Introduction
Cervical cancer is the second most prevalent cancer
in females worldwide (1). Human
papillomavirus (HPV) infection is a sexually transmitted infection
and is a risk for cervical cancer. However, in addition to HPV
infection, other factors exist that influence the risk of
developing cervical cancer (2,3).
Dysregulated activation of many genes, such as CD44, CD24, CD38,
FRA-1 and SOX9 has been implicated in cervical cancer (4–8).
miRNAs are closely related to the occurrence and regulation of
cervical cancer (9). However, the
etiology of cervical carcinoma remains poorly understood.
Cluster of differentiation (CD) 24 was originally
described as a B lymphocyte marker. CD24 is a heavily glycosylated
cell surface protein that appears to be associated with aggressive
cancers involving invasion and metastasis (10,11).
Huang and Lee found that CD24 overexpression is a predictor of
decreased long-term survival in patients with cervical carcinoma
and that CD24 expression is a potential prognostic biomarker for
cervical carcinomas (12). Sung
et al found that CD24 expression is an independent
prognostic marker in patients with cervical squamous cell
carcinoma, even following adjuvant treatment after surgery. The
results showed that new therapeutic strategies targeting CD24
expression stratified by subgroups may have important clinical
implications (13). Kwon et
al suggested that CD24 expression is a significant independent
prognostic factor for distant metastasis-free survival in patients
with uterine cervical squamous cell carcinoma (14). Although some evidence was reported,
the significant roles of CD24 in cervical cancer development are
still elusive.
In the present study, we examined the expression
levels of CD24 in cervical cancer tissues. At the same time, we
studied the influence of CD24 on the cell proliferation and
apoptosis in a cervical cancer cell line and explored the possible
mechanisms.
Materials and methods
Cell culture
One identified general human cervical cancer cell
line, HeLa, was cultured in RPMI-1640 (HyClone, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS) (Gibco by Life
Technologies™, Grand Island, NY, USA), 100 U/ml penicillin and 100
µg/ml streptomycin (GE Healthcare Life Sciences, Logan, UT,
USA) at 37°C in the presence of 5% CO2.
Patient samples
Sixteen participants were recruited at the Cancer
Hospital of Hunan Province, Central South University (Changsha,
Hunan, China). Consent forms were obtained from individual
patients, and experimental protocols were approved by the
Institutional Review Board of the Cancer Hospital of Hunan
Province. All subjects enrolled in the study were Chinese. All
clinical and biological data were available for the samples
(Table I). Cervical cancer tissue
and corresponding non-tumor normal tissue were collected, and each
biopsy sample was divided into two sections; one was submitted for
routine histological diagnosis, and the remaining section was used
for qPCR, immunohistochemistry (IHC) and western blotting
experiments.
 | Table ICharacteristics of the cervical
cancer patients. |
Table I
Characteristics of the cervical
cancer patients.
| Sample | Age, years | HPV type | Histological
diagnosis | Stagea |
|---|
| 1 | 46 | 16 | Cervical
intermediately differentiated squamous cell cancer | IIa2 |
| 2 | 60 | 16 | Cervical
intermediately differentiated squamous cell cancer | IIa2 |
| 3 | 60 | 16, 53, 58 | Cervical poorly
differentiated squamous cell cancer | IIb |
| 4 | 47 | 16 | Cervical
intermediately differentiated squamous cell cancer | Ib2 |
| 5 | 49 | 18 | Cervical
intermediately differentiated squamous cell cancer | IIa1 |
| 6 | 49 | 6 | Cervical highly
differentiated squamous cell cancer | IIb |
| 7 | 43 | 16 | Cervical
intermediately differentiated squamous cell cancer | Ib1 |
| 8 | 48 | 16 | Cervical
intermediately differentiated squamous cell cancer | Ib2 |
| 9 | 40 | 16, CP8304 | Cervical
intermediately differentiated squamous cell cancer | Ib1 |
| 10 | 46 | 16 | Cervical
intermediately differentiated squamous cell cancer | Ib2 |
| 11 | 39 | 16 | Cervical
intermediately differentiated squamous cell cancer | IIb |
| 12 | 56 | (−) | Cervical poorly
differentiated squamous cell cancer | IIa1 |
| 13 | 50 | 16 | Cervical poorly
differentiated squamous cell cancer | IIa2 |
| 14 | 46 | 16 | Cervical
intermediately differentiated squamous cell cancer | IIb |
| 15 | 36 | 16, 58 | Cervical poorly
differentiated squamous cell cancer | IIa1 |
| 16 | 60 | 59 | Cervical
intermediately differentiated squamous cell cancer | IIb |
Total RNA extraction and quantitative
real-time PCR (qRT-PCR) analysis
Total RNA was extracted from the cervical cancer and
corresponding non-tumor normal tissues using TRIzol reagent and
cDNA synthesis was carried out using the RevertAid First Strand
cDNA Synthesis kit (both from CWBio, Beijing, China) according to
the manufacturer's recommendations. qRT-PCR was carried out with
GoTaq qPCR Master Mix (Promega, Fitchburg, WI, USA). For detection
of CD24 mRNA expression levels, GAPDH was amplified in parallel as
an internal control. The sequences of the primers used for qPCR
were as follows: CD24 forward, 5′-acccacgcagatttattcca-3′ and
reverse, 5′-accacgaagagactggctgt-3′; GAPDH forward,
5′-cgaccactttgtcaagctca-3′ and reverse, 5′-actgagtgtggcagggactc-3′.
The expression of mRNA was assessed by evaluated CT values. The CT
values were normalized with the expression levels of GAPDH and the
relative amount of mRNA specific to each of the target genes was
calculated using the 2−ΔΔCt method (15–19).
qPCR was carried out with the Bio-Rad CFK96™ Real-Time System
(Bio-Rad, Hercules, CA, USA). The data were analyzed by Bio-Rad CFK
Manager software (Bio-Rad). Expression of mRNA was assessed by
evaluated CT values and GAPDH was used as an internal control.
IHC and evaluation of staining
IHC was carried out using the peroxidase
anti-peroxidase technique following a microwave antigen retrieval
procedure. The antibody for CD24 was purchased from Boster
Biotechnology (Wuhan, China). The antibody against CD24
(1:100) was overlaid on cervical cancer and corresponding non-tumor
normal tissue sections and incubated overnight at 4°C. Secondary
antibody incubation (Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA) was performed at room temperature for 30 min. Color
reaction was developed using a 3,3′-diaminobenzidine tetrachloride
(DAB) chromogen solution. All slides were counterstained with
hematoxylin. Positive control slides were included in every
experiment in addition to the internal positive controls. The
specificity of the antibody was determined with matched IgG isotype
antibody as a negative control.
