Introduction
Oral squamous cell carcinoma (OSCC) is a common
malignancy in the oral cavity, accounting for ~90% of all oral
tumors, with an estimated 300,000 new cases annually (1). Despite advances in diagnosis and
multimodal therapies, tumors are associated with a poor prognosis
with patient 5-year survival rates of approximately 50%. The
unfavorable prognosis is mainly caused by local invasion and the
earlier regional lymph node metastasis that impedes complete tumor
resection and leads to inevitable postoperative recurrence
(2). Therefore, understanding the
underlying molecular mechanisms involved in the progression of OSCC
and developing a novel therapeutic strategy is imperative.
Integrin-linked kinase (ILK), a ubiquitously
expressed protein with serine/threonine protein kinase activities,
interacts with cytoplasmic domains of β1 and β3 integrins (3,4). As a
multifunctional adaptor protein, ILK has been regarded as a vital
regulator of the phosphoinositide 3-kinase (PI3K) signaling
pathway, which activates protein kinase B (PKB)/Akt activity and
inhibits glycogen synthase kinase-3 (Gsk-3) activity, regulating
cell proliferation, apoptosis, differentiation, migration,
invasion, angiogenesis and epithelial-mesenchymal transition (EMT)
(5,6). EMT is a complex cell process in which
cells lose their epithelial features and acquire a mesenchymal
phenotype, which plays a vital role in embryogenesis, tissue
remodeling, wound healing and tumor metastasis. EMT process is
associated with the reduced expression of epithelial markers such
as E-cadherin, and increased expression of mesenchymal markers such
as C-cadherin (7–9). Overexpression of ILK has been reported
in various types of cancer (10–13),
promoting cell migration and invasion by inducing EMT (12,14–17).
Consistent with the abovementioned findings, our previous study on
OSCC clinical specimens revealed that ILK facilitated the
progression and metastasis of OSCC through the EMT-related
upregulation of Snail and consequent aberrant expression of E- and
N-cadherin (18). Knockdown of ILK
has been reported to inhibit cell proliferation, migration,
invasion, angiogenesis, and promotes apoptosis in different types
of cancer cells, including gastric cancer, bladder cancer, lung
cancer, ovarian carcinoma, and tongue cancer (19–23).
However, the molecular mechanisms by which ILK contributes to the
development and progression of OSCC via EMT remain to be
investigated.
In the present study, a lentivirus-mediated short
hairpin RNA (shRNA) targeting ILK was generated and the effects of
ILK silencing on OSCC cells were investigated. The results revealed
that the knockdown of ILK inhibited cell proliferation, invasion,
blocked the cell cycle, promoted cell apoptosis and suppressed the
EMT process in vitro by regulating the Akt and GSk-3β
signaling pathways. Knockdown of ILK also suppressed tumor growth,
invasion and metastasis in nude mice xenograft models. In summary,
the results indicated that ILK is imperative in the development and
progression of OSCC and has the potential to be an efficient
therapeutic target for OSCC treatment.
Materials and methods
Cell line and culture
The human OSCC BCaCD885 cell line was obtained from
the State Key Laboratory of Oral Diseases, Sichuan University
(Chengdu, China) and cultured in Dulbecco's modified Eagle's medium
(DMEM; Gibco-Life Technologies, Grand Island, NY, USA) supplemented
with 15% fetal bovine serum (FBS; Hyclone, Logan, UT, USA), 1%
penicillin/streptomycin at 37°C in a humidified atmosphere with 5%
CO2. The cells were routinely passaged every 2–3 days by
trypsinization (Gibco-Life Technologies). The cells at the
logarithmic growth phase were used in the present study.
Construction of lentiviral vectors
expressing ILK-specific shRNA and transfection (24,25)
The ILK-specific target sequence was selected
according to online siRNA tools utilized from Invitrogen
(http://www.invitrogen.com/rnai) and the
GenBank (accession no. NM 004517.2). Double-stranded DNA containing
the interference sequences were synthesized according to the
manufacturer's instructions (sequence: 5′-CGA AGC TCA ACG AGA ATC
A-3′) (GeneChem, Shanghai, China), and the negative control shRNA
targeted a random and non-specific sequence with no homology to the
human genome. The sequences were cloned into the pGCSIL-green
fluorescent protein (GFP; GeneChem) to generate the lentiviral
vectors, and then transfected into BCaCD885 cells. On the day of
infection, the culture medium was replaced with the appropriately
titered viral supernatant and incubated at 37°C for 12 h. After 3
days of transfection, the number of GFP-positive cells was
measured, and shRNA ILK-silencing efficiency was determined using
western blotting. The titers were averaged to 8×109
TU/ml. The lentivirus containing the human ILK shRNA-expressing
cassette was used as a positive control and designated as the KD
group. The empty vector was used as a negative control, designated
as the NC group, and the non-transfected as the CON group.
RNA isolation and quantitative PCR
Total RNA was prepared using TRIzol reagent
(Invitrogen-Life Technologies, Carlsbad, CA, USA) and cDNA was
synthesized using the PrimeScript® RT reagent kit
(Takara, Dalian, China). ILK mRNA expression was evaluated
quantitatively by RT-qPCR with SYBR® Premix Ex Taq™
(Takara) and the ABI PRISM 7900HT Real-Time PCR system (ABI,
Vernon, CA, USA). The primers were synthesized (Sangon, China) and
the sequences used were: ILK forward, 5′-ATG GAA CCC TGA ACA AAC
ACT-3′ and reverse, 5′-AGC ACA TTT GGA TGC GAG AAA-3′; and GAPDH
forward, 5′-GCT GTC CCT GTA CGC CTC TG-3′ and reverse, 5′-TGC CGA
TGG TGA TGA CCT GG-3′. GAPDH served as an internal control. The
thermocycler conditions used were: preheating at 95°C for 30 sec,
95°C for 5 min, 30 cycles of 10 sec at 95°C, 20 sec at 60°C, 30 sec
at 72°C. The relative amount of PCR product was evaluated as the
comparative threshold cycle of the sample divided by that of GAPDH
(2−ΔΔCt) (26).
Experiments were carried out in triplicate and repeated three
times.
Protein extraction and western blot
analysis
The cells were washed with ice-cold PBS twice and
treated with cell lysis buffer (50 mmol/l Tris, pH 7.8, 150 mmol/l
NaCl, 1% Nonidet-40), then centrifuged at 175 × g for 10 min, and
the supernatants were collected and stored at −80°C. Protein
concentration was measured using the bicinchoninic acid assay (BCA
Protein Assay kit; Thermo Fisher Scientific lnc., Rockford, IL
USA). Equal amounts (50 µg) of protein were heated at 95°C
for 5 min, separated by 8–12% SDS-PAGE gel, and electrotransferred
onto polyvinylidene fluoride (PVDF) membranes (Bio-Rad, Hercules,
CA, USA). The PVDF membranes were then blocked with 5% skim milk in
Tris-buffered saline and incubated overnight at 4°C with monoclonal
primary rabbit antibody of anti-human ILK, cyclin A, cyclin B1,
cyclin D1, CD31, Snail (Cell Signaling Technology, Danvers, MA,
USA), Akt, p-Akt (Ser473), Gsk-3β and p-Gsk-3β (Ser9) (Bioworld
Technology, St. Louis Park, MN, USA), E-cadherin, Slug, Twist2 and
polyclonal primary mouse antibody of anti-human caspase-3, MMP-9
(1:1,000 dilution; Beijing Zhongshan Biotechnology, Beijing, China)
and washed four times with PBS-T. Subsequently, the membranes were
incubated with HRP-conjugated secondary antibodies of goat
anti-rabbit or goat anti-mouse IgG (1:1,000 dilution) at room
temperature for 1 h and washed four times with PBS-T. The signal
was then visualized with an enhanced chemiluminescence assay
according to the manufacturer's instructions (LumiGLO Peroxidase
Chemiluminescent Substrate kit; KPL, Gaithersburg, MD, USA). The
amounts of proteins were quantified by densitometry and GAPDH of
the same sample served as an internal control.
Colony formation assay
The effect of ILK silencing on the colony formation
of BCaCD885 cells was analyzed using the colony formation assay.
Approximately 3×102 cells were plated in 60-mm dishes
and cultured in 10% FBS DMEM at 37°C, 5% CO2 for 2
weeks. The cell colonies were washed twice with PBS, fixed with
methanol for 5 min and stained with crystal violet for 20 min, and
then washed twice using ddH2O. A colony was defined as
aggregates consisting of ≥50 cells, and manually counted under a
microscope (Leica DM LB2, Wetzlar, Germany). The experiments were
performed in triplicate and repeated three times.
