Introduction
Colorectal cancer (CRC) is one of the most common
malignant tumors of the digestive system and is also one of the
leading causes of cancer-related deaths worldwide (1). Early symptoms of CRC are usually not
detected. Once diagnosed, CRC is often in the advanced stage,
including metastasis to liver in the 20% of cases (2). The principle therapeutic method is
surgery, as well as radiotherapy and chemotherapy (3). Although surgical techniques have been
rapidly developed during the past few years, the 5-year survival
rate of CRC patients has not significantly increased. Non-surgical
therapies, such as chemotherapy and radiotherapy, lack efficacy.
Currently, with advantages and achievements in the clinic, more and
more active antitumor ingredients derived from Traditional Chinese
medicine (TCM) are being developed, studied and used.
Cinnamaldehyde (CA), as shown in Fig. 1A, is a bioactive compound isolated
from the stem bark of Cinnamomum cassia, and has been used
as a TCM herb. Studies have demonstrated that CA displays various
biological activities, including antibacterial, immunomodulatory,
cytotoxic and anti-angiogenic activities (4–7). It is
also known to possess marked antitumor effects in vitro and
in vivo (8–11). However, the signaling mechanisms
involved in the inhibition of CRC cell growth by CA are poorly
understood.
The molecular mechanisms involved in the
tumorigenesis and metastasis of CRC are not entirely clear.
Carcinogenesis of CRC is complex and requires the accumulated
alteration of multiple genes and pathways. The
phosphoinositide-3-kinase (PI3K)/AKT signaling pathway is
indispensable to intracellular signal transduction which involves
cell metabolism, apoptosis, survival, differentiation and
proliferation processes. Therefore, it is closely related to the
occurrence, development and metastasis of many types of tumors
(12). AKT and p-AKT are expressed
in CRC tissues at a significantly higher level than those noted in
normal tissues (13). This causes
not only cell malignant transformation, but also tumor cell
migration, adhesion and extracellular matrix degradation (14,15).
Therefore, the PI3K/Akt pathway is considered to be a potential
target for cancer treatment and needs further research. Compounds
that inhibit PI3K/AKT-related genes with drug-like properties
blocking the activation of multiple downstream anti-apoptotic
effector molecules and promoting cell apoptosis are highly
anticipated.
In the present study, we investigated the effect of
CA on cell proliferation, invasion and adhesion, and apoptosis of
CRC cells. In addition, we aimed to study the mechanisms behind the
effect of CA on the PI3K/AKT signaling pathway in order to provide
an experimental foundation for the present research and a possible
treatment of CRC.
Materials and methods
Drugs and antibodies
CA was purchased from the China National Institute
for the Control of Pharmaceutical and Biological Products (purity
99%) and dissolved in dimethylsulfoxide (DMSO). It was dissolved in
DMSO at a stock solution (200 µg/ml) and stored at −80°C.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Annexin
V conjugated to fluorescein-isothiocyanate (Annexin V-FITC)
apoptosis detection kit was purchased from KeyGen Biotech. Co. Ltd.
(Nanjing, China). Insulin-like growth factor-I (IGF-I) was obtained
from PeproTech China (Suzhou, China). Rabbit anti-human antibodies
against MMP-9, MMP-2, E-cadherin, Bax, Bcl-2, PARP, cleaved-PARP,
PI3K p-PI3K, AKT, p-AKT and LY294002 (the PI3K inhibitor) were
purchased from Cell Signaling Technology (Beverly, MA, USA).
Fluorescein-conjugated secondary antibodies were purchased from
Odyssey (LI-COR, Lincoln, NE, USA). All other chemicals used in the
experiment were of the highest purity grade available.
Cell lines and culture
Human CRC cell lines LoVo, SW480 and HCT116 were
obtained from the Type Culture Collection, Chinese Academy of
Sciences (Shanghai, China). All the cells were cultured in
RPMI-1640 medium containing 10% heat-inactivated fetal bovine serum
(FBS) (both from Gibco-BRL, Gaithersburg, MD, USA) in a humidified
incubator with 5% CO2 at 37°C.
MTT assay
Cells in the logarithmic growth phase were firstly
seeded into 96-well culture plates at 6×103 cells/well
and then incubated overnight following treatment of CA at
concentrations of 0, 20, 40, 60 and 80 µg/ml for 24, 48 and
72 h. Afterwards, 20 µl of MTT solution (5 mg/ml) was added
to each well to incubate the cells at 37°C for 4 h. In order to
dissolve the resultant formazan crystals, 100 µl DMSO was
added to each well. Absorbance was detected at 490 nm using an
ELx800 microplate reader (BioTek, Winooski, VT, USA). Cell growth
inhibition rate was calculated using the following formula: 1 −
ODexperiment/ODcontrol.
Cell apoptosis assay
Apoptosis was monitored using the Annexin
V/propidium iodide (PI) method as previously described (16), and its rate was detected by flow
cytometry with an Annexin V-FITC apoptosis detection kit. The cells
were seeded in 6-well plates and treated with CA (0, 20, 40 and 80
µg/l) for 24 h, and then harvested by trypsinization. After
washing twice with cold phosphate-buffered saline (PBS), the cells
were re-suspended in 500 µl binding buffer at
1×106 cells/ml. Annexin V-FITC (5 µl) PI was
subsequently added for incubation at room temperature for 15
min.
