Introduction
Increasing evidence indicates that a small
population of cancer stem cells (CSCs) exists in many solid tumors
including colorectal cancer (CRC) (1–3). CSCs
possess the capacities for continuous self-renewal, multi-lineage
differentiation and natural resistance to conventional therapies,
resulting in cancer relapse and metastasis and eventually the
failure of clinical anticancer treatment (4,5). The
characteristics of CSCs are highly regulated by multiple cellular
signaling cascades including the Notch, Wnt and Hedgehog pathways
(6–8). The Notch signaling pathway is a
fundamental signaling system essential for embryonic development
and tissue homeostasis (9,10). Activation of Notch signaling is
triggered by the interaction of a family of ligands with their
specific transmembrane-spanning Notch receptors (11). In mammals, five ligands (Jagged1,
Jagged2, Delta1, Delta2 and Delta3) and four Notch proteins
(Notch1, 2, 3 and 4) have been identified, of which Notch1 is the
best studied (12,13). Upon ligand binding, Notch protein is
cleaved by proteolytic processing to release an intracellular
domain of Notch (NICD) from the membrane into the cytoplasm. NICD
in turn translocates into the nucleus to participate in the
transcriptional regulation of target genes mediating many
biological processes such as cell differentiation, proliferation
and apoptosis (14,15). Previous studies have proposed that
aberrant activation of the Notch pathway is tightly associated with
the development of various malignancies including CRC (16–18).
Moreover, Notch1 has been shown to be involved in the control of
CSCs by regulating the expression of downstream gene Hes1; and
targeting Notch1 could result in the decrease in CSC proliferation
(19–22). Therefore, attacking CSCs via
suppressing the Notch pathway may provide an opportunity to improve
the efficacy of conventional cancer therapies (6,7,23).
Traditional Chinese medicine (TCM) has been used for
thousands of years in China to clinically treat various diseases
including cancer. Recently, TCM has attracted great interest in the
fields of stem cell biology since various naturally occurring
components have been shown to possess anti-CSC activities (24). Pien Tze Huang (PZH), a well-known
TCM formula prescribed in the Chinese Ming Dynasty more than 450
years ago, has been used in China and Southeast Asia for centuries
as a folk remedy for cancers. Previously, we demonstrated that PZH
possesses a broad range of anticancer activities through affecting
multiple intracellular targets (25–36).
More interestingly, we recently reported that PZH inhibits the
growth of CSCs isolated from colon carcinoma HT-29 cells (37). To further assess the anti-CSC
activity of PZH and explore the underlying mechanism, in the
present study we isolated stem-like side population (SP) from the
human colorectal cancer SW480 cell line, and evaluated the effect
of PZH on the proliferation, apoptosis and differentiation of
isolated SW480 SP cells as well as on the activation of the Notch1
signaling pathway.
Materials and methods
Materials and reagents
Leibovitz's L-15 medium, serum-free stem cell
culture medium (DMEM)/F12, fetal bovine serum (FbS), b27 supplement
(50X), penicillin-streptomycin, trypsin-EDTA and StemPro Accutase
Cell Dissociation Reagent were purchased from Life Technologies
Corp. (Grand Island, NY, USA). EGF and bFGF were obtained from
Peprotech (rocky Hill, NJ, USA). Hoechst 33342 and verapamil were
purchased from Sigma Chemicals (St. Louis, MO, USA). Antibodies
against Notch1, Hes1, CK20 and GAPDH were purchased from Abcam
(Hong Kong). Horseradish peroxidase (HRP)-conjugated secondary
antibodies were obtained from Cell Signaling Technology (beverly,
MA, USA). bCA protein assay reagent kit and Super-Signal West Pico
Chemiluminescent Substrate were obtained from Pierce (rockford, IL,
USA). TrIzol reagent, PrimeScript rT reagent kit and SYbr Premix Ex
Taq II kit were provided by Takara biotechnology Co., Ltd.
(Dalian, Liaoning, China).
Preparation of PZH
PZH was obtained from and authenticated by Zhangzhou
Pien Tze Huang Pharmaceutical Co., Ltd., China (Chinese FDA
approval no. Z35020242). The stock solution of PZH was freshly
prepared by dissolving the powder in phosphate-buffered saline
(PbS) to a concentration of 20 mg/ml. Working concentrations of PZH
were made by diluting the stock solution with culture medium.
Cell culture
The human colorectal cancer cell line SW480 was
purchased from the Cell bank of the Chinese Academy of Sciences
(Shanghai, China). Cells were cultured in Leibovitz's L-15 medium,
containing 10% (v/v) FbS, 100 U/ml penicillin and 100 µg/ml
streptomycin in a 37°C humidified incubator with 5%
CO2.
