Introduction
Pancreatic cancer is a major cause of cancer
mortality worldwide, with a 5-year survival rate of <5%
(1). The outlook for patients who
undergo surgical resection is improved, and in specialized centers,
resection rates of >15% can be achieved (2). However, surgery cannot guarantee a
cure, and the 5-year survival rate does not exceed 10% following
resection (2). Thus, improvement of
long-term survival in these patients is crucial. Elucidating the
molecular mechanism of proliferation, migration and invasion in
pancreatic cancer may be useful to understand the pathogenesis and
progression of the disease, and to offer new targets for effective
therapies.
Salt-inducible kinase 1 (SIK1), also known as
MSK/SIK/SNF1LK is a member of the AMP-activated protein
kinase-related kinases (AMPK-RKs) (3). Cheng et al previously reported
that SIK1 is a regulator of p53-dependent anoikis through the use
of a kinome-wide loss-of-function screen. Additionally, SIK1 loss
facilitated the metastatic spread and survival of disseminated
cells as micrometastases in lungs and a decreased SIK1 expression
closely correlated with the development of distal metastases in
breast cancers from three independent cohorts (4). However, the roles of SIK1 have not
been reported in pancreatic cancer.
The identification of microRNAs (miRNAs) has
broadened our understanding of the mechanisms that regulate the
gene expression with the addition of an entirely novel level of
regulatory control. miRNAs are regulatory, non-coding RNAs ~18–25
nucleotides in length and are expressed at specific stages of
tissue development or cell differentiation, and have large-scale
effects on the expression of a variety of genes at the
post-transcriptional level. Through base pairing with its targeted
mRNAs, a miRNA induces RNA degradation or translational suppression
of the targeted transcripts (5–10). The
aberrant expression of miRNAs has been reported in various human
cancer types and is characterized to have an oncogenic or
tumor-suppressor role. miRNAs are shown to play key roles in cell
survival, proliferation, apoptosis, migration, invasion and various
other characteristic features that are altered in human cancers
(11,12). It was previously reported that
miR-203 is upregulated in pancreatic cancer tissues and the
elevated expression is associated with poorer survival (13,14).
However, the roles mechanisms of miR-203 as an oncogene are crucial
in pancreatic cancer.
In the present study, we showed that SIK1 is
downregulated and loss of SIK1 was associated with gemcitabine
resistance in pancreatic cancer. In pancreatic cancer cells, SIK1
inhibited proliferation, migration and invasion. We performed an
analysis of potential microRNA target sites using the prediction
algorithms, miRanda, TargetScan and PicTar. The three algorithms
predicted that miR-203 is capable of targeting 3′UTR of SIK1, as
confirmed by subsequent experiments. In addition, miR-203 was found
to promote proliferation, migration and invasion in pancreatic
cancer cells, whereas the restoration of SIK1 abrogated the
regulation of pre-miR-203-mediated proliferation, migration and
invasion.
Materials and methods
Pancreatic tissues
Pancreatic cancer tissues and adjacent normal
tissues were obtained from the Second Department of General
Surgery, Linyi People's Hospital Affiliated to Shandong University.
The tissues were examined histologically, and pathologists
confirmed the diagnosis. The medical Ethics Committee of Linyi
People's Hospital Affiliated to Shandong University approved the
experiments undertaken. The use of human tissue samples was
conducted as per internationally recognized guidelines and local
and national regulations. Informed consent was obtained from each
individual.
Cell culture
L3.6pl, BxPC-3, CFPAC, MiaPaCa-2, ASPC-1, PANC-1,
MPanc96, HPAC, SU86.86 and HS766T pancreatic cancer cell lines were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). Briefly, the cells were maintained in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island,
NY, USA) and penicillin/streptomycin at 37°C in a humidified
atmosphere with 5% CO2.
Western blot analysis
Western blot analysis was performed as previously
described (15). Briefly, the cells
were incubated with the primary antibodies, including anti-SIK1
(1:250), anti-c-myc (1:250), anti-Ki67 (1:250), anti-PCNA (1:250),
anti-p21 (1:250), anti-p53 (1:250), anti-RB (1:250), anti-CDK1
(1:250), anti-CDK2 (1:250), anti-CDK4 (1:250), anti-CDK6 (1:250);
anti-CD44 (1:250), anti-Tspan8 (1:250) and anti-β-actin (1:500)
(all from Abcam, Cambridge, MA, USA) overnight at 4°C. IRDye™-800
conjugated anti-rabbit secondary antibodies (Li-COR Biosciences,
Lincoln, NE, USA) were used for 30 min at room temperature. The
specific proteins were visualized by the Odyssey™ Infrared Imaging
system (Gene Company, Lincoln, NE, USA).
MTT assay
The effect of the cell proliferation was assessed by
the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT;
Sigma, St. Louis, MO, USA) assay, which was performed as previously
described (16). Absorbance was
directly proportional to the number of survival cells.
Cell cycle analysis
Cell cycle analysis was performed as previously
described (16). Cells
(8.0×105 cells) were seeded in a 100-mm culture plate
and allowed to attach overnight. The cells were transfected with
plasmids for 24 h, washed twice with NaCl/Pi, and then centrifuged
at 200 × g at room temperature. The pellet was resuspended in 1 ml
cold NaCl/Pi and fixed in 70% ethanol for at least 12 h at 4°C. The
fixed cells were incubated with 100 µl DNase-free RNase A
(200 µg/ml) for 30 min at 37°C, and then 1 mg/ml propidium
iodide was added. The stained cells were analyzed using a
fluorescence-activated cell sorter (BD Accuri C6; BD Biosciences,
Ann Arbor, MI, USA). The percentages of cells in the G1, S and G2/M
phases of the cell cycle were determined using CellQuest Pro
software (FlowJo, Ashland, OR, USA).
Bromodeoxyuridine labeling and
immunofluorescence
Bromodeoxyuridine labeling and immunofluorescence
was performed as previously described (16). The cells grown on coverslips
(Fisher, Pittsburgh, PA, USA) were incubated with bromodeoxyuridine
(BrdU) for 1 h and stained with anti-BrdU antibody (Upstate,
Temecula, CA, USA) according to the manufacturer's instructions.
Images were obtained under a laser scanning microscope (Axioskop 2
Plus; Carl Zeiss Co., Ltd., Jena, Germany).
Colony formation
Colony formation was performed as previously
described (16). For the colony
formation assay, the cells were transfected as indicated, and then
seeded in a 6-well plate. FBS (0.2 ml) was added per well on day 5.
After 9–10 days incubation, plates were washed with PBS and stained
with 0.1% crystal violet. Colonies with over 50 cells were manually
counted.
