Introduction
Melanoma is a malignant tumor that forms in the skin
and accounts for 75% of skin cancer mortality (1). High mortality in melanoma cases is
caused by the rapid proliferation rate of cells and their potential
to metastasize at an early stage. For example, many melanomas have
already metastasized to the lymph nodes and other organs by the
time of diagnosis. Therefore, new strategies for rapid diagnosis
and treatment of melanoma are urgently required because the
effectiveness of treatment is highly dependent on the removal of
the tumor at an early stage (2).
Multinucleated giant cells (MGCs) and polyploidy are
commonly observed in cancer research and are related to poor
clinical prognosis in, for example, breast cancer, prostate cancer
and melanoma (3,4). In pathologic analysis of MGCs, the
cell and nuclear volumes appear enlarged and the chromosome number
is heteroploid and polyploid (5).
MGCs are often formed by cell fusion and fused cells are commonly
found in metastatic tumor tissue (6).
Melanoma metastasis occurs mainly through
hematogenous spread and is organ-specific, especially to the lungs,
brain, skin and liver (7). Cancer
metastasis requires a series of related processes to proceed.
Cancer cells can spread via the blood or the lymphatic system to
distant organs where tumor cells either adapt to the existing
microenvironment or establish a new one by secreting growth factors
or cytokines that facilitate their proliferation (8,9).
Specific genes play important roles in every step of metastasis.
For example, genes that are specific to the circumstances, such as
PTHRP, IL11, CSF2RB (GM-CSF) and
TNFα, may primarily cause organ-specific metastasis
(10,11). However, the 'seed and soil'
hypothesis also proposes that a tumor (seed) must adapt to the
organ environment (soil) in order to survive and proliferate
(12).
The cell fusion method is used in many fields (for
example, in genome research and the generation of antibodies in
vitro), where the polyethylene glycol (PEG) fusion has been the
traditional method (13).
Electrofusion method is used for high fusion efficiency. In order
to achieve this high fusion efficiency in the BTX ECM2001
electronic fusion device, cells are arranged in a bead chain due to
a high frequency alternating current wave (AC) that facilitates the
cell membrane contact that is required for cell fusion and then
fuse together using a direct current (DC). However, ECM2001
electronic fusion device is not so commonly used and are much more
expensive (14). In this study, we
obtained M-MGCs using a modified phytohaemagglutinin (PHA)-ECM830
electronic fusion method [with an ECM830 instrument for cell fusion
and PHA for agglutination of cells, which facilitates cell membrane
contact (15)].
Microarray analysis is a useful method for cancer
study. Specific oligonucleotides or cDNA fragments are fixed to the
gene chip, and the basic principle of which is hybridization
(16). Tens of thousands of
fragments can be detected simultaneously on gene chips such as the
Affymetrix chip, which contains 22-base pair (bp) length
oligonucleotides synthesized at high density using in situ
lithography technology (17),
making this process continuous, integrated and automatic (16).
In summary, we investigated the metastatic
properties and gene expression of melanoma MGCs (M-MGCs). We found
that they exhibited decreased proliferation potential but increased
metastatic properties. Microarray analysis showed that the
β-tubulin gene group (especially the TUBB2B gene), which is
related to cell invasion and metastasis, was significantly
upregulated in M-MGC cells. Our results suggest the positive role
of M-MGC cells in melanoma metastasis and the related genes in this
process.
Materials and methods
Ethics statement
Animal research was carried out in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals of the Beijing Tiantan Hospital, Capital Medical
University (no. 201201001). The Committee on the Ethics of Animal
Experiments of the Beijing Tiantan Hospital, Capital Medical
University, approved the experimental protocol. All efforts were
made to minimize suffering of the animals.
Cell culture
Mouse melanoma cancer B16-F10 cells, M-MGCs, and
293T lentiviral packaging cell lines were all cultured in
Dulbecco's modified Eagle's medium (DMEM) containing 5%
heat-inactivated fetal bovine serum (FBS) (both from HyClone,
Carlsbad, CA, USA), 100 U/ml penicillin, and 0.1 mg/ml
streptomycin, and in a humidified 5% CO2 incubator at
37°C.
Cell labeling
B16-F10 cells were labeled with pLL3.7 and
pLL3.7-dsRED lentivirus. We purchased pLL3.7, which expresses EGFP
alone, and the packaging plasmids (pMDL, pRev and pVSVG) from
Addgene (Cambridge, MA, USA). The pLL3.7-dsRED plasmid was
constructed by replacing an EGFP fragment of pLL3.7 with a
polymerase chain reaction product of dsRED. Lentiviruses were
generated from the 293T packaging cell line, and the B16-F10 cells
were infected according to the natural protocol of lentivirus
production and purification (18).
ECM830 cell fusion method
B16-F10-GFP (1×107) and B16-F10-RFP
(1×107) cells were mixed and centrifuged at 900 rpm for
5 min. The cells were resuspended in 200 μl of electronic
liquid after the supernatant was completely removed. The cells were
then shocked 3 times with 250 V for 30 μsec, rested for 5
min, and finally placed in media for culture.
PHA-ECM830 cell fusion method
B16-F10-GFP (1×107) and B16-F10-RFP
(1×107) cells were mixed, washed twice with DMEM, and
incubated in 500 μl of PHA in DMEM (50 μg/ml;
Sigma-Aldrich, Carlsbad, CA, USA) at 37°C for 30 min. After
centrifugation (900 rpm, 5 min) and complete removal of the
supernatant, 200 μl of electronic liquid was added. Cells
were gently resuspended in DMEM and transferred to an electric
shock cup where they were shocked 3 times with 250 V for 30
μsec. After resting for 5 min, cells were transferred to
culture media.
Fluorescence-activated cell sorting
(FACS)
The cultured cells were washed twice and subjected
to FACS using a BD FACSAria sorter with DMEM in order to purify
GFP/RFP double-positive cells. The cells were collected in a medium
containing 15% FBS and double antibiotics (100 U/ml penicillin, 0.1
mg/ml streptomycin (Gibco Life Technologies, Carlsbad, CA, USA),
and then immediately cultured in 10% FBS DMEM.
Cell and nuclear volume measurements
Cells were suspended in DMEM at 1×105/ml,
and cell and nuclear volumes were detected and assessed using a
Beckman Coulter counter multisizer 3.