Isolation and culture of side population
(SP) cells
SP cells from SW480 cells were isolated and analyzed
using Moflo XDP cell sorter flow cytometry (beckman Coulter,
Fullerton, CA, USA) as previously described (37). Excitation of Hoechst dye was
performed using a UV laser at 355 nm, and the fluorescence was
measured with a 450±25 nm filter (Hoechst blue) and a 620±15 nm
filter (Hoechst red). Sorted SP cells were cultured in DMEM/F12,
containing b27 (1X), 20 ng/ml EGF and 20 ng/ml bFGF.
In vitro sphere formation assay
SP cells were seeded at a density of 250 cells/well
in 24-well ultra-low attachment plates (Corning, Lowell, MA, USA)
and grown in DMEM/F12 serum-free stem cell culture medium. The
medium was added every 2 days. After 7 days the spheroids were
photographed and quantified. A spheroid with a diameter >100
µm was considered to be a full sphere.
In vivo tumorigenic assay
Athymic female bAbL/c nude mice were purchased from
Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). Mice
were housed under pathogen-free conditions with a 12-h light/dark
cycle. Food and water were given ad libitum. Freshly sorted
500 SP or non-SP cells were resuspended in 50 µl PbS and
then mixed with 50 µl Matrigel (bD biosciences, San Jose,
CA, USA). SP or non-SP cells were respectively injected into the
right or left subaxillary area of the mice. Tumor development was
monitored every 2 days for 7 weeks. All animal treatments were
strictly in accordance with the International Ethics Guidelines and
the National Institutes of Health Guide concerning the Care and Use
of Laboratory Animals, and the experiments were approved by the
Institutional Animal Care and Use Committee of Fujian University of
Traditional Chinese Medicine.
Evaluation of cell viability by MTS
assay
Sorted SW480 SP cells were seeded into 96-well
plates at a density of 1.0×104 cells/well in serum-free
stem cell culture medium DMEM/F12. After cells were treated with
various concentrations of PZH for 48 h, 20 µl MTS was added
to each well, and the samples were incubated for an additional 2 h
at 37°C. The absorbance was measured at 490 nm using an ELISA
reader (Model ELX800; bioTek Instruments, Inc., Winooski, VT,
USA).
Apoptosis analysis with Hoechst
staining
SP cells were seeded into 24-well plates at a
density of 1×105 cells/well in serum-free stem cell
culture medium DMEM/F12. After treatment with various
concentrations of PZH for 48 h, the cells were dissociated by
StemPro Accutase Cell Dissociation reagent and stained with Hoechst
33342 (1 µg/ml) for 10 min. Cell apoptosis was observed
under a fluorescence microscope and photographed at a magnification
of ×400.
RNA extraction and qPCR analysis
SP cells were seeded into 24-well plates at a
density of 1×105 cells/well in serum-free stem cell
culture medium DMEM/F12. After cells were treated with various
concentrations of PZH for 48 h, total RNA was isolated with TRIzol
reagent and reverse-transcribed with PrimeScript RT reagent kit
according to the manufacturer's instructions. The obtained cDNA was
used to determine the expression of Notch1, Hes1 and CK20 by qPCR.
β-actin was used as internal control. Primer sequences used in PCR
are listed in Table I.
 | Table IPrimer sequences for PCR. |
Table I
Primer sequences for PCR.
| Gene | Primers
(5′→3′) |
|---|
| CK20 | F:
CGAGTTGCTTCCCGATACTTC |
| R:
ACTTGCCAGCATACAACCCAA |
| Notch1 | F:
GGTGCCGAACCAATACAACCCTCT |
| R:
TTGCTGCTGCTGGATGTTTGCTG |
| Hes1 | F:
CTGGAGAGGCGGCTAAGGTGTT |
| R:
TGTTGCTGGTGTAGACGGGGAT |
| β-actin | F:
AAGGTGACAGCAGTCGGTTGGAG |
| R:
GAGAAGTGGGGTGGCTTTTAGGAT |
Western blot analysis
SP cells were seeded in 6-well plates at a density
of 4×105 cells/well in serum-free stem cell culture
medium DMEM/F12 and treated with various concentrations of PZH for
48 h. The treated cells were lysed with mammalian cell lysis buffer
containing protease and phosphatase inhibitor cocktails. Total
protein concentrations were determined by bCA assay. A total of 30
µg of total proteins was resolved on 10% SDS-PAGE gels and
electroblotted. The PVDF membranes were blocked with blocking
buffer and probed with primary antibodies against Notch1, Hes1,
CK20 or GAPDH (1:1,000) overnight at 4°C and subsequently incubated
with the appropriate HRP-conjugated secondary antibody (1:5,000)
followed by Super-Signal West Pico chemiluminescence detection.