Reverse transcription-polymerase chain
reaction and quantitative polymerase chain reaction for mRNA
Total RNA was isolated from the cells or tissues
using TRIzol reagent (cat no. 101472; Invitrogen Life Technologies,
Carlsbad, CA, USA). cDNA was synthesized from 1 µg of total
RNA in a 20-µl reverse transcription (RT) system followed by
PCR amplification in a 50-µl PCR system performed using an
RT-PCR kit (cat no. A3500; Promega, Madison, WI, USA). Housekeeping
gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was
used as RNA loading control. The PCR primer sequences used were:
GAPDH forward, 5′-ATTCAACGGCACAGTCAAGG-3′ and reverse,
5′-GCAGAAGGGGCGGAGATGA-3′; PCNA forward, 5′-CTGTAGCGGCGTTGTTGC-3′
and reverse, 5′-TCGTTGATGAGGTCCTTG-3′; Ki67 forward,
5′-CAACTATCCTCGTCTGTCC-3′ and reverse, 5′-GGTCCCTAAAGATGTGCT-3′;
p21 forward, 5′-CCCGTGAGCGATGGAACT-3′ and reverse,
5′-CGAGGCACAAGGGTACA AGA-3′; p53 forward,
5′-CCTCCTCAGCATCTTATCCG-3′ and reverse, 5′-CACAAACACGCACCTCAAA-3′;
Rb forward, 5′-AAGGTTTCAGGGTATCAG -3′ and reverse,
5′-GTGGGTCTGTATGTTGTG-3′; Tspan8 forward,
5′-TCGAATTCTTTCCGAAATGGCAGGTGTGAG-3′ and reverse,
5′-ATGTCGACTGCATCCACAGATTCATTTG TTC-3′; CD44 forward,
5′-AGACATCTACCCCAGCAAC-3′ and reverse, 5′-CGTTGAGTCCACTTGGCTTTC-3′;
CDK2 forward, 5′-AGAAACAAGTTGACGGGAG-3′ and reverse,
5′-GAAGAGGAATGCCAGTGAG-3′; CDK4 forward, 5′-CAGTTCGTGAGGTGGCTTTA-3′
and reverse, 5′-GGGGTGCCTTGTCCAGATA-3′; CDK6 forward,
5′-TGCCCACTGAAACCATAAAGG-3′ and reverse, 5′-ATCCACAGCGTGACGACCA-3′;
and SIK1 forward, 5′-GTCCCTCGGAAGGAACTAGC-3′ and reverse,
5′-CTCGCGTTTTTCCTTAGCTG-3′. PCR was conducted according to the
manufacturer's instructions and the PCR products were analyzed by
agarose gel electrophoresis. The gels were photographed and
densities of the bands were determined with a computerized image
analysis system (Alpha Innotech, San Leandro, CA, USA). The area of
each band was calculated as the integrated density value (IDV).
Quantitaive PCR (qPCR) for SIK1 was performed with a Power
SYBR-Green PCR Master Mix (Applied Biosystems, Carlsbad, CA, USA)
according to the manufacturer's instructions.
qPCR for microRNAs
Total RNA from cultured cells, with efficient
recovery of small RNAs, was isolated using the mirVana miRNA
Isolation kit (cat no. AM2654; Ambion, Austin, TX, USA). Detection
of the mature form of miRNAs was performed using the mirVana
RT-qPCR miRNA Detection kit (cat no. AM7659; Ambion), according to
the manufacturer's instructions. The U6 small nuclear RNA was used
as an internal control.
Methods of bioinformatics
Analysis of the potential microRNA target sites was
performed using the prediction algorithms, miRanda (http://www.microrna.org/), TargetScan (http://www.targetscan.org) and PicTar (http://pictar.bio.nyu.edu).
Immunofluorescence analysis
Immunofluorescence analysis was performed as
previously described (16). For
immunofluorescence analysis, the cells were plated on glass
coverslips in 6-well plates and transfected as indicated. At 36 h
after transfection, the coverslips were stained with the anti-SIK1
antibodies mentioned earlier. Alexa Fluor 488 goat anti-mouse IgG
antibody or goat anti-rabbit IgG antibody was used as secondary
antibody (Invitrogen Life Technologies). Coverslips were
counterstained with DAPI (Invitrogen-Molecular Probes, Eugene, OR,
USA) for the visualization of nuclei. Microscopic analysis was
performed with a confocal laser-scanning microscope (Leica
Microsystems, Bensheim, Germany). Fluorescence intensities were
measured in a few viewing areas for 200–300 cells/coverslip and
analyzed using ImageJ 1.37v software (http://rsb.info.nih.gov/ij/index.html).
Migration and invasion assay
For the Transwell migration assays,
2.5×104–5.3×104 cells were plated in the top
chamber with the non-coated membrane (24-well insert, pore size, 8
mm; BD Biosciences, San Jose, CA, USA). For the invasion assays,
1.25×105 cells were plated in the top chamber with
Matrigel-coated membrane (24-well insert, pore size, 8 mm; BD
