Introduction
Cancer cells in ascites fluid are known to grow
under highly anaerobic conditions. Previous research has shown that
pO2 in animal or patient malignant ascites is very low
(1–3). Established tumor cells in ascites grow
under highly crowded, virtually anoxic conditions (4–6). Under
a hypoxic condition, cancer cells develop an efficient adaptive
metabolic response to ensure their survival and proliferation.
Glutamine and glucose represent the two main carbon sources for
mammalian cells. In hypoxic cancer cells, glucose uptake and
glycolytic activity are upregulated to produce pyruvate, which is
then converted into lactate instead of being oxidized via the
tricarboxylic acid cycle and oxidative phosphorylation (OXP HOS)
(7). More recently, it has been
shown that hypoxic cancer cells also exhibit an elevated glutamine
demand and utilization to support cell proliferation through a
glucose-independent tricarboxylic acid (TCA) cycle pathway
(8–10). Cancer cells in malignant ascites
favor a switch to glucose and glutamine-dependent anaerobic
metabolic pathways, allowing adaptation to this microenvironment
and promoting growth.
Viruses are dependent on the metabolic machinery of
the host cell to supply the energy and molecular building blocks
needed for genome replication, viral protein synthesis and membrane
production. Extensive reprogramming of central carbon metabolism to
induce the glycolytic pathway or glutamine metabolism has been
observed during viral infection to meet virus replication
requirements (11–15). Moreover, various studies have shown
that hypoxia affects the replication of certain viruses (16–19).
These studies demonstrate that the specific host cellular
metabolism may be essential for maximal viral replication.
Vesicular stomatitis virus (VSV) is an oncolytic
virus that replicates rapidly in tumor cell lines. A previous study
showed that VSV can effectively replicate in hypoxic tumor cells
in vitro and in vivo (20). Based on these findings, we asessed
the oncolytic effect of VSV on carcinoma cells in malignant ascites
and explored the impact of cellular metabolism on VSV
replication.
Materials and methods
Vesicular stomatitis virus and cell
lines
Two tumor cell lines were used in the experiments:
hepatocellular carcinoma cell line H22 and retroperitoneal sarcoma
cell line MethA. Both cell lines were obtained from the American
Type Culture Collection (ATCC; Rockville, MD, USA). They were
maintained in RPMI-1640 medium or Dulbecco's modified Eagle's
medium (DMEM) tissue culture medium. Media were supplemented using
10% fetal bovine serum (FBS) (Gemini), 1% glutamine and 1%
antibiotic mixture (Cellgro) unless indicated otherwise. Cells were
grown in a humidified atmosphere containing 5% CO2 at
37°C. These two cell lines have the capacity to grow
intraperitoneally (i.p.) in BALB/c female mice.
∆51M recombinant IFN-inducing mutants of wild-type
VSV were used in the animal study and they showed no toxic and
durable cures in a previous study (21). VSV-∆51 expressing GFP was a
recombinant derivative of VSV-∆51, and kindly provided by Yan-Jun
Wen (Sichuan University). Plaque-forming units (PFU) were used for
calculating infectious titers. Inactivated virus was produced by
exposure to UV light for 45 min. Viruses were propagated in A549
cells (ATCC) and purified as previously described (22).
For viral infection, 24 h after seeding, the medium
containing FBS was removed and replaced by serum-free medium 24 h
before infection at a multiplicity of infection (MOI) of 1.0. One
hour later, the infection medium was removed and replaced by
complete serum-free medium with or without 600 mg/l L-glutamine,
2.5% glucose or 110 mg/l sodium pyruvate as indicated. Culture
media were supernatants and cells were harvested at 0, 12, 24 and
48 h post-infection and processed for metabolites and protein
quantifications. At each time point the cellular proteins were
extracted, and cell supernatants were analyzed.
Plaque assay for VSV
The virus was purified from the supernatants by
passing through a 0.2-µm filter and centrifuging at 30,000 ×
g, and then re-suspension in PBS. After A549 tumor cells were
challenged with VSV at infection of 1.0 PFU/cell for 24 h, the
supernatant was harvested for a progeny virus assay. PFU were used
for calculating infectious titers as previously described (23).
Antibodies and inhibitors
β-actin antibodies were purchased from Sigma
(Milano, Italy). VSV whole-virus antisera was provided by Yan-Jun
Wen (Sichuan University). Sodium oxamate, 2-deoxy-D-glucose (2-DG),
pyruvate, bis-2-(5-phen-ylacetamido-1,3,4-thiadiazol-2-yl)ethyl
sulfide (BPTES) and dimethyl-α-ketoglutarate were purchased from
Sigma. 2-DG and oxamate were directly solubilized in cell culture
medium and BPTES was solubilized in dimethyl sulfoxide (DMSO) to a
stock concentration of 10 mM, used at specified concentrations. In
this research, all of the antibodies were used at the
manufacturer's recommended dilution.
In vitro experiments
After 3 weeks of incubation, ascites cells were
collected, washed with phosphate-buffered saline (PBS) three times,
and used fresh for assays as described previous (24,25).
After washing, the H22 and MethA cells from the ascites were used
for further study immediately or maintained in RPMI-1640 medium.
They were grown in a sealed modular chamber flushed with contained
5% CO2 at 37°C. For in vitro experiments, cells
obtained from ascites were counted to 1×105 cells per
milliliter medium and cultured in 6-well plates, 24-well plates or
10-cm dishes. When the H22 and MethA cells were incubated for 12 h,
they were treated with VSV as previously described (26). In brief, the virus was added to the
culture at an MOI of 1.0 PFU per cell. After VSV incubation for 1 h
at 37°C, the cells were washed once with PBS and 5 ml of RPMI-1640
or DMEM. Following incubation, the cells were maintained for an
additional period of 0–48 h and harvested, washed 3 times with PBS,
scraped into RIPA buffer, centrifuged at 8,000 rpm at 4°C for 10
min and stored at −80°C for further analysis, and the media were
collected to conduct further investigation. At the end of the
incubation period, viable cells in each well were counted using the
trypan blue method and the final results were normalized as
described.
mRNA determinations
Total RNA was isolated by an Isogen RNA extraction
kit (Nippon Gene, Inc., Tokyo, Japan) and reverse-transcribed by
murine leukemia virus reverse transcriptase using a High Pure RNA
Isolation kit (Invitrogen, Sydney, Australia) according to the
manufacturer's protocol. cDNA amplicons were amplified with
specific primers (data not shown). We used the β-actin gene as an
internal control (202 bp; β-actin sense,
5V-CTTCCTGGGCATGGAGTCCT-3V; antisense, 5V-GGAGCAATGATCTTGATCTT-3V).