DNA content and karyotype analysis
Cells (2×106) were collected, washed
twice with phosphate-buffered saline (PBS; HyClone), fixed with 75%
ethanol, treated with 1 mg/ml RNase for 45 min at 37°C, stained
with 0.5 mg/ml propidium iodide (both from Sigma-Aldrich) for 45
min in the dark, and then analyzed for DNA content using flow
cytometry. The DNA content of the subcutaneous and metastasis
samples was analyzed after ex vivo culture. For karyotype
analysis, cells were sent to the Department of Medical Genetics of
the Institute of Basic Medical Sciences (IBMS), treated with
colcemid and stained with Giemsa solution (both from
Sigma-Aldrich).
In vitro proliferation rate curve
Cells (2×105) were plated on 60 mm plates
for proliferation rate analysis. The medium was changed every 2
days and the number of cells was counted daily.
Tumor xenografts and analysis
We used C57BL/6 mice (female, 20–25 g, 8-week old;
Cancer Institute, Chinese Academy of Medical Sciences, Beijing,
China) in all the experiments. In the subcutaneous studies, cells
(5×104) in PBS were injected subcutaneously into the
rear flank of the mice in order to determine tumor growth rate
in vivo. The animals were observed daily and tumor volumes
were measured every 5 day using a vernier caliper. The mice were
euthanized when tumor volumes exceeded 2,000 mm3 or when
>20% of their body mass was lost (measured by using an
electronic balance).
In the metastasis studies, cells (1×104)
in PBS were injected intravenously into the mice, which were then
observed daily. They were euthanized 25 days after this injection
or when they showed signs of morbidity (anorexia, difficulty
moving, and/or weight loss >20%). Pulmonary metastatic samples
were collected and then measured using an electronic balance
(Mettler Toledo AL204).
Histological analysis
Tumor samples and lung tissues were excised, fixed
in 10% neutral-buffered formalin and embedded in paraffin for
hematoxylin and eosin (HE) staining at the Pathology Centre of the
IBMS.
RNA extraction and gene chip
analysis
RNA was extracted using TRIzol reagent (Invitrogen
Life Technologies, Carlsbad, CA, USA) according to the
manufacturer's suggested protocol. RNA samples were then sent to
the central laboratory of the IBMS for subsequent Affymetrix gene
chip analysis. A gene expression ratio of M-MGCs/B10-F10 ≥2 was
considered significant. Gene functions were analyzed using
Ingenuity pathway analysis (IPA) database software.
Western blotting analysis
Cells were harvested in lysis buffer (2% SDS, 0.1
mol/l DTT, 10% glycerol and 60 mmol/l Tris, pH 6.8) and boiled at
98°C for 10 min. The protein lysates were separated by using 4–12%
Bis-Tris SDS-PAGE gel (Invitrogen Life Technologies) and
transferred onto polyvinyl difluoride (PVDF) membranes (Pierce
Chemical Co., Rockford, IL, USA). Non-specific reactivity was
blocked by 10% non-fat dry milk in TBST buffer (20 mmol/l Tris-HCl,
150 mmol/l NaCl, 0.05% Tween-20, pH 7.5) at room temperature for 2
h. The membranes were then incubated with the primary antibodies
for TUBB2B (1:500) and phospho-S6 (1:4,000) (both from Abcam,
Cambridge, UK), and β-actin (1:8,000) at 4°C for 10 h, followed by
another incubation with a horseradish peroxidase (HRP)-conjugated
secondary antibody (both from Santa Cruz Biotechnology, Santa Cruz,
CA, USA) at 1:5,000 dilution and at room temperature for 4 h.
Statistical analysis
All data are presented as the mean ± Sd. Data were
analyzed, and P-values produced, using SPSS 17.0 statistical
software. P<0.05 was considered to indicate a statistically
significant difference.
Results
M-MGCs obtained by a modified PHA-ECM830
electronic fusion method
B16-F10 is a commonly used melanoma cell line with a
high proliferation rate and metastatic potential to the lungs when
injected intravenously (19). We
used these cells to obtain M-MGCs by using cell fusion in
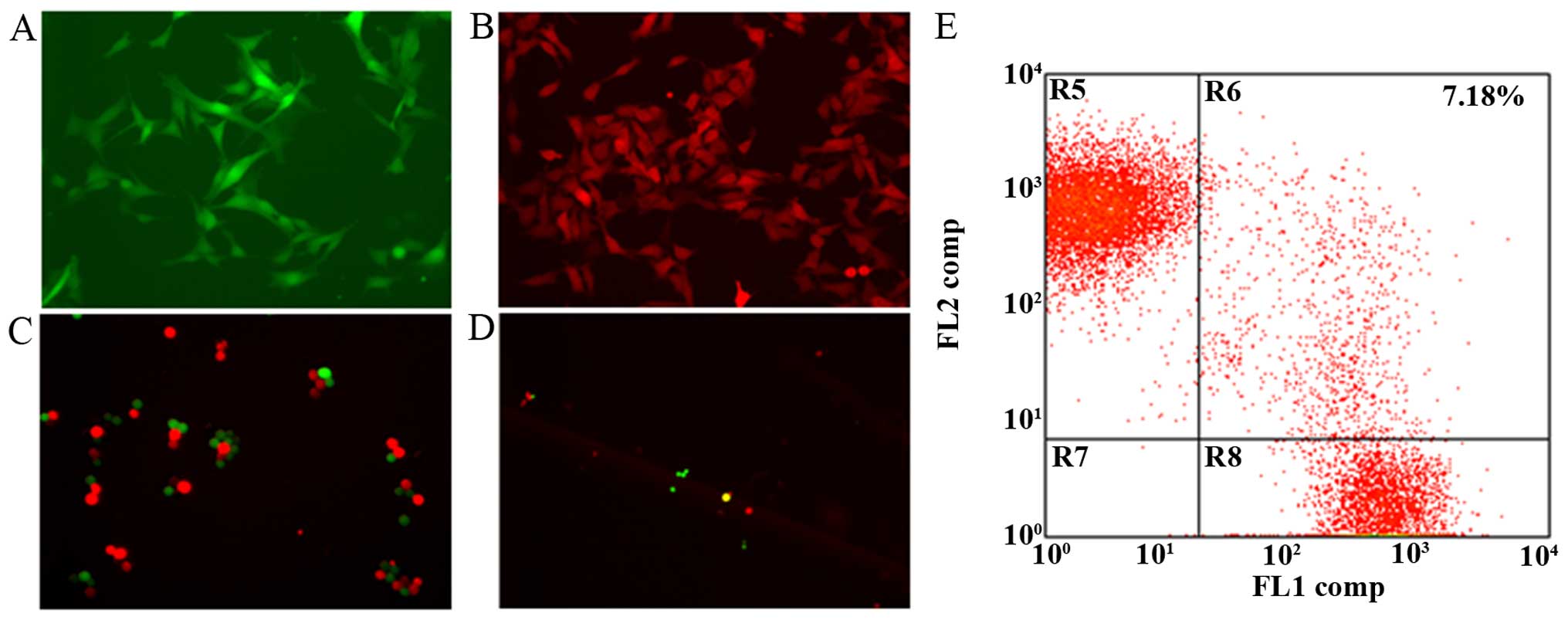
vitro. First, B16-F10 cells were labeled with GFP and RFP
fluorescence (fig. 1A and B) for
easy screening of the after fusion. Initially, we attempted to fuse
the labeled B16-F10 cells using the ECM830 electronic fusion
method; however, the cell fusion efficiency was so limited that
positive fusion cells (those that showed both GFP and RFP and
appeared yellow after overlay) were only partially observed in one
microscopic field. Therefore, we added the cell-agglutinating agent
PHA prior to cell fusion in order to enhance the level of cell
membrane contact, which is an essential part of the process
(fig. 1C). The addition of PHA
significantly increased fusion efficiency, and fusion cells were
clearly observable in one field (fig.
