markedly
overexpressed in laryngeal SCC tissues as compared to normal
tissues (35). The aberrant
overexpression of ADAMTS5 may be associated with the tumorigenesis
of OSCC. To the best of our knowledge, the present study is the
first to identify that the expression of ADAMTS5 was regulated by
Fzd2 in TSCCa cells.
Earlier studies have demonstrated that
β-catenin-dependent (canonical) and β-catenin-independent
(non-canonical) signaling can be activated by Fzd2 (36). Recently, the STAT3 signaling pathway
has been identified as a new non-canonical pathway downstream of
Fzd2 in cancer cell lines (30).
Corresponding to such previous results, we also found that Fzd2
overexpression enhanced OSCC cell migration and invasion at least
partly by promoting STAT3 signaling activation. Notably, it has
been reported that the administration of recombinant Wnt5a promotes
OSCC cell migration through the Wnt/planar cell polarity (PCP)
pathway and/or the Wnt/Ca2+ pathway (17). Given the fact that Wnt5a can be
activated by Fzd2 in an autocrine-positive feedback manner
(30), further studies are being
conducted in our laboratory to investigate whether Fzd2 mediates
the malignant behaviors of OSCC cells through the Wnt/PCP and/or
Wnt/Ca2+ pathway.
In summary, the present study provides evidence that
Fzd2 mediates the migration and invasion of OSCC cells, at least
partly by regulating the STAT3 signaling pathway. These results
suggest Fzd2 is a novel therapeutic target for OSCC.
Acknowledgments
The present study was supported by grants from the
Science and Technology Project of Shenyang City (no. F14-158-9-38),
the Youth Startup Foundation of School of Stomatology, China
Medical University (no. K101593-15-01) and the Department of
Education, Liaoning Province (no.: L2015597).
References
|
1
|
Knopf A, Lempart J, Bas M,
Slotta-Huspenina J, Mansour N and Fritsche MK: Oncogenes and tumor
suppressor genes in squamous cell carcinoma of the tongue in young
patients. Oncotarget. 6:3443–3451. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taguchi T, Nishimura G, Takahashi M,
Komatsu M, Sano D, Sakuma N, Arai Y, Yamashita Y, Shiono O, Hirama
M, et al: Treatment results and prognostic factors for advanced
squamous cell carcinoma of the larynx treated with concurrent
chemoradiotherapy. Cancer Chemother Pharmacol. 72:837–843. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zitelli KB, Zedek D, Ranganathan P and
Amerson EH: Squamous cell carcinoma of the lip associated with
adalimumab therapy for ankylosing spondylitis: A case report and
review of TNF-α inhibitors and cutaneous carcinoma risk. Cutis.
92:35–39. 2013.PubMed/NCBI
|
|
4
|
Dionne KR, Warnakulasuriya S, Zain RB and
Cheong SC: Potentially malignant disorders of the oral cavity:
Current practice and future directions in the clinic and
laboratory. Int J Cancer. 136:503–515. 2015.
|
|
5
|
Wenig BM: Squamous cell carcinoma of the
upper aerodigestive tract: Precursors and problematic variants. Mod
Pathol. 15:229–254. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han S, Chen Y, Ge X, Zhang M, Wang J, Zhao
Q, He J and Wang Z: Epidemiology and cost analysis for patients
with oral cancer in a university hospital in China. BMC Public
Health. 10:1962010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fury MG, Xiao H, Sherman EJ, Baxi S,
Smith-Marrone S, Schupak K, Gewanter R, Gelblum D, Haque S, Schoder
H, et al: A phase II trial of bevacizumab + cetuximab + cisplatin
with concurrent intensity modulated radiation therapy (IMRT) for
patients with stage III/IVB head and neck squamous cell carcinoma
(HNSCC). Head Neck. Mar 17–2015.Epub ahead of print. View Article : Google Scholar
|
|
8
|
Simpson DR, Mell LK and Cohen EE:
Targeting the PI3K/AKT/mTOR pathway in squamous cell carcinoma of
the head and neck. Oral Oncol. 51:291–298. 2015. View Article : Google Scholar
|
|
9
|
Sagara N, Toda G, Hirai M, Terada M and
Katoh M: Molecular cloning, differential expression, and
chromosomal localization of human frizzled-1, frizzled-2, and
frizzled-7. Biochem Biophys Res Commun. 252:117–122. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peifer M and Polakis P: Wnt signaling in
oncogenesis and embryogenesis - a look outside the nucleus.
Science. 287:1606–1609. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ueno K, Hirata H, Hinoda Y and Dahiya R:
Frizzled homolog proteins, microRNAs and Wnt signaling in cancer.
Int J Cancer. 132:1731–1740. 2013. View Article : Google Scholar
|
|
12
|
Lee HC, Kim M and Wands JR: Wnt/Frizzled
signaling in hepatocellular carcinoma. Front Biosci. 11:1901–1915.
