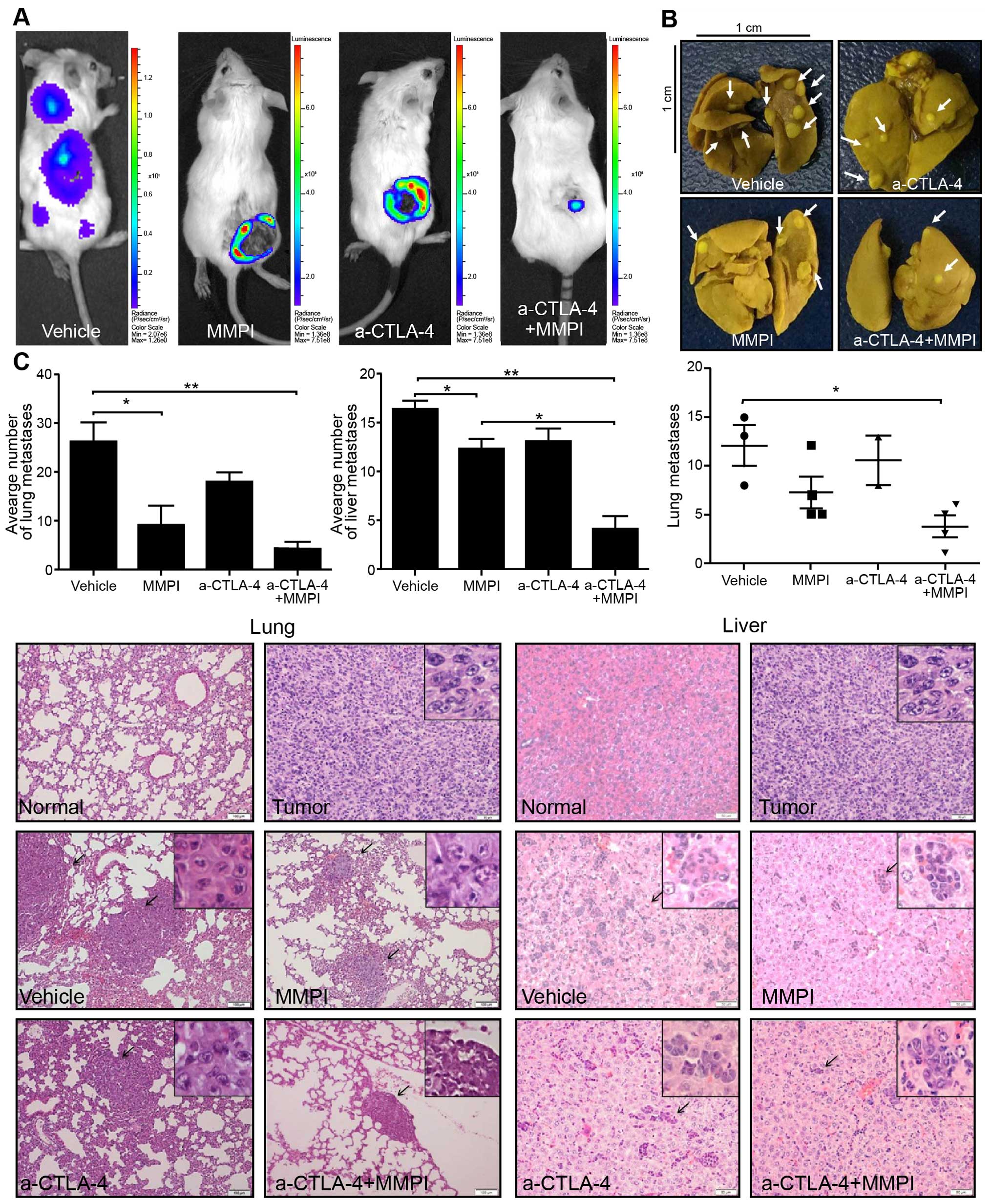
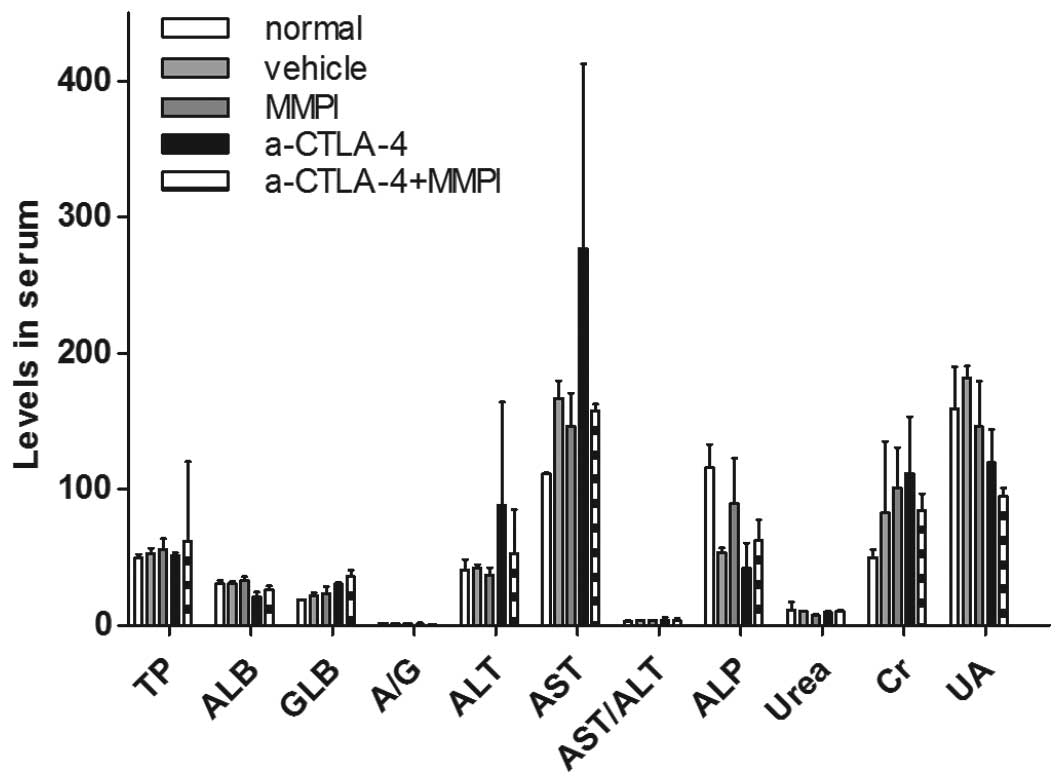
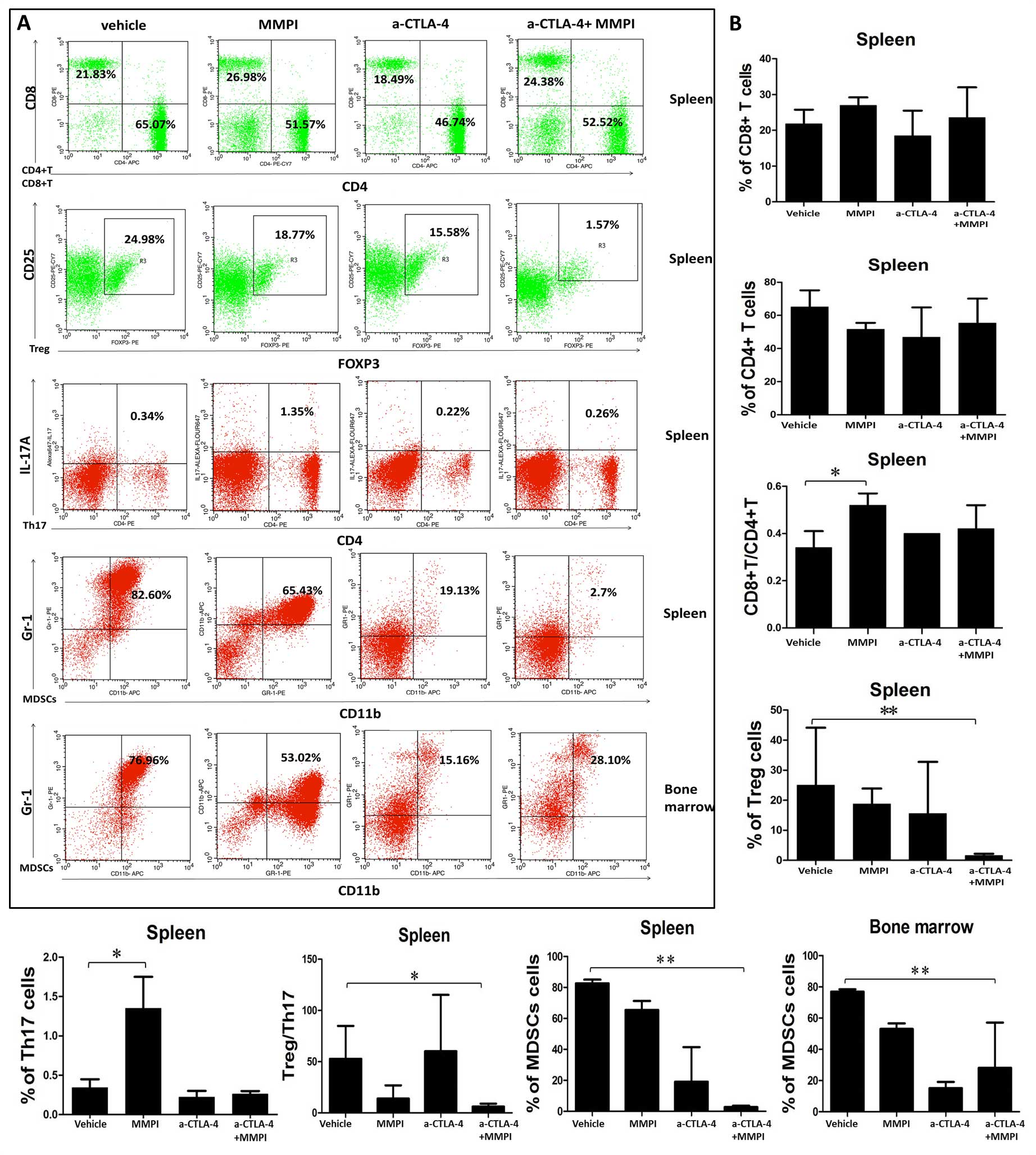
|
1
|
Lotem M, Merims S, Frank S, Ospovat I and
Peretz T: Ctla-4 blockade: A new hope for the immunotherapy of
malignant melanoma. Harefuah. 151:585–588. 6042012.In Hebrew.
|
|
2
|
Prieto PA, Yang JC, Sherry RM, Hughes MS,
Kammula US, White DE, Levy CL, Rosenberg SA and Phan GQ: CTLA-4
blockade with ipilimumab: Long-term follow-up of 177 patients with
metastatic melanoma. Clin Cancer Res. 18:2039–2047. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McAllister SS and Weinberg RA: The
tumour-induced systemic environment as a critical regulator of
cancer progression and metastasis. Nat Cell Biol. 16:717–727. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baginska J, Viry E, Paggetti J, Medves S,