1D). FACS analysis showed that the fusion efficiency was ≤7.18%
(Fig. 1E). The presence of live
fusion cells after selection indicated successful cell fusion.
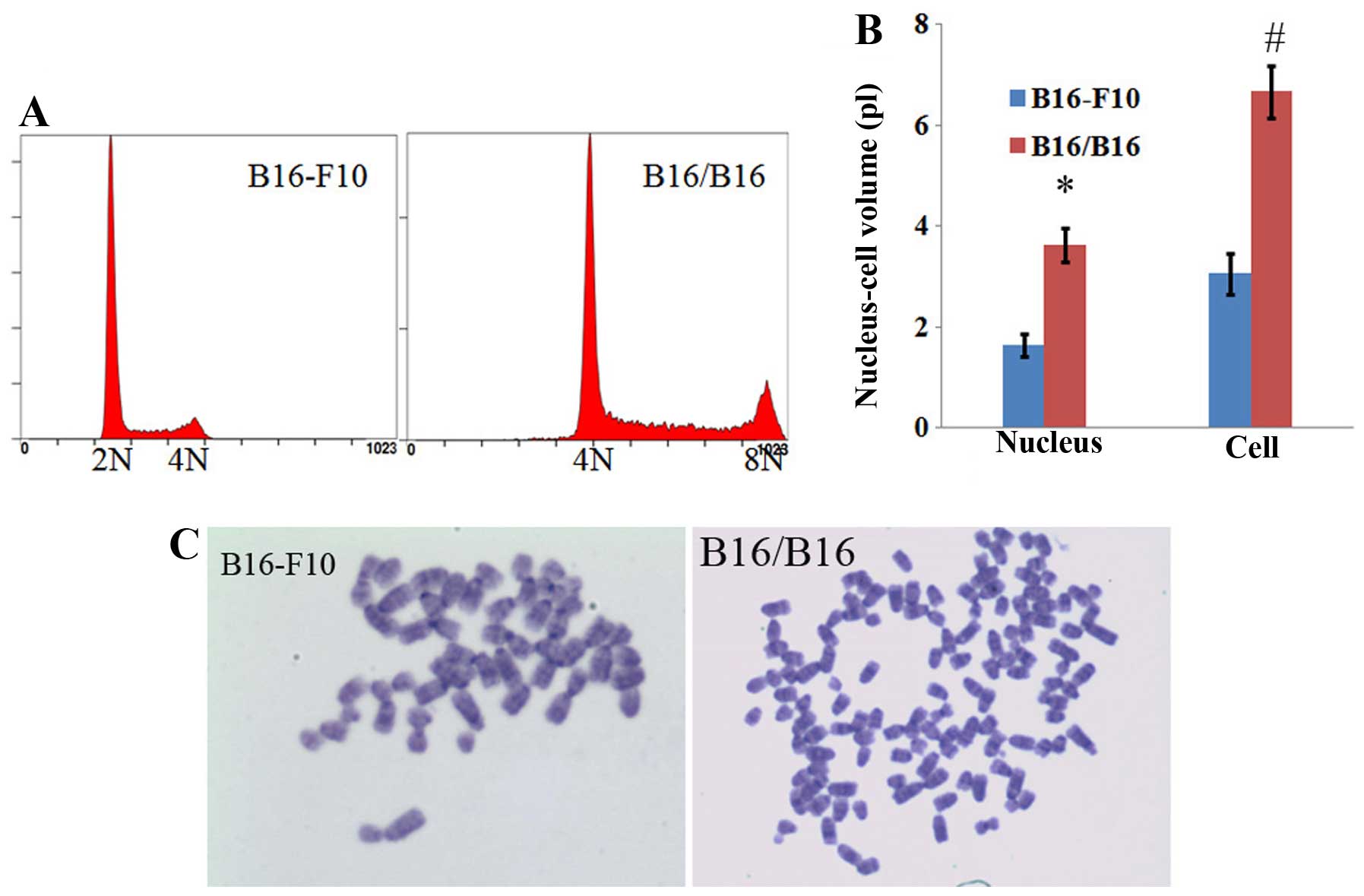
The cell and nuclear sizes of M-MGCs were both twice
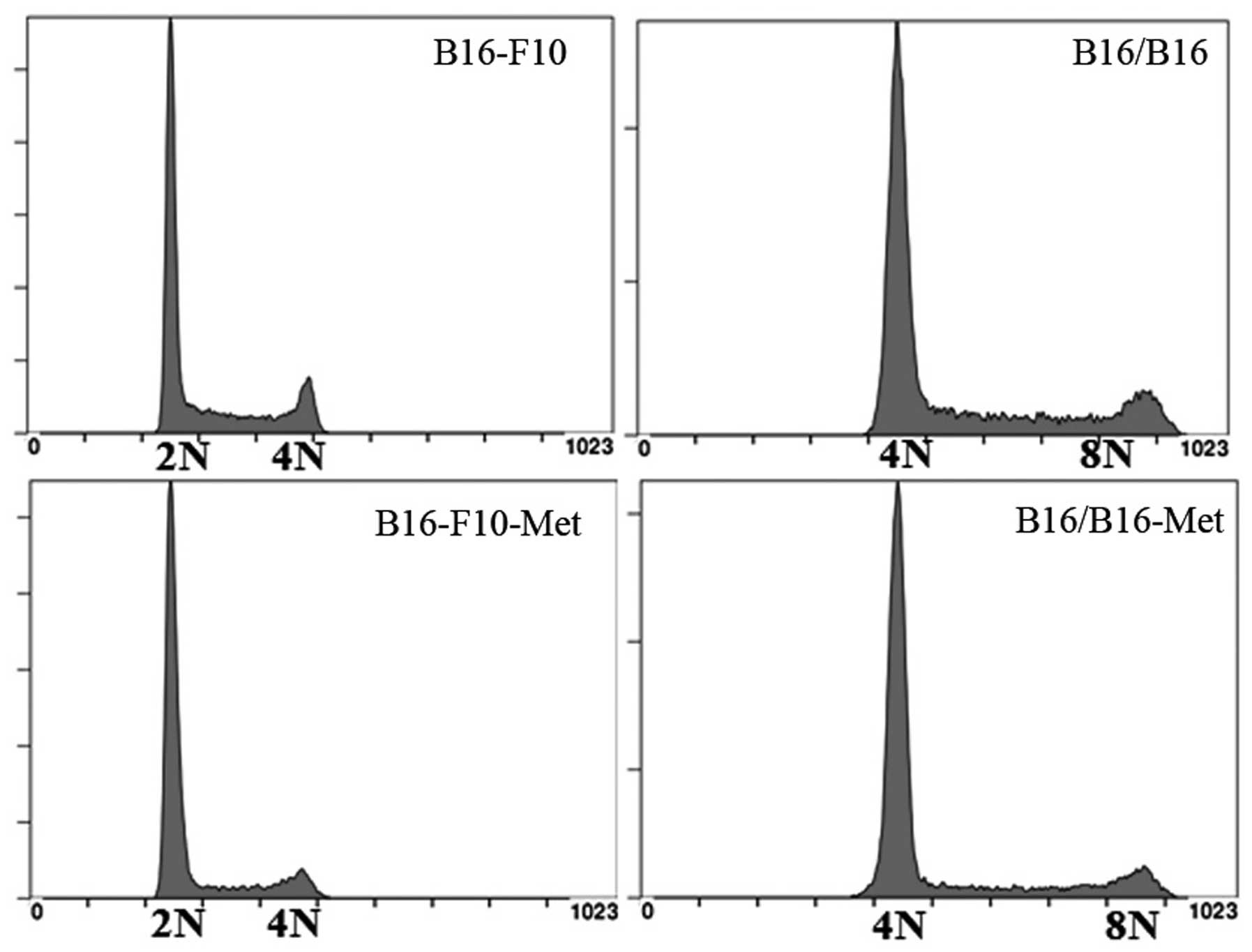
as large as those of the parent B16-F10 cells (Figs. 1D and 2B). DNA content and karyotype analysis