The amplification was carried out in a Palm Cycler. The initial
cDNA synthesis was performed at 54°C for 30 and 5 min at 94°C for
denaturation. This was followed by 30 rounds of amplification of
PCR consisting of 1 min at 94°C, 1 min at 55°C, 2 min at 72°C with
a Gene Amp PCR System 9600 (Perkin Elmer) and Taq DNA
polymerase (Toyobo, Osaka, Japan). The amplified DNA fragments were
visualized by electrophoresis at 120 V on 2% agarosegel in 1X TAE
buffer containing ethidium bromide. DNA molecular weight marker was
also electrophoresed for comparison.
Glucose, glutamine and pyruvate
quantifications
Metabolites were quantified from cell supernatants
using a Glucose (GO) Assay kit, a Glutamine Determination kit
(GLN1) (Sigma-Aldrich, Saint-Quentin Fallavier, France) and a
pyruvate assay kit (BioVision, Nanterre, France). Assays were
performed according to the manufacturer's instructions. At each
time point the cellular proteins were extracted, and cell
supernatants were analyzed. For each assay, concentrations in the
cell culture medium were normalized with total protein amounts in
the well and are expressed as nmol consumed (compared to time zero)
per µg of protein (nmol/µg).
Western blot analysis
For western blot analysis, protein samples were
subjected to 10% sodium dodecylsulfate-polyacrylamide gel
electrophoresis (PAGE) and transferred to a nitrocellulose
membrane. The membrane was blocked with 5% skim milk in TBS [20 mM
Tris and 137 mM NaCl (pH 7.3)] containing 0.1% Tween-20, and then
the membrane was incubated with the specific antibodies. After
being washed, the membrane was incubated with peroxidase-conjugated
goat anti-mouse immunoglobulin G or anti-rabbit immunoglobulin G.
Kodak Molecular Imaging software was used to quantification the
intensity of the bands (Eastman Kodak Co., Rochester, NY, USA).
Quantitative assay of VEGF in ascites
fluid and plasma
After obtaining the cell-free supernatants of
ascites and plasma of mice, VEGF levels were measured with ELISA
kits (Quantikine; R&D Systems, Minneapolis, MN, USA) as
described by the manufacturer's protocol.
Flow cytometry
Malignant ascites H22 and MethA or cells obtained
from ascites or cultured in a dish were infected with GFP-VSV as
previously described. To evaluate infectivity, GFP fluorescence
intensity was measured by flow cytometry 48 h after incubation;
data were analyzed using FLOWJO software. For analysis of
apoptosis, flow cytometric analysis (FCM) was performed to detect
sub-G1/apoptotic cells in hypotonic buffer as previously described
(24).
TUNEL
Fluorescent in situ terminal deoxynucleotidyl
transferase dUTP nick end labeling assay was used to analyze the
apoptotic cells within malignant ascites or cultured cancer cells
after VSV infection using a commercially available apoptotic cell
detection kit (Promega, Madison, WI, USA). After the cells were
incubated with the VSV for 48 h, the cells were rinsed with PBS 3
times. The samples were counted to 1×106 cells and
subsequently spread on a slide to perform TUNEL assay following the
manufacturer's protocol.
Murine tumor models
BALB/c female mice were used to establish H22 and
MethA cell models, respectively. Before injection, the mice were
housed in autoclaved microisolator cages in specific pathogen-free
conditions and fed autoclaved pellets and sterile water ad
libitum. All animal research was approved by the Animal Care
and Use Committee of Sichuan University and was in compliance with
all regulatory guidelines. The health status of each animal was
monitored daily. Following a week of acclimatization, the mice were
injected i.p. with 10×106 H22 and MethA cells. Malignant
ascites was collected three weeks later, red blood cells were
schizolysed, and ascites fluid free of red blood cells was washed
with PBS three times and centrifuged at 300 × g at 4°C for 5 min.
Each type of tumor cell (1×106) suspended in 100
µl of PBS was then injected i.p. into the mice with a
14-gauge needle.
Treatment and sample collection
After approximately 7 days of cancer cell
incubation, ascites formation was observed. On day 10, 12 and 14,
the mice were treated with 1×108 VSV-∆51 or
1×108 PFU equivalent of UV-inactivated VSV-∆51 by
intra-peritoneal injection. Five mice from each group were left to
develop ascites, and the weight of each mouse was recorded every
other day until day 24 after implantion when the mice were
euthanized. After euthanasia, the ascites fluid was collected, and
the numbers of tumor cells and red blood cells were counted per
milliliter per mouse. Before sacrifice, a blood sample was
collected through the ophthalmic artery; ascites fluid was
completely aspirated as previously described (24). The other five mice of each group
were monitored on a daily basis for tumor burden, cachexia and
abdominal distension. Mice were euthanized when their abdominal
circumference reached 9.5 cm or they were becoming moribund. The
survival time of each animal was calculated from the day of the
beginning of the treatment to euthanasia. The ascites volume of
each mouse was calculated as previously described (24). All of the specimens including cells,
cell-free ascites fluid, tumors dissected from the peritoneal
cavity and the plasma were stored in liquid nitrogen for further
analysis. Ascites fluids of the control group were centrifuged and
supernatants were liquidated into 1 ml samples and stored at −80°C
until needed.
Assessment of toxicity
The relevant indices concerning side effect, such as
diarrhea, anorexia, skin ulceration, or cachexia were observed
every day. The heart, liver, lung, spleen and kidney were harvested
and fixed in 4% paraformaldehyde in PBS, then sectioned and stained
with H&E. All of the sections were observed by two different
pathologists.
Statistical analysis
All data are reported as the means ± SE. Comparison
of the different group samples was carried out by analysis of
variance (ANOVA) and an unpaired Student's t-test. The level of
significance was set as P<0.05. We used standard statistical
software SPSS, version 13, for all statistical analyses.