Statistical analysis
All data are presented as the averages of three
determinations and were analyzed using the SPSS package for Windows
(version 18.0). Statistical analysis of the data was performed with
Student's t-test and ANOVA.
Results
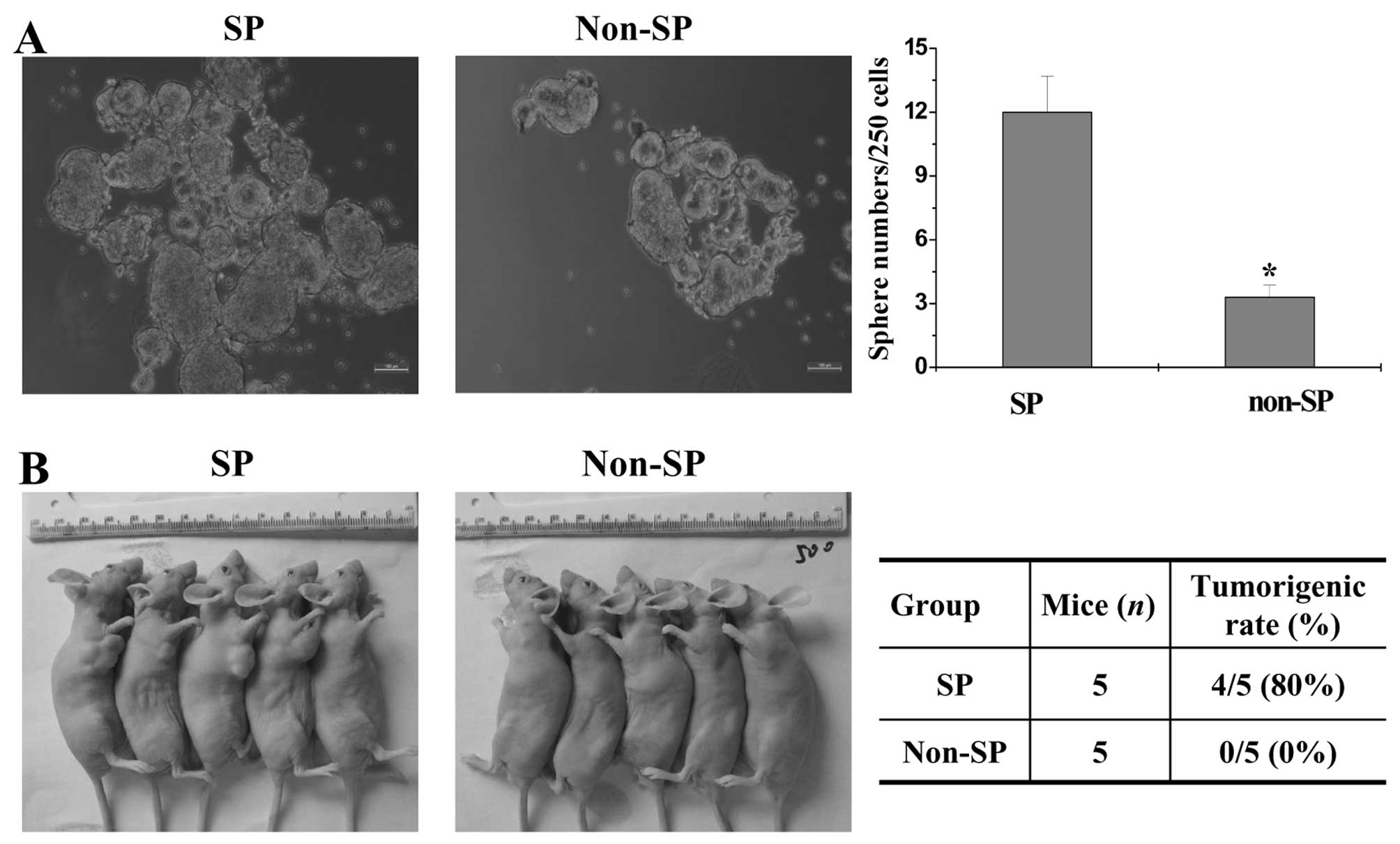
SP cells sorted from the SW480 cell line
exhibit high tumorigenic capacity in vivo and in vitro
Spheroid formation and tumorigenicity assays were
performed to assess the tumorigenic capacity of SP and non-SP in
vitro and in vivo. The numbers of tumor spheroids formed
from the SP and non-SP cells were 12±1.6 and 3±1, respectively
(P<0.05 vs. the SP group), suggesting that the SP cells
exhibited higher tumor spheroid formation capacity when compared
with the non-SP cells. (Fig. 1A).