Hoechst 33258 staining
Hoechst 33258 staining was performed as previously
described (17). HCT116, LoVo and
SW480 cells were fixed with 4% paraformaldehyde for 30 min after
treatment with CA for 24 h at room temperature and washed once with
PBS. Cells were incubated in Hoechst 33258 (50 ng/ml) for 30 min at
room temperature and then washed with PBS. Apoptotic cells were
identified by the condensation and fragmentation of their nuclei
and photographed by a Zeiss Axioplan 2 fluorescence microscope
(Jena, Germany).
Invasion assay
The cell migration assay was performed using
Transwell membrane filter inserts (pore size, 8-µm; Costar,
Corning, NY, USA) in 24-well dishes. The cells were pretreated for
24 h with different concentrations of CA. Approximately
1×104 cells in 200 µl of serum-free medium were
placed in the upper chamber, and 300 µl of the medium
containing 10% bovine serum was placed in the lower chamber. The
plates were incubated for 24 h at 37°C in 5% CO2, and
the cells were then fixed in 4% paraformaldehyde for 5 min and
stained with 0.05% crystal violet (Sigma-Aldrich, St. Louis, MO,
USA) in PBS for 15 min. Cells on the upper side of the filters were
removed with cotton-tipped swabs, and those on the underside of the
filters were examined and counted under a microscope as previously
described (18).
Cell-matrix adhesion assay
The cell adhesion assay was performed as previously
described (19). Briefly, CRC cells
were treated with or without CA (20, 40 or 80 µg/ml) for 24
h, and then harvested and resuspended in RPMI-1640 medium.
Approximately 2×105 live cells were seeded into
precoated 96-well plates with 2.5 µg/ml fibronectin (FN).
Each concentration group contained 12-wells and every 3-wells were
washed twice after 30, 60, 90 and 120 min to remove the
non-adherent cells. After washing with PBS, the adherent cells were
measured by an MTT assay. Similar to the MTT assay, optical density
(OD) values were measured and the cell adhesion inhibition rates
were calculated based on the means of three wells.
Western blot analysis
Western blot analysis was performed according to the
method reported by Li et al with slight modification
(20). The cells were lysed in RIPA
buffer in an ice bath for 20 min, and centrifuged at 12,000 × g for
10 min at 4°C. The supernatant was stored at −80°C until analyses.
The protein concentration was measured using the BCA method
(Beyotime). An equal amount of proteins was loaded onto 10%
SDS-polyacrylamide gel for electrophoresis and transferred by
electroblotting to a polyvinylidene difluoride membrane (Millipore,
Boston, MA, USA), which was blocked with 5% BSA, and then incubated
with the indicated primary antibodies against MMP-9, MMP-2,
E-cadherin, Bax, Bcl-2, PARP, cleaved-PARP, PI3K AKT, (1:1,000),
p-PI3K and p-AKT (1:500) at 4°C overnight. After washing with
Tris-buffered saline with Tween-20 (TBST) for three times (5
min/time), secondary fluorescent antibody (1:2,000 dilutions) was
added to the membrane at room temperature for 1 h. The signal
intensity of the OD of each band on the membrane was detected by
Odyssey (LI-COR) ImageJ analyzer software; β-actin was used as
loading control and for normalization.
Statistical analysis
All data are presented as means ± standard deviation
(SD). Statistical analysis was performed by SPSS 19.0 software with
one-way ANOVA followed by Dunnet's test to compare the treatment
and the control groups. A p-value of ≤0.05 was considered to
indicate a statistically significant result.
Results
Effects of CA on the cell inhibition rate
of human CRC cells
It is well-known that uncontrolled proliferation is
the major malignant characteristic of cancer cells. In the present
study, we first tested whether CA inhibits CRC cell proliferation
in vitro. After treatment with 0, 10, 20, 30, 40, 60 and 80
µg/ml of CA for 12, 24 and 48 h, the effect of different
concentrations on the inhibition rate of the cells was observed
using the MTT assay. As shown in Fig.
1B–D, the inhibition rates of the cells were significantly
higher compared with the control group at the same time points
(p<0.01), and the inhibition by CA was exhibited in an
approximate dose- and time-dependent manner. Twenty-four hours were
found to be the optimal administration time for the next study. The
IC50 values of CA inhibition of HCT116, LoVo and SW480
cell growth at 24 h were 30.7, 30.6 and 35.69 µg/ml,
respectively.
CA induces the apoptosis of human CRC
cells
Apoptosis is a highly regulated process and is
regulated by a series of genes and cell-signaling pathways. To
further confirm whether CA induces apoptosis in CRC cells, we
evaluated the effects of CA on CRC cells using Annexin V-FITC and
observed the nuclear morphological changes in cells using Hoechst
33258 staining. The results (Fig.