Cell cycle analysis
BCaCD885 cells were collected and washed with
ice-cold PBS, fixed with 70% ethanol for 24 h, then centrifuged at
600 × g for 5 min, and resuspended in propidium iodide (PI; Sigma,
St. Louis, MO, USA) supplemented with RNase A and incubated at 37°C
in the dark for 30 min, then analyzed using a flow cytometer
(Beckman Coulter, Brea, CA, USA) to measure the cell cycle
distribution. The relative proportions of the cells in the
individual cell-cycle phase fraction were determined from the flow
cytometry data. The experiments were performed in triplicate and
repeated three times.
Cell apoptosis analysis
After the cells were harvested, 5×105
cells were rinsed twice with PBS, and centrifuged at 600 × g for 5
min and resuspended in 500 µl binding buffer. The cells were
then incubated for 15 min with 1 µl of Annexin V-PE and 5
µl 7-AAD for 15 min at room temperature in the dark
according to the manufacturer's instructions (Annexin V-PE/7-AAD
Apoptosis Detection kit; Keygentec, China). Cell apoptosis was
analyzed using flow cytometry (Beckman Coulter). The experiments
were carried out in triplicate and repeated three times.
Transwell invasion assay
After reaching 80% confluence, the BCaCD885 cells
were digested with 0.05% trypsin and resus-pended at
5×104 cells/100 µl. Prior to cell incubation, the
cells (300 µl) and 200 µl of serum-free DMEM were
added to the in the inner chamber, and DMEM medium (500 µl)
with 10% FBS to the lower chamber, between which the Matrigel gel
(BD Biosciences, Billerica, MA, USA) was placed on the surface of
the filtration membrane of the Transwell micropore with an aperture
of 8 µm. Following incubation at 37°C for 24 h, the
non-invading cells in the inner chamber and the extracellular
Matrigel were gently removed and washed with PBS. The number of
invasive cells that migrated through the gel to the lower surface
of the membrane were stained with 1% crystal violet for 20 min and
images were captured under a phase contrast microscope in five
random fields. Results were presented as the mean percentage of the
control (control OD at 562 nm assigned as 100%). Each experiment
was performed in triplicate and repeated three times.
Cell adhesion assays
Prior to cell cultivation, 50 µl of
extracellular matrix gel (ECM gel; Sigma) mixed with serum-free
DMEM (1:1) and 50 µl serum-free DMEM containing 10 g/l
bovine serum albumin (BSA; Sigma) were added to 96-well plates and
incubated at 37°C for 30 min. BCaCD885 cells were trypsinized and
resuspended at 5×104 cells/100 µl in DMEM medium.
Cells (100 µl) were added to the 96-well plate and cultured
for 4 h. After incubation, the non-adherent cells were removed by
PBS washing, and adherent cells were fixed and stained with 1%
crystal violet for 20 min at 37°C. Absorbances at 590 nm were
determined using a microplate reader (Bio-Rad).
Animals and tumor xenograft treatment
model
Fifteen 6-week-old BALB/C-nu/nu mice were purchased
from Sichuan University Experimental Animal Center (Sichuan,
China), bred and held under specific pathogen-free conditions. The
mice were allowed access to food and water ad libitum prior
to surgery under optimal conditions (12-h light/dark with humidity
at 60±5%, 22±3°C). The protocol was approved by the Ethics
Committee of the State Key Laboratory of Oral Diseases, Sichuan
University. Animal procedures were performed according to the
internationally accepted ethical guidelines.
Single-cell suspension of 100 µl containing
2×106 BCaCD885 cells was injected subcutaneously into
the skin of the two armpits for each mouse. When tumors formed on
day 7 after inoculation, the mice were randomly divided into three
treatment groups, n=5 mice per group. Different treatments were
performed on the three groups over four consecutive days. For group
1, one side was injected with 1×107 TU (50 µl)
lentivirus-encoded shRNA targeting ILK (KD) and the other side was
injected with 50 µl sterilizing saline (CON). Mice in group
2 were injected on one side with KD and the other side with
1×107 TU (50 µl) negative control lentivirus
(empty vector, NC). Mice in group 3 were injected on one side with
NC and the other side with CON. The time of tumor formation was
recorded, and 28 days after injection, the mice were sacrificed.
The xenograft tumors were excised and weighed, fixed in 10%
buffered formalin at room temperature for pathology analysis, and
the other tumor tissues were immediately placed in liquid nitrogen
for the frozen section of immunofluorescence assay.
Histology and immunohistochemistry
The tumor tissue, lymph node and vital organs
harvested from mice xenograft were fixed in 10% buffered formalin
and embedded in paraffin, and 5-µm sections were stained
with hematoxylin and eosin. The microvessels were counted from 10
different fields under the microscope at a magnification of ×200,
corresponding to areas with the highest density of vessels.
After being fixed and embedded, the samples were
dewaxed twice in xylene for 15 min and rehydrated in gradient
concentration of ethanol (approximately 10–15 ml) at room
temperature. The sections were treated with 3% hydrogen peroxide
for 30 min at room temperature and rinsed three times with PBS to
block non-specific antibodies. The samples were incubated in
primary antibodies of rabbit anti-human ILK (1:100 dilution; Cell
Signaling Technology), Snail (1:200 dilution) and E-cadherin (1:100
dilution) (both from Abcam, Cambridge, MA, USA), caspase-3 (1:100
dilution), Ki67 (1:100 dilution) and MMP-9 (1:100 dilution)
(Beijing Zhongshan Biotechnology) separately at 37°C for 60 min and
incubated overnight at 4°C. The sections were subsequently washed
three times with PBS, and incubated in secondary antibodies for 40
min at 37°C using DAB staining (DAB Horseradish Peroxidase Color
Development kit; Beijing Zhongshan Biotechnology). The sections
were then counterstained with hematoxylin, dehydrated and mounted.
To detect tumor angiogenesis, the sections were incubated in the
monoclonal primary mouse anti-human CD31 (1:100 dilution; BD
Biosciences) overnight at 4°C, and the remainder of the procedure
was performed as mentioned above. Observations were performed under
an Olympus multifunction microscope (Olympus, Tokyo, Japan) and
fluorescence microscope (Leica DM LB2). Each section was examined
independently by two pathologists and five fields of view were
randomly selected under an optical microscope at magnification of
×200.
Statistical analysis
Data were presented as the means ± SD. Differences
between groups were analyzed by one-way ANOVA. Statistical analysis
was performed using the SPSS 13.0 software package (SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate statistical
significance.