Results
In vivo inhibition of malignant
ascite formation
To evaluate the effect of VSV on malignant ascite
formation, mice bearing i.p. H22 and MethA tumor cells were treated
with VSV 3 times on day 10, 12 and 14 after incubation. Using a
well-established animal model of malignant ascites formation, here
we showed an inhibitory effect of VSV on intraperitoneal tumor
growth, malignant ascites formation. Fig. 1A shows the survival curves of the
mice bearing H22 or MethA tumor cells from the three groups. In
several mice, VSV treatment abolished the malignant ascites
completely; nearly 80% survived the full intended length of study
(40 days). In contrast, all of the mice from the NS and
UV-VSV-treated group died by day 40, before the treatment period
was over. At the end of the treatment period, the mean volume of
ascites in the control groups was 7.44±1.45 and 4.76±0.63 ml per
mouse in the H22 and MethA model, respectively. The mean volume of
ascites in the VSV-treated groups were 1.09±0.45 and 1.06±0.66 ml
per mouse (P<0.05); ascites in several mice were abolished
completely (Fig. 1B). The health
condition of the control mice was poorer than that of the treated
group and exhibited anemia, asthenia or asitia. VSV treatment
significantly reduced the number of floating tumor cells and the
red blood cells (P<0.05) in the peritoneal cavity (Fig. 1C). In the H22 and MethA models only
41.6±4.21×106 and 23.43±3.37×106 tumor cells
and 21.6±4.21×106 and 35.43±3.37×106 red
blood cells per milliliter were found in the mice treated with VSV,
while in the controls and UV-VSV group there were nearly 2-fold
more tumor cells and 8-fold more red blood cells than that in the
VSV-treated group. Therefore, treatment of VSV in the malignant
models reduced the tumor burden and improved the health condition
of the mice. Differences between the VSV and UV-VSV group achieved
statistical significance (P<0.05). These results suggest that
administration of VSV within the peritoneal cavity was sufficient
to suppress malignant ascite formation and prolong survival time.
This higher level of inhibition suggests that VSV is efficient for
the treatment of ascites particularly in the late phase.
VSV decreases the VEGF level in plasma
and ascites
VEGF is the most widely implicated growth factor
involved in the initiation and progression of malignant ascite
formation. We therefore measured VEGF (Fig. 1D) concentrations in plasma and
ascites and found a significant decrease in the concentration
levels in the treated mice. Compared to the values in the control
group, plasma and ascite VEGF levels were significantly reduced. In
the VSV-treated group total ascite VEGF levels decreased from
637.33±118.72 to 133.40±46.45 pg/ml (P<0.05) in the H22 group
and 372.12±6.78l to 104.25±39.44 pg/ml (P<0.05) in the MethA
group, respectively. Furthermore, the plasma VEGF level was
significantly reduced from 257.72±43.26 in the control mice to
34.53±15.68 pg/ml (P<0.05) in the H22 group and from
175.45±26.76 in the control mice to 23.22±13.12 pg/ml (P<0.05)
in the MethA group. Together, these results showed that VSV
effectively blocks cancer and hypoxia-induced elevation of VEGF
levels in these mice. This level of VEGF suppression is correlation
with significant regression of disease within the peritoneal
cavity.
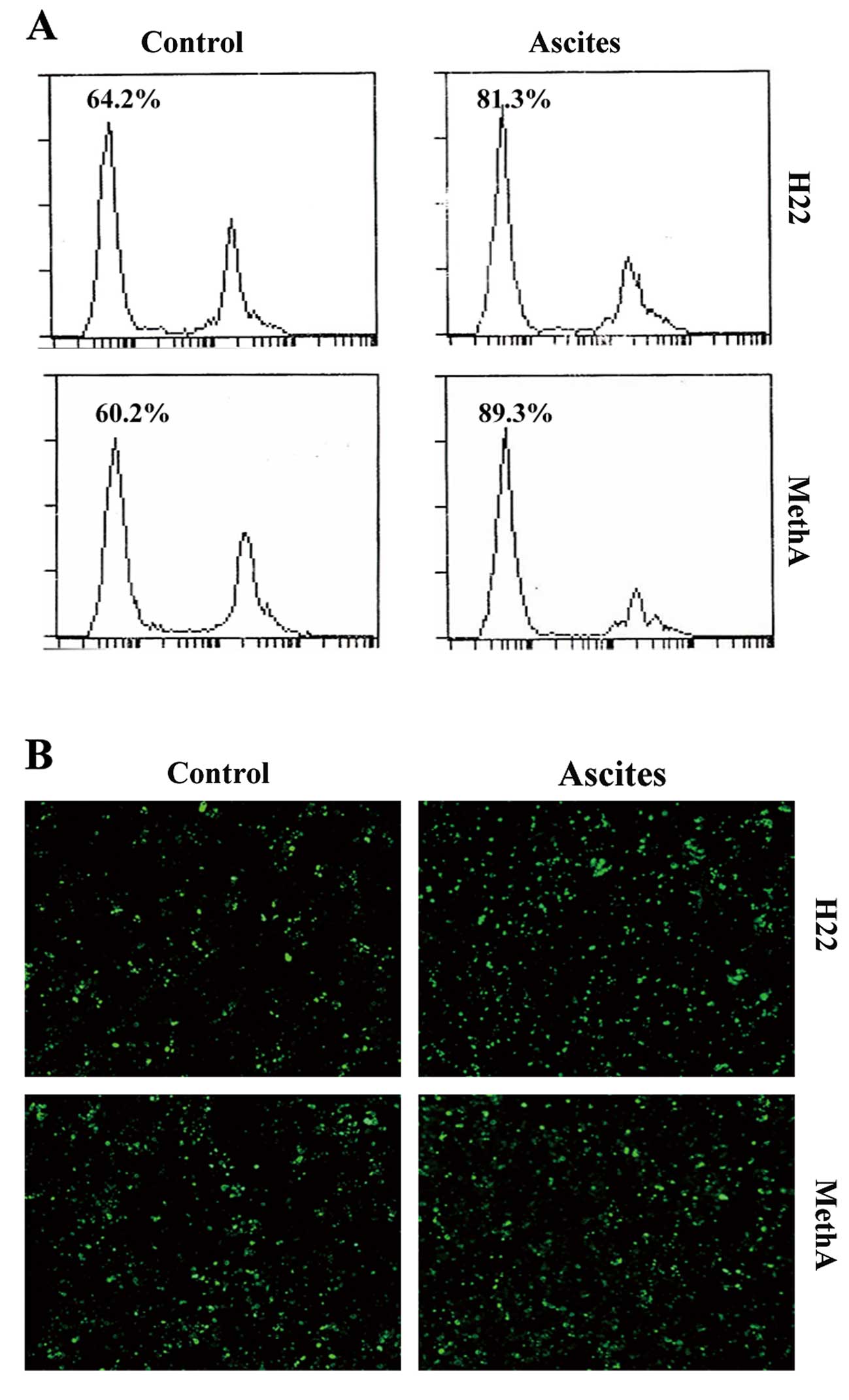
VSV induces the apoptosis of H22 and
MethA cells in malignant ascites
To investigate the apoptotic effect of VSV on the
malignant ascites cells, we treated ascites cells and cultured
cells with VSV. We used flow cytometry to assess the percentage of
sub-G1 phase cells in order to estimate the number of apoptotic
cells. Cells from the ascites exhibited increased apoptosis
compared to the cells cultured in a dish treated with VSV (Fig. 2A). In malignant ascites of late
phase, we showed that VSV induced cell apoptosis by 64.2% in the
cultured H22 cells whereas in ascites H22 cells the rate of
apoptosis was 81.3% after 48 h of infection. Regarding the MethA
cells, the rate of apoptosis was 60.2% in the cultured cells
whereas the rate was 89.3% in the ascites cells. Furthermore, TUNEL
assay was also carried out to detect early DNA fragmentation
associated with apoptosis (Fig.