Sections were blindly evaluated by two investigators
in an effort to provide a consensus on staining patterns by light
microscopy (Olympus). CD24 staining was assessed according to the
methods described by Hara and Okayasu (20) with minor modifications. Each case
was rated according to a score that added a scale of intensity of
staining to the area of staining. At least 10 high-power fields
were randomly chosen, and >1,000 cells were counted for each
section. The intensity of staining was graded on the following
scale: 0, no staining; 1+, mild staining; 2+, moderate staining;
and 3+, intense staining. The area of staining was evaluated as
follows: 0, no staining of cells in any microscopic fields; 1+,
<30% of the tissue stained positive; 2+, between 30 and 60% of
the tissue stained positive; 3+, >60% of the tissue stained
positive. The minimum score when summed (extension + intensity)
was, therefore, 0; and the maximum, 6. A combined staining score
(extension + intensity) of ≤2 was considered to be negative
staining (low staining); a score between 3 and 4 was considered to
be moderate staining; whereas a score between 5 and 6 was
considered to be strong staining. An optimal cut-off level was
identified as follows: a staining index score of 0–2 was used to
define tumors with negative expression and 3–7 indicated positive
expression of these two proteins. Agreement between the two
evaluators was 95%, and all scoring discrepancies were resolved
through discussion between the two evaluators.
Construction of the pEGFP-N1-CD24 vector
and cell transfection
The coding region of the CD24 gene was generated by
PCR with the primer pair: 5′-tattatctcgagatgggcagagcaatggt ggc-3′
and 5′-ggcggcgaattcttaagagtagagatgcagaa-3′. PCR was performed under
the following conditions: one cycle for 5 min at 94°C, 30 cycles
for 45 sec at 94°C, 45 sec at 55°C, and 90 sec at 72°C, and ended
with 10 min at 72°C. The fragments were cloned into the TA vector
(Promega) and used to transform E. coli JM109 (Takara,
Dalian, China). Following selection and propagation, the pure
plasmid DNA was prepared by standard methods. The DNA fragments
were removed from the TA vector by restriction enzyme digestion
with XhoI and EcoR1 (Promega) to subclone into the
pEGFP-N1 vector. The fusion sequences were verified by DNA
sequencing using ABI 3730. To establish a stable CD24-expressing
cell line, the plasmid pEGFP-N1/CD24 or control empty vector
pEGFP-N1 was transfected into the HeLa cells, using Lipofectamine
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturers' instructions, followed by G418 selection. The stable
transfectants, HeLa/CD24 and HeLa/vector, were isolated and the
transcription of CD24 protein was determined by qPCR and western
blot experiments.
In vitro CCK-8 assay for cellular
viability
Cell viability was measured with Cell Counting Kit-8
(CCK-8) assay (7Sea Pharmatech Co., Ltd., Shanghai, China). Cells
were prepared in 96-well cell culture plates at a cellular density
of 5×103 cells/well for the HeLa/CD24, HeLa/vector and
HeLa cells at 37°C for 24 h. The cell monolayer was washed three
times with phosphate-buffered saline (PBS) containing 1.2 mM
CaCl2 and 0.7 mM MgCl2. Then a 1:10 diluted
CCK-8 solution in RPMI-1640 was added to the cells and incubated
for 2 h at 37°C. The results were measured by a microplate reader
at 450 nm and are expressed as percentages of the control values.
All experiments were conducted in triplicate.
Colony formation assay
Approximately 500 HeLa/CD24, HeLa/vector and HeLa
cells were plated in a 6-well plate with triplicate repeats for
each cell group to detect the colony formation efficiency (CFE).
When the clone contained >50 cells, we washed the plate with PBS
and fixed the cells in 4% paraformaldehyde at room temperature for
10 min. The cells were washed twice and stained with crystal violet
for 20 min and then the clone number was counted. The CFE was
calculated as: Ratio = (the clone number/the number of planted
cell) × 100%.
Invasion assay
A total of 2×104 HeLa/CD24, HeLa/vector
and HeLa cells were re-suspended in serum-free RPMI-1640 medium and
were seeded into a Matrigel (BD Biosciences, Franklin Lakes, NJ,
USA.)-coated polycarbonate membrane in the upper chambers of a
Transwell apparatus (Corning, Corning, NY, USA). The lower chambers
were loaded with 15% FBS RPMI-1640 medium. Forty-eight hours later,
some of the cells invaded to the lower surface of the upper
chambers. Cells on the top surface of the upper chambers were
removed by a cotton swab. Cells that invaded to the lower surface
were fixed in 10% formaldehyde, and stained with crystal violet.
The cells were observed under a microscope and counted in five
different fields (magnification, ×100). The assay was repeated in
at least three independent experiments.
Effect of CD24 on cervical cancer cell
apoptosis
Cell apoptosis was analyzed by flow cytometric
analysis using a MoFlo™ XDP High-Performance Cell Sorter (Beckman
Coulter, Brea, CA, USA), propidium iodide (PI) and Hoechst 33342
double staining (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China).
Briefly, the HeLa cells (HeLa, HeLa/vector and HeLa/CD24) were
seeded at a density of 1×106 cells/well into 6-well
culture plates. The cells were collected in an Eppendorf tube at 24
h and washed twice with PBS by centrifugation. The supernatants
were discarded. To detect apoptosis, 500 µl PBS, 5 µl
Hoechst 33342 and 5 µl PI were added to each tube, and the
contents of the tube were mixed in the dark at room temperature for
15 min, followed by FCM testing (Beckman Coulter). Data were
acquired and analyzed using Summit v5.2 software (Beckman
Coulter).
Detection of mitochondrial membrane
potential by JC-1
The impact of CD24 was measured by flow cytometry
using the sensitive and relatively mitochondrion-specific
lipophilic cationic probe fluorochrome JC-1. JC-1 accumulates to
form J-aggregates and emits red fluorescence in the mitochondria
with high membrane potential, yet dissociates into monomers and
emits green fluorescence in those that lose cross-membrane
electrochemical gradient. The cells were suspended in 1 ml of warm
staining buffer at ~1×106 cells/ml and were incubated at
37°C for 5 min. Then 1 µl of 2 mM JC-1 (2 µM final
concentration) was added and the cells were incubated at 37°C in 5%
CO2 for 15–30 min. The cells were pelleted by
centrifugation, resuspended by gently flicking the tubes, and 500
µl PBS was added to each tube. Cells were analyzed with a
MoFlo™ XDP High-Performance cell sorter. Data were acquired and
analyzed using Summit v5.2 software.
Intracellular ROS measurement
The production of intracellular reactive oxygen
species (ROS) was measured by performing flow cytometry using the
oxidation-sensitive probe, 2′,7′-dichlorofluorescein diacetate
(DCFH-DA) (Applygen, Beijing, China). Briefly, 10 mM DCFH-DA stock
solution (in methanol) was diluted 4,000-fold in cell culture
medium without serum or other additives to yield a 2.5 mM working
solution. After the exposure of human umbilical endothelial cells
(HUVECs) to silica nanoparticles for 3 and 24 h, respectively, the
cells in 6-well plates were washed twice with PBS and incubated in
2 ml working solution of DCFH-DA at 37°C in the dark for 30 min.