Biosciences, San Jose, CA, USA). In the two assays, the cells were
plated in medium without serum or growth factors, and medium
supplemented with serum was used as a chemoattractant in the lower
chamber. The cells were incubated for 24 h and cells that did not
migrate or invade through the pores were removed by a cotton swab.
Cells on the lower surface of the membrane were stained with the
Diff-Quick Staining Set (Dade) and counted.
Wound-healing assay
Cells (5×105) were seeded onto each 35-mm
glass bottom dish (MatTek Co., Ashland, MA, USA) and cultured at
37°C with 5% CO2 for 24 h. The confluent monolayer of
the cells was wounded. Monolayers of cells were wounded with yellow
pipette tips. After washing with warm PBS, the cells were incubated
in fresh culture medium. The wounded areas were photographed at
different time-points using a Nikon inverted microscope (Eclipse
TE2000-U) equipped with a video camera (DS-U1) (both from Nikon,
Japan).
Statistical analysis
Data are presented as mean ± SEM. The Student's
t-test (two-tailed) was used to compare two groups (P<0.05 was
considered significant).
Results
SIK1 is downregulated and its loss is
associated with gemcitabine resistance in pancreatic cancer
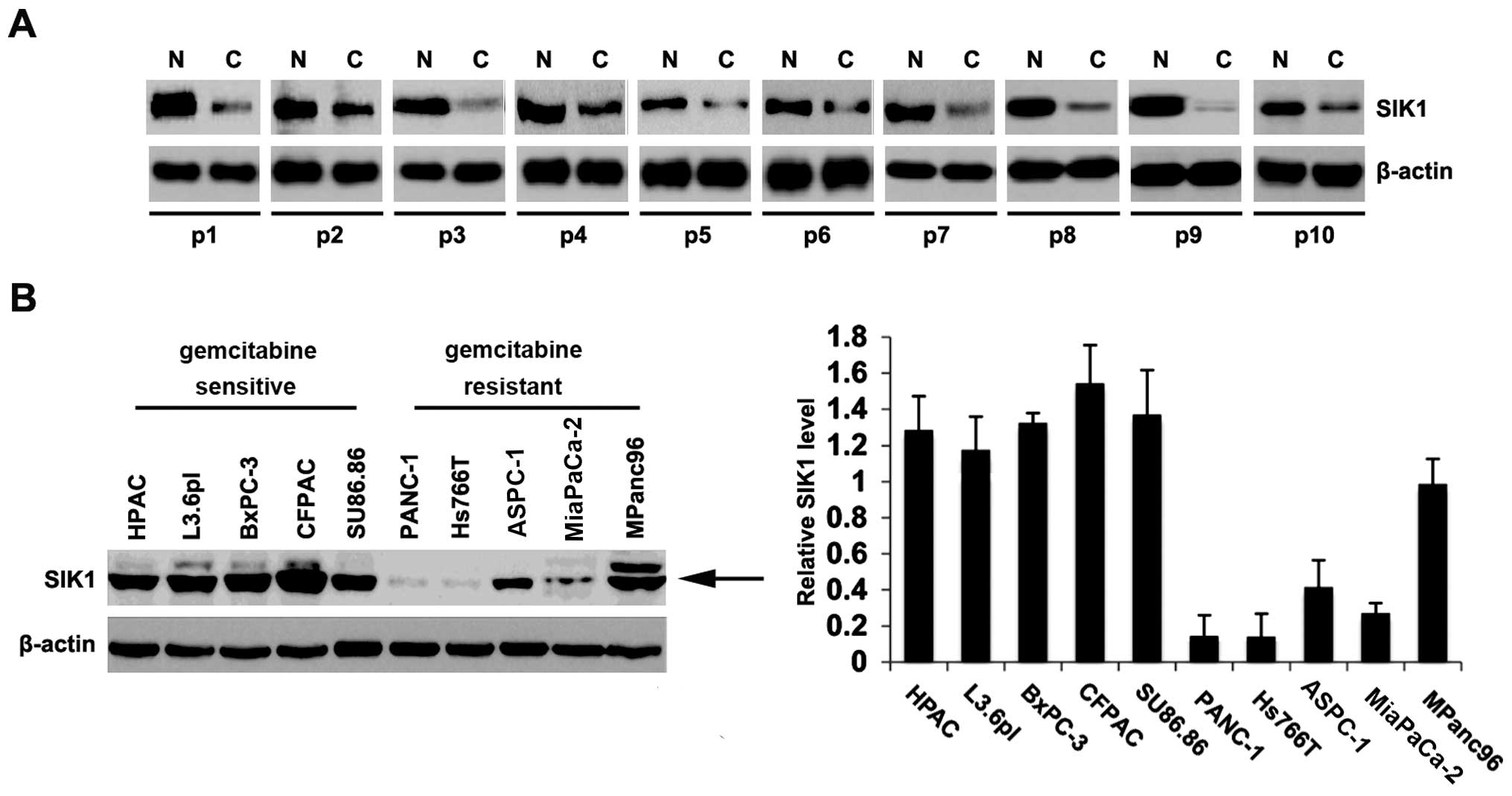
To identify SIK1 expression between pancreatic
cancer tissues and adjacent normal tissues, we performed western
blot analysis in cancer versus normal tissues. Protein was isolated
from 10 pairs of pancreatic cancer tissues and normal tissues
(patient nos. 1–10). We found that SIK1 protein was significantly
decreased in cancer tissues, compared with adjacent normal tissues
(Fig. 1A), suggesting that SIK1 is
a tumor-suppressive gene in pancreatic cancer. To identify the SIK1
protein expression among different pancreatic cancer cell lines, we
performed western blot analysis in the HPAC, L3.6pl, CFPAC, BxPC-3,
SU86.86, PANC-1, Hs766T, ASPC-1, MiaPaCa-2 and MPanc96 pancreatic
cancer cell lines. PANC-1, Hs766T, ASPC-1, MiaPaCa-2 and MPanc96
cells are gemcitabine-resistant cells and HPAC, L3.6pl, CFPAC,
BxPC-3, SU86.86 cells are gemcitabine-sensitive cells (17,18).
Protein isolated from the 10 types of cell lines were detected by
western blot analysis and the results showed that the expression of
SIK1 was lower in gemcitabine-resistant cells (PANC-1, Hs766T,
ASPC-1, MiaPaCa-2 and MPanc96 cells) than gemcitabine-sensitive
cells (HPAC, L3.6pl, CFPAC, BxPC-3, SU86.86) (Fig. 1B, left panel). A quantitative image
analysis was performed to analyze the SIK1 protein expression in
these tissue sections and we found that SIK1 expression was indeed
downregulated in gemcitabine-resistant pancreatic cancer (Fig. 1B, right panel). These results
suggested that SIK1 is downregulated in pancreatic cancer tissues
and associated with gemcitabine resistance.
SIK1 inhibits proliferation in pancreatic
cancer
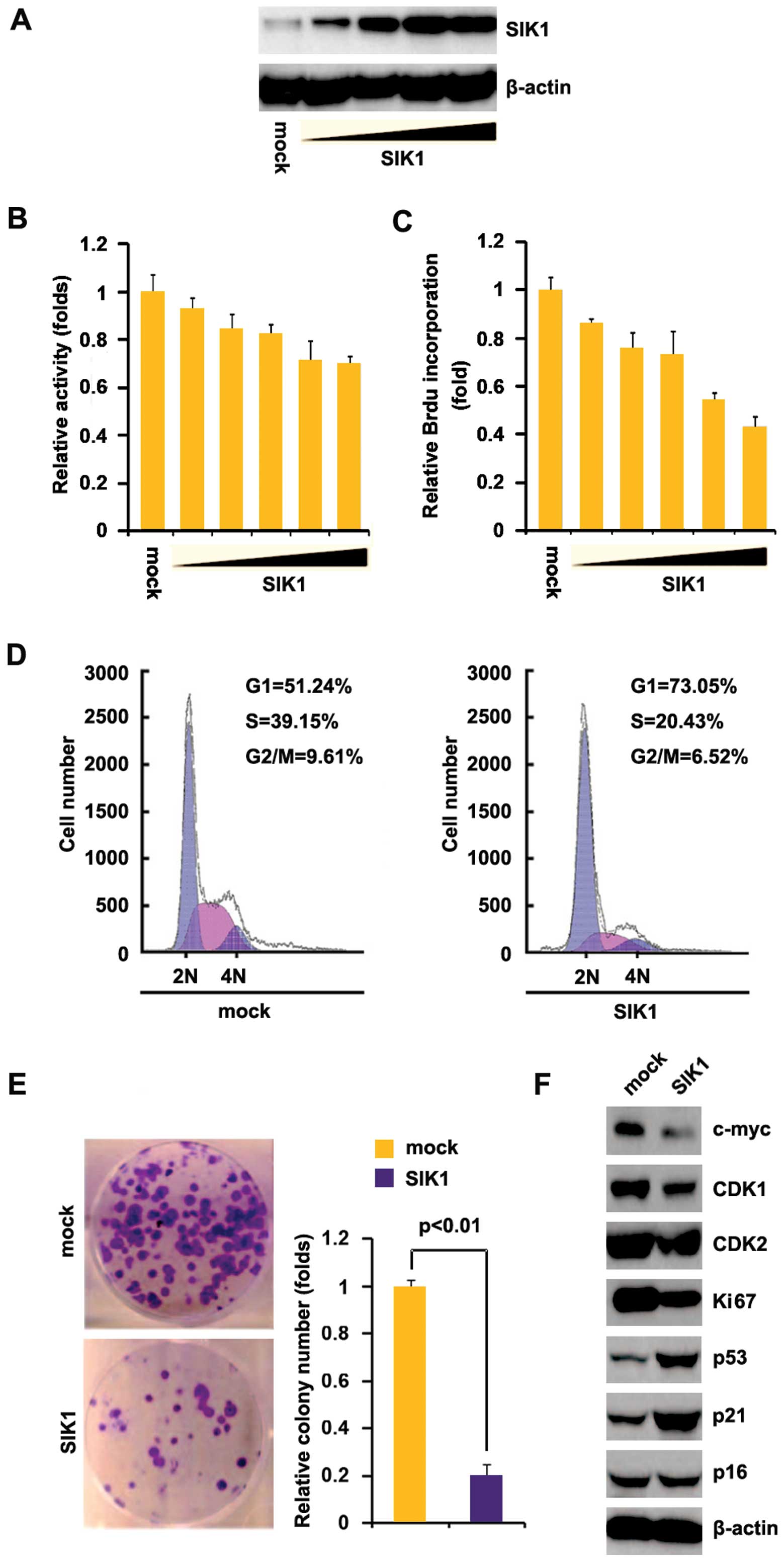
To investigate whether SIK1 affected the
proliferation of pancreatic cancer cells, we examined whether
SIK1-expressing plasmids stably expressed SIK1 protein in Hs766T
cells, using western blot analysis. The results showed that SIK1
protein was significantly increased by SIK1-expressing plasmids in
the cells (Fig. 2A). In addition,
we performed an MTT assay to detect the proliferation of Hs766T
cells transfected with SIK1-expressing plasmids. The results showed
that SIK1 inhibited proliferation in Hs766T cells after 48 h of
transfection and the inhibition was dose-dependent (Fig. 2B). To determine the effects of SIK1
on proliferation, we performed a Brdu incorporation assay to detect
DNA synthesis in the cells. The results confirmed that SIK1
significantly inhibited DNA synthesis in the cells (Fig. 2C). To identify whether DNA synthesis
inhibition contributed to lower S-phase fractions in Hs766T cells
transfected with SIK1, we performed cell cycle analysis to analyze
its effects on the cell cycle. The results showed lower S-phase
fractions in HS766T cells transfected with SIK1 than in the cells
transfected with empty vector (Fig.