2) indicated that the rates of early and late apoptosis in the
cells were increase by CA, and the apoptotic rates following
treatment with 20, 40 and 80 µg/ml of CA were significantly
higher than the rates in the control group. Cell morphological
change was assessed by fluorescence microscopy after staining with
Hoechst 33258. The apoptotic cells (Fig. 3, indicated with arrows) exhibited
highly fluorescent condensed chromatin. In the treated cells, we
observed small, fragmented and condensed nuclei with typical
apoptotic morphology in contrast with normal symmetrical, blue
nuclei (Fig. 3).
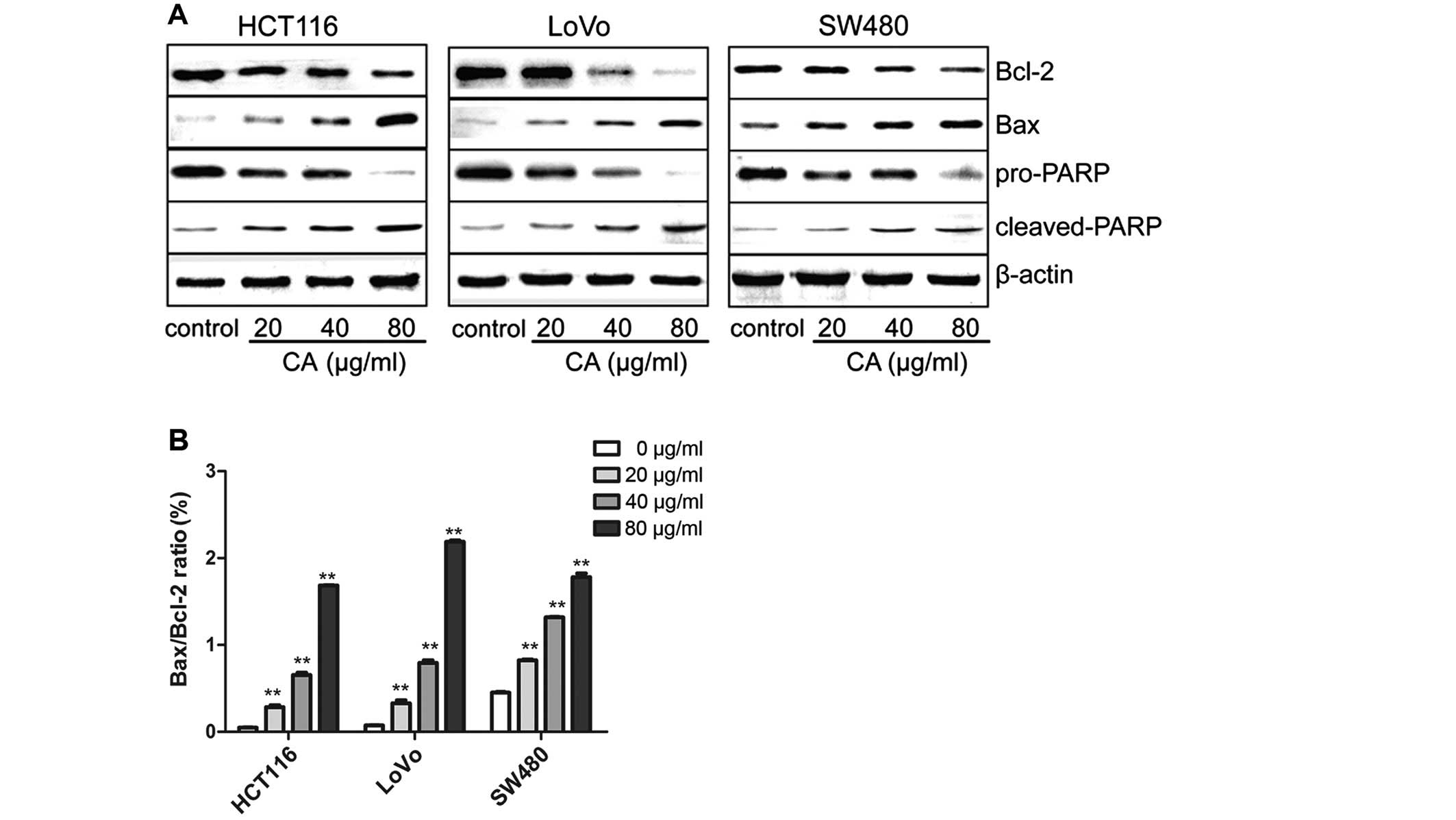
CA induces apoptosis via regulation of
apoptosis-related genes in human CRC cells
It is well-known that proteins of the Bcl-2 family
and PARP are influential in the apoptotic process (21). Targeting the proteins of the Bcl-2
family is a common practice for many anticancer agents inducing
apoptosis and the ratio of Bax/Bcl-2 also plays a critical role
(22). In order to study the
molecular mechanisms behind the CA-induced apoptosis of human CRC
cells, the expression levels of apoptosis-related proteins were
evaluated by western blot analysis after treatment with various
concentrations of CA for 24 h. Western blot analysis revealed that
the Bax and cleaved-PARP expression was obviously increased,
whereas PARP and Bcl-2 expression was decreased, leading to an
upregulation in the ratio of Bax/Bcl-2 (Fig. 4). This may be one of the molecular
mechanisms by which CA induces apoptosis in CRC cells.
CA inhibits CRC cell invasion and
adhesion
We used Transwell chamber and cell-matrix adhesion
assays to test whether CA inhibits CRC cell invasion and adhesion.