showed that the obtained M-MGCs had converted from being
hyperdiploid to hypertetraploid (Fig.
2A and C).
M-MGCs exhibit increased metastatic
potential
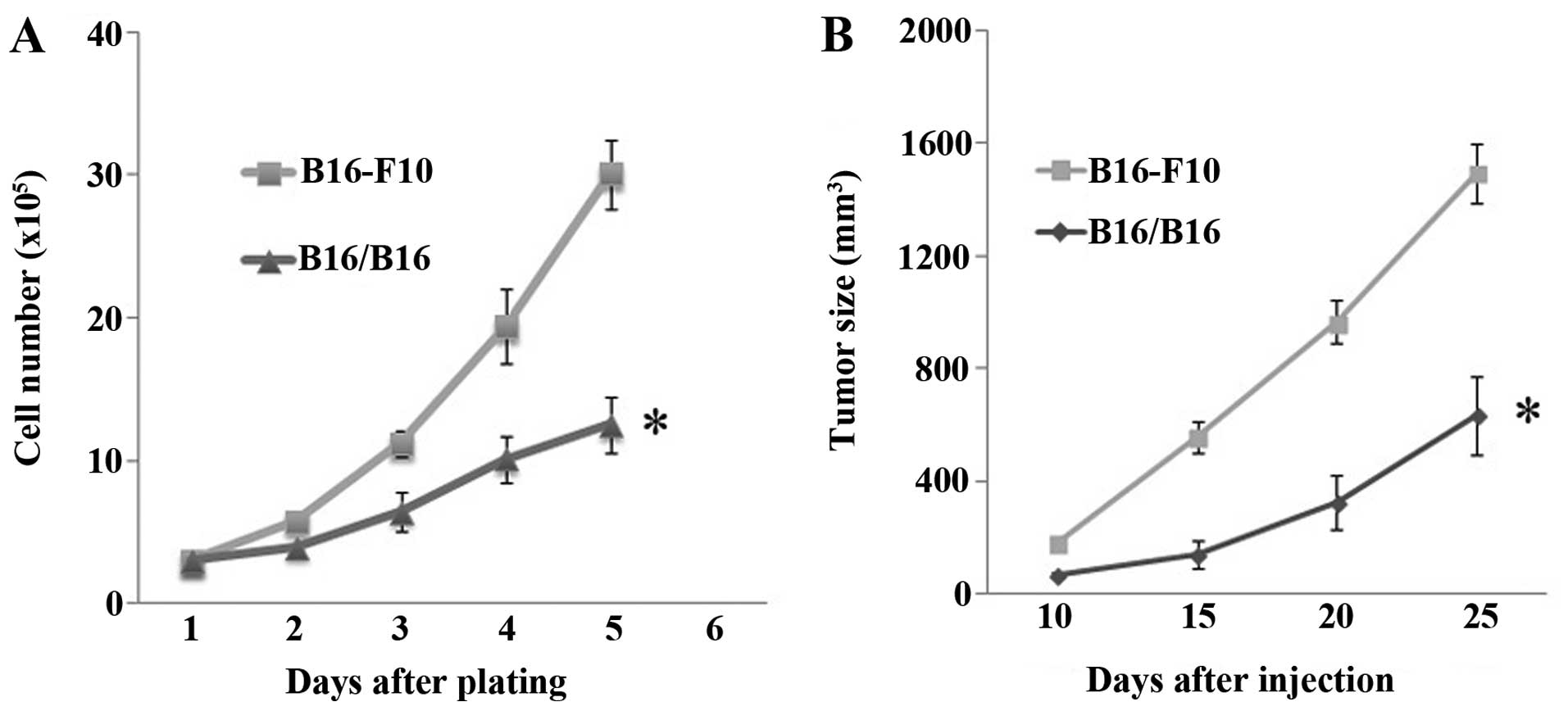
The M-MGCs showed a decreased rate of proliferation
in the cell culture. Both their proliferation rate in vitro
(Fig. 3A) and the growth rate in
vivo (Fig. 3B) decreased.