Results
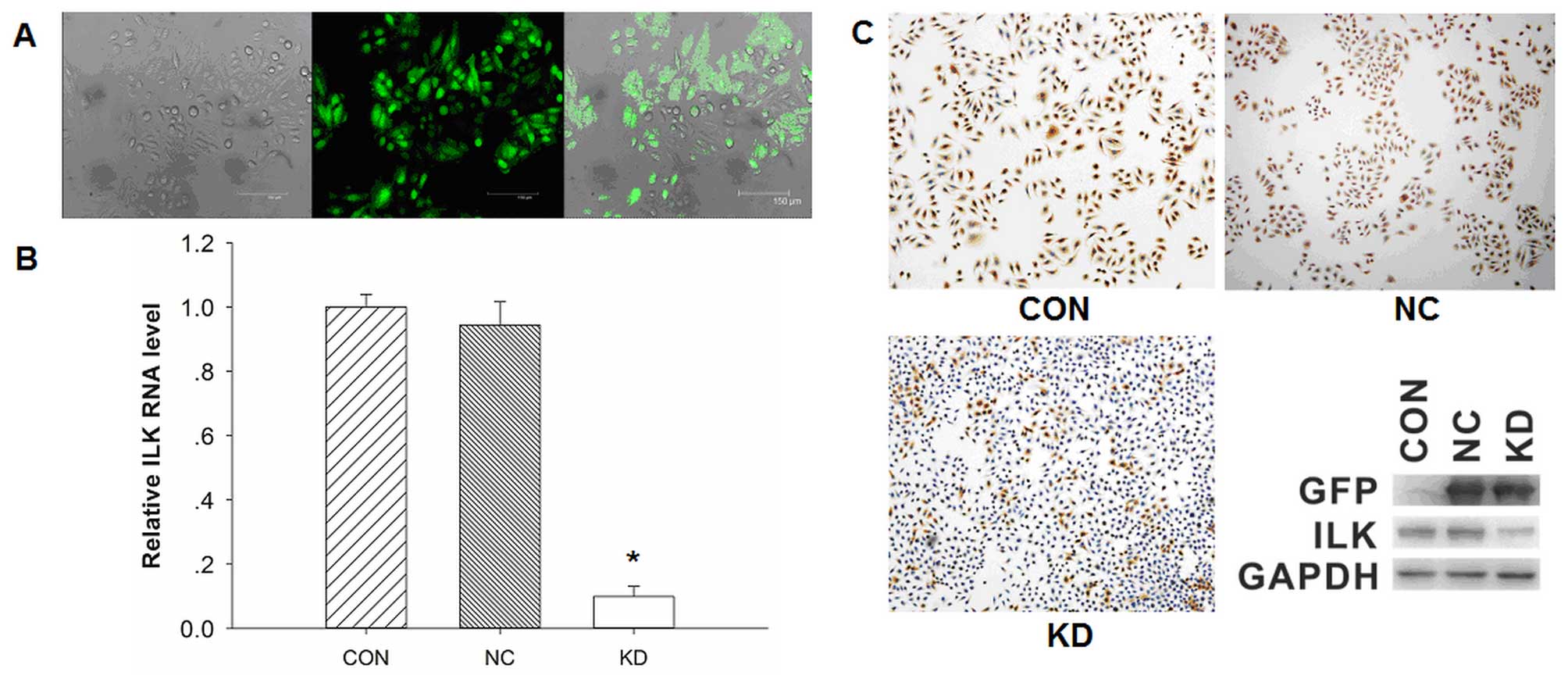
Observation of transfection efficiency
and identification of ILK expression
The transfection efficiency was determined under a
fluorescence microscope. A large number of cells exhibited bright
green fluorescence, which represented high transfection efficiency,
while no green fluorescence was detected in non-transfected cells
(Fig. 1A). The transfected cells
were isolated by G418 selection, and then cultured and identified
using qPCR, western blotting and an immuno-fluorescence assay. The
mRNA expression of ILK was significantly downregulated in BCaCD885
ILK knockdown cells (KD group) compared with the BCaCD885 vector
cells (NC group) and BCaCD885 cells (CON group), and the results
were statistically significant (P<0.05, Fig. 1B). By contrast, there was no
significant difference on ILK mRNA expression between CON and NC
cells.
Immunofluorescence images showed that ILK was
significantly weaker in KD cells compared with the control cells
(Fig. 1C). The protein expression
of ILK was significantly reduced by 80.0% in the KD cells compared
with the NC cells.
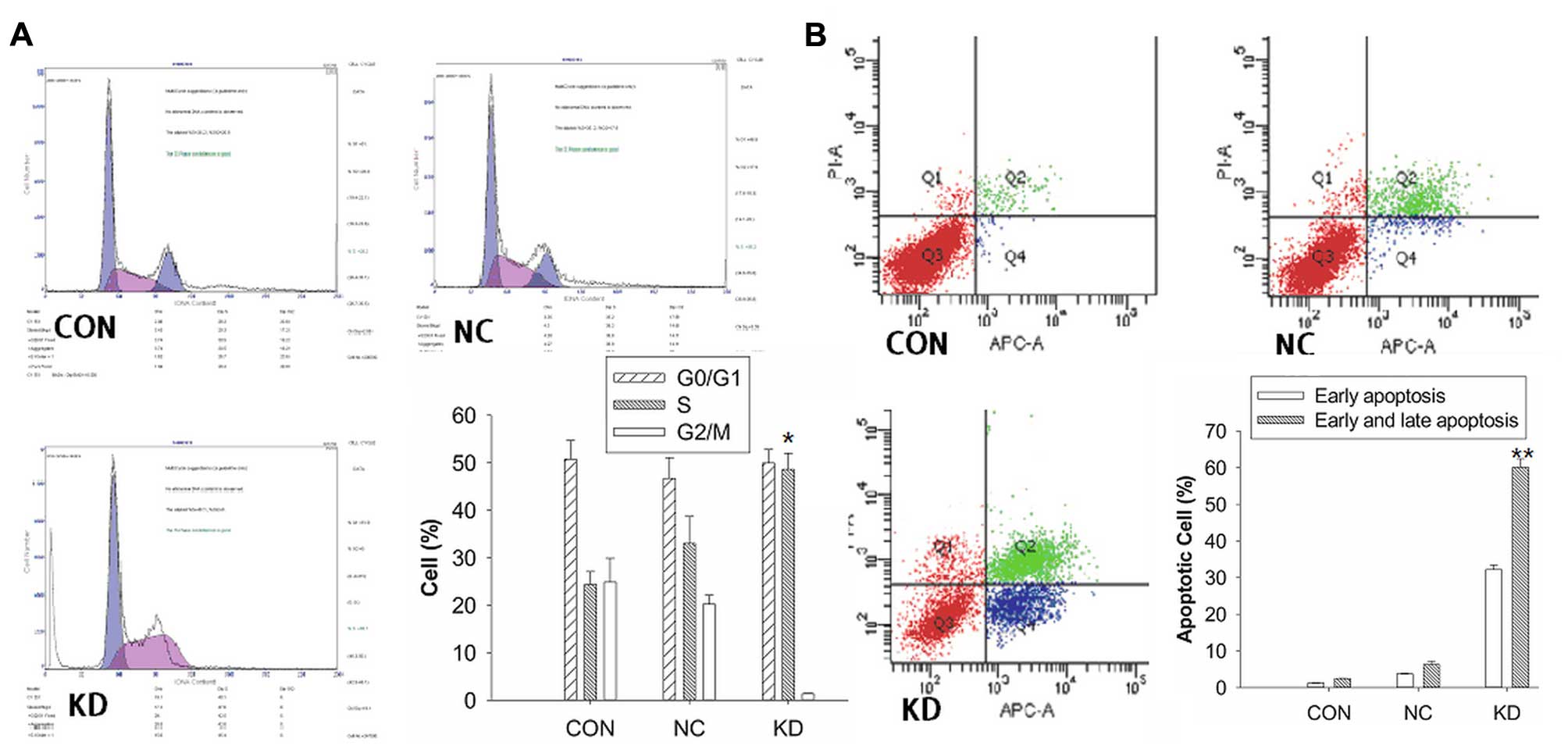
Effects of ILK knockdown on cell cycle
and cell apoptosis
The cell cycle profile was analyzed by flow
cytometry (Fig. 2A). The data
revealed that the percentage of KD cells blocked in the S phase
(48.67±3.36%) was significantly higher compared with the CON cells
(24.36±2.75%) and NC cells (33.03±5.86%), respectively (P<0.05,
Fig. 2B). The results suggested
that the knockdown of BCaCD885 ILK cells were arrested at the
restriction point of the cell cycle and failed to enter mitosis,
leading to cell apoptosis.
The cell apoptotic rate was evaluated by flow
cytometry. As shown in Fig. 2B, the
results showed that the early apoptotic cells (APC+,
PI−, 32.2%) and late apoptotic cells (APC+,
PI+, 27.9%) in the KD group were significantly higher
than the control groups (P<0.01), which indicated that the
inhibition of cell proliferation may be caused by apoptotic cell
death.