As shown in Fig. 5, the result of
the Transwell assay showed that after treatment with different
concentrations of CA (0, 20, 40 and 80 µg/ml) for 24 h, the
number of the cells that invaded across the 8-µm diameter
pores to the lower chamber was markedly decreased compared with the
number of invasive cells in the control group, indicating that CA
suppresses the invasion of CRC cells in a dose-dependent manner
(Fig. 5B). The results indicated
that increasing CA concentrations promoted an increased inhibition
of cell invasion.
Meanwhile, similar to the results of the Transwell
invasion assay, the adhesion rates of the cells treated with CA at
all time points were lower than those of the control group. Further
data demonstrated that the inhibitory rate of adhesion escalated as
the concentrations of CA increased (Fig. 6). Consequently, CA markedly
inhibited the adhesion of CRC cells to FN and the inhibition rate
exhibited a dose- and time-dependent trend.
Effects of CA on expression of invasion-
and adhesion-related genes in human CRC cells
We investigated the expression of E-cadherin and
MMP-2 and MMP-9 in the CRC cells to explore whether changes in the
expression were involved in the inhibition of invasion and adhesion
of these cells. E-cadherin, a member of the cadherin superfamily,
is involved in maintaining cell polarity and organizing the
epithelium by strengthening intercellular adhesion; and is also
closely associated to invasion in an MMP2-dependent manner
(23). In addition to adhesion
molecules, matrix metalloproteinases (MMPs) have the ability to
degrade extracellular matrix (ECM) proteins and influence cell
behaviors, including invasion and differentiation. Among the MMPs
mentioned above, the activity of two gelatinases, MMP-2 and MMP-9,
was found to be closely related with tumor metastasis in particular
(24–26). Western blotting was used to analyze
the levels of E-cadherin, MMP-2 and MMP-9. As shown in Fig. 7, CA significantly reduced the
expression of MMP-2 and MMP-9 in a concentration-dependent manner.
Expression of E-cadherin was upregulated as the dose of CA
increased which may be responsible for the reduction in cell
invasion and adhesion observed in the CRC cells.
Effects of CA on the PI3K/Akt signaling
pathway in human CRC cells
The PI3K/Akt signaling pathway participates in
regulating cell biological behaviors. It has been reported to
inhibit cellular apoptosis and promote cell survival (27). Thus, for many chemotherapeutic
drugs, their antitumor effects are achieved by blocking this
pathway (28,29). Phosphorylated PI3K and Akt are
attractive molecular targets as they contribute to the development
of human CRC and resistance to conventional therapies. Therefore,
the potential role of CA on the PI3K/Akt signaling pathway was
examined.
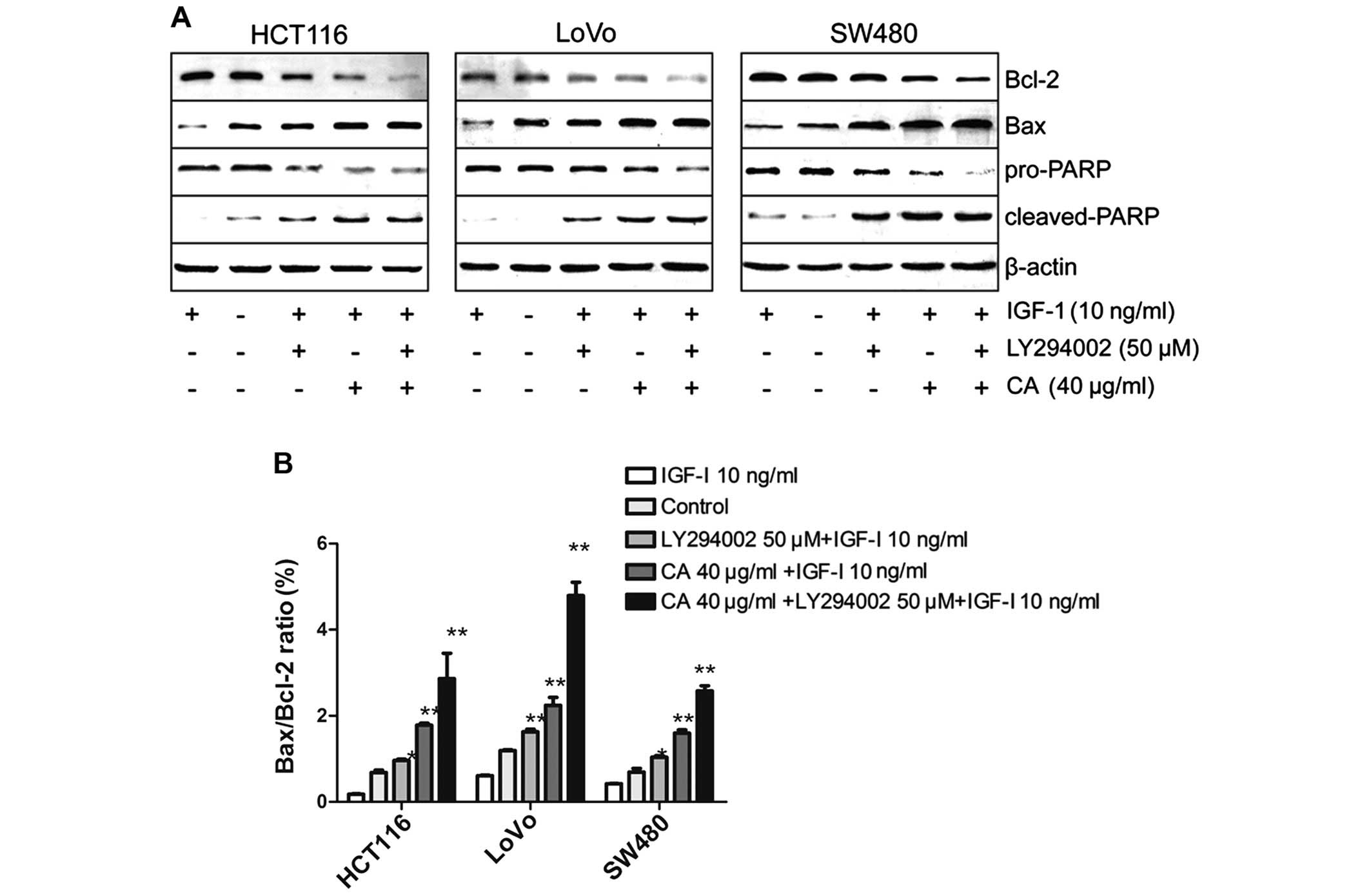
In the present study, the cells were pretreated with
insulin-like growth factor-1 (IGF-1) (one of the most potent
activators of the PI3K/Akt signaling pathway) for 2 h in order to
activate the PI3K/Akt signaling pathway, and then treated with 40
µg/ml CA for 24 h (30). To
further confirm the results, CRC cells were pretreated with IGF-I
for 2 h, followed by exposure to the well-characterized
pharmacological inhibitor LY294002 (50 µM) (a specific
inhibitor of PI3K) for 24 h as a positive control (31). Western blot analysis indicated that
CA inhibited IGF-1-induced expression of p-AKT protein. The total
p-PI3K level was also inhibited although the total PI3K, total Akt