However, the downregulation of the M-MGCs growth rate in
vivo was not that obvious compared to the change of the
proliferation rate in vitro. This discrepancy may have
arisen because of the enlarged tumor size of the M-MGCs. After the
tumor cells were injected intravenously, the potential for M-MGCs
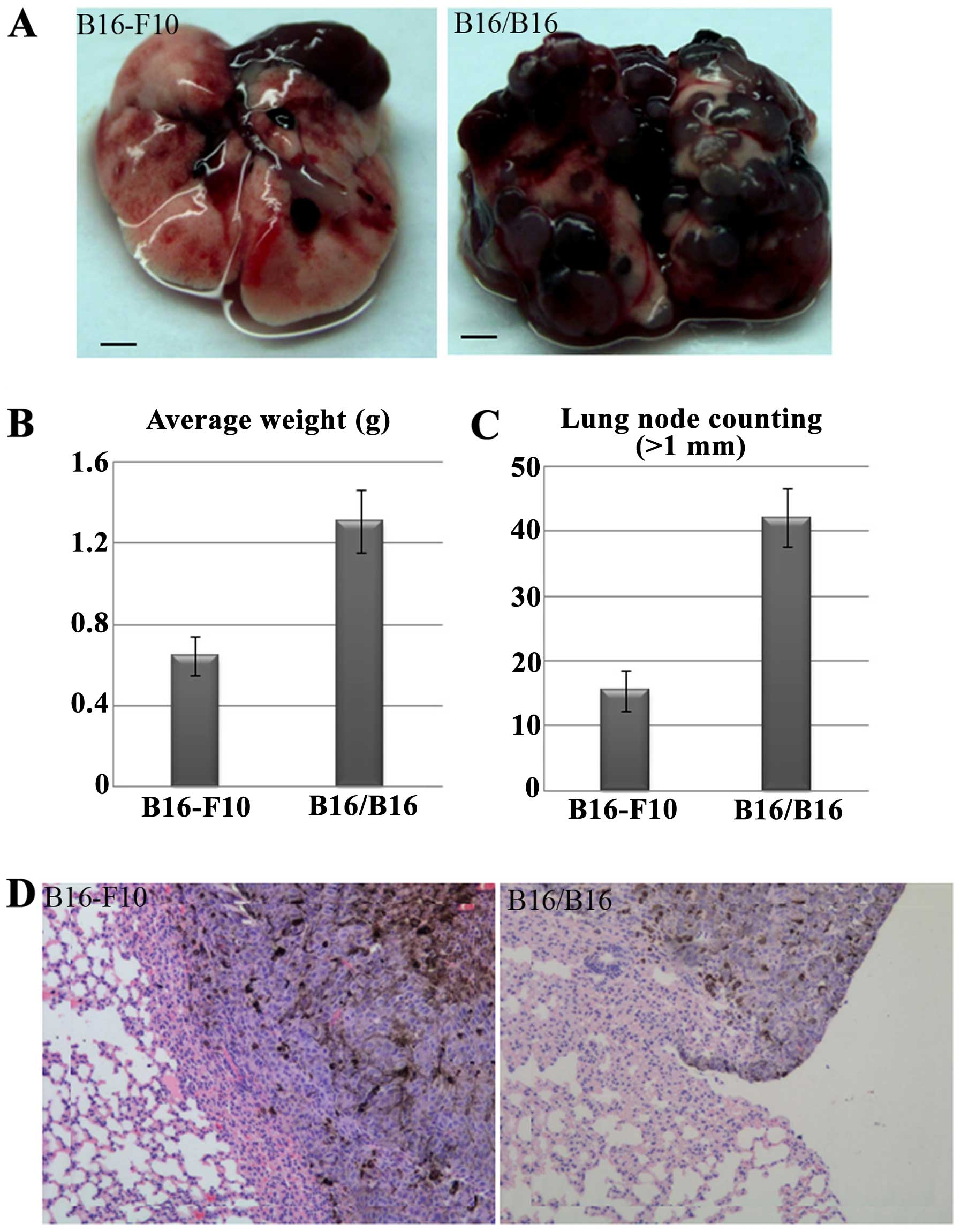
to metastasize to the lung was enhanced (Fig. 4A). The weight of lung containing
metastatic tumors and the metastatic nodules on the lung surface
were counted, and the results also showed the enhanced metastatic
potential of M-MGCs to the lungs (Fig.
4B and C). Hematoxylin-eosin (HE) analysis showed the
metastatic M-MGCs to the lungs (Fig.
4D). However, HE analysis also showed the M-MGCs did not
metastasize to other organs, such as the liver, kidneys, spleen and
brain.