2B). Although the apoptotic effects of VSV were variable among
the different cells, cells from ascites illustrated increased
apoptosis. Taken together, these results suggest that VSV induces a
high rate of apoptosis in cells from malignant ascites fluid.
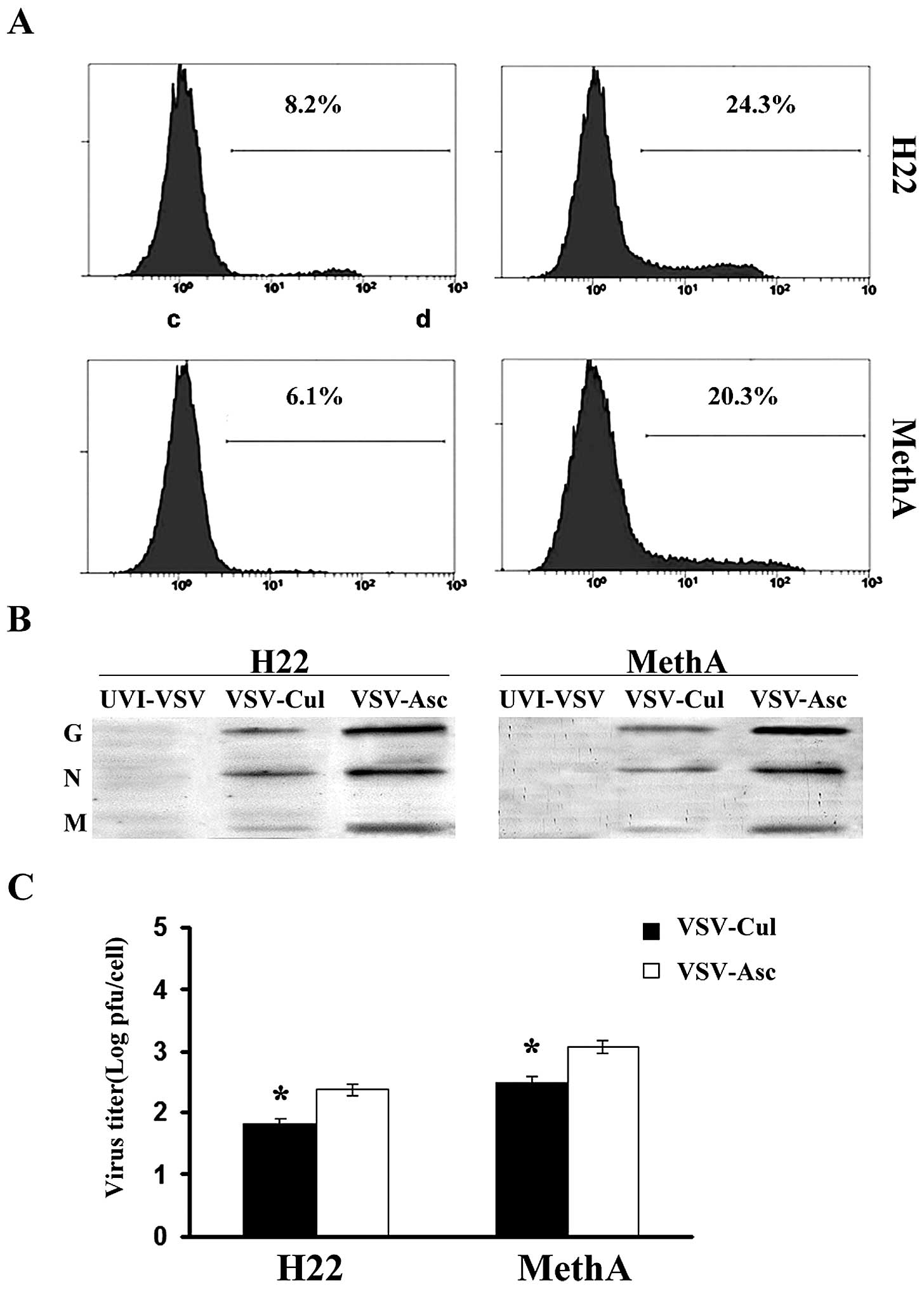
VSV replication is enhanced in the H22
and MethA cells from malignant ascites
To quantitatively analyze the expression of viral
and cellular proteins, we performed viral infection at an MOI of
1.0 in the following experiments. We assessed the effect of VSV
replication in the H22 and MethA cancer cells from the malignant
ascites. H22 and MethA cells were infected with VSV-∆ 51-GFP as
previously described. The expression of VSV-∆ 51-GFP in the H22 and
MethA cells was examined using flow cytometry (Fig. 3A). We observed that VSV replication
in malignant ascites H22 and MethA cancer cells was enhanced.