2D). To identify the effect of SIK1 on colony formation, we
performed the colony formation assay. The results showed that
overexpression of SIK1 significantly suppressed the colony
formation rate of Hs766T cells following transfection (Fig. 2E). In addition, we performed western
blot analysis to confirm that SIK1 affected proliferation markers.
The results of the western blot analysis demonstrated that c-myc,
CDK1 and Ki67 expression were downregulated while p53 and p21 were
upregulated by SIK1 (Fig. 2F).
SIK1 suppresses migration and
invasion
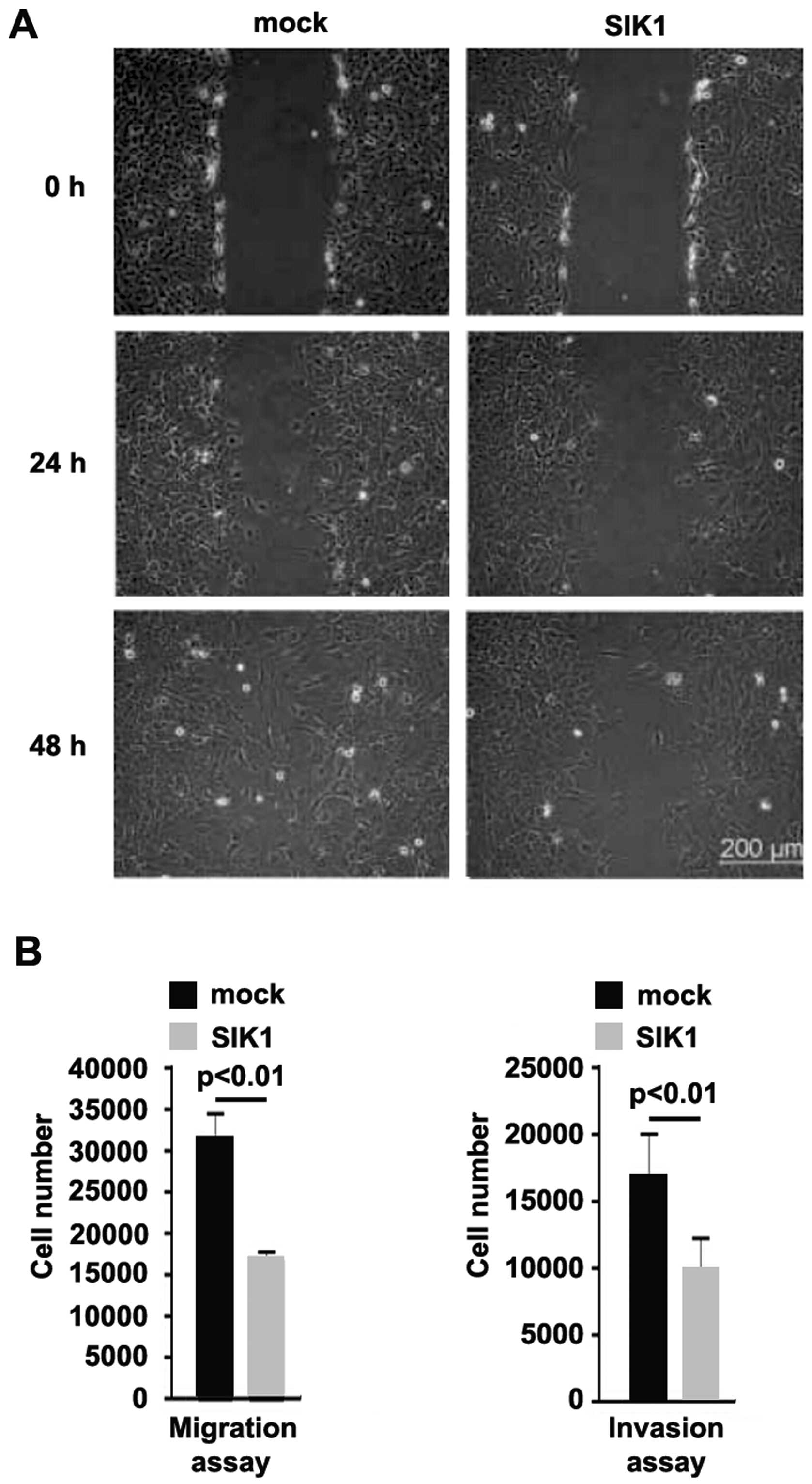
To identify the role of SIK1 in regulating the
migration and invasion of Hs766T cells, a wound-healing assay was
performed. The wound-healing assay showed that SIK1 significantly
inhibited motility in the cells (Fig.
3A) (P<0.05). To confirm the results, we performed a
migration and invasion assay to detect the migration and invasion
of Hs766T cells transfected with SIK1-expressing plasmids and empty
vectors. Ectopic SIK1 inhibited motility and invasion by ~2-fold in
the cells (Fig. 3B).
miR-203 degrades SIK1 in pancreatic
cancer cells
Having demonstrated that SIK1 expression is
specifically downregulated in pancreatic cancer (Fig. 1A) and it suppressed proliferation,
migration and invasion in vitro, we examined the mechanisms
suppressing SIK1 expression in pancreatic cancer. MicroRNAs (miRs)
are a class of small non-coding RNAs (~22 nucleotides) and
negatively regulate the protein-coding gene expression by targeting
mRNA degradation or translation inhibition (6–8).
Upregulation of specific miRNA can contribute to the downregulation
of tumor-suppressive gene (19–21).
Thus, SIK1 was downregulated by the overexpression of specific
miRNA in pancreatic cancer.