level in each experimental group did not obviously change (Fig. 8). Taken together, CA inhibited the
PI3K/Akt signaling pathway by downregulating p-PI3K and p-AKT.
To confirm whether the apoptosis of CRC cells by CA
was mainly regulated through the PI3K/Akt pathway, IGF-1 and
LY294002 were used to evaluate the underlying mechanisms. Recent
studies indicate that IGF-1 not only acts as an insulin and
mediates the growth hormone action, but also can inhibit apoptosis
(32). The PI3K/Akt pathway is
implicated in the entire spectrum of IGF-1-induced anti-apoptotic
mechanisms (33). The results
indicated that the IGF-1-induced anti-apoptotic effect was
inhibited by LY294002 and CA (Fig.
9). Taken together, CA downregulate PI3K/Akt-dependent
transcriptional activity, resulting in the regulation of
apoptosis-related gene expression.
Discussion
In recent years, with advances in treatment, the
survival rate of colorectal cancer (CRC) patients has increased.
However, many patients still relapse after surgery, radiotherapy
and chemotherapy. Thus preventing the recurrence and metastasis of
CRC is one of the focuses of the present study. Resistance of cell
death, increased invasion and metastasis ability, and
self-sufficiency in growth signals are intrinsic characteristics of
all types of carcinomas (34),
including CRC. Based on these facts, three types of CRC cells with
different differentiation stage and invasive ability were selected
for the present research.
CA has attracted a great deal of research interest
for its anticancer properties. It has been used to inhibit the
growth and induce the apoptosis of cancer cells as a natural
bioactive substance in several studies. Its potential in the
development of an effective anticancer and chemopreventive agent
has been a focus in previous studies (35–37).
However, research on the mechanism of CA against colon cancer is
rare. Thus, we explored the effect of CA on apoptosis, invasion and
metastasis of CRC cells and the related molecular mechanisms.
Resisting apoptosis is the principal mechanism by
which tumor cells resist death, and it is an important point for
the development of anticancer drugs. In the present study, we
applied Annexin V-FITC/PI double staining and FCM (Fig. 2) and Hoechst staining (Fig. 3) to investigate the effects of CA on
the apoptosis of human CRC cells. The results indicated that CA
induced apoptosis at both the early and late stages.
Apotosis can be triggered by two signaling pathways,
the extrinsic pathway (death receptor pathway) and the intrinsic
pathway (the mitochondrial pathway) (38). Poly(ADP-ribose) polymerase (PARP) is
a key signaling nuclear protein involved in apoptosis. Activated
caspase protein cuts it into cleaved poly(ADP-ribose) polymerase
which is a marker of the progression of apoptosis (39,40).
To further explore the molecular mechanisms, western blot analysis
was used (Fig. 4). Then, we found
that the protein level of PARP was decreased in a dose-dependent
manner, while the c-PARP protein level was increased. The Bcl-2
family plays a significant part in apoptosis (41). Particularly, the stoichiometries of
Bax (pro-apoptotic gene) and Bcl-2 (anti-apoptotic gene) are
influential factors for the downstream activation of caspase
protein (42,43). Therefore, we continued to detect the
expression change in Bcl-2 family members. We found that the ratio
of Bax/Bcl-2 increased with increasing doses of CA. These results
suggest that CA can regulate the ratio of Bax/Bcl-2 to induce
apoptosis in human CRC cells.