Moreover, in the in vivo study, SP cells initiated tumors
with 500 cells in 4 of 5 mice (80%), whereas non-SP cells failed to
form tumors (0 of 5 mice) (0%) (Fig.
1b), indicating that SP cells displayed significantly stronger
tumorigenicity in vivo, demonstrating that the isolated
SW480 SP cells possessed characteristics similar to cancer stem
cells.
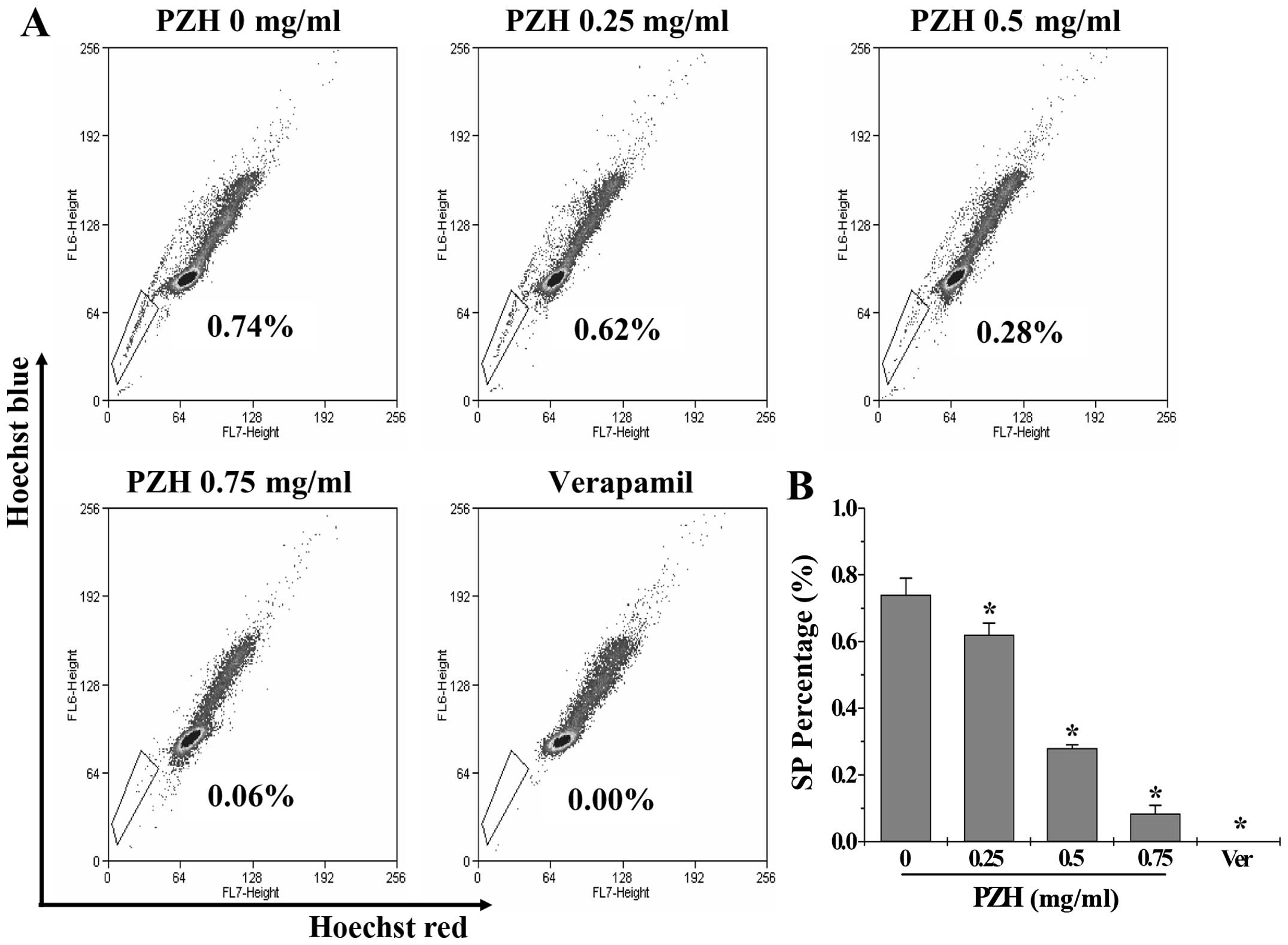
PZH decreases the percentage of SP cells
in the SW480 cells
To evaluate the effect of PZH on cancer stem cells,
we determined the percentage of SP cells. Similar to the positive
control verapamil, PZH significantly and dose-dependently decreased
the SP proportion from 0.74±0.05 to 0.08±0.02% in the SW480 cells
(P<0.05 vs. the control group) (Fig.