Then the cells were washed twice with cold PBS and resuspended in
the PBS for analysis of intracellular ROS by FACS (Beckman
Coulter).
Intracellular Ca2+
concentration assay
Intracellular Ca2+ concentration was
measured by means of the fluorescent Ca2+ chelator
Fura-2 AM, which permeates into cells where it is cut into Fura-2,
resorting within the cells. Fura-2 combines with intracellular
Ca2+ to form a fluorescent compound, whose fluorescent
intensity is determined at excitation wavelength 340 nm and
emission wavelength 510 nm in FACS. After treatment, the cells were
harvested and rinsed with PBS. The harvested cells were suspended
in PBS and incubated with 5 µM Fura-2 AM for 60 min at 37°C.
During the session of incubation with Fura-2 AM, cell cultures were
mildly shaken at intervals of 10 min aimed to facilitate the
combination of Fura-2 and Ca2+ to form the fluorescent
compound. Then, the cells were washed twice and resuspended in PBS
for FACS measurement. Data were acquired and analyzed using Summit
v5.2 software.
Western blot analysis
The cervical cancer tissues, corresponding non-tumor
normal tissues, and HeLa cells were lysed in RIPA buffer (CWBio),
and the total protein concentration was determined using
Pierce® BCA protein assay kit (Thermo Scientific, Inc.,
Rockford, IL, USA). Extracts containing 50 µg of proteins
were separated on 10% SDS-PAGE gels and electroblotted onto
nitrocellulose membranes (HyClone Laboratories). The membranes were
inhibited using tris-buffered saline/Tween-20 (25 mM Tris-HCl, 150
mM NaCl, pH 7.5 and 0.05% Tween-20) containing 5% non-fat milk
followed by overnight incubation at 4°C with the primary antibodies
[rabbit anti-human p38 antibody (1:200; catalog no. B7178; Anbo
Biotechnology Company, Changzhou, China); rabbit anti-human JNK2
antibody (catalog no. sc-827) and rabbit anti-human c-Jun antibody
(1:500; catalog no. sc-1694; Santa Cruz Biotechnology, Inc.); and
rabbit anti-Fra-1 antibody (1:300; ImmunoWay Biotechnology Co.)].
Following three washes, the membranes were incubated with a
horseradish peroxidase-conjugated secondary antibody (Santa Cruz
Biotechnology, Inc.), and the specific signals were visualized
using an ECL detection system. Anti-GAPDH antibody (1:3,000; Santa
Cruz Biotechnology, Inc.) was used as a loading control.
Statistical analysis
Differences in non-parametric variables were
analyzed by the Mann-Whitney U test. Differences in the
quantitative variables between groups were analyzed by Student's
t-test using SPSS 11.0 program (SPSS, Inc., Chicago, IL, USA). A
value of p<0.05 was considered to indicate a statistically
significant result.
Results
CD24 is highly expressed in cervical
cancer tissues
To detect the mRNA expression levels of the CD24
molecule in the cervical cancer and adjacent non-cancerous tissues,
16 samples of each were selected to perform qPCR of the CD24 gene.
The data were analyzed using the 2−ΔΔCt method and the
fold-change in the expression of these genes relative to the
internal control gene, GAPDH, was analyzed. The expression of the
CD24 gene was higher in the cervical cancer samples compared with
the adjacent non-cancerous tissues, and the normalized CD24 gene
expression in cervical cancer was upregulated by 3.29-fold
(Table II).
 | Table IImRNA expression level of CD24 in the
cervical cancer and adjacent non-cancerous tissues by qPCR. |
Table II
mRNA expression level of CD24 in the
cervical cancer and adjacent non-cancerous tissues by qPCR.
| Gene | Sample | n | CD24 (mean ±
SD) | GAPDH CT (mean ±
SD) | ΔCT (mean ±
SD) | ΔΔCT (mean ±
SD) | Folda |
|---|
| CD24 | Cervical
cancer | 16 | 28.31±1.05 | 18.67±0.64 | 9.64±0.43 | −1.72±0.38 | 3.29 |
| Non-cancerous
tissues | 16 | 29.24±1.12 | 17.88±0.58 | 11.36±0.45 | | (2.53–4.28) |
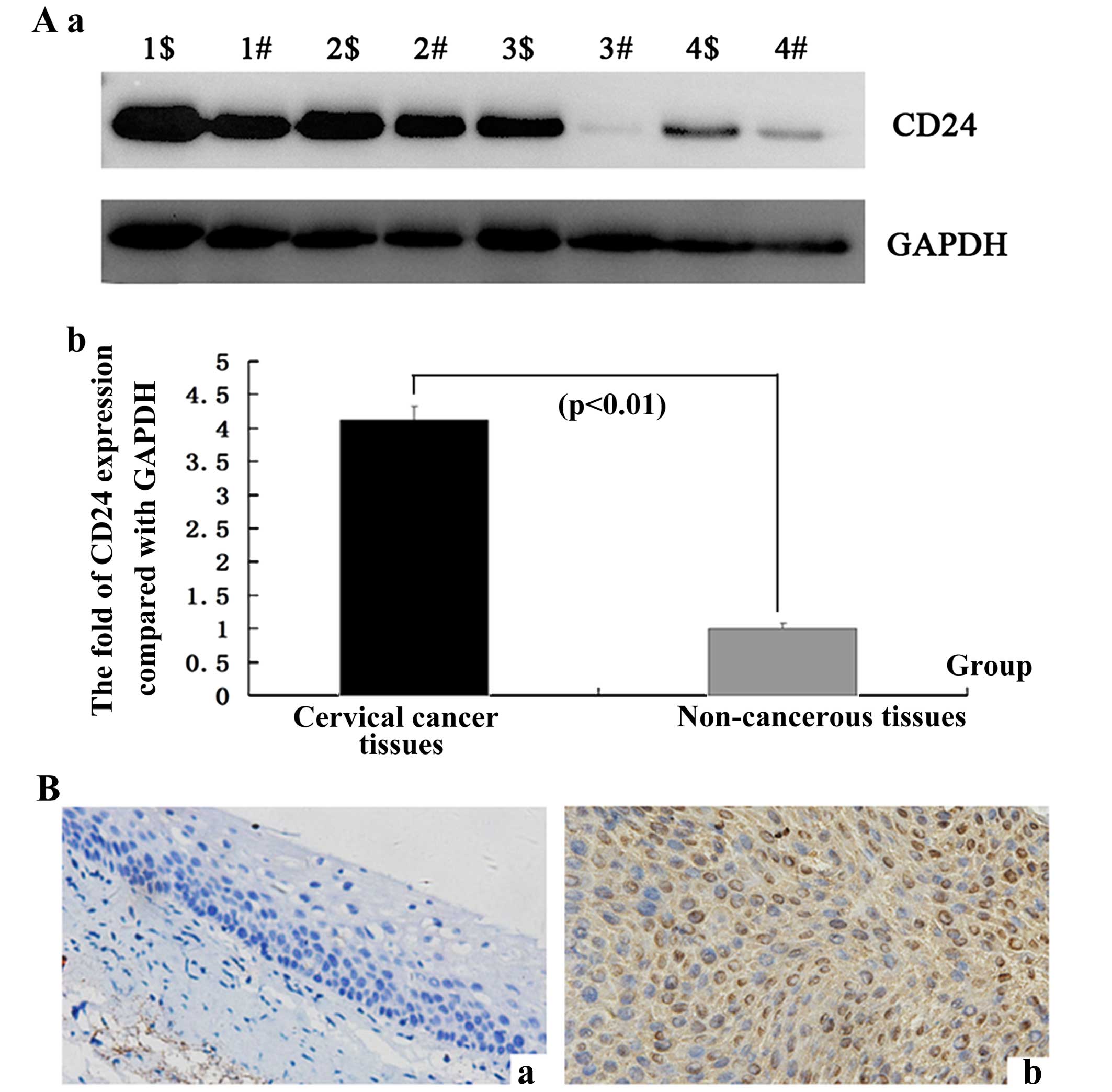
To determine whether the CD24 gene is expressed at a
higher level in cervical cancer compared with the adjacent
non-cancerous tissues, the protein expression levels of CD24 were
further examined by western blotting in 1–4 samples (p<0.01;
Fig. 1A). In comparison with the
adjacent non-cancerous tissues, the expression level was identified
to be greater in the cervical cancer tissues, which corresponded
with the qPCR results.