Berchem G, Moussay E and Janji B: The critical role of the tumor
microenvironment in shaping natural killer cell-mediated anti-tumor
immunity. Front Immunol. 4:4902013. View Article : Google Scholar
|
|
5
|
Whiteside TL: Induced regulatory T cells
in inhibitory microenvironments created by cancer. Expert Opin Biol
Ther. 14:1411–1425. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Borriello L and DeClerck YA: Tumor
microenvironment and therapeutic resistance process. Med Sci
(Paris). 30:445–451. 2014.In French. View Article : Google Scholar
|
|
7
|
Hida K, Akiyama K, Ohga N, Maishi N and
Hida Y: Tumour endothelial cells acquire drug resistance in a
tumour microenvironment. J Biochem. 153:243–249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shuman Moss LA, Jensen-Taubman S and
Stetler-Stevenson WG: Matrix metalloproteinases: Changing roles in
tumor progression and metastasis. Am J Pathol. 181:1895–1899. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thomas D, Ritz MF, Malviya AN and Gaillard
S: Intracellular acidification mediates the proliferative response
of PC12 cells induced by potassium ferricyanide and involves MAP
kinase activation. Int J Cancer. 68:547–552. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martinus RD, Linnane AW and Nagley P:
Growth of rho 0 human Namalwa cells lacking oxidative
phosphorylation can be sustained by redox compounds potassium
ferricyanide or coenzyme Q10 putatively acting through the plasma
membrane oxidase. Biochem Mol Biol Int. 31:997–1005.