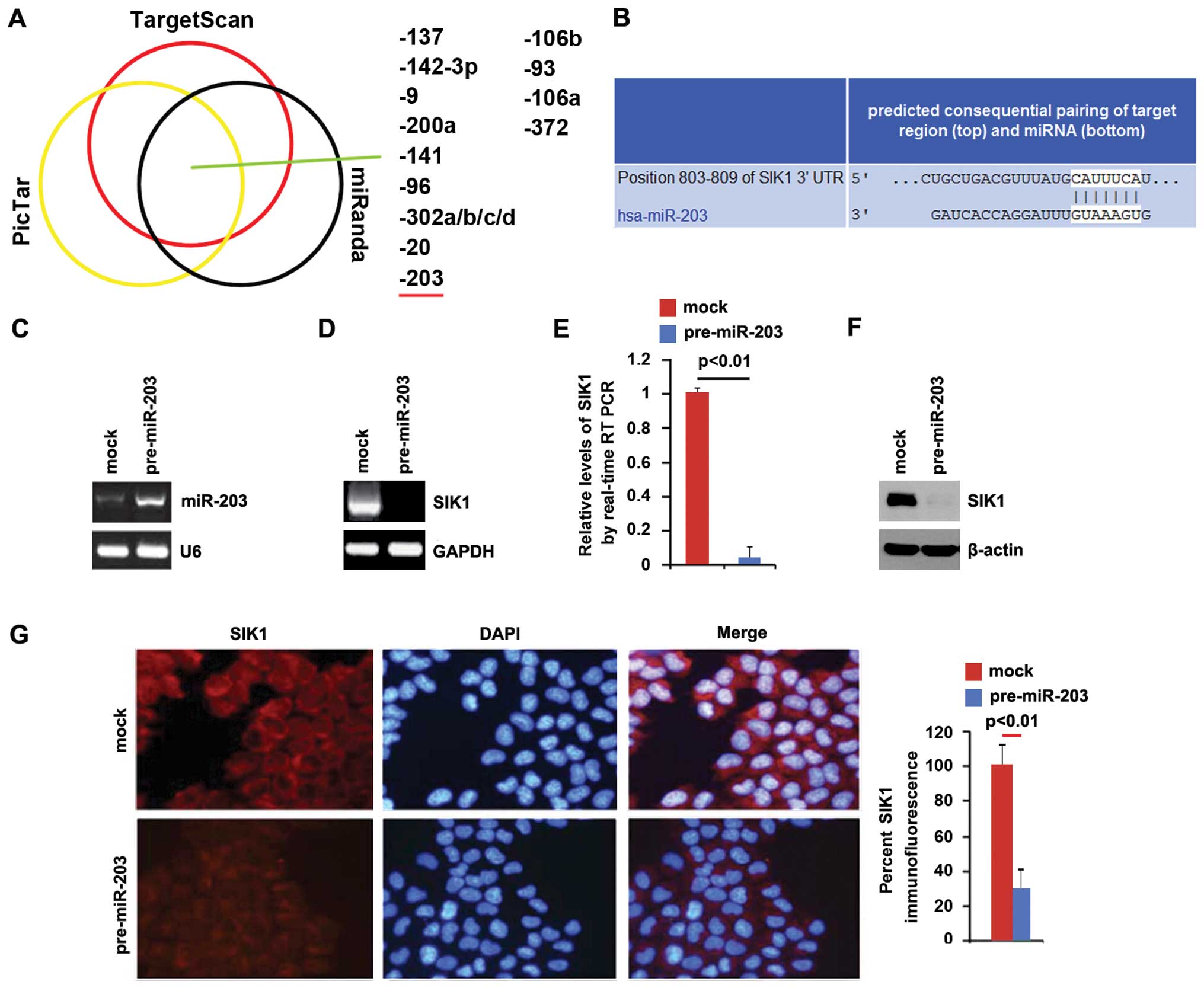
To confirm this finding, the prediction algorithms,
miRanda (http://www.microrna.org/microrna/home.do), TargetScan
(http://www.targetscan.org) and PicTar
(http://pictar.mdc-berlin.de/) were used
to analyze 3′UTR of SIK1. The three algorithms predicted that
miR-137, miR-142-3p, miR-9, miR-200a, miR-141, miR-96,
miR-302a/b/c/d, miR-20, miR-203, miR-106b, miR-93, miR-106a and
miR-372 were able to target 3′UTR of SIK1 (Fig. 4A). An elevated expression of miR-203
in pancreatic tumors is associated with poorer survival (13). Target sites on 3′UTR of SIK1 are
shown in Fig. 4B. The result showed
that miR-203 downregulated SIK1 expression by targeting its 3′UTR
in pancreatic cancer and that SIK1 was downregulated in pancreatic
cancer cells, due to the overexpression of miR-203. To identify the
role of miR-203 in regulating SIK1 expression in pancreatic cancer
cells, BxPC-3 cells were transfected with pre-miR-203 and control
miR. Following transfection, miR-203 expression was detected by
qPCR and the results showed that miR-203 was significantly
increased by pre-miR-203 in the cells (Fig. 4C). To confirm the result obtained,
we performed RT-PCR to detect SIK1 mRNA expression in BxPC-3 cells
transfected with pre-miR-203 or control miR, and identified that
SIK1 mRNA was degraded (Fig. 4D).
Consistent with the results of RT-PCR, the qPCR results
demonstrated that SIK1 mRNA was reduced in BxPC-3 cells transfected
with pre-miR-203, compared to the control miR-transfected groups
(Fig. 4E). To detect whether SIK1
protein was affected by miR-203, we performed a western blot
analysis in BxPC-3 cells transfected with pre-miR-203 or control
miR. The results showed that SIK1 protein was evidently suppressed
in the cells transfected with pre-miR-203 (Fig. 4F). We also performed
immunoflurescence analysis to detect SIK1 expression in BxPC-3
cells transfected with pre-miR-203 or control miR. The results
showed that SIK1 protein (Fig. 4G)
was significantly downregulated in the cells transfected with
pre-miR-203.
miR-203 promotes proliferation in
pancreatic cancer cells
We identified that SIK1 was downregulated in
pancreatic cancer tissues and inhibited proliferation, migration
and invasion in pancreatic cancer cells, and that miR-203 degraded
SIK1 in pancreatic cancer cells. Additionally, an elevated
expression of miR-203 in pancreatic tumors was associated with
poorer survival (13). Thus,
contrary to SIK1, miR-203 may promote proliferation, migration and
invasion in pancreatic cancer.
To investigate whether miR-203 contributed to the
proliferation of pancreatic cancer cells, we examined whether
pre-miR-203 was stably expressed in BxPC-3 cells using qPCR. The
results showed that miR-203 was significantly increased by
pre-miR-203 in the cells (Fig.
5A).