Immunoblot analysis using anti-VSV serum also revealed higher
levels of expression of VSV proteins in the cells from the ascites
than that in cultured cells at 24 h after VSV infection (Fig. 3B). Twenty-four hours following
infection, the number of VSV-infected cells increased 2- to 4-fold
in the H22 cells from the malignant ascites and 2- to 4-fold in the
MethA cells from the malignant ascites (Fig. 3C) compared to the cultured cells.
These results indicate that the production of viral proteins and
the viral replication were enhanced in cancer cells from the
malignant ascites.
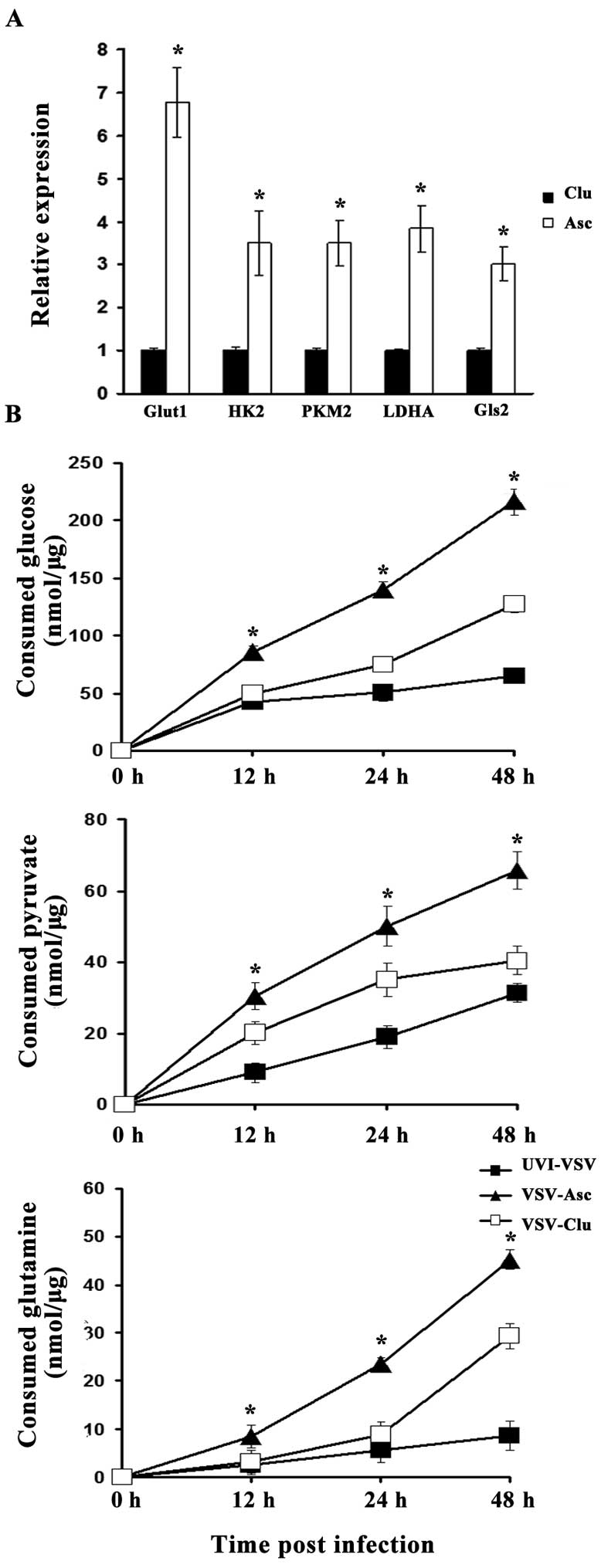
H22 and MethA cells from the malignant
ascites exhibit increased aerobic glycolysis and glutamine
metabolism
Hypoxia promotes the shift from OXP HOS to a
glycolytic mode, leading to increased glucose capture and lactic
acid production by tumor cells (anaerobic 'glycolysis'). Here, we
aimed to gain further insight into the glycolytic activity of
malignant ascites H22 cells and compare this activity to that of
normoxic cultured cells. We first investigated the expression of
genes involved in glycolytic metabolism such as hexokinase 2 (Hk2),
PKM2 and lactate dehydrogenasea (LDHA) as well as glucose
transporters such as glucose transporter 1 (Glut1). We also
determined the expression of glutaminase GLS2, the key enzyme which
catalyzes glutamate production from glutamine in a hypoxic
condition. All glycolytic activity was standardized to that noted
in the nomoxia dish-cultured cells. The ascites H22 cells exhibited
a relatively higher level of expression of all these genes than
levels noted in the control cells cultured under normoxia,
resulting in high glycolytic activity and glutamine metabolism.
Statistical significance was found for GLUT1, PKM2, Hk2, GLS2 and
LDHA (P<0.05). Of note, GLUT1 and LDHA are known to be
responsible for increased glucose uptake and consumption via
anaerobic glycolysis but not oxidative phosphorylation. The result
indicates that cancer cells in malignant ascites are highly
glycolytic and the glucose and glutamine consumption are elevated
(Fig. 4A).
Glycolysis production and glutamine are
consumed for efficient infectious VSV replication
It is known that viruses make use of cell nutrition
for replication. We next assessed the three main carbon sources of
hypoxia cells: glucose, glutamine and pyruvate. We then measured
key metabolite consumption within the supernatants of the ascites
or cultured H22 cells infected with VSV (MOI 1.0) or UVI-VSV at
different times post-infection in the medium with indicated
concentrations of glucose pyruvate lactate or glutamine (i.e., 0,
12, 24 and 48 h). Following infection with VSV, the consumption of
glucose, glutamine and pyruvate was elevated because of virus
replication (Fig. 4B).
Interestingly, the consumption of glucose, pyruvate and glutamine
in the VSV-infected ascites cancer cells was more rapid than that
noted in the VSV-infected cultured cells. Moreover, the gap in
consumption between the VSV infection and UVI-VSV infection in
ascites cancer cells was much more obvious than that in the
cultured cancer cells (data not shown).