To confirm the pattern of CD24 in cervical cancer,
IHC was carried out with antibodies against CD24 protein in the
cervical cancer and the adjacent non-cancerous tissues. CD24 was
identified as being differentially expressed between the cervical
cancer tissues and the adjacent non-cancerous tissues. IHC showed a
similar pattern in protein expression with the western blot
results. A high score for CD24 was noted in 56.25% (9/16) of the
cervical cancer tissues and 18.75% (3/16) of the adjacent
non-cancerous tissues. A low score was found in 12.50% (2/16) and
68.75% (11/16) of the cervical cancer and the adjacent
non-cancerous tissues, respectively (p=0.0097<0.01) (Fig. 1B and Table III). This corresponded with the
qPCR results.
 | Table IIIThe difference in CD24 expression
between the cervical cancer and the adjacent non-cancerous
tissues. |
Table III
The difference in CD24 expression
between the cervical cancer and the adjacent non-cancerous
tissues.
| n | Score
| χ2 | P-value |
|---|
| Low (0–2) | Moderate (3–4) | High (5–6) |
|---|
| n (%) | n (%) | n (%) |
|---|
| Cervical
cancer | 16 | 2 (12.50) | 5 (31.25) | 9 (56.25) | 9.26 | 0.0097<0.01 |
| Non cancerous
tissues | 16 | 11 (68.75) | 4 (25.00) | 3 (18.75) | | |
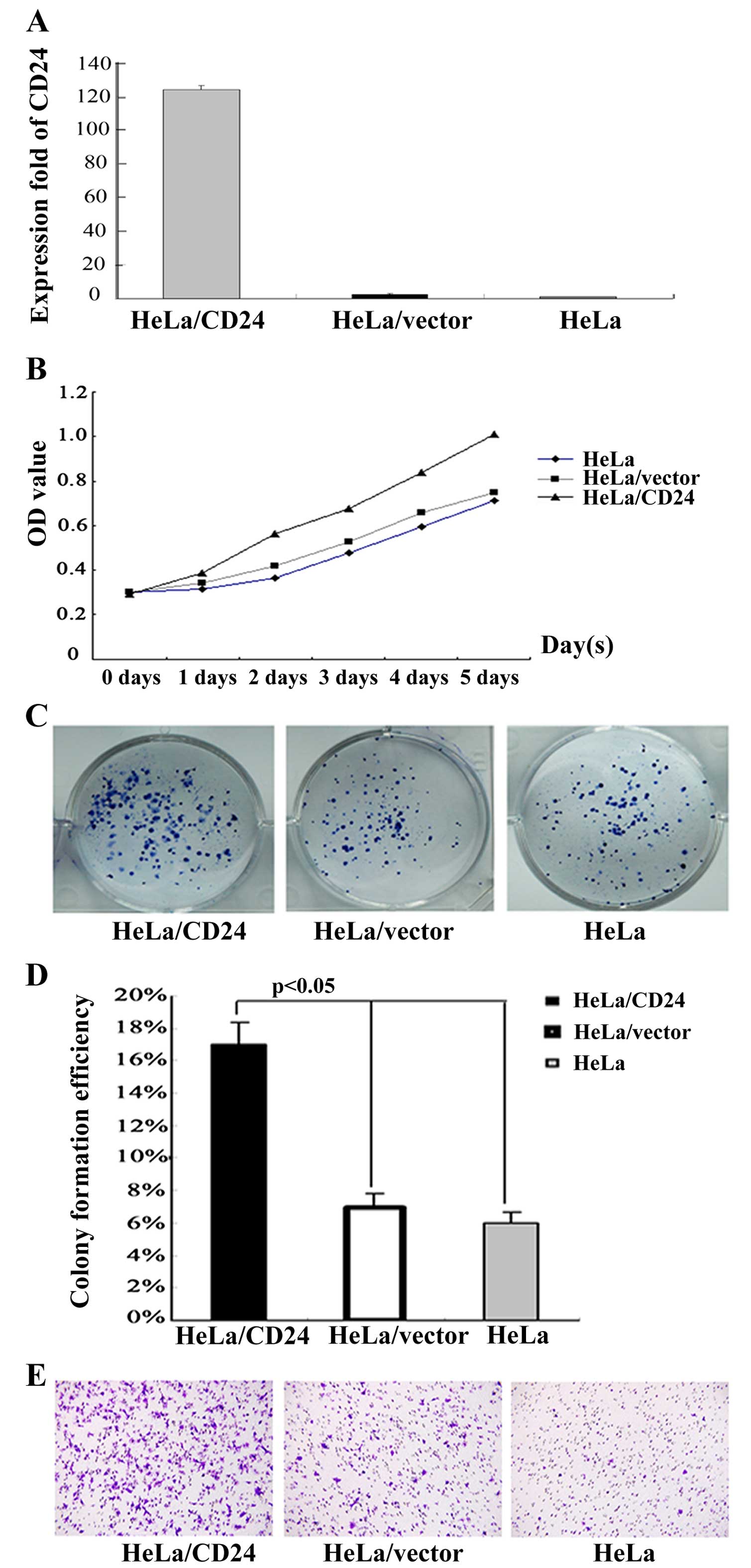
CD24 promotes the growth and invasion of
cervical cancer cells in vitro
To elucidate the function of CD24 in the growth of
cervical cancer cells, the HeLa cells were transfected with the
plasmid pEGFP-N1/CD24 or the control vector to generate CD24-stable
expressing HeLa/CD24 or control HeLa/vector cell lines. After
demonstrating CD24 mRNA transcription by RT-PCR (Fig. 2A), the spontaneous proliferation of
HeLa, HeLa/vector and HeLa/CD24 cells was determined by the CCK-8
assay (Fig. 2B). Clearly, CD24
significantly promoted the proliferation of HeLa cells. Therefore,
endogenous CD24 overexpression promoted the proliferation of HeLa
cells in vitro.