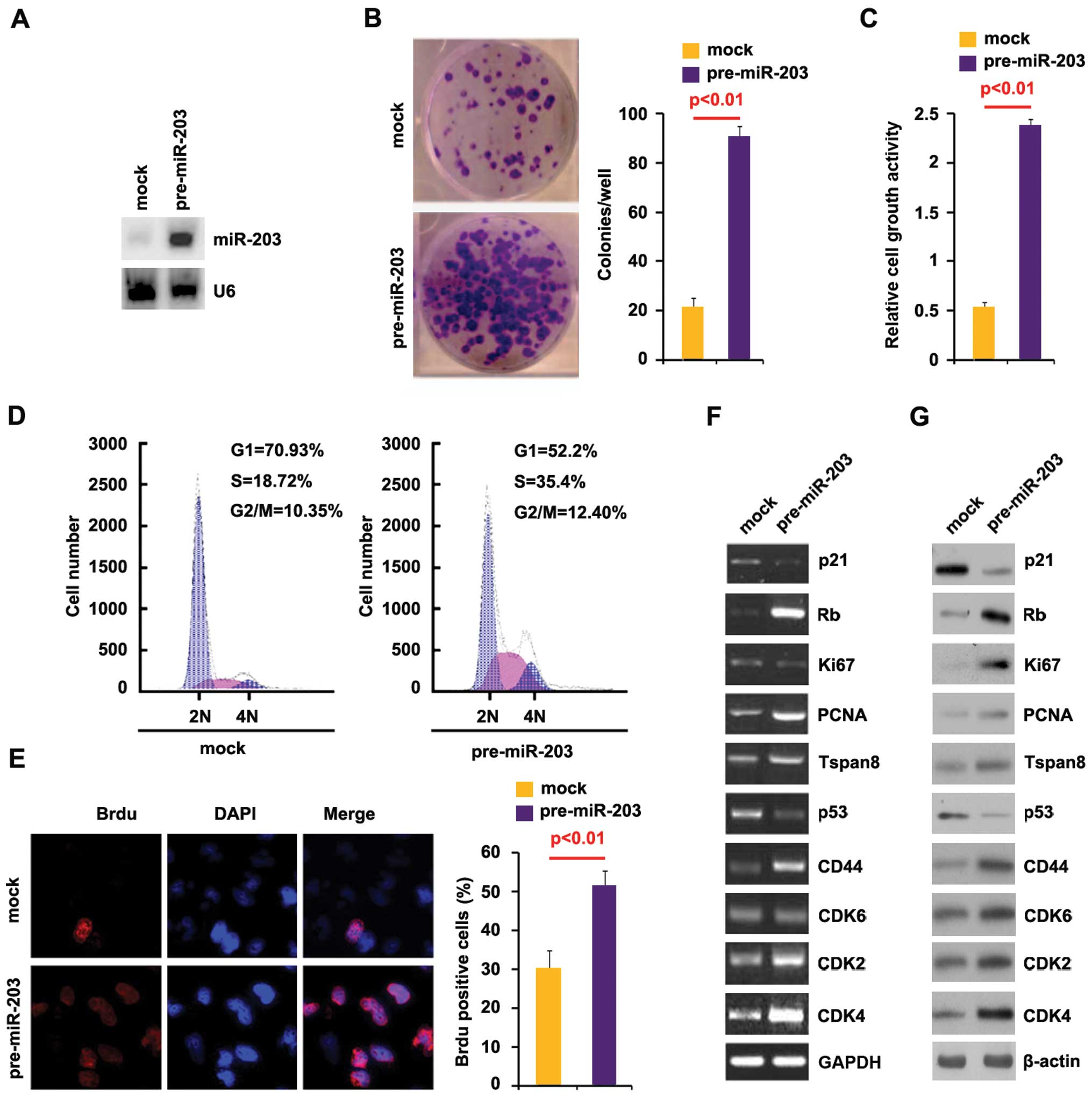 | Figure 5miR-203 promotes proliferation in
pancreatic cancer cells. (A) qPCR for miR-203 in BxPC-3 cells.
BxPC-3 cells were transfected with pre-miR-203 or control miR
(mock). U6 was used as the loading control; n=3. (B) Colony
formation assay for BxPC-3 cells transfected with pre-miR-203 or
control miR (mock). Colonies with >50 cells were counted.
Representative micrographs (left panel) and quantification of
colonies (right panel) following transfection with pre-miR-203 or
control miR (mock); n=3. (C) MTT assay for BxPC-3 cells. BxPC-3
cells were transfected as indicated and then cell viability was
measured by MTT assay; n=3. (D) Cell cycle analysis for BxPC-3
cells transfected with pre-miR-203 or control miR (mock).
Histograms of DNA contents obtained by FACS analysis. The
percentages of each cell cycle stages are shown in the inset of the
histograms; n=3. (E) Brdu incorporation assay for BxPC-3 cells.
Representative micrographs (left panel) and quantification (right
panel) of BrdU-incorporating cells following transfection with
pre-miR-203 or control miR (mock); n=3. (F) RT-PCR for p21, Rb,
Ki67, PCNA, Tspan8, p53, CD44, CDK4, CDK6 and CDK2 in BxPC-3 cells
infected with pre-miR-203 or control miR (mock). GAPDH was used as
the loading control; n=3. (G) Western blot analysis for p21, Rb,
Ki67, PCNA, Tspan8, p53, CD44, CDK4, CDK6 and CDK2 in BxPC-3 cells
infected with pre-miR-203 or control miR (mock). β-actin was used
as the loading control; n=3. |
To identify the effect of miR-203 on colony
formation, we performed the colony formation assay. The results
showed that the overexpression of miR-203 significantly increased
the colony formation rate of BxPC-3 cells following transfection
(Fig. 5B).
In addition, an MTT assay was performed to detect
the proliferation of BxPC-3 cells transfected with pre-miR-203. The
results showed that pre-miR-203 promoted proliferation in the cells
after 48 h of transfection (Fig.
5C). To determine the effects of pre-miR-203 on proliferation,
we performed a cell cycle analysis to analyze its effects on the
cell cycle. The results showed higher S-phase fractions in BxPC-3
cells transfected with pre-miR-203 than in BxPC-3 cells transfected
with control miR (Fig. 5D). To
identify whether DNA synthesis promotion contributed to higher
S-phase fractions in BxPC-3 cells transfected with pre-miR-203, we
performed the BrdU incorporation assay to detect DNA synthesis in
the cells. The results confirmed that pre-miR-203 significantly
promoted DNA synthesis in the cells and representative micrographs
(left panel) and quantification (right panel) of BrdU-incorporating
cells following transfection with pre-miR-203 or control miR (mock)
was identified (Fig. 5E). In
subsequent studies, we performed RT-PCR to identify whether the
mRNA of the proliferation markers was also affected by pre-miR-203
in the cells. The results of RT-PCR showed that Rb, PCNA, Tspan8,
CD44, CDK2 and CDK4 expression was upregulated while p21 and p53
expression was downregulated by pre-miR-203 in the cells (Fig. 5F). In addition, we performed western
blot analysis to confirm that pre-miR-203 regulated these markers.
The results of the western blot analysis demonstrated that Rb,
Ki67, PCNA, Tspan8, CD44, CDK2, CDK4 and CDK6 expression was
upregulated while p21 and p53 expression was downregulated by
pre-miR-203 (Fig. 5G). These
results suggested that pre-miR-203 promotes proliferation in BxPC-3
cells.