Microarray analysis show changes to
metastasis in M-MGCs associated with β-tubulin gene group
The A260/280 ratio of extracted RNA was ~1.8. There
were clear 28S, 18S and 5S RNA bands in the 1.2% formaldehyde
agarose gel, and the 28S/18S ratio was ~2, which indicated the
integrity of the RNA fragments. Microarray analysis showed that
changes in the AKT network were associated with proliferation and
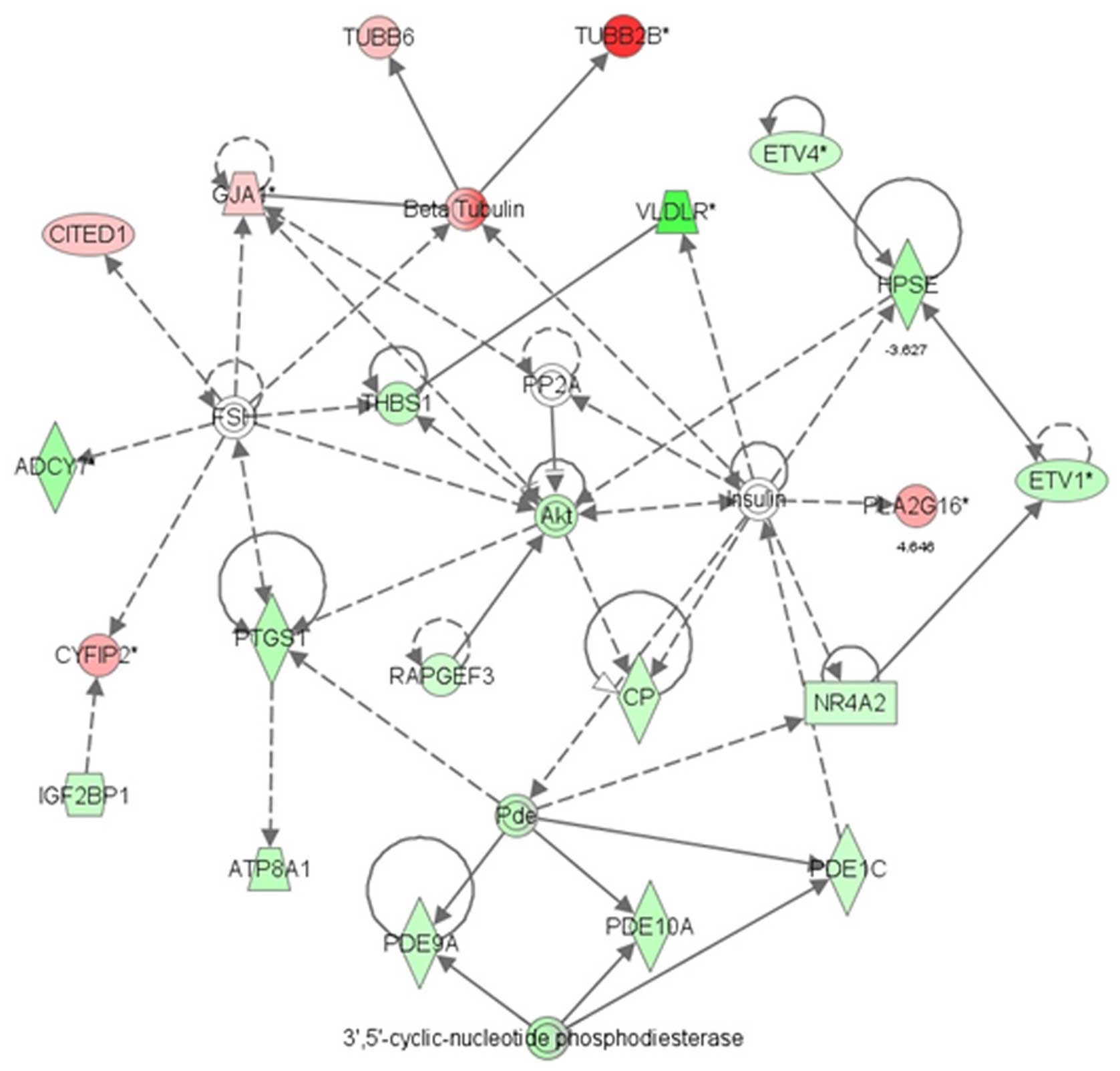
metastasis in the M-MGCs (Fig. 5).
At the centre of this network, the AKT gene was significantly
downregulated. Other genes in the PI3K-AKT pathway that play an
important role in cell proliferation, such as PI3K and pS6, were
also downregulated (Table I).
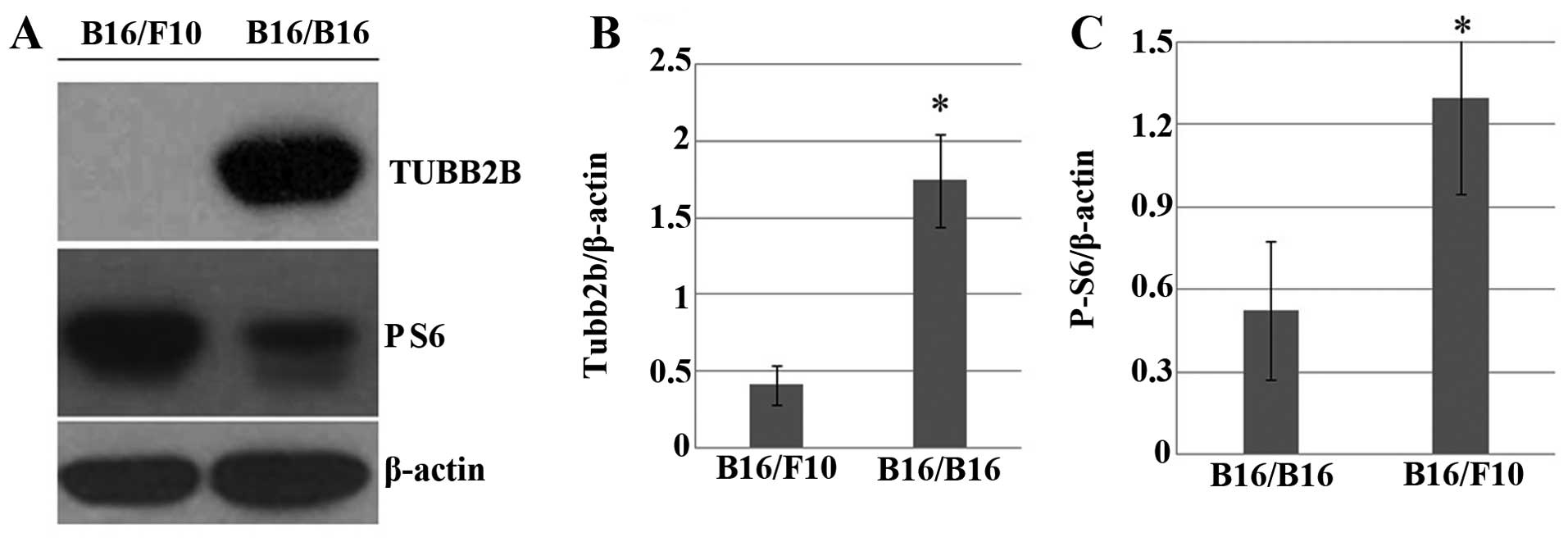
Western blot analysis confirmed the downregulation of pS6,
downstream of the PI3K-AKT pathway in M-MGCs (Fig. 6A and C). The β-tubulin gene group,
which is related to cell migration and invasion, was upregulated in
M-MGCs (Table II). The
TUBB2B gene, which is also part of the AKT network (Fig. 5), was significantly upregulated in
M-MGCs, and this was also confirmed by western blot analysis
(Fig. 6A and B).