In regards to invasion, it is the one of most
critical and fatal characteristics of malignant tumors. Adhesion of
tumor cells is an essential and initial step for tumor invasion and
metastasis (44,45). Research has shown that invasion and
adhesion are mediated largely by MMPs, and among the MMPs, MMP-2
and MMP-9 are critical factors (46). However, no study has extensively
explored the underlying mechanisms of the anti-adhesive,
anti-invasive effects of CA. To test whether CA inhibits invasion
and adhesion of CRC cells, Transwell chamber and cell-matrix
adhesion assays were applied. We found that CA inhibited cell
invasion (Fig. 5) and adhesion
(Fig. 6) of human CRC cells in
vitro. Meanwhile, we also found that the effectiveness of CA on
cell invasion and adhesion was influenced by the inhibition of
MMP-2 and MMP-9 activities in a dose-dependent manner (Fig. 7).
Self-sufficiency in growth signals is another
characteristic of tumor cells. As one of the most important
intracellular pathways, the PI3K/Akt pathway is frequently
activated in a number of cancers and is responsible for cell
growth, metabolism, proliferation, survival, motility and invasion.
Furthermore, previous research found that high PI3K expression is
associated with CRC metastasis (47–50).
In the present study, it was discovered that CA effectively
inhibited activation of the PI3K/AKT pathway (Fig. 8).
In previous research, many scholars found that IGF-1
is highly expressed in the serum of patients with CRC. Moreover,
IGF-1 plays an important role in the pathogenesis and metastasis of
CRC (51,52). In the present study, we found that
IGF-1 activated the PI3K/AKT signaling pathway in these three cell
lines. To further confirm this, we treated the cells with CA or
LY294002 (a specific inhibitor of PI3K). The results revealed that
CA effectively inhibited activation of the PI3K/AKT pathway, and
the efficiency of CA can basically approach the the efficacy of
LY294002 (Fig. 9).
In conclusion, CA induces apoptosis and inhibits
invasion and adhesion in CRC cells by inhibiting activation of the
PI3K/Akt pathway, and it is capable of antagonizing the activation
of the PI3K/AKT signaling pathway by IGF-1. These findings provide
an experimental basis for CA to be used as a drug for colon cancer;
however, more basic research is needed to verify the antitumor
activity of CA.
Acknowledgments
The present study was supported by the National
Natural Scientific Foundation of China (no. 81473605), the Priority
Academic Program Development of Jiangsu Higher Education
Institutions (PAPD), the Special Grants for Leading Principal
Investigators (LJ200908) from Jiangsu Administration of Traditional
Chinese Medicine, and the Jiangsu Provincial Special Program of
Medical Science (BL2014100).
References
|
1
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nosher JL, Ahmed I, Patel AN, Gendel V,
Murillo PG, Moss R and Jabbour SK: Non-operative therapies for
colorectal liver metastases. J Gastrointest Oncol. 6:224–240.
2015.PubMed/NCBI
|
|
3
|
Jawed I, Wilkerson J, Prasad V, Duffy AG
and Fojo T: Colorectal cancer survival gains and novel treatment
regimens: A systematic review and analysis. JAMA Oncol. 1:787–795.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang ST, Chen PF and Chang SC:
Antibacterial activity of leaf essential oils and their
constituents from Cinnamomum osmophloeum. J Ethnopharmacol.
77:123–127. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koh WS, Yoon SY, Kwon BM, Jeong TC, Nam KS
and Han MY: Cinnamaldehyde inhibits lymphocyte proliferation and
modulates T-cell differentiation. Int J Immunopharmacol.
20:643–660. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ka H, Park HJ, Jung HJ, Choi JW, Cho KS,
Ha J and Lee KT: Cinnamaldehyde induces apoptosis by ROS-mediated
mitochondrial permeability transition in human promyelocytic
leukemia HL-60 cells. Cancer Lett. 196:143–152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
López P, Sánchez C, Batlle R and Nerín C:
Solid- and vapor-phase antimicrobial activities of six essential
oils: Susceptibility of selected foodborne bacterial and fungal
strains. J Agric Food Chem. 53:6939–6946. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Imai T, Yasuhara K, Tamura T, Takizawa T,
Ueda M, Hirose M and Mitsumori K: Inhibitory effects of
cinnamaldehyde on
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung
carcinogenesis in rasH2 mice. Cancer Lett. 175:9–16. 2002.