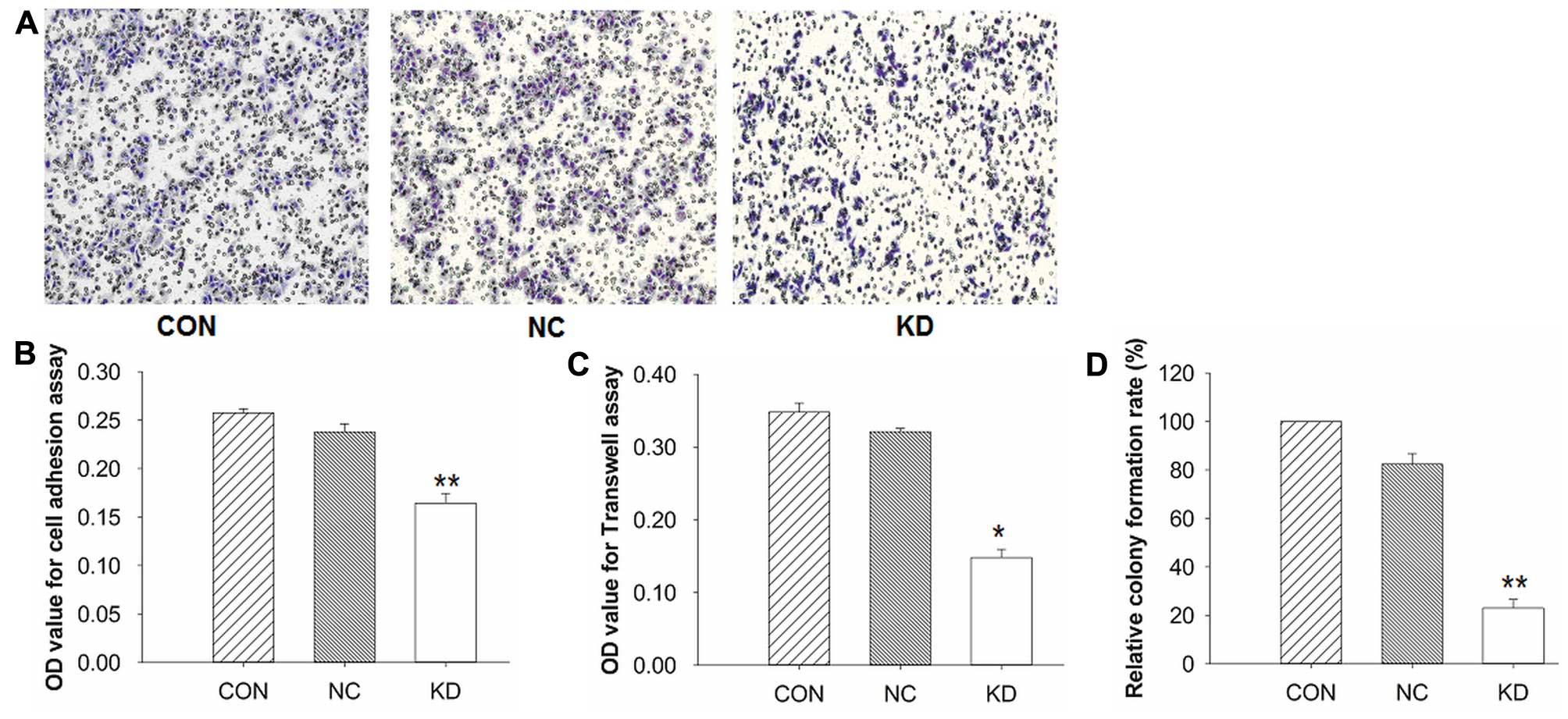
Knockdown of ILK suppressed cell
adhesion, invasion and cell proliferation of BCaCD885 cells
We examined the effects of ILK silencing on the cell
adhesion and invasion of BCaCD885 cells. The cell adhesion assay
showed that the number of KD cells adhering to the extracellular
cell matrix gel was markedly decreased compared to the remaining
groups (P<0.01), and there was no significant difference between
the NC and CON groups (P>0.05, Fig.
3B). Furthermore, to determine the effect of ILK knockdown on
cell invasion ability, a Transwell assay was applied. As shown in
Fig. 3A, the cells cultured in
Transwell chambers were allowed to migrate through the 8-µm
pores of membranes coated with Matrigel. The penetrating cells in
the KD group were fewer than those in the control groups
(P<0.05, Fig. 3C), which was
consistent with the adhesion assays. These results demonstrated
that the knockdown of ILK suppressed the abilities of adhesion and
invasion of BCaCD885 cells.
The colony formation assay was applied for the
detection of cell proliferation, and the results showed that the KD
cells had a significantly lower cell proliferation rate than the
remaining cells (P<0.01). However, no significant difference was
observed between the NC and CON groups (P>0.05, Fig. 3D).
Effects of downregulating ILK on the
expression of proteins associated with cell proliferation,
invasion, apoptosis and EMT
To determine the underlying mechanism of
downregulating ILK on cell proliferation, invasion, apoptosis and
EMT, western blot analysis was applied to detect the expression of
the corresponding proteins. Images showed that the expression of
cyclin A, cyclin B1 and cdc2, key proteins regulating the cell
cycle from S phase to G2/M phase, were significantly down-regulated
in KD cells compared with the control cells (Fig. 4A). The expression of cyclin D1,
which was involved in regulating the cell cycle from G1 to S phase,
was only slightly down-regulated (Fig.
4A). Fig. 4A also showed that
the expression of MMP-9, which refers to the ability of cell
invasion, was inhibited in ILK knockdown cells compared with the
control cells. Notably, the expression of key regulating proteins
of EMT, including Snail, Twist2 and Slug, were also reduced in KD
cells. A protein band of cleaved Snail was observed in ILK
knockdown cells (Fig. 4A). At the
same time, the expression of E-cadherin was slightly upregulated in
the KD cells. An increase of caspase-3 protein was detected in KD
cells, indicating that ILK may promote cell apoptosis by
upregulating caspase-3. The findings suggested that downregulating
ILK suppressed EMT and the invasiveness of OSCC cells.
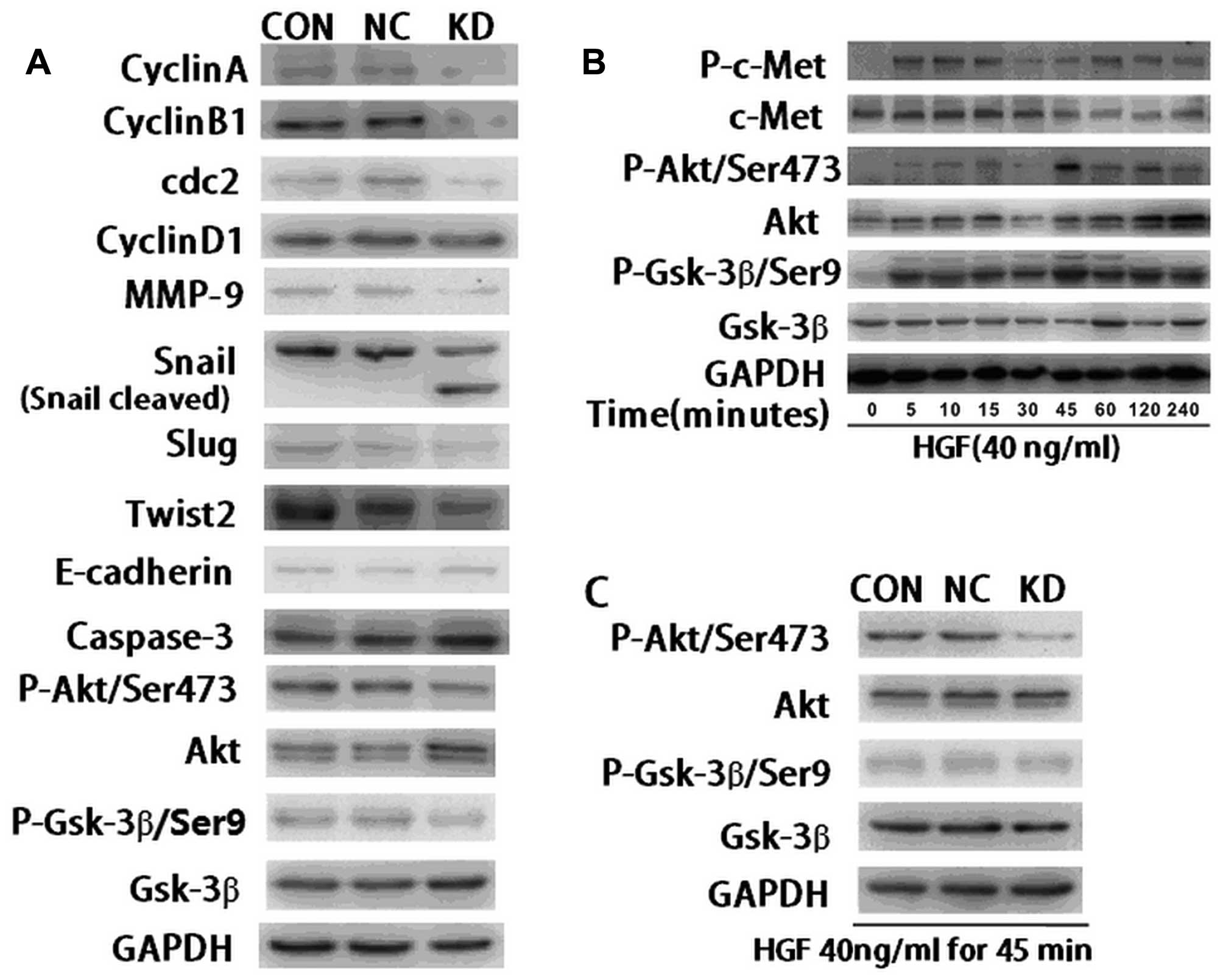 | Figure 4Effects of ILK knockdown on the
protein expression of genes associated with cell proliferation,
invasion, apoptosis, EMT and downstream signaling molecules in
BCaCD885 cells. (A) Western blot analysis of relative proteins in
KD, NC and CON cells. Cyclin A, cyclin B1, cyclin D1, cdc2, MMP-9,
Snail, Twist2, Slug, E-cadherin and caspase-3 proteins were
examined by western blot analysis, using GAPDH protein as the
control. The results show that the protein expression of E-cadherin
and caspase-3 was increased, while the protein expression of cyclin
A, cyclin B1, cyclin D1, cdc2, MMP-9, Snail, Twist2, Slug, p-Akt
and p-Gsk-3β was suppressed in the KD group compared with the
control groups. (B) BCaCD885 cells were treated with 40 ng/ml HGF
and the expression of Akt, Gsk-3β, c-Met and their phosphorylations
were detected by western blot analysis. The expression of
phosphorylation of the HGF receptor c-Met was observed after 5 min
and the expression of p-Akt and p-Gsk-3β reached its peak at 45 min
following treatment with 40 ng/ml HGF. (C) After cells were treated
with 40 ng/ml HGF for 45 min, the expression of p-Akt and p-Gsk-3β
was evidently weaker in ILK knockdown cells compared with the
remaining cells. ILK, integrin-linked kinase; Gsk-3, glycogen
synthase kinase-3; EMT, epithelial-mesenchymal transition; HGF,
hepatocyte growth factor. |
Effects of ILK knockdown on the
expression of downstream signaling target molecules
To examine the ILK downstream targets and molecular
mechanisms of the effects that ILK knockdown exerted on BCaCD885
cells as mentioned above, we investigated the protein levels of
Akt, Gsk-3β and their phosphorylated formation. Western blot
analysis showed that expression of p-Akt (Ser473) and p-Gsk-3β
(Ser9) were inhibited in ILK knockdown cells compared with the
control cells (Fig. A). However,
the expression of Akt and Gsk-3β showed no difference in the three
groups (Fig. 4A).