Glycolysis is required for optimal
infectious VSV production
Since pyruvate, glucose and glutamine are consumed
for virus production, we hypothesized that glycolysis and glutamine
metabolism are necessary for efficient viral replication. We
investigated the impact of both glucose and glutamine deprivation
on VSV replication. As expected, the replication of the virus was
restrained in medium without glutamine or glucose, as shown in
Fig. 5A, and replication of the
virus was restored when glutamine or glucose was added. Thus, we
next examined VSV replication following treatment with oxamate and
2-DG, a glycolytic inhibitor. Oxamate is a pyruvate analog that can
block the conversion of pyruvate to lactate or acetyl-CoA (27). 2-DG, a glucose analog that inhibits
hexokinase, is the first enzyme in the glycolytic pathway. We first
investigated the effect of 2-DG on glycolysis in the ascites H22
cancer cells. Glucose uptake, ATP production and pyruvate
production were increased (data not shown) in the ascites cancer
cells. VSV-infected ascites H22 cells treated with oxamate or 2DG
exhibited a decrease in the release of extracellular infectious
virus (Fig. 5B). Notably, the
replication of the VSV was restored, when the medium was
supplemented with 4 mM pyruvate. These data indicate that
glycolysis is a critical metabolic pathway for optimal VSV
replication.
Glutamine is involved in VSV replication
in malignant ascites H22 cells
It has been shown that hypoxic cancer cells also use
glutamine as a carbon fuel source for survival (28–30).
Viruses require exogenous glutamine for efficient replication, and
inhibition of glutamine metabolism blocks certain virus protein
synthesis (14). We examined
infectious virus replication following pharmacological inhibition
of glutamine metabolism. VSV-infected cells were treated with BPTES
to inhibit glutaminase, the key enzyme that catalyzes the first
step of glutaminolysis, which converts glutamine to glutamate.
Fig. 5C shows that blockage of
glutaminolysis reduced viral replication. Moreover, virus
production in the BPTES-treated cells was significantly recovered
by α-ketoglutarate supplementation.
Discussion
We examined the effect of VSV on the suppression of
malignant ascites accumulation, survival time and tumor burden in a
H22 and MethA cell malignant ascites model. We found that VSV
profoundly suppressed VEGF secretion and induced the apoptosis of
ascites cancer cells. Furthermore, we found that the replication of
VSV was enhanced in malignant ascites. We showed that cancer cells
in malignant ascites increased the 'glycolytic' switch to produce
pyruvate and elevate usage of glutamine as a carbon fuel source.
The curative effect of VSV on malignant ascites may lie in the
hypoxia-driven metabolic adaptive processes, such as high
glycolysis rate, hexosamine biosynthetic pathway activation, and
glutamine metabolism favoring vesicular stomatitis virus
replication.
Virus replication depends on the metabolic machinery
of the host cell to supply the energy and metabolize nutrients. In
support of our metabolomic data, we showed that when the ascites
H22 cells were infected by VSV, the consumption of glycolysis
production and glutamine increased. In glucose-free media or after
glutamine starvation, VSV replication was markedly increased in the
ascites H22 cells. Interestingly, the blockage of glycolysis or
glutamine metabolism was able to restain VSV replication, while
addition of pyruvate or glutamine metabolite increased the VSV
titer. Although the requirement of increased glycolysis production
and glutamine metabolite for virus replication is clear, how
glycolysis is utilized for viral replication remains to be
determined. It is possible that enhanced glycolysis directly
supports other replication needs, such as the generation of ATP and
NADH.
More recently, it has been shown that hypoxic cancer
cells also use glutamine as a carbon fuel source for survival.
Previous research found that glutamine metabolism is considerably
altered during viral infection and is necessary to anaplerotically
replenish the TCA cycle (14,31),
raising the possibility that glutamine also serves as an
anaplerotic substrate for the TCA cycle during VSV infection.
However, glutamine is consumed in multiple metabolic pathways,
providing the nitrogen for nucleotide biosynthesis. The observed
blockage of virus production under conditions of glutamine
deprivation could also be due to the fact that VSV requires
glutamine as both a carbon source and a nitrogen source to support
the replicative needs. Metabolic carbon and nitrogen flux analysis
is warranted to gain further insight into global glucose and
glutamine usage during VSV infection.
The data presented here demonstrated that inhibition
of the glycolytic pathway via oxamate or 2-DG treatment resulted in
a significant reduction in VSV replication. Our data coincided with
other studies that found that the glycolytic pathway of glucose
utilization is specifically altered during viral infection. Other
laboratories have previously described that many human viruses
activate glycolysis and dysregulate the expression and/or activity
of GLUT1 and Hk2 after infection to facilitate glycolysis (12,32–36).
Moreover, inhibition of the glycolytic pathway has been shown to
restrict HCMV and HSV-1 replication and induce apoptosis in cells
latently infected with KSHV (37–39).
Viral replication may increase the demand of critical metabolite
matters, which are provided by glycolytic pathway and glutaming
metabolism to support their life cycles.
Under a hypoxic condition, a shifting of cellular
metabolism to enhance the anaerobic state was observed. Activation
of the PI3K/Akt pathway, Myc transcription factor and
hypoxia-inducible factor 1 (HIF-1) under a hypoxic condition plays
important metabolic roles in enhancing glycolysis or utilization of
glutamine which upregulates genes involved in glucose uptake
(GLUT1), anaerobic glycolysis (LDH-A, Hk2 and PKM2) and enhances
the expression of glutaminase (GLS). It has been shown in previous
studies that VSV selectively invades tumor cells with p53, Ras and
Myc gene mutations (40,41). Those genes were transcriptional
activate Hk2, pyruvate kinase under hypoxia in a HIF-independent
manner. The requirement of glycolysis and glutamine metabolism in
VSV replication made VSV metabolism targeted to hypoxia cancer
cells. Furthermore, the oncolytic virus has been used in the
treatment of peritoneally planted ascites tumor cells and an
orthotropic model of bladder cancer (21,42,43),
all of which were under a hypoxic environment. Intraperitoneal
administration was well tolerated, and no severe adverse effects
were observed, even using immunocompromised hosts. VSV instillation
therapy in an orthotopic model of bladder cancer showed promising
antitumor activity and safety (43).
VSV is expected to be applicable for cancer therapy
as an oncolytic virus since the viral replication of VSV is
enhanced in cancer cells compared with normal tissues. In the
present study, replication of VSV was enhanced in ascites H22 and
MethA cells. Enhancement of the viral replication in cancer cells
from ascites is considered to be due to upregulation of glycolysis
and glutamine metabolism in the hypoxic condition found in ascites.