1993.PubMed/NCBI
|
|
12
|
Stockmann C, Schadendorf D, Klose R and
Helfrich I: The impact of the immune system on tumor: Angiogenesis
and vascular remodeling. Front Oncol. 4:692014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tao K, Fang M, Alroy J and Sahagian GG:
Imagable 4T1 model for the study of late stage breast cancer. BMC
Cancer. 8:2282008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Demaria S, Kawashima N, Yang AM, Devitt
ML, Babb JS, Allison JP and Formenti SC: Immune-mediated inhibition
of metastases after treatment with local radiation and CTLA-4
blockade in a mouse model of breast cancer. Clin Cancer Res.
11:728–734. 2005.PubMed/NCBI
|
|
15
|
Fink K and Boratyński J: The role of
metalloproteinases in modification of extracellular matrix in
invasive tumor growth, metastasis and angiogenesis. Postepy Hig Med
Dosw Online. 66:609–628. 2012.In Polish. View Article : Google Scholar
|
|
16
|
Benson CS, Babu SD, Radhakrishna S,
Selvamurugan N and Ravi Sankar B: Expression of matrix
metalloproteinases in human breast cancer tissues. Dis Markers.
34:395–405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Overall CM and Kleifeld O: Tumour
microenvironment - opinion: Validating matrix metalloproteinases as
drug targets and anti-targets for cancer therapy. Nat Rev Cancer.
6:227–239. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li NG, Tang YP, Duan JA and Shi ZH: Matrix
metalloproteinase inhibitors: A patent review (2011–2013). Expert
Opin Ther Pat. 24:1039–1052. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li W, Saji S, Sato F, Noda M and Toi M:
Potential clinical applications of matrix metalloproteinase
inhibitors and their future prospects. Int J Biol Markers.
28:117–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Srivastava MK, Zhu L, Harris-White M,
Huang M, St John M, Lee JM, Salgia R, Cameron RB, Strieter R,
Dubinett S, et al: Targeting myeloid-derived suppressor cells
augments antitumor activity against lung cancer. Immunotargets
Ther. 2012:7–12. 2012.PubMed/NCBI
|
|
21
|
Radosavljević GD, Jovanović IP, Kanjevac
TV and Arsenijević NN: The role of regulatory T cells in the
modulation of anti-tumor immune response. Srp Arh Celok Lek.
141:262–267. 2013.In Serbian. View Article : Google Scholar
|
|
22
|
Prabhala RH, Pelluru D, Fulciniti M,
Prabhala HK, Nanjappa P, Song W, Pai C, Amin S, Tai YT, Richardson
PG, et al: Elevated IL-17 produced by TH17 cells promotes myeloma
cell growth and inhibits immune function in multiple myeloma.
Blood. 115:5385–5392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Becker JC, Andersen MH, Schrama D and Thor
Straten P: Immune-suppressive properties of the tumor
microenvironment. Cancer Immunol Immunother. 62:1137–1148. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Walker LS: Treg and CTLA-4: Two
intertwining pathways to immune tolerance. J Autoimmun. 45:49–57.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nagaraj S, Youn JI and Gabrilovich DI:
Reciprocal relationship between myeloid-derived suppressor cells
and T cells. J Immunol. 191:17–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Z, Li D, Tsun A and Li B:
FOXP3+ regulatory T cells and their functional
regulation. Cell Mol Immunol. 12:558–565. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar S, Pan CC, Bloodworth JC, Nixon AB,
Theuer C, Hoyt DG and Lee NY: Antibody-directed coupling of
endoglin and MMP-14 is a key mechanism for endoglin shedding and
deregulation of TGF-β signaling. Oncogene. 33:3970–3979. 2014.
View Article : Google Scholar :
|
|
28
|
Krstic J and Santibanez JF: Transforming
growth factor-beta and matrix metalloproteinases: Functional
interactions in tumor stroma-infiltrating myeloid cells. Sci World
J. 2014:5217542014. View Article : Google Scholar
|
|
29
|
Guedez L, Jensen-Taubman S, Bourboulia D,
Kwityn CJ, Wei B, Caterina J and Stetler-Stevenson WG: TIMP-2
targets tumor-associated myeloid suppressor cells with effects in
cancer immune dysfunction and angiogenesis. J Immunother.
35:502–512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fan Q, Gu D, Liu H, Yang L, Zhang X, Yoder
MC, Kaplan MH and Xie J: Defective TGF-β signaling in bone
marrow-derived cells prevents hedgehog-induced skin tumors. Cancer
Res. 74:471–483. 2014. View Article : Google Scholar :
|
|
31
|
Xiang X, Poliakov A, Liu C, Liu Y, Deng
ZB, Wang J, Cheng Z, Shah SV, Wang GJ, Zhang L, et al: Induction of
myeloid-derived suppressor cells by tumor exosomes. Int J Cancer.