2).
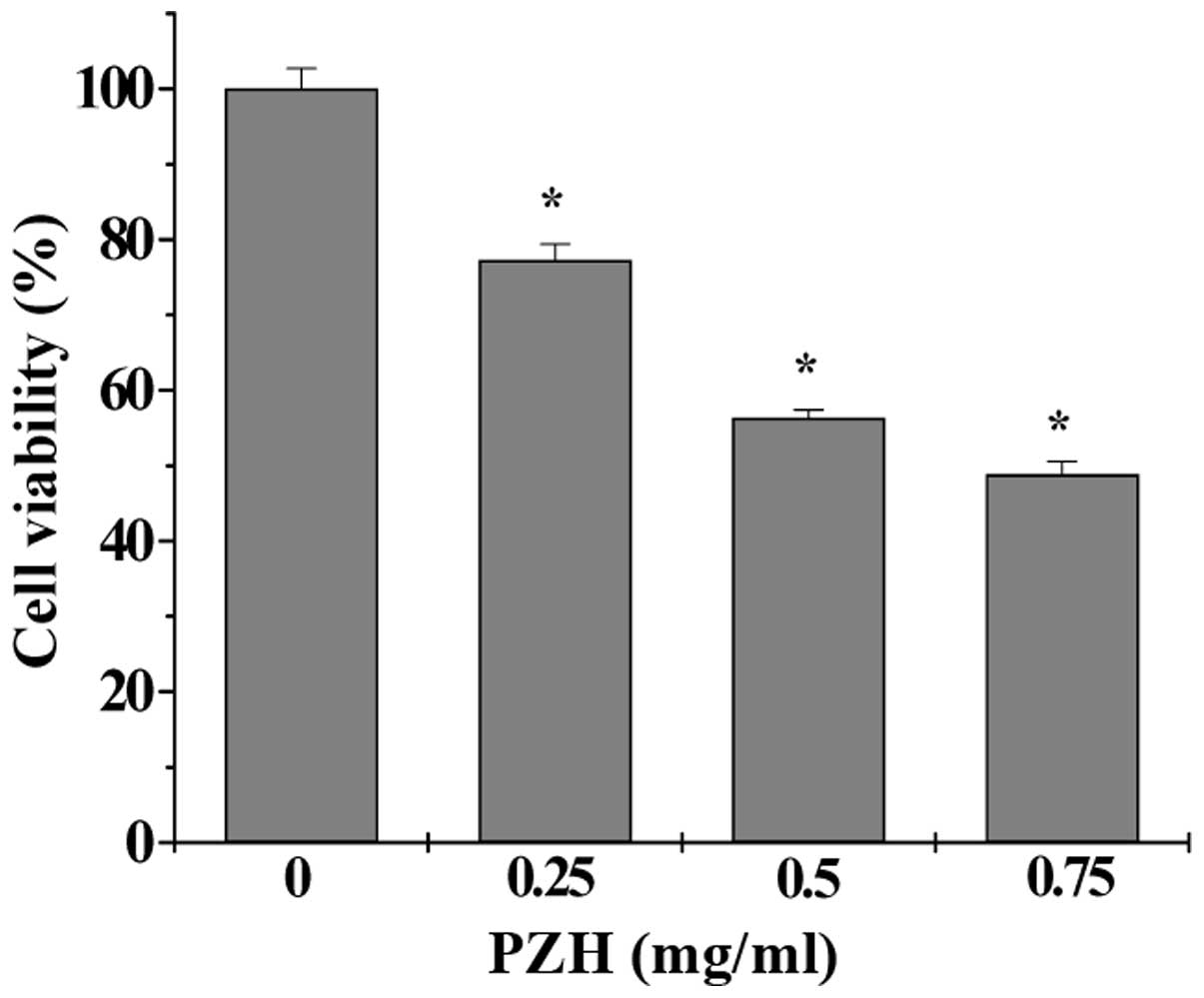
PZH inhibits the proliferation of the
sorted SW480 SP cells
In order to assess the effect of PZH on the
proliferation of CSCs, the viability of the sorted SW480 SP cells
was measured by MTS assay. PZH treatment significantly and
dose-dependently decreased the SP viability by 22.75–51.16%
compared with the control (P<0.05) (Fig. 3).
PZH enhances the apoptosis of sorted
SW480 SP cells
Cell apoptosis was determined by Hoechst staining.
PZH-treated cells displayed typical apoptotic morphological
characteristics including condensed chromatin and fragmented
nuclear morphology, while the untreated cell nuclei were
homogeneously stained and less intense (Fig. 4).