View Article : Google Scholar
|
|
9
|
Huang TC, Chung YL, Wu ML and Chuang SM:
Cinnamaldehyde enhances Nrf2 nuclear translocation to upregulate
phase II detoxifying enzyme expression in HepG2 cells. J Agric Food
Chem. 59:5164–5171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu SJ, Ng LT and Lin CC: Effects of
vitamin E on the cinnamaldehyde-induced apoptotic mechanism in
human PLC/PRF/5 cells. Clin Exp Pharmacol Physiol. 31:770–776.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chew EH, Nagle AA, Zhang Y, Scarmagnani S,
Palaniappan P, Bradshaw TD, Holmgren A and Westwell AD:
Cinnamaldehydes inhibit thioredoxin reductase and induce Nrf2:
Potential candidates for cancer therapy and chemoprevention. Free
Radic Biol Med. 48:98–111. 2010. View Article : Google Scholar
|
|
12
|
Suman S, Kurisetty V, Das TP, Vadodkar A,
Ramos G, Lakshmanaswamy R and Damodaran C: Activation of AKT
signaling promotes epithelial-mesenchymal transition and tumor
growth in colorectal cancer cells. Mol Carcinog. 53(Suppl 1):
E151–E160. 2014. View
Article : Google Scholar
|
|
13
|
Johnson SM, Gulhati P, Rampy BA, Han Y,
Rychahou PG, Doan HQ, Weiss HL and Evers BM: Novel expression
patterns of PI3K/Akt/mTOR signaling pathway components in
colorectal cancer. J Am Coll Surg. 210:767–778. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: Its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Wang SJ, Xia W, Rahman K, Zhang Y,
Peng H, Zhang H and Qin LP: Effects of tatariside G isolated from
Fagopyrum tataricum roots on apoptosis in human cervical cancer
HeLa cells. Molecules. 19:11145–11159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu C, Sun G, Yuan G, Wang R and Sun X:
Effects of platycodin D on proliferation, apoptosis and PI3K/Akt
signal pathway of human glioma U251 cells. Molecules.
19:21411–21423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hulkower KI and Herber RL: Cell migration
and invasion assays as tools for drug discovery. Pharmaceutics.
3:107–124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu N, Luo J, Jiang B, Wang L, Wang S, Wang
C, Fu C, Li J and Shi D: Marine bromophenol
bis(2,3-dibromo-4,5-dihydroxy-phenyl)-methane inhibits the
proliferation, migration, and invasion of hepatocellular carcinoma
cells via modulating β1-integrin/FAK signaling. Mar Drugs.
13:1010–1025. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Gu JF, Zou X, Wu J, Zhang MH, Jiang
J, Qin D, Zhou JY, Liu BX, Zhu YT, et al: The anti-lung cancer
activities of steroidal saponins of P. polyphylla Smith var.
chinensis (Franch.) Hara through enhanced immunostimulation in
experimental Lewis tumor-bearing C57BL/6 mice and induction of
apoptosis in the A549 cell line. Molecules. 18:12916–12936. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zinkel S, Gross A and Yang E: BCL2 family
in DNA damage and cell cycle control. Cell Death Differ.
13:1351–1359. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gupta S, Afaq F and Mukhtar H: Involvement
of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle
arrest and apoptosis by apigenin in human prostate carcinoma cells.
Oncogene. 21:3727–3738. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bae GY, Choi SJ, Lee JS, Jo J, Lee J, Kim
J and Cha HJ: Loss of E-cadherin activates EGFR-MEK/ERK signaling,
which promotes invasion via the ZEB1/MMP2 axis in non-small cell
lung cancer. Oncotarget. 4:2512–2522. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park KS, Kim SJ, Kim KH and Kim JC:
Clinical characteristics of TIMP2, MMP2, and MMP9 gene
polymorphisms in colorectal cancer. J Gastroenterol Hepatol.
26:391–397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cavdar Z, Canda AE, Terzi C, Sarioglu S,
Fuzun M and Oktay G: Role of gelatinases (matrix metalloproteinases
2 and 9), vascular endothelial growth factor and endostatin on
clinicopathological behaviour of rectal cancer. Colorectal Dis.
13:154–160. 2011. View Article : Google Scholar
|
|
26
|
Bauvois B: New facets of matrix
metalloproteinases MMP-2 and MMP-9 as cell surface transducers:
Outside-in signaling and relationship to tumor progression. Biochim
Biophys Acta. 1825:29–36. 2012.
|
|
27
|
Wang H, Duan L, Zou Z, Li H, Yuan S, Chen
X, Zhang Y, Li X, Sun H, Zha H, et al: Activation of the
PI3K/Akt/mTOR/p70S6K pathway is involved in S100A4-induced
viability and migration in colorectal cancer cells. Int J Med Sci.
11:841–849. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ryu YL, Jung KH, Son MK, Yan HH, Kim SJ,
Shin S, Hong S and Hong SS: Anticancer activity of HS-527, a novel
inhibitor targeting PI3-kinase in human pancreatic cancer cells.
Cancer Lett. 353:68–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang X, Li XR and Zhang J: Current status
and future perspectives of PI3K and mTOR inhibitor as anticancer
drugs in breast cancer. Curr Cancer Drug Targets. 13:175–187. 2013.
View Article : Google Scholar
|
|
30
|
Elumalai P, Arunkumar R, Benson CS,
Sharmila G and Arunakaran J: Nimbolide inhibits IGF-I-mediated
PI3K/Akt and MAPK signalling in human breast cancer cell lines
(MCF-7 and MDA-MB-231). Cell Biochem Funct. 32:476–484.