Thus, VSV metabolism is targeted to hypoxic cancer cells, implying
that this oncolytic virus exhibits its anticancer ability
efficiently under a hypoxic condition.
Acknowledgments
The present study was supported, in part, by the
Project of the National Natural Science Foundation of China (no.
81071862).
Abbreviations:
|
NS
|
0.9% NaCl solution
|
|
i.p.
|
intraperitoneally
|
|
TUNEL
|
fluorescent in situ terminal
deoxynucleotidyl transferase dUTP nick end labeling
|
|
VSV
|
∆51M recombinant IFN-inducing mutant
of wild-type vesicular stomatatis virus (Indiana strain)
|
|
PFU
|
plaque-forming units
|
|
RBC
|
red blood cell
|
References
|
1
|
Del Monte U: Considerations on factors
influencing the oxygenation of ascites tumours. Eur J Cancer.
5:639–640. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng B, Williams M and Chance B: Effects
of glucose, anoxia, and adriamycin on the chemiluminescence of
Ehrlich Ascites cells. FEBS Lett. 160:169–172. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Inoue M, Mukai M, Hamanaka Y, Tatsuta M,
Hiraoka M and Kizaka-Kondoh S: Targeting hypoxic cancer cells with
a protein prodrug is effective in experimental malignant ascites.
Int J Oncol. 25:713–720. 2004.PubMed/NCBI
|
|
4
|
Kuemmerle A, Decosterd LA, Buclin T,
Liénard D, Stupp R, Chassot PG, Mosimann F and Lejeune F: A phase I
pharma-cokinetic study of hypoxic abdominal stop-flow perfusion
with gemcitabine in patients with advanced pancreatic cancer and
refractory malignant ascites. Cancer Chemother Pharmacol.
63:331–341. 2009. View Article : Google Scholar
|
|
5
|
Li XF, Carlin S, Urano M, Russell J, Ling
CC and O'Donoghue JA: Visualization of hypoxia in microscopic
tumors by immunofluorescent microscopy. Cancer Res. 67:7646–7653.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Driessen A, Landuyt W, Pastorekova S,
Moons J, Goethals L, Haustermans K, Nafteux P, Penninckx F, Geboes
K, Lerut T, et al: Expression of carbonic anhydrase IX (CA IX), a
hypoxia-related protein, rather than vascular-endothelial growth
factor (VEGF), a pro-angiogenic factor, correlates with an
extremely poor prognosis in esophageal and gastric adenocarcinomas.
Ann Surg. 243:334–340. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wise DR, Ward PS, Shay JE, Cross JR,
Gruber JJ, Sachdeva UM, Platt JM, DeMatteo RG, Simon MC and
Thompson CB: Hypoxia promotes isocitrate dehydrogenase-dependent
carboxylation of α-ketoglutarate to citrate to support cell growth
and viability. Proc Natl Acad Sci USA. 108:19611–19616. 2011.
View Article : Google Scholar
|
|
9
|
Metallo CM, Gameiro PA, Bell EL, Mattaini
KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L,
et al: Reductive glutamine metabolism by IDH1 mediates lipogenesis
under hypoxia. Nature. 481:380–384. 2012.
|
|
10
|
Le A, Lane AN, Hamaker M, Bose S, Gouw A,
Barbi J, Tsukamoto T, Rojas CJ, Slusher BS, Zhang H, et al:
Glucose-independent glutamine metabolism via TCA cycling for
proliferation and survival in B cells. Cell Metab. 15:110–121.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fontaine KA, Sanchez EL, Camarda R and
Lagunoff M: Dengue virus induces and requires glycolysis for
optimal replication. J Virol. 89:2358–2366. 2015. View Article : Google Scholar :
|
|
12
|
Munger J, Bajad SU, Coller HA, Shenk T and
Rabinowitz JD: Dynamics of the cellular metabolome during human
cytomegalovirus infection. PLoS Pathog. 2:e1322006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ripoli M, D'Aprile A, Quarato G,
Sarasin-Filipowicz M, Gouttenoire J, Scrima R, Cela O, Boffoli D,
Heim MH, Moradpour D, et al: Hepatitis C virus-linked mitochondrial
dysfunction promotes hypoxia-inducible factor 1 alpha-mediated
glycolytic adaptation. J Virol. 84:647–660. 2010. View Article : Google Scholar :
|
|
14
|
Fontaine KA, Camarda R and Lagunoff M:
Vaccinia virus requires glutamine but not glucose for efficient
replication. J Virol. 88:4366–4374. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao L, Hu ZY, Dong X, Tan Z, Li W, Tang
M, Chen L, Yang L, Tao Y, Jiang Y, et al: Targeting Epstein-Barr
virus oncoprotein LMP1-mediated glycolysis sensitizes
nasopharyngeal carcinoma to radiation therapy. Oncogene.
33:4568–4578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pillet S, Le Guyader N, Hofer T,
NguyenKhac F, Koken M, Aubin JT, Fichelson S, Gassmann M and
Morinet F: Hypoxia enhances human B19 erythrovirus gene expression
in primary erythroid cells. Virology. 327:1–7. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vassilaki N, Kalliampakou KI, Kotta-Loizou
I, Befani C, Liakos P, Simos G, Mentis AF, Kalliaropoulos A, Doumba
PP, Smirlis D, et al: Low oxygen tension enhances hepatitis C virus
replication. J Virol. 87:2935–2948. 2013. View Article : Google Scholar :
|
|
18
|
Aghi MK, Liu TC, Rabkin S and Martuza RL:
Hypoxia enhances the replication of oncolytic herpes simplex virus.
Mol Ther. 17:51–56. 2009. View Article : Google Scholar
|
|
19
|
Haque M, Davis DA, Wang V, Widmer I and
Yarchoan R: Kaposi's sarcoma-associated herpesvirus (human
herpesvirus 8) contains hypoxia response elements: Relevance to
lytic induction by hypoxia. J Virol. 77:6761–6768. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Connor JH, Naczki C, Koumenis C and Lyles
DS: Replication and cytopathic effect of oncolytic vesicular
stomatitis virus in hypoxic tumor cells in vitro and in vivo. J
Virol. 78:8960–8970. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stojdl DF, Lichty BD, tenOever BR,
Paterson JM, Power AT, Knowles S, Marius R, Reynard J, Poliquin L,
Atkins H, et al: VSV strains with defects in their ability to
shutdown innate immunity are potent systemic anti-cancer agents.