miR-203 overexpression promotes migration
and invasion in pancreatic cancer
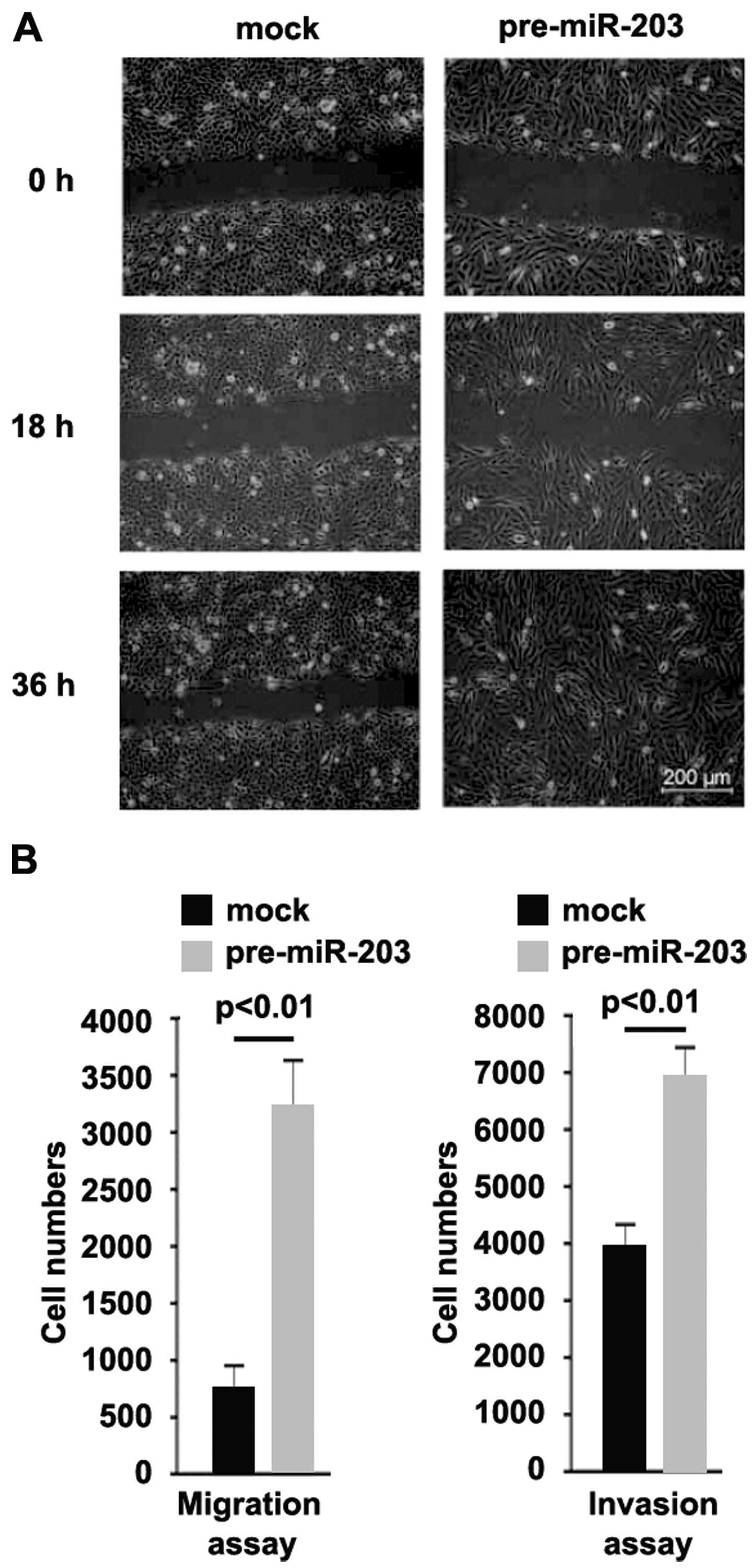
To identify whether miR-203 affected migration and
invasion, we performed a wound-healing assay, and the results
showed that miR-203 significantly promoted migration in BxPC-3
cells (Fig. 6A). We also performed
a migration and invasion assay to detect the role of miR-203,
consistent with the wound-healing assay, and the results
demonstrated that the ability of migration and invasion was
increased in the cells transfected with pre-miR-203 (Fig. 6B).
SIK1 abrogates pre-miR-203-mediated
proliferation, migration and invasion regulation
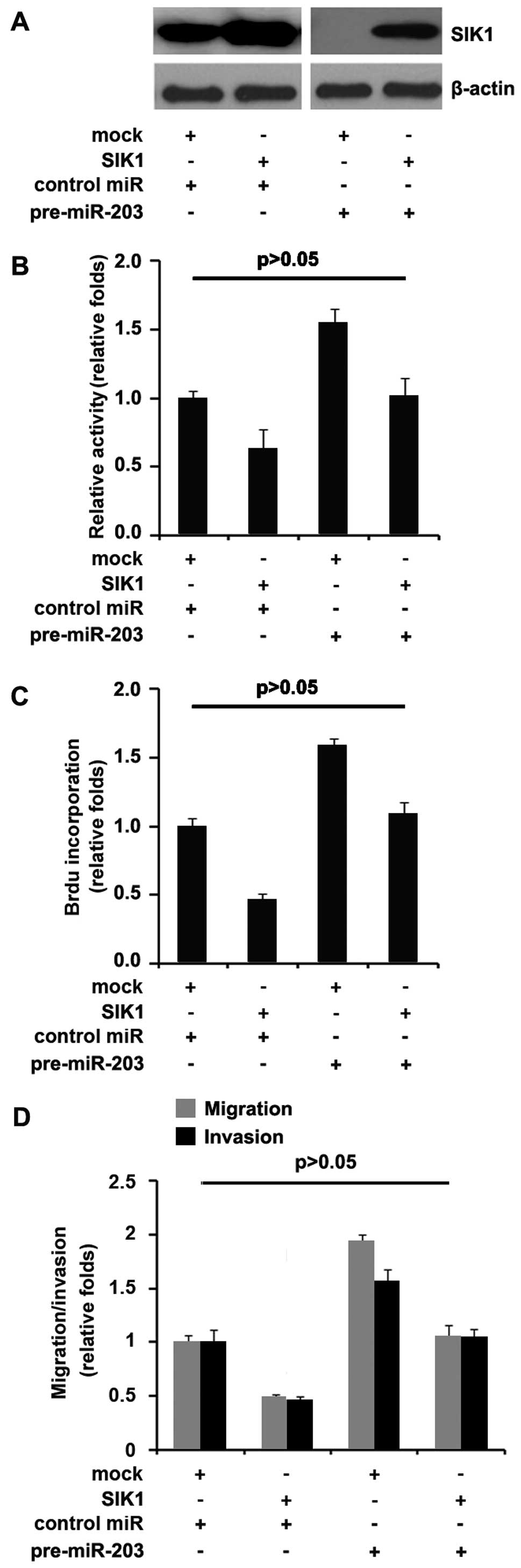
As miR-203 degraded SIK1, we aimed to identify
whether the ectopic expression of SIK1 by transfection of the cDNA
that did not contain the predicted target of 3′UTR would escape the
regulation of miR-203 and thus attenuate or eliminate miR-203
function. To this end, we transfected SIK1-expressing plasmids or
empty vector (mock) into control miR or pre-miR-203-treated BxPC-3
cells. Immunoblotting revealed that the transfection of
SIK1-expressing plasmids eliminated the effect of miR-203 on SIK1
protein (Fig. 7A). The
overexpression of miR-203 in BxPC-3 cells promoted proliferation,
motility and invasion. To identify whether SIK1 abrogated or
attenuated the roles of miR-203 on proliferation, motility and
invasion, control miR or pre-miR-203-treated BxPC-3 cells were
transfected with SIK1-expressing plasmids or empty vectors (mock),
we performed MTT, Brdu incorporation, and migration and invasion
assays and then determined whether pre-miR-203-treated BxPC-3 cells
exhibited a >50% increase in proliferation, migration and
invasion as compared to control miR-treated cells (Fig. 7B–D). Restoration of SIK1 sufficed to
reverse the increase of migration and invasion (Fig. 7B–D) observed in pre-miR-203-treated
cells. Thus, miR-203 promoted proliferation, migration and invasion
by degrading SIK1 expression.
Discussion
The AMP-activated protein kinases (AMPKs) are
important in regulating metabolism and cell growth (22–24).
SIK1 is a member of AMPK-RKs (3).
Clinical studies have shown that reduced levels of SIK1 are
associated with distal metastases and poor outcome in breast
cancer, and SIK1 expression has been associated with a
tumor-suppressor function (4,25–27).
In the present study we have shown that SIK1 was downregulated and
loss of SIK1 was associated with gemcitabine resistance in
pancreatic cancer. Thus, it is probable that SIK1 is a
tumor-suppressive gene that plays an important role in gemcitabine
resistance.
We identified that SIK1 was able to suppress
proliferation in pancreatic cancer cells. We found that the
clonogenic ability was significantly suppressed by SIK1. There is a
connection between cancer stem cells and cologenic ability
(28–31). The results showed that SIK1
inhibited sphere growth in pancreatic cancer (data not published).
Moreover, consistent with a previous report that SIK1 couples LKB1
to p53-dependent anoikis and suppresses metastasis in breast cancer
(4), we demonstrated that SIK1
overexpression significantly upregulated the p53 level and
ectopically expressed SIK1-inhibited migration and invasion in
pancreatic cancer cells.
Upregulation of specific miRNAs can contribute to
the downregulation of tumor-suppressive genes (19–21).