 | Table IThe PI3K-AKT pathway is downregulated
in melanoma multinucleated giant cells. |
Table I
The PI3K-AKT pathway is downregulated
in melanoma multinucleated giant cells.
| Gene symbol | Gene title | Fold-change |
|---|
| Pik3r1 |
Phosphatidylinositol 3-kinase, regulatory
subunit, polypeptide 1 (p85α) | −2.05297a |
| Akt3 | Thymoma viral
proto-oncogene 3 | −2.65325a |
| Rps6ka1 | Ribosomal protein
S6 kinase polypeptide 1 | −2.67889a |
 | Table IIThe β-tubulin gene group was
upregulated in melanoma multinucleated giant cells. |
Table II
The β-tubulin gene group was
upregulated in melanoma multinucleated giant cells.
| Gene symbol | Gene title | Fold-change |
|---|
| Tubb2b | Tubulin, β2b | 10.8058a |
| Tubb6 | Tubulin, β6 | 3.39871a |
|
Tubb2a-ps2 | Tubulin, β2a,
pseudogene 2 | 2.82951a |
M-MGCs show DNA stability following
culture in vitro and ex vivo
M-MGCs were cultured for 6 months ex vivo
after being derived from metastatic tumor sites. DNA content
analysis showed that the genotype of M-MGCs was consistent after
ex vivo and in vitro passage, indicating the genomic
stability of M-MGCs (Fig. 7).
Discussion
MGCs are commonly observed in melanoma pathological
analysis, especially at metastatic tumor sites, and they are
usually associated with poor prognosis (3,4,20).
Cell fusion, which is considered a cause of cancer metastasis, is
one process by which MGCs are formed (20). For the formation of MGCs, cancer
cells can fuse with many types of somatic cells, including stromal
and epithelial cells, as well as other cancer cells. In the present
study, we obtained the MGCs in vitro by fusing melanoma
cells with each other (21–23).
In the present study, an ECM830 electronic fusion
device was used to achieve melanoma cell fusion. However, the
fusion efficiency was very limited, and so PHA was used to promote
contact between the cells by agglutination, thereby promoting cell
fusion (13). PHA significantly
increased fusion efficiency (>10 times); therefore, our study
demonstrates a convenient and effective fusion method (PHA-ECM830
fusion) that could be used for future cancer research. The MGCs
obtained by this method could be used, for example, in studies of
organ-specific cancer metastasis and genome stability research. We
also found that the MGCs obtained in this study exhibited stable
genome characteristics. Although the mechanism of this property was
not revealed in our study and awaits further research, these fusion
cells could potentially provide a useful model for genome stability
research.
Cell proliferation and metastasis are two of the
most important characteristics of cancer cells. However, previous
studies have shown that proliferation rate is reduced when cancer
cells are fused with fibroblast cells (24). In our study, the M-MGCs we obtained
showed decreased proliferation and increased metastasis to the
lungs. These effects could be due to tumor suppressor genes, such
as p53 (24,25). Another possible explanation is that
a decreased rate of proliferation enables cancer cells to execute
other functions, such as cancer metastasis. However, the function
of MGCs in cancer malignancy is complicated, and further research
will be necessary to validate these hypotheses.
Our microarray analysis indicated that the PI3K-AKT
pathway includes the primary set of differentially expressed genes
associated with cell proliferation in M-MGCs. We also found
decreased protein expression in this pathway, indicating that it
probably plays an important role in melanoma cell proliferation. In
the PI3K-AKT pathway, the upstream molecules RTK-PI3K-AKT activate
the downstream molecules mTOR-pS6 and further promote the
eukaryotic initiation factor 4E (eIF4E) to regulate protein
translation (26). AKT can also
directly act on transcription factors, such as p27 and p21, to
regulate the cell cycle and cell proliferation. There are many
specific inhibitors in this pathway; for example, wortmannin and
rapamycin specifically inhibit the activity of PI3K and mTOR,
respectively (26–28). The PI3K-AKT pathway is highly active
in melanoma cells, which proliferate rapidly (27), and AKT3 reportedly significantly
inhibits the proliferation rate of these cells.
Cancer metastasis involves a series of processes,
including the metastasis of the primary melanoma cells to a distant
organ through blood or lymph vessels where they adhere to the
capillary and invade the new environment. The cancer cells adapt to
the new environment and promote cell proliferation by secreting
cell growth factors or cytokines (9). In our study, metastasis of melanoma
was specific to the lungs, and this may be because MGCs only
express lung-specific metastasis genes and are, therefore, a good
fit for the lung environment. In future studies, it will be
necessary to investigate the mechanisms of cell adhesion, cell
invasion, and cell growth in the new organ environment.
Microtubulin is a part of the globular protein
family, of which α-tubulin and β-tubulin are members. These two
proteins have a similar dimensional structure and they form tightly
integrated dimers to become the subunits of microtubules. β-tubulin
can be constructed by several subunits including TUBB, TUBB1,
TUBB2A, TUBB2B AND TUBB2C, of which TUBB2B plays an important role
in cell invasion and migration. In Drosophila, salivary
gland development is inhibited when the TUBB2B gene is
knocked out (29). In rats,
knockdown of TUBB2B inhibits the migration of the cortical
neurons (30). In our study,
TUBB2B was significantly upregulated in M-MGCs, which
indicates that it may play an important role in melanoma
metastasis.