At the same time, HeLa, HeLa/vector and HeLa/CD24
cells were cultured into a 6-well plate and CFE was observed during
12 days of culture. After the 12 days, HeLa/CD24 cells grew well
and most clones had reached >50 cells, but HeLa and HeLa/vector
cells had fewer cells attached to the plates and formed smaller
clones compared to the HeLa/CD24 cells. We counted the number of
clones and the statistical analysis showed significant differences
in CFE among the HeLa, HeLa/vector and HeLa/CD24 cells (p<0.01;
Fig. 2C and D).
Cell invasion is an important step during tumor
metastasis. Thus, we detected the invasion ability of the HeLa,
HeLa/vector and HeLa/CD24 cells by Transwell assay in vitro.
We found that more HeLa/CD24 cells migrated through the
Matrigel-coated polycarbonate membrane (Fig. 2E). The results showed that HeLa/CD24
cells had higher invasive ability compared to the HeLa and
HeLa/vector cells.
CD24 inhibits the apoptosis of cervical
cancer cells in vitro
We found that CD24 was highly expressed in the
cervical cancer tissues by qPCR, western blotting and IHC
technologies. To elucidate the function of CD24 in the apoptosis of
cervical cancer cells, the apoptosis of HeLa, HeLa/vector and
HeLa/CD24 cells was tested. We performed a Hoechst 33342/PI
double-staining experiment to test the rate of apoptosis in the
HeLa, HeLa/vector and HeLa/CD24 cells. A considerable decrease in
the percentage of apoptotic cells was observed for HeLa/CD24
(3.67±0.28%), HeLa/vector (9.41±0.83%) and HeLa cells (10.25±0.92%)
(Fig. 3A).
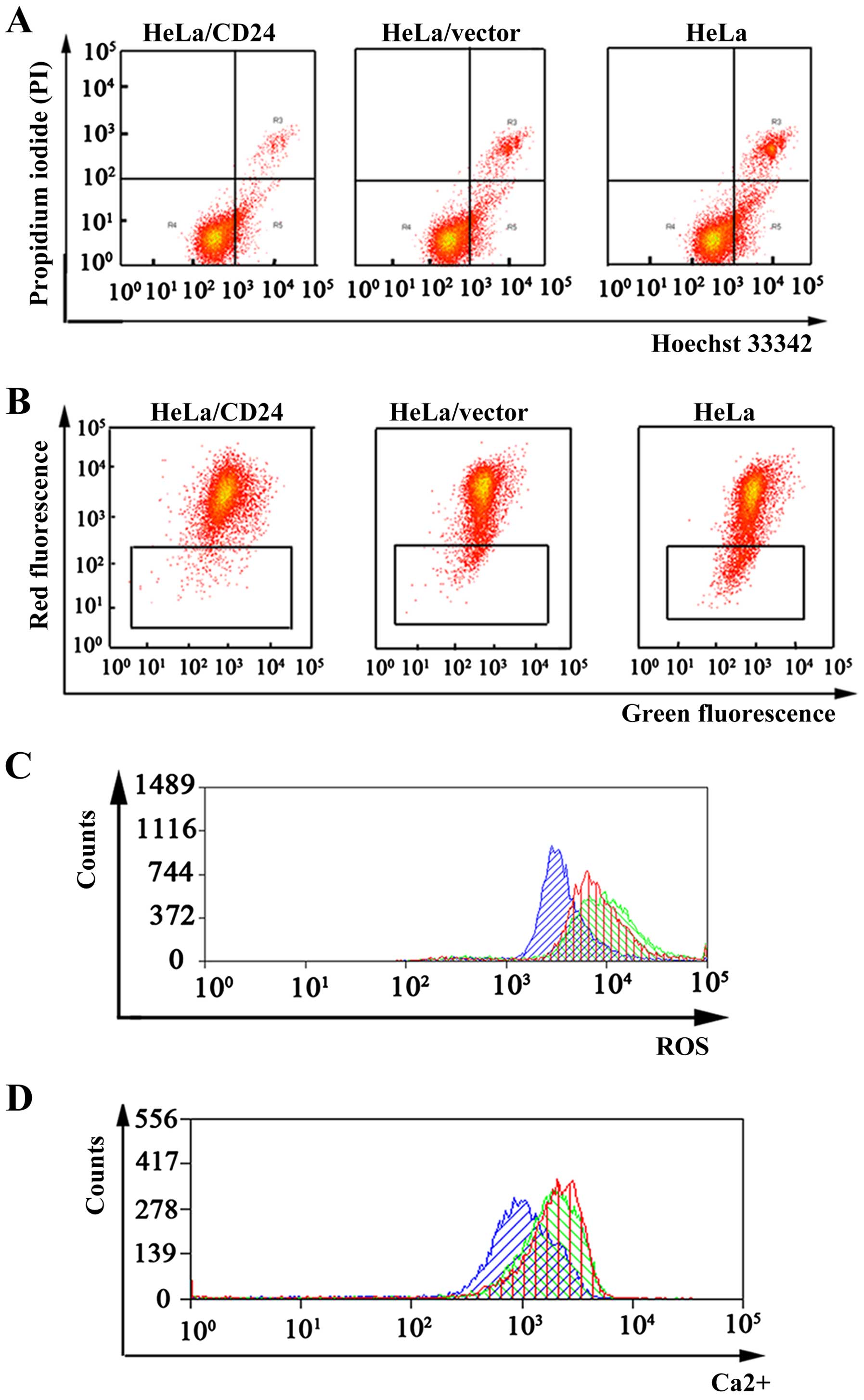 | Figure 3Cell apoptosis, mitochondrial
membrane potential (ΔΨm), intracellular reactive oxygen species
(ROS) and calcium ion (Ca2+) concentrations in the HeLa,
HeLa/vector and HeLa/CD24 cells. (A) Cell apoptosis analysis of
HeLa, HeLa/vector and HeLa/CD24 cells was tested by flow cytometry.