To determine the effects of ILK knockdown on the
phosphorylation of Akt and Gsk-3β induced by exogenous growth
factors, we used hepatocyte growth factor (HGF) as an intervention,
which was capable of activating PI3K and was present in the tumor
microenvironment. The phosphorylation levels of Akt and Gsk-3β were
detected by western blot analysis. The results showed that 5 min
after BCaCD885 cells were treated with HGF at 40 ng/ml, c-Met, the
phosphorylation of HGF receptor was observed. After 45 min, the
phosphorylation level of Akt and Gsk-3β reached its peak (Fig. 4B). The phosphorylation level of Akt
and Gsk-3β in ILK knockdown cells was not as significant as in the
control cells. These results suggested that ILK was able to inhibit
HGF-induced Akt and Gsk-3β phosphorylation to a certain extent
(Fig. 4C).
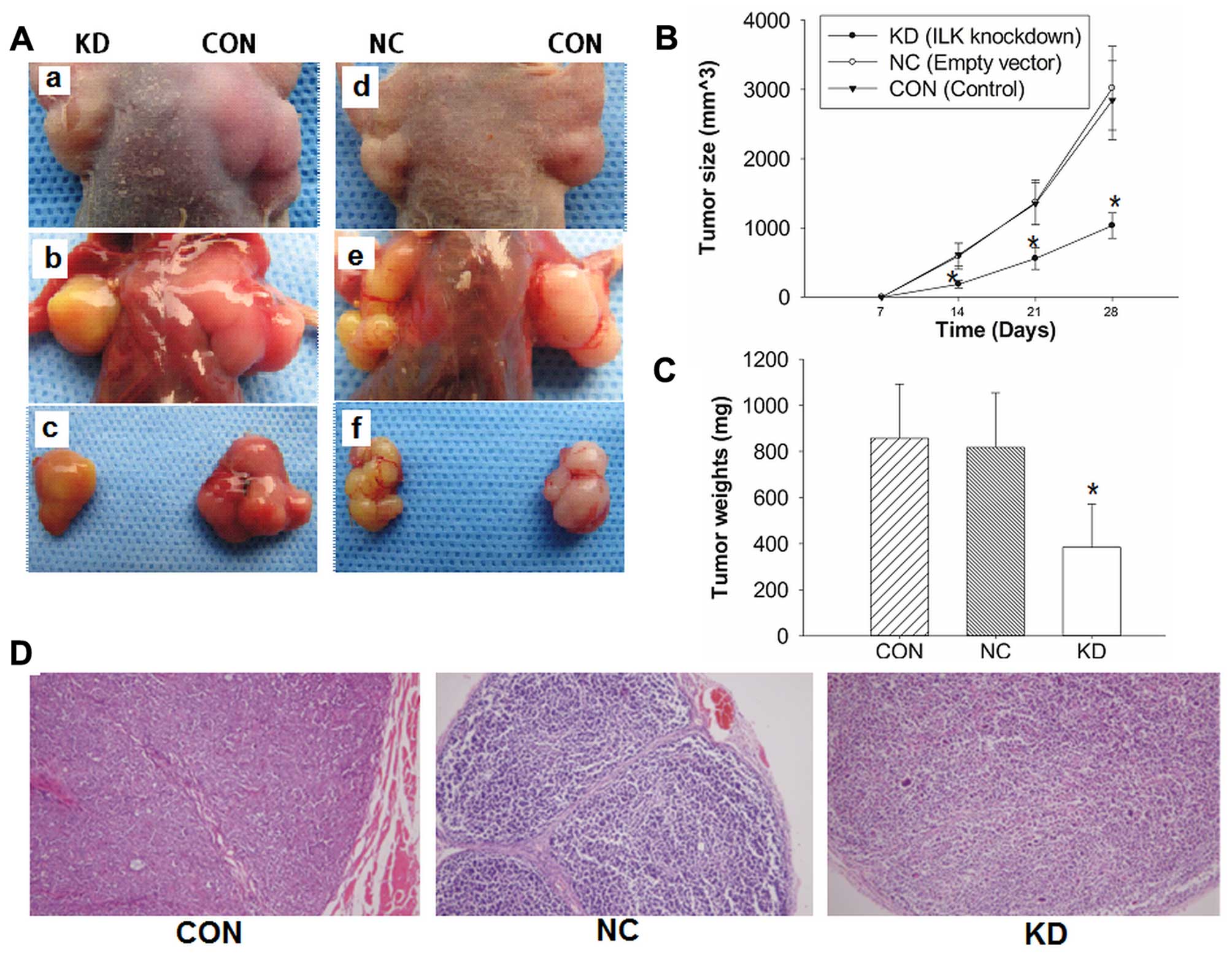
Knockdown of ILK inhibits tumor growth
and metastasis of BALB/C nude mouse xenograft model
To investigate whether ILK shRNA inhibited the
growth of tumors in a nude mouse xenograft model, 2×106
cells in 0.1 ml PBS were implanted subcutaneously into the armpits
of BALB/C nude mice. The growth of mouse xenograft tumors was
significantly inhibited in the KD group compared with the control
groups (Fig. 5A). Mice were
sacrificed and the tumors were collected and stained with H&E
(magnification, ×100) and immunohistochemistry (Fig. 5D). The average tumor sizes were
1,032±182 mm3 in the KD group, 3,021±606 mm3
in the NC group and 2,835±571 mm3 in the CON group
(Fig. 5B), and the average tumor
weights were 302±188 mg in the KD group, 817±237 mg in the NC group
and 857±235 mg in the CON group (Fig.
5C). The inhibiting rate of tumor growth was 55.56 or 53.57%,
respectively. Statistical analysis revealed significant differences
between the KD group and the remaining groups (P<0.05).