Our study demonstrates the changes in cell
metastatic ability in M-MGCs are associated with β-tubulin gene
group. However, the specific genes for the metastasis of melanoma
cells and M-MGCs are not explicit, further study can be done to
verify the results. For the organ specific metastasis, cell fusion
method is a special useful tool (for example, different organ
specific metastatic cells can be fused together to see the genotype
and the following metastatic phenotype changes of the fusion
cells). Collectively, our results provide novel insights into the
properties of melanoma and they could contribute towards the design
of new strategies for rapid treatment of this cancer.
Acknowledgments
The present study was supported by a grant from the
National Natural Science Foundation of China (nos. 81302186,
81372354 and 81271563), the Beijing Natural Science Foundation
(nos. 7132034 and 7151002), the Beijing Health System High-level
Personnel Building Foundation (no. 2013-3-018), the Beijing
Laboratory of Biomedical Materials Foundation and the Natural
Science Foundation of Beijing Neurosurgical Institute. We thank
Professor Hongbing Zhang, Professor Yuqin Liu and Jianhui Ma for
their helpful advice and support. We also would like to thank
Editage (www.editage.com) for English language
editing.
References
|
1
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Damsky WE, Theodosakis N and Bosenberg M:
Melanoma metastasis: New concepts and evolving paradigms. Oncogene.
33:2413–2422. 2014. View Article : Google Scholar
|
|
3
|
Abdalla F, Boder J, Markus R, Hashmi H,
Buhmeida A and Collan Y: Correlation of nuclear morphometry of
breast cancer in histological sections with clinicopathological
features and prognosis. Anticancer Res. 29:1771–1776.
2009.PubMed/NCBI
|
|
4
|
Rajagopalan H and Lengauer C: Aneuploidy
and cancer. Nature. 432:338–341. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mossbacher U, Knollmayer S, Binder M,
Steiner A, Wolff K and Pehamberger H: Increased nuclear volume in
metastasizing 'thick' melanomas. J Invest dermatol. 106:437–440.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pawelek JM and Chakraborty AK: Fusion of
tumour cells with bone marrow-derived cells: A unifying explanation
for metastasis. Nat Rev Cancer. 8:377–386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fidler IJ: The role of the organ
microenvironment in brain metastasis. Semin Cancer Biol.
21:107–112. 2011. View Article : Google Scholar
|
|
8
|
Langley RR and Fidler IJ: The seed and
soil hypothesis revisited - the role of tumor-stroma interactions
in metastasis to different organs. Int J Cancer. 128:2527–2535.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: from dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nguyen DX and Massagué J: Genetic
determinants of cancer metastasis. Nat Rev Genet. 8:341–352. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gupta GP, Minn AJ, Kang Y, Siegel PM,
Serganova I, Cordón-Cardo C, Olshen AB, Gerald WL and Massagué J:
Identifying site-specific metastasis genes and functions. Cold
Spring Harb Symp Quant Biol. 70:149–158. 2005. View Article : Google Scholar
|
|
12
|
Fidler IJ: The pathogenesis of cancer
metastasis: The 'seed and soil' hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lentz BR: PEG as a tool to gain insight
into membrane fusion. Eur biophys J. 36:315–326. 2007. View Article : Google Scholar
|
|
14
|
Kandušer M and Ušaj M: Cell electrofusion:
Past and future perspectives for antibody production and cancer
cell vaccines. Expert Opin Drug Deliv. 11:1885–1898. 2014.
View Article : Google Scholar
|
|
15
|
Veselý P, Entlicher G and Kocourek J: Pea
phytohemagglutinin selective agglutination of tumour cells.
Experientia. 28:1085–1086. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Russo G, Zegar C and Giordano A:
Advantages and limitations of microarray technology in human
cancer. Oncogene. 22:6497–6507. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dalma-Weiszhausz DD, Warrington J,
Tanimoto EY and Miyada CG: The affymetrix GeneChip platform: An
overview. Methods Enzymol. 410:3–28. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kutner RH, Zhang XY and Reiser J:
Production, concentration and titration of pseudotyped HIV-1-based
lentiviral vectors. Nat Protoc. 4:495–505. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fidler IJ and Nicolson GL: organ
selectivity for implantation survival and growth of b16 melanoma
variant tumor lines. J Natl Cancer Inst. 57:1199–1202.
1976.PubMed/NCBI
|
|
20
|
Wang X, Willenbring H, Akkari Y, Torimaru
Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S and
Grompe M: Cell fusion is the principal source of
bone-marrow-derived hepatocytes. Nature. 422:897–901. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chakraborty AK, Sodi S, Rachkovsky M,
Kolesnikova N, Platt JT, Bolognia JL and Pawelek JM: A spontaneous
murine melanoma lung metastasis comprised of host x tumor hybrids.
Cancer Res. 60:2512–2519. 2000.PubMed/NCBI
|
|
22
|
Duelli D and Lazebnik Y: Cell-to-cell
fusion as a link between viruses and cancer. Nat Rev Cancer.
7:968–976. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duelli D and Lazebnik Y: Cell fusion: A
hidden enemy? Cancer Cell. 3:445–448. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harris H: The analysis of malignancy by
cell fusion: The position in 1988. Cancer Res. 48:3302–3306.
1988.PubMed/NCBI
|
|
25
|
Green DR and Kroemer G: Cytoplasmic
functions of the tumour suppressor p53. Nature. 458:1127–1130.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Babchia N, Calipel A, Mouriaux F, Faussat
AM and Mascarelli F: The PI3K/Akt and mTOR/P70S6K signaling
pathways in human uveal melanoma cells: Interaction with B-Raf/ERK.
Invest Ophthalmol Vis Sci. 51:421–429. 2010. View Article : Google Scholar
|
|
27
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wong KK, Engelman JA and Cantley LC:
Targeting the PI3K signaling pathway in cancer. Curr Opin Genet
Dev. 20:87–90. 2010. View Article : Google Scholar :
|
|
29
|
Jattani R, Patel U, Kerman B and Myat MM:
deficiency screen identifies a novel role for beta 2 tubulin in
salivary gland and myoblast migration in the Drosophila embryo. Dev
Dyn. 238:853–863. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jaglin XH, Poirier K, Saillour Y, Buhler
E, Tian G, Bahi-Buisson N, Fallet-bianco C, Phan-Dinh-Tuy F, Kong
XP, Bomont P, et al: Mutations in the beta-tubulin gene TUBB2B
result in asymmetrical polymicrogyria. Nat Genet. 41:746–752. 2009.
View Article : Google Scholar : PubMed/NCBI
|