The percentage of cell apoptosis for the HeLa/CD24 cells was 3.54,
3.68 and 3.79% in three independent experiments; the percentage of
cell apoptosis for the HeLa/vector cells was 9.24, 9.47 and 9.53%;
and the percentage of cell apoptosis for the HeLa cells was 9.89,
10.11 and 10.74% (p<0.05). (B) ΔΨm of HeLa, HeLa/vector and
HeLa/CD24 cells was tested by flow cytometry. The percent of
HeLa/CD24 was 1.58, 1.63 and 1.72% in three independent
experiments, the HeLa/vector was 3.48, 3.56 and 3.74%, the HeLa was
7.89, 7.11 and 7.74% (p<0.05). (C) ROS test of HeLa, HeLa/vector
and HeLa/CD24 cells was tested by flow cytometry. Blue indicates
HeLa/CD24, red indicates HeLa/vector and green indicates HeLa
cells. (D) Ca2+ concentrations test of HeLa, HeLa/vector
and HeLa/CD24 cells was tested by flow cytometry. Blue indicates
HeLa/CD24, red indicates HeLa/vector and green indicates HeLa
cells. Data are representative of three independent
experiments. |
CD24 affects the mitochondrial membrane
potential (ΔΨm), ROS and calcium ion (Ca2+)
concentrations of cervical cancer cells in vitro
Cell apoptosis is closely related with a reduction
in ΔΨm and an increase in intracellular ROS and Ca2+
concentrations. Thus, we tested the influence of CD24 on these
three parameters. Our results showed that overexpression of CD24 in
cervical cancer HeLa cells, led to an increase in ΔΨm and
inhibition of cell apoptosis (Fig.
3B). ROS results showed that overexpression of CD24 in the HeLa
cells, led to a decrease in intracellular ROS (Fig. 3C). Ca2+ experiment
results showed that overexpression of CD24 in the HeLa cells, led
to a decrease in Ca2+ concentrations and suppressed the
apoptosis of cervical cancer cells (Fig. 3D).
CD24 is correlated with dysregulation of
the MAPK signaling pathway in cervical cancer tissues
To uncover the possible mechanism of CD24 in
cervical cancer, we tested the expression levels of key molecules
in the MAPK signaling pathway by western blotting. p38, JNK2 and
c-Jun were upregulated in the cervical cancer compared with levels
in the adjacent non-cancerous tissues (Fig. 4A). Since CD24 was highly expressed
in the cervical cancer tissues, we inferred that CD24 was
correlated with dysregulation of the MAPK signaling pathway in
cervical cancer tissues in vitro. A positive correlation may
exist between the expression of CD24 and the MAPK signaling pathway
in cervical cancer.
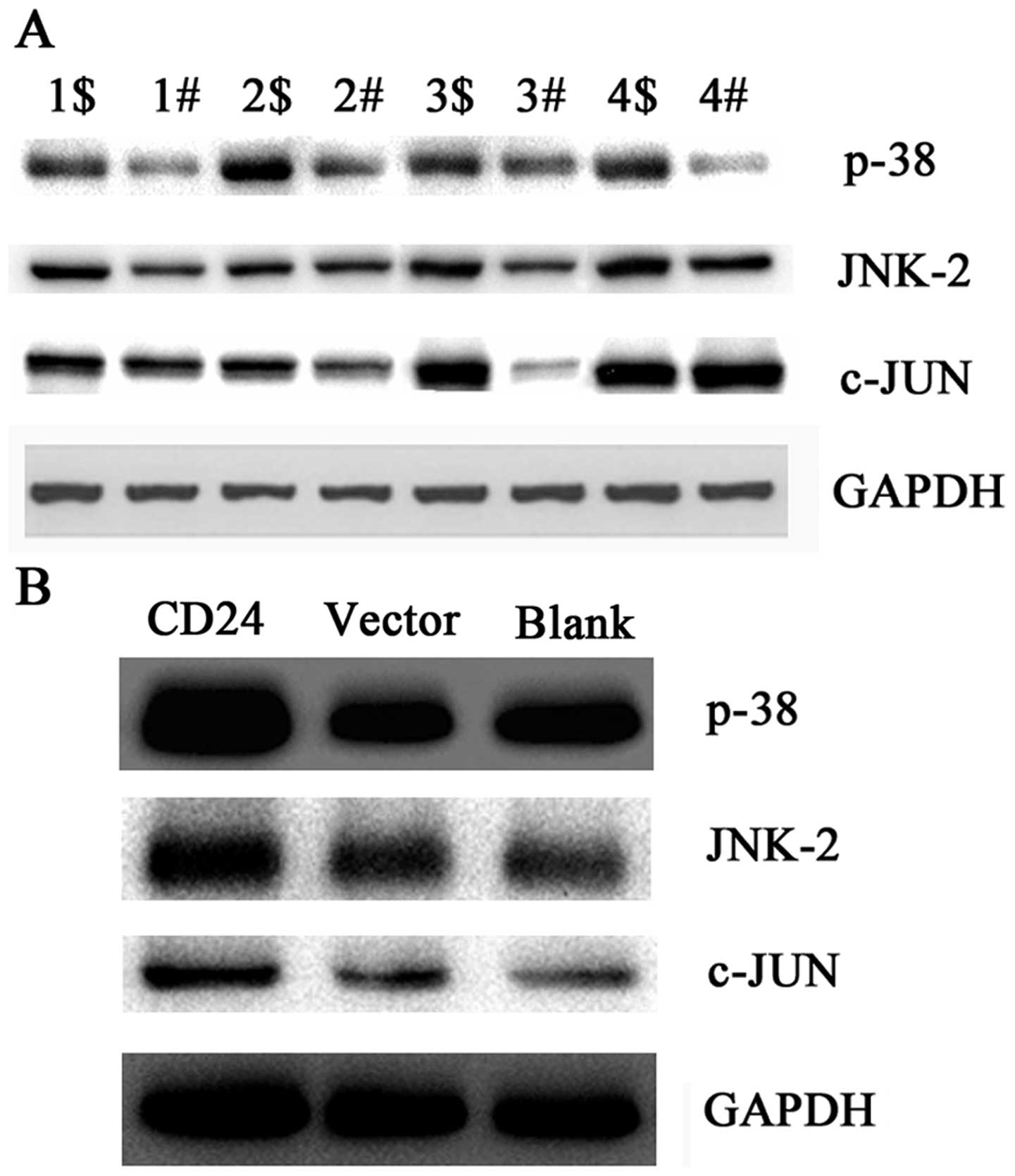 | Figure 4Expression levels of p38, JNK2 and
c-Jun protein in cervical cancer tissues and in HeLa, HeLa/vector
and HeLa/CD24 cells. (A) Cervical cancer and normal adjacent
tissues used in the detection of CD24 mRNA expression levels by
qPCR were selected to detect the expression levels of p38, JNK2 and
c-Jun protein by western blot analysis. 1$–4$, cervical cancer
tissues; 1#–4#, adjacent non-cancerous tissues. Data are
representative of three independent experiments. (B) Expression
levels of p38, JNK2 and c-Jun protein in HeLa, HeLa/vector and
HeLa/CD24 cells. CD24, HeLa cells transfected with pEGFP-N1-CD24.
Vector, HeLa cells transfected with pEGFP-N1. Blank, HeLa cells not
transfected with the plasmid. Data are representative of three
independent experiments. |
CD24 overexpression affects the
expression of p38, JNK2 and c-Jun in vitro
To confirm whether CD24 affects the expression of
p38, JNK2 and c-Jun in vitro, we detected the expression
levels of p38, JNK2 and c-Jun in HeLa, HeLa/vector and HeLa/CD24
cells by western blotting. The results showed that p38, JNK2 and
c-Jun proteins were highly expressed in the HeLa/CD24 cells
compared with levels in the HeLa and HeLa/vector cells (Fig. 4B). These results suggest that CD24
overexpression affects the expression of p38, JNK2 and c-Jun in
vitro.