Mice from the KD group also showed a significant
inhibition of the spontaneous lymph node or distant metastasis, in
which no metastasis was observed, whereas lymph node metastasis
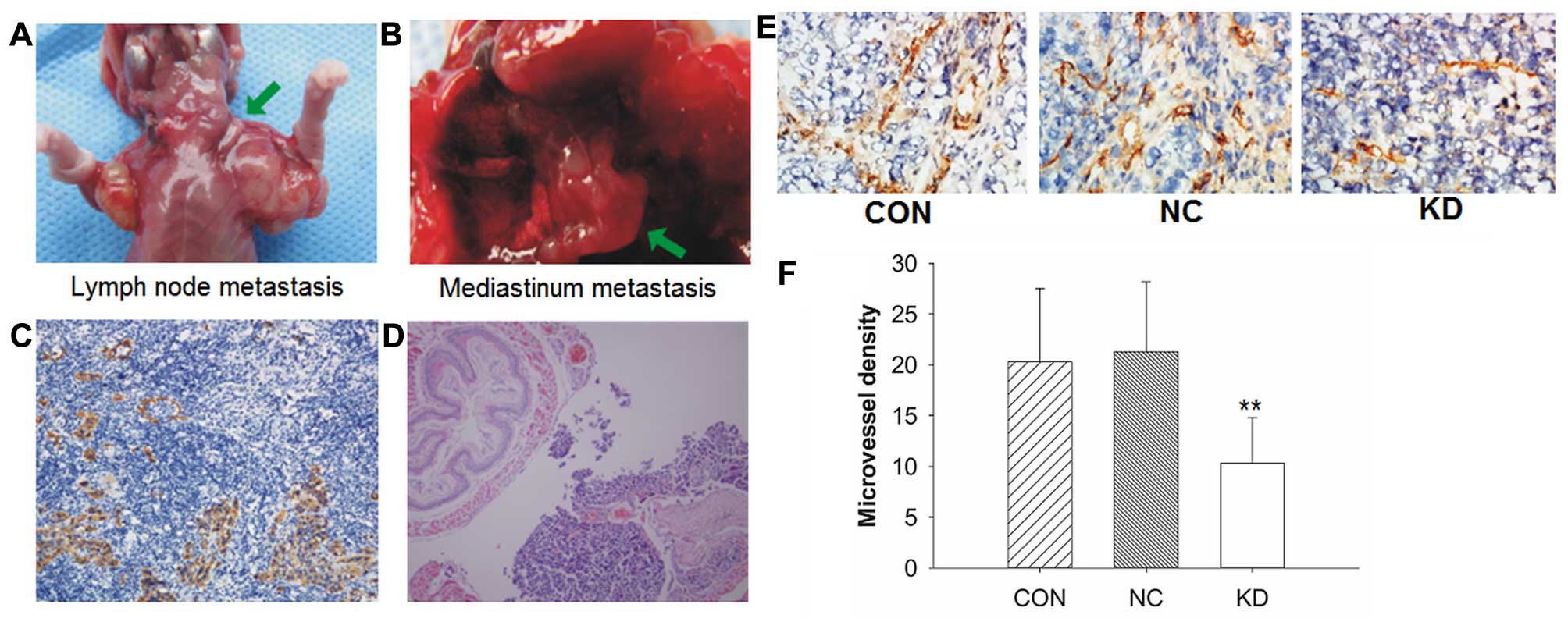
(Fig. 6A) was detected in 2/10 of
the mice injected with negative control lentivirus and in 3/10 of
the mice injected sterilizing saline. One case of mediastinum
metastasis (Fig. 6B) was detected
in the CON group. Fig. 6C and D
show the representative images of immunohistochemical staining of
cytokeratin and H&E sections (magnification, ×100).
Immunohistochemical staining of CD31 was applied to
detect the correlation between new blood vessel formation and tumor
growth. Data revealed that the KD group showed a low CD31
expression and apparent inhibition of angiogenesis in the tumor
tissue, while intense CD31 expression and a higher number of
vessels were observed in the control groups (Fig. 6E). The KD group showed less vessels
compared with the control groups (P<0.01, Fig. 6F).
Immunohistochemical assays were performed to confirm
ILK expression and its impact on some of its downstream signaling
target molecules associated with EMT in xenograft tumors (Fig. 7). The results indicated that the KD
group had an apparent decreased expression of ILK, Ki67, MMP-9,
Snail as well as a higher caspase-3 and E-cadherin expression in
tumor tissue, compared with the control groups (Fig. 7). The results were in concordance
with assays in vitro and supported the suggestion that the
knockdown of ILK suppressed OSCC tumor growth, metastasis and EMT
in vitro and in vivo.
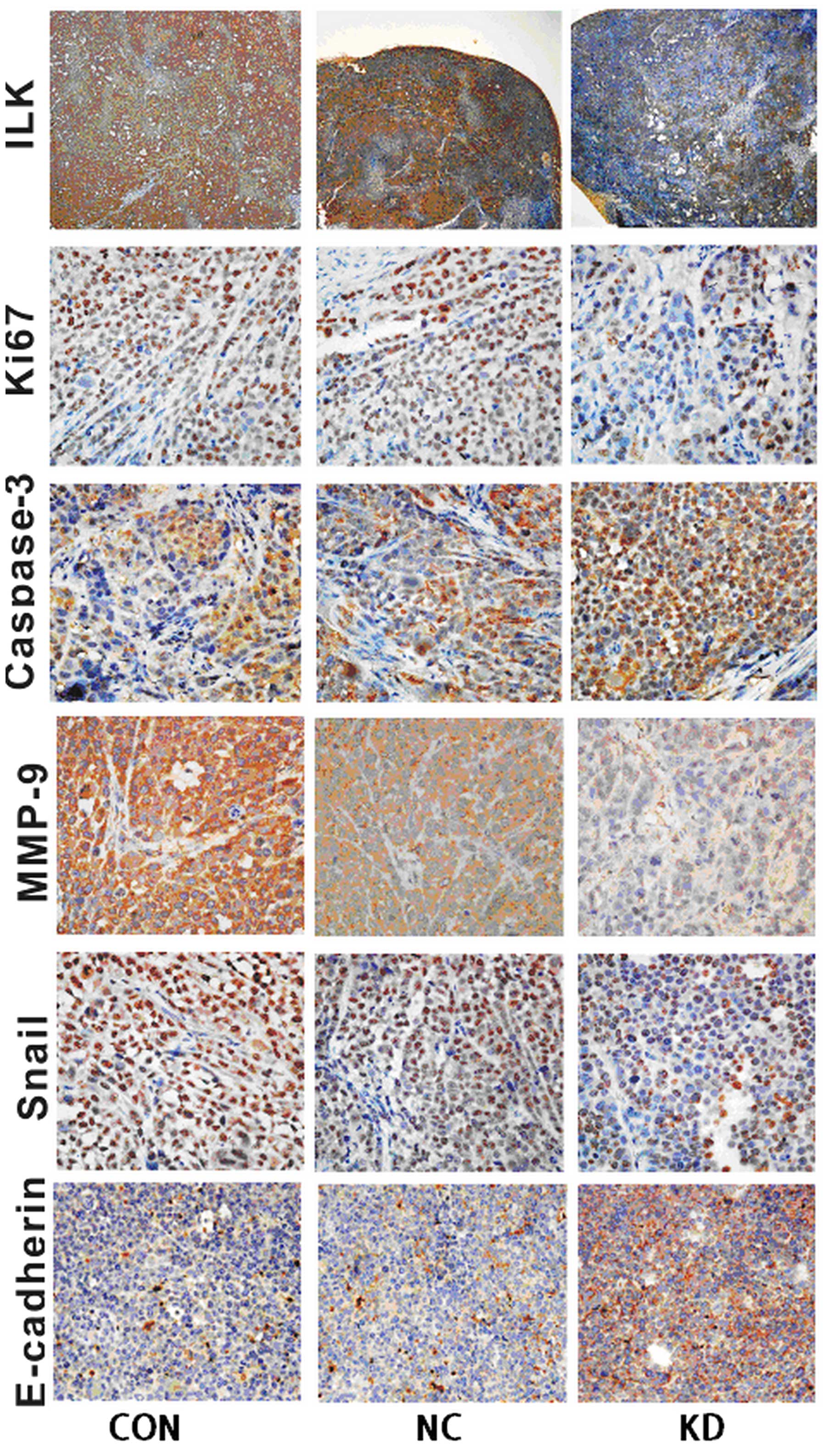 | Figure 7Effects of ILK knockdown on the
expression of ILK proteins and the impact on its downstream
signaling target molecules associated with EMT in xenograft tumors.
Immunohistochemical staining assay of antibodies of rabbit
anti-human ILK, Ki67, Snail, MMP-9, caspase-3 and E-cadherin,
respectively, the nuclei were counterstained using hema-toxylin
(ILK, magnification ×40; others, magnification ×400). Typical
images demon strated that the KD group had a strong positive
staining for E-cadherin and caspase-3, but weak positive staining
for ILK, Ki67, MMP-9 and Snail compared with the control groups.