2014.PubMed/NCBI
|
|
31
|
Yamaguchi K, Lee SH, Kim JS, Wimalasena J,
Kitajima S and Baek SJ: Activating transcription factor 3 and early
growth response 1 are the novel targets of LY294002 in a
phosphatidylinositol 3-kinase-independent pathway. Cancer Res.
66:2376–2384. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Samani AA, Yakar S, LeRoith D and Brodt P:
The role of the IGF system in cancer growth and metastasis:
Overview and recent insights. Endocr Rev. 28:20–47. 2007.
View Article : Google Scholar
|
|
33
|
Mitsiades CS, Mitsiades N, Poulaki V,
Schlossman R, Akiyama M, Chauhan D, Hideshima T, Treon SP, Munshi
NC, Richardson PG, et al: Activation of NF-kappaB and upregulation
of intracellular anti-apoptotic proteins via the IGF-1/Akt
signaling in human multiple myeloma cells: Therapeutic
implications. Oncogene. 21:5673–5683. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou L, Lu Y, Yang G and Wu J: Research on
tumorigenicity of cinnamaldehyde in melanoma cell lines and its
mechanism. Tumour Biol. 35:5717–5722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yun M, Lee D, Park MN, Kim EO, Sohn EJ,
Kwon BM and Kim SH: Cinnamaldehyde derivative (CB-PIC) sensitizes
chemo-resistant cancer cells to drug-induced apoptosis via
suppression of MDR1 and its upstream STAT3 and AKT signalling. Cell
Physiol Biochem. 35:1821–1830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liao BC, Hsieh CW, Liu YC, Tzeng TT, Sun
YW and Wung BS: Cinnamaldehyde inhibits the tumor necrosis
factor-alpha-induced expression of cell adhesion molecules in
endothelial cells by suppressing NF-kappaB activation: Effects upon
IkappaB and Nrf2. Toxicol Appl Pharmacol. 229:161–171. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Matthews GM, Newbold A and Johnstone RW:
Intrinsic and extrinsic apoptotic pathway signaling as determinants
of histone deacetylase inhibitor antitumor activity. Adv Cancer
Res. 116:165–197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jagtap P and Szabó C: Poly(ADP-ribose)
polymerase and the therapeutic effects of its inhibitors. Nat Rev
Drug Discov. 4:421–440. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Soldani C and Scovassi AI:
Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: An update.
Apoptosis. 7:321–328. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Su CC, Chen JY, Din ZH, Su JH, Yang ZY,
Chen YJ, Wang RY and Wu YJ: 13-Acetoxysarcocrassolide induces
apoptosis on human gastric carcinoma cells through
mitochondria-related apoptotic pathways: p38/JNK activation and
PI3K/AKT suppression. Mar Drugs. 12:5295–5315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Reed JC: Regulation of apoptosis by bcl-2
family proteins and its role in cancer and chemoresistance. Curr
Opin Oncol. 7:541–546. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yin C, Knudson CM, Korsmeyer SJ and Van
Dyke T: Bax suppresses tumorigenesis and stimulates apoptosis in
vivo. Nature. 385:637–640. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yates CM, McGettrick HM, Nash GB and
Rainger GE: Adhesion of tumor cells to matrices and endothelium.
Methods Mol Biol. 1070:57–75. 2014. View Article : Google Scholar
|
|
45
|
Liu SQ, Su YJ, Qin MB, Mao YB, Huang JA
and Tang GD: Sphingosine kinase 1 promotes tumor progression and
confers malignancy phenotypes of colon cancer by regulating the
focal adhesion kinase pathway and adhesion molecules. Int J Oncol.
42:617–626. 2013.
|
|
46
|
Gao XH, Yang XQ, Wang BC, Liu SP and Wang
FB: Overexpression of twist and matrix metalloproteinase-9 with
metastasis and prognosis in gastric cancer. Asian Pac J Cancer
Prev. 14:5055–5060. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Samuels Y and Ericson K: Oncogenic PI3K
and its role in cancer. Curr Opin Oncol. 18:77–82. 2006. View Article : Google Scholar
|
|
48
|
Richardson CJ, Schalm SS and Blenis J:
PI3-kinase and TOR: PIKTORing cell growth. Semin Cell Dev Biol.
15:147–159. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dunlap J, Le C, Shukla A, Patterson J,
Presnell A, Heinrich MC, Corless CL and Troxell ML:
Phosphatidylinositol-3-kinase and AKT1 mutations occur early in
breast carcinoma. Breast Cancer Res Treat. 120:409–418. 2010.
View Article : Google Scholar
|
|
50
|
Paradiso A, Mangia A, Azzariti A and
Tommasi S: Phosphatidylinositol 3-kinase in breast cancer: Where
from here? Clin Cancer Res. 13:5988–5990. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wu Y, Yakar S, Zhao L, Hennighausen L and
LeRoith D: Circulating insulin-like growth factor-I levels regulate
colon cancer growth and metastasis. Cancer Res. 62:1030–1035.
2002.PubMed/NCBI
|
|
52
|
Akagi Y, Liu W, Zebrowski B, Xie K and
Ellis LM: Regulation of vascular endothelial growth factor
expression in human colon cancer by insulin-like growth factor-I.
Cancer Res. 58:4008–4014. 1998.PubMed/NCBI
|