Discussion
Cervical cancer is the second most common
gynecological malignancy threatening the health of women worldwide
and remains a leading cause of cancer-related deaths in women in
developing countries (1–3,21–23).
One major cause is persistent infection of HPV, leading to abnormal
epithelial lesions, with progression to precancerous and invasive
cancer (24,25). Dysregulated activation of other
genes, such as CD24 and CD44, influences the risk of developing
cervical cancer (4–8). Thus, sensitive and specific biomarkers
for the early detection of cervical cancer are urgently required to
reduce the high morbidity and mortality of this disease.
In the present study, we found that the expression
of the CD24 gene was higher in the cervical cancer samples when
compared with that in the adjacent non-cancerous tissues and the
normalized CD24 gene expression in cervical cancer was upregulated
by 3.29-fold. There was a similar tendency noted by western blot
experiments. IHC showed a similar pattern in protein expression
with qPCR and western blot results. A high score of CD24 was noted
in 56.25% (9/16) of the cervical cancer tissues and 18.75% (3/16)
of the adjacent non-cancerous tissues. Huang and Lee found that
CD24 was overexpressed in invasive cervical carcinoma (12). Kwon et al results showed that
positive staining of CD24 expression was found in 58.9% of the
cases (14). Our results
corresponded with their results.
To uncover the potential mechanism of CD24 in
cervical cancer, we studied the effect of CD24 on the
proliferation, invasion and apoptosis of cervical cancer HeLa
cells. Our results showed that there was a considerable increase in
proliferation and invasion of HeLa cells with CD24 overexpession.
Compared with the HeLa cells, the rate of apoptotic cells was
decreased in the HeLa cells with CD24 overexpession. Overexpression
of CD24 in laryngeal squamous cell carcinoma is associated with
invasiveness, metastatic potential and high tumor proliferation
status (26). Wang et al
found that CD24 was associated with enhanced invasiveness of
gastric carcinogenesis and a poor prognosis (27). Leelawat et al showed that
CD24 expression is linked to the aggressiveness of
cholangiocarcinoma cells and the adverse prognosis of
cholangiocarcinoma patients (28).
CD24 enhanced DNA damage-induced apoptosis by modulating NF-κB
signaling in CD44-expressing breast cancer cells (29). Moreover, CD24 affects the occurrence
and development of malignant tumors by cell proliferation, cell
invasion and cell apoptosis.
Furthermore, to explore the possible mechanism of
CD24 in cervical cancer, we tested the expression levels of key
molecules (p38, JNK2 and c-Jun) in the MAPK signaling pathway.
Compared with the adjacent non-cancerous tissues, p38, JNK2 and
c-Jun were upregulated in the cervical cancer. Thus, a positive
correlation between the expression of CD24 and key molecules of the
MAPK signaling pathway exist in cervical cancer tissues. At the
same time, we studied the effect of the overexpression of CD24 on
p38, JNK2 and c-Jun in vitro. The results showed that
overexpression of CD24 may upregulate the expression of p38, JNK2
and c-Jun in HeLa cells. MAPKs transduce extracellular signals into
a variety of cellular processes, such as cell proliferation,
survival, death and differentiation (30). JNK is a family of protein kinases,
which are activated by stress stimuli and regulate various cellular
processes including proliferation, apoptosis and survival (31). In A549 human non-small cell lung
cancer cells, JNK may be specifically required in vivo for
the maintenance of the tumor-initiating population of tumor cells
rather than for proliferation and survival of the entire cell
population (32). Based on the
comprehensive literature and our results, we infer that CD24 plays
its role by affecting the expression of p38, JNK2 and c-Jun.
In summary, our results suggest that CD24 promoted
the proliferation and inhibited the apoptosis of cervical cancer
cells through the MAPK signaling pathway.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (81272975, 81172302, 81402270
and 81402307), the Key Project of Hunan Provincial Natural Science
Foundation (12JJ2044), the Project of Hunan Provincial Natural
Science Foundation (12JJ3121), the Project of Hunan Provincial
Development and Reform Commission, the Planned Science and
Technology Project of Hunan Province (2010FJ3088, 2012FJ2014 and
2015JC3024), the Planned Project of Department of Health of Hunan
Province (B2015-042), and the Hunan Provincial Innovation
Foundation for Postgraduats.
Abbreviations:
|
CD24
|
CD24 molecule
|
|
HPV
|
human papillomavirus
|
|
MAPK
|
mitogen-activated protein kinase
|
|
ROS
|
reactive oxygen species
|
|
FBS
|
fetal bovine serum
|
|
PI
|
propidium iodide
|
|
CFE
|
colony formation efficiency
|
|
DCFH-DA
|
2′,7′-dichlorofluorescein
diacetate
|
|
IHC
|
immunohistochemistry
|
|
GADPH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
References
|
1
|
Parish SL, Swaine JG, Son E and Luken K:
Determinants of cervical cancer screening among women with
intellectual disabilities: Evidence from medical records. Public
Health Rep. 128:519–526. 2013.PubMed/NCBI
|
|
2
|
Duvlis S, Popovska-Jankovic K, Arsova ZS,
Memeti S, Popeska Z and Plaseska-Karanfilska D: HPV E6/E7 mRNA
versus HPV DNA biomarker in cervical cancer screening of a group of
Macedonian women. J Med Virol. 87:1578–1586. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu Z, Zhu D, Wang W, Li W, Jia W, Zeng X,
Ding W, Yu L, Wang X, Wang L, et al: Genome-wide profiling of HPV
integration in cervical cancer identifies clustered genomic hot
spots and a potential microhomology-mediated integration mechanism.
Nat Genet. 47:158–163. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiao S, Zhou Y, Yi W, Luo G, Jiang B, Tian
Q, Li Y and Xue M: Fra-1 is downregulated in cervical cancer
tissues and promotes cervical cancer cell apoptosis by p53
signaling pathway in vitro. Int J Oncol. 46:1677–1684.
2015.PubMed/NCBI
|
|
5
|
Wobus M, Kuns R, Wolf C, Horn LC, Köhler
U, Sheyn I, Werness BA and Sherman LS: CD44 mediates constitutive
type I receptor signaling in cervical carcinoma cells. Gynecol.
83:227–234. 2001.
|
|
6
|
Liao S, Xiao S, Zhu G, Zheng D, He J, Pei
Z, Li G and Zhou Y: CD38 is highly expressed and affects the
PI3K/Akt signaling pathway in cervical cancer. Oncol Rep.
32:2703–2709. 2014.PubMed/NCBI
|
|
7
|
Wu JH, Liang XA, Wu YM, Li FS and Dai YM:
Identification of DNA methylation of SOX9 in cervical cancer using
methylated-CpG island recovery assay. Oncol Rep. 29:125–132.