ILK, integrin-linked kinase; EMT, epithelial-mesenchymal
transition. |
Discussion
OSCC is a lethal epithelial cancer occurring in the
oral cavity. The disease is associated with a poor prognosis
resulting from its local invasion and early regional lymph node
meta stases (27). Previously, by
analyzing OSCC clinical specimens, we revealed that ILK
overexpression was associated with the progression and metastasis
of OSCC, possibly through EMT-related upregulation of Snail and the
consequent aberrant expression of E- and N-cadherin (15). The underlying mechanisms of invasion
and metastasis of OSCC remain to be elucidated, although previous
findings have (28) demonstrated
that EMT may be of great importance.
ILK is a widely expressed multifunctional
intracellular effector of cell-matrix interactions that play a
vital role in the regulation of numerous cell processes including
EMT. EMT or EMT-like phenomena are considered a prerequisite for
metastasis, in certain cases (29).
The functional loss of E-cadherin and presence of N-cadherin are
regarded as characteristics of the EMT program (30). The downregulation of E-cadherin, an
epithelial cell marker and a potent suppressor of tumor cell
invasion and metastasis, is mediated through several repressors,
such as Snail, Slug and Twist (31).
In the present study, we have demonstrated that the
knockdown of ILK inhibited the EMT process with increased
E-cadherin expression and decreased N-cadherin expression in
vitro and in vivo, which is the hallmark of EMT
(30). Transcriptional repression
is a major mechanisms leading to the downregulation of E-cadherin,
which is suppressed by repressors such as Snail, Slug, Twist, ZEB1
and vimentin (32,33). Our findings showed that the
knockdown of ILK inhibited EMT-associated transcription factors
such as Snail, Slug and Twist2, as well as MMP-9 expression in
vitro and in vivo. We also demonstrated that
downregulation of ILK inhibited cell proliferation, adhesion and
invasion, arrested the cell cycle, and promoted cell apoptosis
in vitro. Radeva et al (34) reported that ILK signaling pathways
increased the expression of cyclin D1, activation of
cyclin-dependent kinase 4 and cyclin E-associated kinases,
promoting the cell cycle from the G1 to the S phase. Inhibition of
ILK induced cell cycle arrest at the G1 phase (35,36).
However, the results showed that the percentage of KD cells blocked
in the S phase (48.67±3.36%) was significantly higher, as compared
with the control groups, suggesting the cell cycle was arrested at
the restriction point and failed to enter mitosis, leading to cell
apoptosis. Cell apoptosis analysis revealed that the early
apoptotic cells (APC+, PI−, 32.2%) and late
apoptotic cells (APC+, PI+, 27.9%) in the KD
group were significantly increased compared with the control
groups. Western blotting images demonstrated that the protein
expression of cyclin A, cyclin B1 and Cdc2, key proteins regulating
cell cycle from the S phase to G2/M phase, decreased significantly,
while the cyclin D1 protein expression slightly inhibited the KD
group. Studies by Fielding et al (37–39)
indicated that, ILK played a critical and unexpected role in the
organization of centrosomal protein complexes during mitotic
spindle assembly and DNA segregation. Thus, the knockdown of ILK
resulted in mitosis failure and the cell cycle was arrested at the
S phase. These findings suggest that downregulation of ILK
suppressed the development and progression of OSCC cells.
Furthermore, the knockdown of ILK inhibited the
phosphorylation of its downstream targets Akt on Ser473 and Gsk-3β
on Ser9, respectively, and Gsk-3β downregulated Snail
phosphorylation on Ser246. The knockdown of ILK inhibited the
phosphorylation of Akt, and inhibited Akt activity (40). Thus, ILK and Akt phosphorylation
deactivated Gsk-3β, and subsequently inhibited Snail
phosphorylation, leading to EMT (41). Kalra et al (42) reported that the knockdown of ILK
suppressed p-Akt, p-Gsk-3β, β-catenin, Snail, MMP-9 and Twist. We
have demonstrated that the knockdown of ILK inhibited p-Akt and
p-Gsk-3β although no significant changes were identified in Akt and
Gsk-3β expression. The results also supported the above findings,
indicating that ILK plays a vital role in the EMT process through
the Akt/Gsk-3β/Snail pathway, which was in agreement with the
previous study (23). To
investigate the effects of the ILK knockdown on the p-Akt and
p-Gsk-3β induced by exogenous growth factors, HGF was applied prior
to detection, which existed in the tumor micro-environment and
activated PI3K. The results revealed that ILK was able to inhibit
HGF-induced Akt and Gsk-3β phosphorylation to a certain extent.
In addition, the animal experiment showed that the
knockdown of ILK significantly inhibited the tumor growth in
xenograft models, with tumor inhibition rates of 55.56 or 53.57%,
respectively. ILK knockdown also suppressed spontaneous lymph node
or distant metastasis in vivo. Compared with the control
groups, the KD group exhibited lower micro vascular density, which
is necessary for tumor growth and metastasis. Cell proliferation
and prevention of necrosis requires that tumor growth be dependent
on adequate oxygen and nutrients, as induced by tumor angiogenesis
(43). Immunohistochemical images
of xenograft tumor tissue confirmed that ILK knockdown suppressed
OSCC tumor growth, metastasis and EMT.
In conclusion, the findings of the present study
have demonstrated that the knockdown of ILK inhibited the
proliferation and metastasis of OSCC cells in vitro and
in vivo. The present results suggest that ILK is crucial in
cell progression, metastasis and EMT, by regulating key proteins
such as Akt, Gsk-3β, MMP-9, Snail, E-cadherin and caspase-3, which
were essential for cell proliferation, apoptosis, metastasis,
angio-genesis as well as EMT. Thus, ILK should be regarded as a
newly proven oral cancer metastasis regulator and a potential
therapeutic molecular target for OSCC.
Acknowledgments
This study was supported by grants from the Sichuan
Science Foundation (contract grant nos. 2011SZ0156 and 2014SZ0204)
and the National Natural Science Foundation of China (contract
grant no. 81202134).
References
|
1
|
Scully C and Felix DH: Oral medicine -
update for the dental practitioner oral cancer. Br Dent J.
200:13–17. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bello IO, Soini Y and Salo T: Prognostic
evaluation of oral tongue cancer: Means, markers and perspectives
(II). Oral Oncol. 46:636–643. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hannigan GE, Leung-Hagesteijn C,
Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC and Dedhar
S: Regulation of cell adhesion and anchorage-dependent growth by a
new beta 1-integrin-linked protein kinase. Nature. 379:91–96. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zervas CG, Psarra E, Williams V, Solomon
E, Vakaloglou KM and Brown NH: A central multifunctional role of
integrin-linked kinase at muscle attachment sites. J Cell Sci.
124:1316–1327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hannigan G, Troussard AA and Dedhar S:
Integrin-linked kinase: A cancer therapeutic target unique among
its ILK. Nat Rev Cancer. 5:51–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Somasiri A, Howarth A, Goswami D, Dedhar S
and Roskelley CD: Overexpression of the integrin-linked kinase
mesenchymally transforms mammary epithelial cells. J Cell Sci.
114:1125–1136. 2001.PubMed/NCBI
|
|
7
|
Wu C, Keightley SY, Leung-Hagesteijn C,
Radeva G, Coppolino M, Goicoechea S, McDonald JA and Dedhar S:
Integrin-linked protein kinase regulates fibronectin matrix
assembly, E-cadherin expression, and tumorigenicity. J Biol Chem.
273:528–536. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nawshad A, Lagamba D, Polad A and Hay ED:
Transforming growth factor-β signaling during
epithelial-mesenchymal transformation: Implications for
embryogenesis and tumor metastasis. Cells Tissues Organs.
179:11–23. 2005. View Article : Google Scholar
|
|
9
|
Foroni C, Broggini M, Generali D and Damia
G: Epithelial-mesenchymal transition and breast cancer: Role,
molecular mechanisms and clinical impact. Cancer Treat Rev.
38:689–697. 2012. View Article : Google Scholar
|
|
10
|
Li R, Liu B, Yin H, Sun W, Yin J and Su Q:
Overexpression of integrin-linked kinase (ILK) is associated with
tumor progression and an unfavorable prognosis in patients with
colorectal cancer. J Mol Histol. 44:183–189. 2013. View Article : Google Scholar
|
|
11
|
Li J, Yang ZL, Ren X, Zou Q, Yuan Y, Liang
L, Chen M and Chen S: ILK and PRDX1 are prognostic markers in
squamous cell/adenosquamous carcinomas and adenocarcinoma of
gallbladder. Tumour Biol. 34:359–368. 2013. View Article : Google Scholar
|
|
12
|
Chen D, Zhang Y, Zhang X, Li J, Han B, Liu
S, Wang L, Ling Y, Mao S and Wang X: Overexpression of
integrin-linked kinase correlates with malignant phenotype in
non-small cell lung cancer and promotes lung cancer cell invasion
and migration via regulating epithelial-mesenchymal transition
(EMT)-related genes. Acta Histochem. 115:128–136. 2013. View Article : Google Scholar
|
|
13
|
Watzka SB, Setinek U, Stubenberger EB,
Tötsch M, Dekan G, Marcher M, Fleck T and Müller MR:
Integrin-linked kinase, phosphorylated AKT and the prognosis of
malignant pleural mesothelioma. Eur J Cardiothorac Surg.