PZH promotes differentiation of sorted
SW480 SP cells
Differentiation of CSCs was evaluated by the
expression of differentiation marker CK20. Data from qPCr and
western blot analyses showed that PZH profoundly increased the mRNA
and protein expression of CK20 in the SP cells (Fig. 5).
PZH suppresses the Notch1 pathway in the
sorted SW480 SP cells
The activation of Notch1 signaling was analyzed by
examining the expression of key mediators, Notch1 and Hes1. PZH
markedly reduced the expression of Notch1 and Hes1 in the isolated
SW480 SP cells, at both the transcriptional and translational
levels (Fig. 5).
Discussion
CRC is one of the most common cancers worldwide,
accounting for more than one million new cases and over a half
million deaths each year (38,39).
Chemotherapy is an important modality of CRC treatment. However,
drug resistance and cytotoxicity limit the effectiveness of
currently used chemotherapies (40). Mounting evidence suggests that the
existence of CSCs is one of the major causative factors for cancer
initiation and progression, and eventually leading to the failure
of clinical treatment. On the other hand, natural products,
particularly TCM, have attracted great attention in the field of
cancer research due to their definite therapeutic efficacy and
fewer adverse effects. Therefore, discovering naturally occurring
agents and/or targeting CSCs have become promising strategies for
cancer therapies. The well-known TCM formula PZH has long been used
in China to clinically treat various malignancies including CRC. We
previously demonstrated that PZH contains a broad range of
anticancer activities including an inhibitory effect on CSCs
(25–37), suggesting that PZH could be
developed as a novel multi-potent therapeutic agent for cancer
treatment.
Isolation and identification are critical steps for
CSC study. A commonly used approach to isolate stem cells is SP
analysis using fluorescence-activated cell sorting (FACS), which is
based on the ability of CSCs to expel incorporated Hoechst dyes due
to the overexpression of multidrug resistance (MDr) genes and
ATP-binding cassette (AbC) transporters (41,42).
SP cells have been identified in various types of cancer and are
shown to be correlated with tumor grade and patient prognosis. One
strategy of functional identification for isolated CSCs is
implanting SP cells in immune-deficient mice to assess their in
vivo tumor-initiating capacity (43). Another approach to verify the
stemness of isolated CSCs is in vitro sphere-forming assay,
with a special serum-free culture system where only CSC-enriched
cells can survive and form three-dimensional spheres (44). In the present study, we isolated
stem-like SP cells from human CrC SW480 cells and confirmed that
the sorted SP cells possessed characteristics of CSCs since SP
cells displayed stronger capacities of spheroid formation in
vitro and tumorigenicity in vivo, as compared with the
non-SP cells. More importantly, here we demonstrated that PZH
significantly decreased the percentage of SP cells in a
dose-dependent manner. Moreover, PZH treatment significantly and
dose-dependently inhibited the proliferation, and promoted the
apoptosis and differentiation of the isolated SW480 SP cells.
The Notch pathway plays important roles in many
biological processes including tissue patterning and morphogenesis,
cell differentiation, proliferation and apoptosis, which therefore
is essential for embryonic development and tissue homeostasis in
adulthood (9,10). Alterations in the components of the
Notch pathway have been strongly associated with cancer and appear
to be involved in the regulation of CSCs (19–22).
Thus, inhibition of the Notch pathway may provide a target for the
development of new anti-CSC therapeutic agents. Using qPCR and
western blot analyses, we found that PZH treatment markedly
suppressed the expression of Notch1 and Hes1 in the isolated SW480
SP cells, two mediators in the Notch pathway.
In conclusion, PZH may negatively modulate the
characteristics of CSCs through suppression of the Notch1 signaling
pathway.
Acknowledgments
The present study was sponsored by the National
Natural Science Foundation of China (81403390), the Research Fund
of the Education bureau of Fujian Province (JA14162), the
Joint-Project of Education and Health bureaus of Fujian Province
(WKJ-FJ-21), and the Developmental Fund of Chen Keji Integrative
Medicine (CKJ2013012, CKJ2013013 and CKJ2014004).
Abbreviations:
|
CRC
|
colorectal cancer
|
|
PZH
|
Pien Tze Huang
|
|
TCM
|
traditional Chinese medicine
|
|
CSC
|
cancer stem cell
|
|
SP
|
side population
|
References
|
1
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells - perspectives on current status and future directions:
AACR Workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: Models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar
|
|
4
|
Costea DE, Tsinkalovsky O, Vintermyr OK,
Johannessen AC and Mackenzie IC: Cancer stem cells - new and
potentially important targets for the therapy of oral squamous cell
carcinoma. Oral Dis. 12:443–454. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Locke M, Heywood M, Fawell S and Mackenzie
IC: Retention of intrinsic stem cell hierarchies in
carcinoma-derived cell lines. Cancer Res. 65:8944–8950. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takebe N, Harris PJ, Warren RQ and Ivy SP:
Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog
pathways. Nat Rev Clin Oncol. 8:97–106. 2011. View Article : Google Scholar
|
|
7
|
Bolós V, Blanco M, Medina V, Aparicio G,
Díaz-Prado S and Grande E: Notch signalling in cancer stem cells.