2013.
|
|
8
|
Dobo C, Stavale JN, Lima FO, Ribeiro DA,
Arias V, Gomes TS and Oshima CT: HSP27 is commonly expressed in
cervical intraepithelial lesions of Brazilian women. Asian Pac J
Cancer Prev. 14:5007–5010. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Wang Q, Liu H, Shao N, Tan B,
Zhang G, Wang K, Jia Y, Ma W, Wang N, et al: The association of
miR-146a rs2910164 and miR-196a2 rs11614913 polymorphisms with
cancer risk: A meta-analysis of 32 studies. Mutagenesis.
27:779–788. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujikuni N, Yamamoto H, Tanabe K, Naito Y,
Sakamoto N, Tanaka Y, Yanagihara K, Oue N, Yasui W and Ohdan H:
Hypoxia-mediated CD24 expression is correlated with gastric cancer
aggressiveness by promoting cell migration and invasion. Cancer
Sci. 105:1411–1420. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu C, Zheng S, Shen H, Xu K, Chen J, Li
H, Xu Y, Xu A, Chen B, Kaku H, et al: Clinical significance of CD24
as a predictor of bladder cancer recurrence. Oncol Lett. 6:96–100.
2013.PubMed/NCBI
|
|
12
|
Huang LW and Lee CC: Cluster of
differentiation 24 expression is an independent prognostic factor
of adverse outcome in cervical carcinoma. Int J Gynecol Cancer.
23:325–330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sung CO, Park W, Choi YL, Ahn G, Song SY,
Huh SJ, Bae DS, Kim BG and Lee JH: Prognostic significance of CD24
protein expression in patients treated with adjuvant radiotherapy
after radical hysterectomy for cervical squamous cell carcinoma.
Radiother Oncol. 95:359–364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kwon GY, Ha H, Ahn G, Park SY, Huh SJ and
Park W: Role of CD24 protein in predicting metastatic potential of
uterine cervical squamous cell carcinoma in patients treated with
radiotherapy. Int J Radiat Oncol Biol Phys. 69:1150–1156. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods.
25:402–408. 2001. View Article : Google Scholar
|
|
16
|
Zhou Y, Wang W, Zheng D, Peng S, Xiong W,
Ma J, Zeng Z, Wu M, Zhou M, Xiang J, et al: Risk of nasopharyngeal
carcinoma associated with polymorphic lactotransferrin haplotypes.
Med Oncol. 29:1456–1462. 2012. View Article : Google Scholar
|
|
17
|
Zheng D, Liao S, Zhu G, Luo G, Xiao S, He
J, Pei Z, Li G and Zhou Y: CD38 is a putative functional marker for
side population cells in human nasopharyngeal carcinoma cell lines.
Mol Carcinog. Jan 28–2015.Epub ahead of print. View Article : Google Scholar
|
|
18
|
Zhu W, Li J, Su J, Li J, Li J, Deng B, Shi
Q, Zhou Y and Chen X: FOS-like antigen 1 is highly expressed in
human psoriasis tissues and promotes the growth of HaCaT cells in
vitro. Mol Med Rep. 10:2489–2494. 2014.PubMed/NCBI
|
|
19
|
Zheng D, Zhu G, Liao S, Yi W, Luo G, He J,
Pei Z, Li G and Zhou Y: Dysregulation of the PI3K/Akt signaling
pathway affects cell cycle and apoptosis of side population cells
in nasopharyngeal carcinoma. Oncol Lett. 10:182–188.
2015.PubMed/NCBI
|
|
20
|
Hara A and Okayasu I: Cyclooxygenase-2 and
inducible nitric oxide synthase expression in human astrocytic
gliomas: Correlation with angiogenesis and prognostic significance.
Acta Neuropathol. 108:43–48. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun Y, Zhang R, Zhou S and Ji Y:
Overexpression of Notch1 is associated with the progression of
cervical cancer. Oncol Lett. 9:2750–2756. 2015.PubMed/NCBI
|
|
22
|
Han J and Guo J: Current evidence and
potential mechanisms of therapeutic action of PEDF in cervical
cancer treatment. Curr Mol Med. 15:446–455. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ali SF, Ayub S, Manzoor NF, Azim S, Afif
M, Akhtar N, Jafery WA, Tahir I, Farid-Ul-Hasnian S and Uddin N:
Knowledge and awareness about cervical cancer and its prevention
amongst interns and nursing staff in Tertiary Care Hospitals in
Karachi, Pakistan. PLoS One. 5:e110592010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Swangvaree SS, Kongkaew P and Ngamkham J:
Frequency and type-distribution of human papillomavirus from
paraffin-embedded blocks of high grade cervical intraepithelial
neoplasia lesions in Thailand. Asian Pac J Cancer Prev.
14:1023–1026. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Origoni M, Carminati G, Rolla S, Clementi
M, Sideri M, Sandri MT and Candiani M: Human papillomavirus viral
load expressed as relative light units (RLU) correlates with the
presence and grade of preneoplastic lesions of the uterine cervix
in atypical squamous cells of undetermined significance (ASCUS)
cytology. Eur J Clin Microbiol Infect Dis. 31:2401–2406. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi Y, Gong HL, Zhou L, Tian J and Wang Y:
CD24: A novel cancer biomarker in laryngeal squamous cell
carcinoma. ORL J Otorhinolaryngol Relat Spec. 74:78–85. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang YC, Wang JL, Kong X, Sun TT, Chen HY,
Hong J and Fang JY: CD24 mediates gastric carcinogenesis and
promotes gastric cancer progression via STAT3 activation.
Apoptosis. 19:643–656. 2014. View Article : Google Scholar
|
|
28
|
Leelawat K, Keeratichamroen S, Leelawat S
and Tohtong R: CD24 induces the invasion of cholangiocarcinoma
cells by upregulating CXCR4 and increasing the phosphorylation of
ERK1/2. Oncol Lett. 6:1439–1446. 2013.PubMed/NCBI
|
|
29
|
Ju JH, Jang K, Lee KM, Kim M, Kim J, Yi
JY, Noh DY and Shin I: CD24 enhances DNA damage-induced apoptosis
by modulating NF-κB signaling in CD44-expressing breast cancer
cells. Carcinogenesis. 32:1474–1483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang P, Han J and Hui L: MAPK signaling
in inflammation-associated cancer development. Protein Cell.
1:218–226. 2010. View Article : Google Scholar
|
|
31
|
You H, Lei P and Andreadis ST: JNK is a
novel regulator of intercellular adhesion. Tissue Barriers.
1:e268452013. View Article : Google Scholar
|
|
32
|
Okada M, Shibuya K, Sato A, Seino S,
Watanabe E, Suzuki S, Seino M and Kitanaka C: Specific role of JNK
in the maintenance of the tumor-initiating capacity of A549 human
non-small cell lung cancer cells. Oncol Rep. 30:1957–1964.
2013.PubMed/NCBI
|