39:180–184. 2011. View Article : Google Scholar
|
|
14
|
Serrano I, McDonald PC, Lock FE and Dedhar
S: Role of the integrin-linked kinase (ILK)/Rictor complex in
TGFβ-1-induced epithelial-mesenchymal transition (EMT). Oncogene.
32:50–60. 2013. View Article : Google Scholar
|
|
15
|
Becker-Santos DD, Guo Y, Ghaffari M,
Vickers ED, Lehman M, Altamirano-Dimas M, Oloumi A, Furukawa J,
Sharma M, Wang Y, et al: Integrin-linked kinase as a target for
ERG-mediated invasive properties in prostate cancer models.
Carcinogenesis. 33:2558–2567. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan Z, Yin H, Wang R, Wu D, Sun W, Liu B
and Su Q: Overexpression of integrin-linked kinase (ILK) promotes
migration and invasion of colorectal cancer cells by inducing
epithelial-mesenchymal transition via NF-κB signaling. Acta
Histochem. 116:527–533. 2014. View Article : Google Scholar
|
|
17
|
Liang F, Zhang S, Wang B, Qiu J and Wang
Y: Overexpression of integrin-linked kinase (ILK) promotes glioma
cell invasion and migration and down-regulates E-cadherin via the
NF-κB pathway. J Mol Histol. 45:141–151. 2014. View Article : Google Scholar
|
|
18
|
Zhao D, Tang XF, Yang K, Liu JY and Ma XR:
Over-expression of integrin-linked kinase correlates with aberrant
expression of Snail, E-cadherin and N-cadherin in oral squamous
cell carcinoma: Implications in tumor progression and metastasis.
Clin Exp Metastasis. 29:957–969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao G, Guo LL, Xu JY, Yang H, Huang MX
and Xiao G: Integrin-linked kinase in gastric cancer cell
attachment, invasion and tumor growth. World J Gastroenterol.
17:3487–3496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao J, Zhu J, Li HY, Pan XY, Jiang R and
Chen JX: Small interfering RNA targeting integrin-linked kinase
inhibited the growth and induced apoptosis in human bladder cancer
cells. Int J Biochem Cell Biol. 43:1294–1304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Q, Xiao L, Yuan D, Shi X and Li P:
Silencing of the inte-grin-linked kinase gene induces the apoptosis
in ovarian carcinoma. J Recept Signal Transduct Res. 32:120–127.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo X, Wang W, Hu J, Feng K, Pan Y, Zhang
L and Feng Y: Lentivirus-mediated RNAi knockdown of NUPR1 inhibits
human nonsmall cell lung cancer growth in vitro and in vivo. Anat
Rec (Hoboken). 295:2114–2121. 2012. View
Article : Google Scholar
|
|
23
|
Xing Y, Qi J, Deng S, Wang C, Zhang L and
Chen J: Small interfering RNA targeting ILK inhibits metastasis in
human tongue cancer cells through repression of
epithelial-to-mesenchymal transition. Exp Cell Res. 319:2058–2072.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Federico Maurizio: Lentivirus Gene
Engineering Protocols (Methods in Molecular Biology). Humana Press;
229. pp. 69–85. 2003
|
|
25
|
Zheng YP, Liu H, Zeng H, Xiong L, Feng ZH
and Sun NX: Downregulation of lentivirus-mediated ILK RNAi on
tractional force generation in human retinal Müller cells. Acta
Pharmacol Sin. 30:1625–1633. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leong DT, Gupta A, Bai HF, Wan G, Yoong
LF, Too HP, Chew FT and Hutmacher DW: Absolute quantification of
gene expression in biomaterials research using real-time PCR.
Biomaterials. 28:203–210. 2007. View Article : Google Scholar
|
|
27
|
Choi S and Myers JN: Molecular
pathogenesis of oral squamous cell carcinoma: Implications for
therapy. J Dent Res. 87:14–32. 2008. View Article : Google Scholar
|
|
28
|
Qiao B, Johnson NW and Gao J:
Epithelial-mesenchymal transition in oral squamous cell carcinoma
triggered by transforming growth factor-β1 is Snail
family-dependent and correlates with matrix metalloproteinase-2 and
-9 expressions. Int J Oncol. 37:663–668. 2010.PubMed/NCBI
|
|
29
|
Voulgari A and Pintzas A:
Epithelial-mesenchymal transition in cancer metastasis: Mechanisms,
markers and strategies to overcome drug resistance in the clinic.
Biochim Biophys Acta. 1796:75–90. 2009.PubMed/NCBI
|
|
30
|
Araki K, Shimura T, Suzuki H, Tsutsumi S,
Wada W, Yajima T, Kobayahi T, Kubo N and Kuwano H: E/N-cadherin
switch mediates cancer progression via TGF-β-induced
epithelial-to-mesenchymal transition in extrahepatic
cholangio-carcinoma. Br J Cancer. 105:1885–1893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shin SY, Rath O, Zebisch A, Choo SM, Kolch
W and Cho KH: Functional roles of multiple feedback loops in
extracellular signal-regulated kinase and Wnt signaling pathways
that regulate epithelial-mesenchymal transition. Cancer Res.
70:6715–6724. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peinado H, Portillo F and Cano A:
Transcriptional regulation of cadherins during development and
carcinogenesis. Int J Dev Biol. 48:365–375. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Radeva G, Petrocelli T, Behrend E,
Leung-Hagesteijn C, Filmus J, Slingerland J and Dedhar S:
Overexpression of the integrin-linked kinase promotes
anchorage-independent cell cycle progression. J Biol Chem.
272:13937–13944. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Persad S, Attwell S, Gray V, Delcommenne
M, Troussard A, Sanghera J and Dedhar S: Inhibition of
integrin-linked kinase (ILK) suppresses activation of protein
kinase B/Akt and induces cell cycle arrest and apoptosis of
PTEN-mutant prostate cancer cells. Proc Natl Acad Sci USA.
97:3207–3212. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
D'Amico M, Hulit J, Amanatullah DF,
Zafonte BT, Albanese C, Bouzahzah B, Fu M, Augenlicht LH, Donehower
LA, Takemaru K, et al: The integrin-linked kinase regulates the
cyclin D1 gene through glycogen synthase kinase 3beta and
cAMP-responsive element-binding protein-dependent pathways. J Biol
Chem. 275:32649–32657. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fielding AB, Dobreva I, McDonald PC,
Foster LJ and Dedhar S: Integrin-linked kinase localizes to the
centrosome and regulates mitotic spindle organization. J Cell Biol.
180:681–689. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fielding AB, Lim S, Montgomery K, Dobreva
I and Dedhar S: A critical role of integrin-linked kinase, ch-TOG
and TACC3 in centrosome clustering in cancer cells. Oncogene.
30:521–534. 2011. View Article : Google Scholar
|
|
39
|
Fielding AB and Dedhar S: The mitotic
functions of integrin-linked kinase. Cancer Metastasis Rev.
28:99–111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Koul D, Shen R, Bergh S, Lu Y, de Groot
JF, Liu TJ, Mills GB and Yung WK: Targeting integrin-linked kinase
inhibits Akt signaling pathways and decreases tumor progression of
human glioblastoma. Mol Cancer Ther. 4:1681–1688. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Doble BW and Woodgett JR: Role of glycogen
synthase kinase-3 in cell fate and epithelial-mesenchymal
transitions. Cells Tissues Organs. 185:73–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kalra J, Sutherland BW, Stratford AL,
Dragowska W, Gelmon KA, Dedhar S, Dunn SE and Bally MB: Suppression
of Her2/neu expression through ILK inhibition is regulated by a
pathway involving TWIST and YB-1. Oncogene. 29:6343–6356. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Muramatsu M, Yamamoto S, Osawa T and
Shibuya M: Vascular endothelial growth factor receptor-1 signaling
promotes mobilization of macrophage lineage cells from bone marrow
and stimulates solid tumor growth. Cancer Res. 70:8211–8221. 2010.
View Article : Google Scholar : PubMed/NCBI
|