Cancer Cell. 4:263–275. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nguyên TL, Abdelbary H, Arguello M,
Breitbach C, Leveille S, Diallo JS, Yasmeen A, Bismar TA, Kirn D,
Falls T, et al: Chemical targeting of the innate antiviral response
by histone deacetylase inhibitors renders refractory cancers
sensitive to viral oncolysis. Proc Natl Acad Sci USA.
105:14981–14986. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cave DR, Hendrickson FM and Huang AS:
Defective interfering virus particles modulate virulence. J Virol.
55:366–373. 1985.PubMed/NCBI
|
|
24
|
Zhou Y, Wen F, Zhang P, Tang R and Li Q:
Matrix protein of vesicular stomatitis virus: A potent inhibitor of
vascular endothelial growth factor and malignant ascites formation.
Cancer Gene Ther. 20:178–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pourgholami MH, YanCai Z, Lu Y, Wang L and
Morris DL: Albendazole: a potent inhibitor of vascular endothelial
growth factor and malignant ascites formation in H22 tumor-bearing
nude mice. Clin Cancer Res. 12:1928–1935. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Q, Wei YQ, Wen YJ, Zhao X, Tian L, Yang
L, Mao YQ, Kan B, Wu Y, Ding ZY, et al: Induction of apoptosis and
tumor regression by vesicular stomatitis virus in the presence of
gemcitabine in lung cancer. Int J Cancer. 112:143–149. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Papaconstantinou J and Colowick SP: The
role of glycolysis in the growth of tumor cells. I Effects of
oxamic acid on the metabolism of Ehrlich ascites tumor cells in
vitro. J Biol Chem. 236:278–284. 1961.PubMed/NCBI
|
|
28
|
Guillaumond F, Leca J, Olivares O, Lavaut
MN, Vidal N, Berthezène P, Dusetti NJ, Loncle C, Calvo E, Turrini
O, et al: Strengthened glycolysis under hypoxia supports tumor
symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma.
Proc Natl Acad Sci USA. 110:3919–3924. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Metallo CM, Gameiro PA, Bell EL, Mattaini
KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L,
et al: Reductive glutamine metabolism by IDH1 mediates lipogenesis
under hypoxia. Nature. 481:380–384. 2012.
|
|
30
|
Scott DA, Richardson AD, Filipp FV,
Knutzen CA, Chiang GG, Ronai ZA, Osterman AL and Smith JW:
Comparative metabolic flux profiling of melanoma cell lines: Beyond
the Warburg effect. J Biol Chem. 286:42626–42634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Delgado T, Carroll PA, Punjabi AS,
Margineantu D, Hockenbery DM and Lagunoff M: Induction of the
Warburg effect by Kaposi's sarcoma herpe svirus is required for the
maintenance of latently infected endothelial cel ls. Proc Natl Acad
Sci USA. 107:10696–10701. 2010. View Article : Google Scholar
|
|
32
|
Vastag L, Koyuncu E, Grady SL, Shenk TE
and Rabinowitz JD: Divergent effects of human cytomegalovirus and
herpes simplex virus-1 on cellular metabolism. PLoS Pathog.
7:e10021242011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Diamond DL, Syder AJ, Jacobs JM, Sorensen
CM, Walters KA, Proll SC, McDermott JE, Gritsenko MA, Zhang Q, Zhao
R, et al: Temporal proteome and lipidome profiles reveal hepatitis
C virus-associated reprogramming of hepatocellular metabolism and
bioenergetics. PLoS Pathog. 6:e10007192010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ramière C, Rodriguez J, Enache LS, Lotteau
V, André P and Diaz O: Activity of hexokinase is increased by its
interaction with hepatitis C virus protein NS5A. J Virol.
88:3246–3254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Loisel-Meyer S, Swainson L, Craveiro M,
Oburoglu L, Mongellaz C, Costa C, Martinez M, Cosset FL, Battini
JL, Herzenberg LA, et al: Glut1-mediated glucose transport
regulates HIV infection. Proc Natl Acad Sci USA. 109:2549–2554.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gonnella R, Santarelli R, Farina A,
Granato M, D'Orazi G, Faggioni A and Cirone M: Kaposi sarcoma
associated herpesvirus (KSHV) induces AKT hyperphosphorylation,
bort-ezomib-resistance and GLUT-1 plasma membrane exposure in THP-1
monocytic cell line. J Exp Clin Cancer Res. 32:792013. View Article : Google Scholar
|
|
37
|
McArdle J, Schafer XL and Munger J:
Inhibition of calmodulin-dependent kinase kinase blocks human
cytomegalovirus-induced glycolytic activation and severely
attenuates production of viral progeny. J Virol. 85:705–714. 2011.
View Article : Google Scholar :
|
|
38
|
Radsak KD and Weder D: Effect of
2-deoxy-D-glucose on cytomegalovirus-induced DNA synthesis in human
fibroblasts. J Gen Virol. 57:33–42. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Courtney RJ, Steiner SM and
Benyesh-Melnick M: Effects of 2-deoxy-D-glucose on herpes simplex
virus replication. Virology. 52:447–455. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Balachandran S, Porosnicu M and Barber GN:
Oncolytic activity of vesicular stomatitis virus is effective
against tumors exhibiting aberrant p53, Ras, or myc function and
involves the induction of apoptosis. J Virol. 75:3474–3479. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Balachandran S, Roberts PC, Kipperman T,
Bhalla KN, Compans RW, Archer DR and Barber GN: Alpha/beta
interferons potentiate virus-induced apoptosis through activation
of the FADD/Caspase-8 death signaling pathway. J Virol.
74:1513–1523. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu HL and Chen J: Oncolytic virus as an
agent for the treatment of malignant ascites. Cancer Biother
Radiopharm. 24:99–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hadaschik BA, Zhang K, So AI, Fazli L, Jia
W, Bell JC, Gleave ME and Rennie PS: Oncolytic vesicular stomatitis
viruses are potent agents for intravesical treatment of high-risk
bladder cancer. Cancer Res. 68:4506–4510. 2008. View Article : Google Scholar : PubMed/NCBI
|