Clin Transl Oncol. 11:11–19. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu S, Dontu G, Mantle ID, Patel S, Ahn
NS, Jackson KW, Suri P and Wicha MS: Hedgehog signaling and bmi-1
regulate self-renewal of normal and malignant human mammary stem
cells. Cancer Res. 66:6063–6071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Noggle SA, Weiler D and Condie BG: Notch
signaling is inactive but inducible in human embryonic stem cells.
Stem Cells. 24:1646–1653. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyamoto S and Rosenberg DW: role of Notch
signaling in colon homeostasis and carcinogenesis. Cancer Sci.
102:1938–1942. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohishi K, Varnum-Finney B, Flowers D,
Anasetti C, Myerson D and Bernstein ID: Monocytes express high
amounts of Notch and undergo cytokine specific apoptosis following
interaction with the Notch ligand, Delta-1. blood. 95:2847–2854.
2000.PubMed/NCBI
|
|
13
|
Bolós V, Grego-bessa J and de la Pompa JL:
Notch signaling in development and cancer. Endocr Rev. 28:339–363.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hansson EM, Lendahl U and Chapman G: Notch
signaling in development and disease. Semin Cancer biol.
14:320–328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iso T, Kedes L and Hamamori Y: HES and
HERP families: Multiple effectors of the Notch signaling pathway. J
Cell Physiol. 194:237–255. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Li B, Ji ZZ and Zheng PS: Notch1
regulates the growth of human colon cancers. Cancer. 116:5207–5218.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reedijk M, Odorcic S, Zhang H, Chetty R,
Tennert C, Dickson BC, Lockwood G, Gallinger S and Egan SE:
Activation of Notch signaling in human colon adenocarcinoma. Int J
Oncol. 33:1223–1229. 2008.PubMed/NCBI
|
|
18
|
Miele L, Golde T and Osborne B: Notch
signaling in cancer. Curr Mol Med. 6:905–918. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qiu M, Peng Q, Jiang I, Carroll C, Han G,
Rymer I, Lippincott J, Zachwieja J, Gajiwala K, Kraynov E, et al:
Specific inhibition of Notch1 signaling enhances the antitumor
efficacy of chemotherapy in triple negative breast cancer through
reduction of cancer stem cells. Cancer Lett. 328:261–270. 2013.
View Article : Google Scholar
|
|
20
|
Sharma A, Paranjape AN, Rangarajan A and
Dighe RR: A monoclonal antibody against human Notch1 ligand-binding
domain depletes subpopulation of putative breast cancer stem-like
cells. Mol Cancer Ther. 11:77–86. 2012. View Article : Google Scholar
|
|
21
|
Wang J, Wang C, Meng Q, Li S, Sun X, Bo Y
and Yao W: sirNA targeting Notch-1 decreases glioma stem cell
proliferation and tumor growth. Mol biol rep. 39:2497–2503. 2012.
View Article : Google Scholar
|
|
22
|
Fre S, Huyghe M, Mourikis P, Robine S,
Louvard D and Artavanis-Tsakonas S: Notch signals control the fate
of immature progenitor cells in the intestine. Nature. 435:964–968.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ward RJ and Dirks PB: Cancer stem cells:
At the headwaters of tumor development. Annu Rev Pathol. 2:175–189.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Efferth T: Stem cells, cancer stem-like
cells, and natural products. Planta Med. 78:935–942. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin JM, Wei LH, Chen YQ, Liu XX, Hong ZF,
Sferra TJ and Peng J: Pien Tze Huang induced apoptosis in human
colon cancer HT-29 cells is associated with regulation of the bcl-2
family and activation of caspase 3. Chin J Integr Med. 17:685–690.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhuang Q, Hong F, Shen A, Zheng L, Zeng J,
Lin W, Chen Y, Sferra TJ, Hong Z and Peng J: Pien Tze Huang
inhibits tumor cell proliferation and promotes apoptosis via
suppressing the STAT3 pathway in a colorectal cancer mouse model.