124:2621–2633. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hida K, Kawamoto T, Ohga N, Akiyama K,
Hida Y and Shindoh M: Altered angiogenesis in the tumor
microenvironment. Pathol Int. 61:630–637. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goubran HA, Kotb RR, Stakiw J, Emara ME
and Burnouf T: Regulation of tumor growth and metastasis: The role
of tumor microenvironment. Cancer growth Metastasis. 7:9–18. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mittal K, Ebos J and Rini B: Angiogenesis
and the tumor microenvironment: Vascular endothelial growth factor
and beyond. Semin Oncol. 41:235–251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chauhan VP, Stylianopoulos T, Martin JD,
Popović Z, Chen O, Kamoun WS, Bawendi MG, Fukumura D and Jain RK:
Normalization of tumour blood vessels improves the delivery of
nanomedicines in a size-dependent manner. Nat Nanotechnol.
7:383–388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Trédan O, Lacroix-Triki M, Guiu S,
Mouret-Reynier MA, Barrière J, Bidard FC, Braccini AL, Mir O,
Villanueva C and Barthélémy P: Angiogenesis and tumor
microenvironment: Bevacizumab in the breast cancer model. Target
Oncol. 10:189–198. 2015. View Article : Google Scholar
|
|
37
|
Sounni NE, Paye A, Host L and Noël A:
MT-MMPS as regulators of vessel stability associated with
angiogenesis. Front Pharmacol. 2:1112011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pucino V, De Rosa V, Procaccini C and
Matarese G: Regulatory T cells, leptin and angiogenesis. Chem
Immunol Allergy. 99:155–169. 2014. View Article : Google Scholar
|
|
39
|
D'Alessio FR, Zhong Q, Jenkins J,
Moldobaeva A and Wagner EM: Lung angiogenesis requires CD4 (+)
forkhead homeobox protein-3 (+) regulatory T cells. Am J Respir
Cell Mol Biol. 52:603–610. 2015. View Article : Google Scholar
|
|
40
|
Mucha J, Majchrzak K, Taciak B, Hellmén E
and Król M: MDSCs mediate angiogenesis and predispose canine
mammary tumor cells for metastasis via IL-28/IL-28RA (IFN-λ)
signaling. PLoS One. 9:e1032492014. View Article : Google Scholar
|
|
41
|
Finke J, Ko J, Rini B, Rayman P, Ireland J
and Cohen P: MDSC as a mechanism of tumor escape from sunitinib
mediated anti-angiogenic therapy. Int Immunopharmacol. 11:856–861.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang B, Kang H, Fung A, Zhao H, Wang T and
Ma D: The role of interleukin 17 in tumour proliferation,
angiogenesis, and metastasis. Mediators Inflamm. 2014:6237592014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Numasaki M, Fukushi J, Ono M, Narula SK,
Zavodny PJ, Kudo T, Robbins PD, Tahara H and Lotze MT:
Interleukin-17 promotes angiogenesis and tumor growth. Blood.
101:2620–2627. 2003. View Article : Google Scholar
|
|
44
|
Ager EI, Kozin SV, Kirkpatrick ND, Seano
G, Kodack DP, Askoxylakis V, Huang Y, Goel S, Snuderl M, Muzikansky
A, et al: Blockade of MMP14 activity in murine breast carcinomas:
Implications for macrophages, vessels, and radiotherapy. J Natl
Cancer Inst. 107:1072015. View Article : Google Scholar
|
|
45
|
Romanchikova N, Trapencieris P, Zemītis J
and Turks M: A novel matrix metalloproteinase-2 inhibitor
triazolylmethyl aziridine reduces melanoma cell invasion,
angiogenesis and targets ERK1/2 phosphorylation. J Enzyme Inhib Med
Chem. 29:765–772. 2014. View Article : Google Scholar
|
|
46
|
Weisshardt P, Trarbach T, Dürig J, Paul A,
Reis H, Tilki D, Miroschnik I, Ergün S and Klein D: Tumor vessel
stabilization and remodeling by anti-angiogenic therapy with
bevacizumab. Histochem Cell Biol. 137:391–401. 2012. View Article : Google Scholar
|