2006. View Article : Google Scholar
|
|
13
|
Hlubek F, Spaderna S, Schmalhofer O, Jung
A, Kirchner T and Brabletz T: Wnt/FZD signaling and colorectal
cancer morphogenesis. Front Biosci. 12:458–470. 2007. View Article : Google Scholar
|
|
14
|
Gurney A, Axelrod F, Bond CJ, Cain J,
Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, et
al: Wnt pathway inhibition via the targeting of Frizzled receptors
results in decreased growth and tumorigenicity of human tumors.
Proc Natl Acad Sci USA. 109:11717–11722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ding LC, Huang XY, Zheng FF, Xie J, She L,
Feng Y, Su BH, Zheng DL and Lu YG: FZD2 inhibits the cell growth
and migration of salivary adenoid cystic carcinomas. Oncol Rep. Feb
19–2015.Epub ahead of print. View Article : Google Scholar
|
|
16
|
Rhee CS, Sen M, Lu D, Wu C, Leoni L, Rubin
J, Corr M and Carson DA: Wnt and frizzled receptors as potential
targets for immunotherapy in head and neck squamous cell
carcinomas. Oncogene. 21:6598–6605. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prgomet Z, Axelsson L, Lindberg P and
Andersson T: Migration and invasion of oral squamous carcinoma
cells is promoted by WNT5A, a regulator of cancer progression. J
Oral Pathol Med. Dec 2–2014.Epub ahead of print. PubMed/NCBI
|
|
18
|
Schefe JH, Lehmann KE, Buschmann IR, Unger
T and Funke-Kaiser H: Quantitative real-time RT-PCR data analysis:
Current concepts and the novel 'gene expression's CT difference'
formula. J Mol Med Berl. 84:901–910. 2006. View Article : Google Scholar
|
|
19
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
van Goor H, Melenhorst WB, Turner AJ and
Holgate ST: Adamalysins in biology and disease. J Pathol.
219:277–286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bonnans C, Chou J and Werb Z: Remodelling
the extracellular matrix in development and disease. Nat Rev Mol
Cell Biol. 15:786–801. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mochizuki S and Okada Y: ADAMs in cancer
cell proliferation and progression. Cancer Sci. 98:621–628. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signalling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Díaz Prado SM, Medina Villaamil V,
Aparicio Gallego G, Blanco Calvo M, López Cedrún JL, Sironvalle
Soliva S, Valladares Ayerbes M, García Campelo R and Antón Aparicio
LM: Expression of Wnt gene family and frizzled receptors in head
and neck squamous cell carcinomas. Virchows Arch. 455:67–75. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sano D and Myers JN: Metastasis of
squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev.
26:645–662. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang YX, Yu SB, Ou-Yang JP, Xia D, Wang M
and Li JR: Effect of protein kinase C alpha, caspase-3, and
survivin on apoptosis of oral cancer cells induced by
staurosporine. Acta Pharmacol Sin. 26:1365–1372. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang RY, Guilford P and Thiery JP: Early
events in cell adhesion and polarity during epithelial-mesenchymal
transition. J Cell Sci. 125:4417–4422. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gujral TS, Chan M, Peshkin L, Sorger PK,
Kirschner MW and MacBeath G: A noncanonical Frizzled2 pathway
regulates epithelial-mesenchymal transition and metastasis. Cell.
159:844–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Orgaz JL, Pandya P, Dalmeida R,
Karagiannis P, Sanchez-Laorden B, Viros A, Albrengues J, Nestle FO,
Ridley AJ, Gaggioli C, et al: Diverse matrix metalloproteinase
functions regulate cancer amoeboid migration. Nat Commun.
5:42552014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chiang WC, Wong YK, Lin SC, Chang KW and
Liu CJ: Increase of MMP-13 expression in multi-stage oral
carcinogenesis and epigallocatechin-3-gallate suppress MMP-13
expression. Oral Dis. 12:27–33. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Henriques AC, de Matos FR, Galvão HC and
Freitas RA: Immunohistochemical expression of MMP-9 and VEGF in
squamous cell carcinoma of the tongue. J Oral Sci. 54:105–111.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ruokolainen H, Pääkkö P and
Turpeenniemi-Hujanen T: Tissue and circulating immunoreactive
protein for MMP-2 and TIMP-2 in head and neck squamous cell
carcinoma - tissue immunoreactivity predicts aggressive clinical
course. Mod Pathol. 19:208–217. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Filou S, Stylianou M, Triantaphyllidou IE,
Papadas T, Mastronikolis NS, Goumas PD, Papachristou DJ, Ravazoula
P, Skandalis SS and Vynios DH: Expression and distribution of
aggrecanases in human larynx: ADAMTS-5/aggrecanase-2 is the main
aggrecanase in laryngeal carcinoma. Biochimie. 95:725–734. 2013.
View Article : Google Scholar
|
|
36
|
Grumolato L, Liu G, Mong P, Mudbhary R,
Biswas R, Arroyave R, Vijayakumar S, Economides AN and Aaronson SA:
Canonical and noncanonical Wnts use a common mechanism to activate
completely unrelated coreceptors. Genes Dev. 24:2517–2530. 2010.
View Article : Google Scholar : PubMed/NCBI
|