Int J Oncol. 40:1569–1574. 2012.PubMed/NCBI
|
|
27
|
Shen AL, Hong F, Liu LY, Lin JM, Zhuang
QC, Hong ZF and Peng J: Effects of Pien Tze Huang on angiogenesis
in vivo and in vitro. Chin J Integr Med. 18:431–436. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen A, Hong F, Liu L, Lin J, Wei L, Cai
Q, Hong Z and Peng J: Pien Tze Huang inhibits the proliferation of
human colon carcinoma cells by arresting G1/S cell cycle
progression. Oncol Lett. 4:767–770. 2012.PubMed/NCBI
|
|
29
|
Shen A, Chen Y, Hong F, Lin J, Wei L, Hong
Z, Sferra TJ and Peng J: Pien Tze Huang suppresses IL-6-inducible
STAT3 activation in human colon carcinoma cells through induction
of SOCS3. Oncol Rep. 28:2125–2130. 2012.PubMed/NCBI
|
|
30
|
Shen A, Lin J, Chen Y, Lin W, Liu L, Hong
Z, Sferra TJ and Peng J: Pien Tze Huang inhibits tumor angiogenesis
in a mouse model of colorectal cancer via suppression of multiple
cellular pathways. Oncol Rep. 30:1701–1706. 2013.PubMed/NCBI
|
|
31
|
Huang M, Zhao H, Xu W, Chu K, Hong Z, Peng
J and Chen L: Rapid simultaneous determination of twelve major
components in Pien Tze Huang by ultra-performance liquid
chromatography coupled with triple quadrupole mass spectrometry. J
Sep Sci. 36:3866–3873. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen H, Shen A, Zhang Y, Chen Y, Lin J,
Lin W, Sferra T and Peng J: Pien Tze Huang inhibits hypoxia-induced
epithelial-mesenchymal transition in human colon carcinoma cells
through suppression of the HIF-1 pathway. Exp Ther Med.
7:1237–1242. 2014.PubMed/NCBI
|
|
33
|
Shen A, Chen H, Chen Y, Lin J, Lin W, Liu
L, Sferra TJ and Peng J: Pien Tze Huang overcomes multidrug
resistance and epithelial-mesenchymal transition in human
colorectal carcinoma cells via suppression of TGF-β pathway. Evid
based Complement Alternat Med. 2014:6794362014. View Article : Google Scholar
|
|
34
|
Lin W, Zhuang Q, Zheng L, Cao Z, Shen A,
Li Q, Fu C, Feng J and Peng J: Pien Tze Huang inhibits liver
metastasis by targeting TGF-β signaling in an orthotopic model of
colorectal cancer. Oncol Rep. 33:1922–1928. 2015.PubMed/NCBI
|
|
35
|
Chen H, Feng J, Zhang Y, Shen A, Chen Y,
Lin J, Lin W, Sferra TJ and Peng J: Pien Tze Huang inhibits
hypoxia-induced angiogenesis via HIF-1 α/VEGF-A pathway in
colorectal cancer. Evid based Complement Alternat Med.
2015:4542792015. View Article : Google Scholar
|
|
36
|
Shen A, Lin W, Chen Y, Liu L, Chen H,
Zhuang Q, Lin J, Sferra TJ and Peng J: Pien Tze Huang inhibits
metastasis of human colorectal carcinoma cells via modulation of
TGF-β1/ZEb/mir-200 signaling network. Int J Oncol. 46:685–690.
2015.
|
|
37
|
Wei L, Chen P, Chen Y, Shen A, Chen H, Lin
W, Hong Z, Sferra TJ and Peng J: Pien Tze Huang suppresses the
stem-like side population in colorectal cancer cells. Mol Med Rep.
9:261–266. 2014.
|
|
38
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Markowitz SD and Bertagnolli MM: Molecular
origins of cancer: Molecular basis of colorectal cancer. N Engl J
Med. 361:2449–2460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Longley DB, Allen WL and Johnston PG: Drug
resistance, predictive markers and pharmacogenomics in colorectal
cancer. biochim biophys Acta. 1766:184–196. 2006.PubMed/NCBI
|
|
41
|
Golebiewska A, Brons NH, Bjerkvig R and
Niclou SP: Critical appraisal of the side population assay in stem
cell and cancer stem cell research. Cell Stem Cell. 8:136–147.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Scharenberg CW, Harkey MA and Torok-Storb
B: The AbCG2 transporter is an efficient Hoechst 33342 efflux pump
and is preferentially expressed by immature human hematopoietic
progenitors. blood. 99:507–512. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nicolis SK: Cancer stem cells and
'stemness' genes in neuro-oncology. Neurobiol Dis. 25:217–229.
2007. View Article : Google Scholar
|
|
44
|
Pastrana E, Silva-Vargas V and Doetsch F:
Eyes wide open: A critical review of sphere-formation as an assay
for stem cells. Cell Stem Cell. 8:486–498. 2011. View Article : Google Scholar : PubMed/NCBI
|