Thus, we examined whether SIK1 was downregulated by the
overexpression of specific miRNAs in pancreatic cancer tissues and
gemcitabine-resistant cell lines. miR-203 is upregulated in
pancreatic cancer tissues and the elevated expression is associated
with poor survival (13,14). Three prediction algorithms were
utilized to analyze 3′UTR of SIK1, and the results showed that
miR-203 was able to target 3′UTR of SIK1, as confirmed by
subsequent studies. However, further studies determining whether
miR-203 and the tumor-suppressor gene SIK1 are inversely expressed
and whether miR-203 suppression mediates the upregulation of SIK1
protein in pancreatic cancer are to be conducted.
In line with previous report that miR-203 is
upregulated in pancreatic cancer tissues and the elevated
expression is associated with poorer survival (13,14),
we found that miR-203 promotes proliferation, invasion and
migration in pancreatic cancer cells.
CD44 is a marker of cancer stem cells in pancreatic
cancer (32,33). In thre present study, the results
showed that cologenic ability was significantly increased, and that
CD44 was significantly upregulated by miR-203. The results suggest
that miR-203 was associated with cancer stem cells. However, the
roles of miR-203 in cancer stem cells of pancreatic cancer remain
to be determined.
To conclude, in this study we characterized a
function of SIK1 in pancreatic cancer. The results have shown that
SIK1 is regulated by miR-203 and that miR-203 functions via SIK1 in
pancreatic cancer.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wagner M, Redaelli C, Lietz M, Seiler CA,
Friess H and Büchler MW: Curative resection is the single most
important factor determining outcome in patients with pancreatic
adenocarcinoma. Br J Surg. 91:586–594. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bright NJ, Thornton C and Carling D: The
regulation and function of mammalian AMPK-related kinases. Acta
Physiol (Oxf). 196:15–26. 2009. View Article : Google Scholar
|
|
4
|
Cheng H, Liu P, Wang ZC, Zou L, Santiago
S, Garbitt V, Gjoerup OV, Iglehart JD, Miron A, Richardson AL, et
al: SIK1 couples LKB1 to p53-dependent anoikis and suppresses
metastasis. Sci Signal. 2:ra352009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pasquinelli AE, Reinhart BJ, Slack F,
Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B,
Müller P, et al: Conservation of the sequence and temporal
expression of let-7 heterochronic regulatory RNA. Nature.
408:86–89. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reinhart BJ, Slack FJ, Basson M,
Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR and Ruvkun G:
The 21-nucleotide let-7 RNA regulates developmental timing in
Caenorhabditis elegans. Nature. 403:901–906. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Farh KK, Grimson A, Jan C, Lewis BP,
Johnston WK, Lim LP, Burge CB and Bartel DP: The widespread impact
of mammalian microRNAs on mRNA repression and evolution. Science.
310:1817–1821. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Calin GA and Croce CM: MicroRNA-cancer
connection: The beginning of a new tale. Cancer Res. 66:7390–7394.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Greither T, Grochola LF, Udelnow A,
Lautenschläger C, Würl P and Taubert H: Elevated expression of
microRNAs 155, 203, 210 and 222 in pancreatic tumors is associated
with poorer survival. Int J Cancer. 126:73–80. 2010. View Article : Google Scholar
|
|
14
|
Ikenaga N, Ohuchida K, Mizumoto K, Yu J,
Kayashima T, Sakai H, Fujita H, Nakata K and Tanaka M: MicroRNA-203
expression as a new prognostic marker of pancreatic adenocarcinoma.
Ann Surg Oncol. 17:3120–3128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan ZQ, Sun M, Feldman RI, Wang G, Ma X,
Jiang C, Coppola D, Nicosia SV and Cheng JQ: Frequent activation of
AKT2 and induction of apoptosis by inhibition of
phosphoinositide-3-OH kinase/Akt pathway in human ovarian cancer.
Oncogene. 19:2324–2330. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang L, Chen F, Pang EJ, Zhang ZQ, Jin BW
and Dong WF: MicroRNA-182 inhibits proliferation through targeting
oncogenic ANUBL1 in gastric cancer. Oncol Rep. 33:1707–1716.
2015.PubMed/NCBI
|
|
17
|
Li Y, VandenBoom TG II, Kong D, Wang Z,
Ali S, Philip PA and Sarkar FH: Up-regulation of miR-200 and let-7
by natural agents leads to the reversal of
epithelial-to-mesenchymal transition in gemcitabine-resistant
pancreatic cancer cells. Cancer Res. 69:6704–6712. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arumugam T, Ramachandran V, Fournier KF,
Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey
DJ and Choi W: Epithelial to mesenchymal transition contributes to
drug resistance in pancreatic cancer. Cancer Res. 69:5820–5828.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu S, Wu H, Wu F, Nie D, Sheng S and Mo
YY: MicroRNA-21 targets tumor suppressor genes in invasion and
metastasis. Cell Res. 18:350–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu S, Si ML, Wu H and Mo YY: MicroRNA-21
targets the tumor suppressor gene tropomyosin 1 (TPM1). J Biol
Chem. 282:14328–14336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shackelford DB and Shaw RJ: The LKB1-AMPK
pathway: Metabolism and growth control in tumour suppression. Nat
Rev Cancer. 9:563–575. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu A and Screaton RA: Using kinomics to
delineate signaling pathways: Control of CRTC2/TORC2 by the AMPK
family. Cell Cycle. 7:3823–3828. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mirouse V, Swick LL, Kazgan N, St Johnston
D and Brenman JE: LKB1 and AMPK maintain epithelial cell polarity
under energetic stress. J Cell Biol. 177:387–392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Klijn JG, Zhang Y, Sieuwerts AM,
Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME and
Yu J: Gene-expression profiles to predict distant metastasis of
lymph-node-negative primary breast cancer. Lancet. 365:671–679.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chin K, DeVries S, Fridlyand J, Spellman
PT, Roydasgupta R, Kuo WL, Lapuk A, Neve RM, Qian Z, Ryder T, et
al: Genomic and transcriptional aberrations linked to breast cancer
pathophysiologies. Cancer Cell. 10:529–541. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu X, Lu X, Wang ZC, Iglehart JD, Zhang X
and Richardson AL: Predicting features of breast cancer with gene
expression patterns. Breast Cancer Res Treat. 108:191–201. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ross AA, Cooper BW, Lazarus HM, Mackay W,
Moss TJ, Ciobanu N, Tallman MS, Kennedy MJ, Davidson NE, Sweet D,
et al: Detection and viability of tumor cells in peripheral blood
stem cell collections from breast cancer patients using
immunocytochemical and clonogenic assay techniques. Blood.
82:2605–2610. 1993.PubMed/NCBI
|
|
29
|
Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO,
Zheng BJ and Guan XY: Identification and characterization of
tumorigenic liver cancer stem/progenitor cells. Gastroenterology.
132:2542–2556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu C, Kelnar K, Liu B, Chen X,
Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et
al: The microRNA miR-34a inhibits prostate cancer stem cells and
metastasis by directly repressing CD44. Nat Med. 17:211–215. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li C, Heidt DG, Dalerba P, Burant CF,
Zhang L, Adsay V, Wicha M, Clarke MF and Simeone DM: Identification
of pancreatic cancer stem cells. Cancer Res. 67:1030–1037. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hermann PC, Huber SL, Herrler T, Aicher A,
Ellwart JW, Guba M, Bruns CJ and Heeschen C: Distinct populations
of cancer stem cells determine tumor growth and metastatic activity
in human pancreatic cancer. Cell Stem Cell. 1:313–323. 2007.
View Article : Google Scholar
|