Introduction
Cancer is one of the most deadly diseases, and
chemotherapy is still the main treatment option (1). Alkylating agents are a class of
anticancer drugs which act by inhibiting the transcription of DNA
into RNA thereby halting protein synthesis. Nitrogen mustard was
the first used DNA alkylating agent, which launched the modern era
of cancer chemotherapy, while the discovery of cisplatin
cis-diamminedichloroplatinum (II) was one of the most
significant events due to its high effectiveness in treating a
variety of cancers (2). However the
side-effects of these agents (toxicity to the bone marrow and
gastrointestinal tract) limit their wide use. Thus, identification
of new anticancer drugs to improve therapeutic effectiveness and
selectivity is currently a 'hot' topic in medicine science. To
minimize the side-effects, different strategies have been proposed,
such as conjugation with an encapsulation of drugs into albumin
nanoparticles (3),
antibody-directed conjugates (4)
and DNA-directed conjugates (5,6) in an
attempt to improve the selectivity of drugs, but few have achieved
successful clinical results.
Many drugs used in the clinic have potent chelating
ability, such as doxorubicin, ciprofloxacin, mercaptopurine and
chloroquine, and have been shown to form metal complexes in
vitro or in vivo (7–9). In
vitro studies have demonstrated that the formed metal complexes
exhibit enhanced (synergism) antitumor activity (10), indicating that their cytotoxicity at
least partially is related to their metal chelating ability.
Moreover, this circumstance was also found in many other metal
complexes, in which the ligands have or do not have biological
activity (8,10,11).
Normally, we use the ambiguous term, ʻsynergismʼ to describe this
phenomenon; however, whether or not the metal complexes exhibit
enhanced activity via a mechanism similar to the ligands has
received less attention. This has hampered our understanding and
identification of various drugs. Therefore, investigation of the
differences in these mechanisms is crucial.
In a previous study, we conjugated DNA alkylating
agent (benzaldehyde nitrogen mustard) with a metal chelator
(pyridinecarboxylic acid hydrazide) to utilize cell cycle
non-specific nitrogen mustard and ROS generation of the chelator
upon capturing iron from a labile-iron pool. Thus, it may enhance
their effectiveness by a multi-target mode of action (12,13).
As predicted, the conjugates indeed had stronger antitumor activity
compared to the chelator or the DNA alkylating agent. Notably, we
found that the copper complexes formed by the conjugates had
excellent antitumor activities, as found in numerous studies
(14–16). Unfortunately, few related studies
have revealed the underlying mechanism (14,17).
In order to extend our previous study, in the present study the
antitumor mechanism of action of benzaldehyde nitrogen mustard
2-pyridine carboxylic acid hydrazone (BNMPH) and its copper complex
against different cell lines was investigated. The results
indicated that both agents shared a similar mechanism of action via
ROS generation, cell cycle arrest, apoptosis and autophagy. Yet,
the difference in ROS formation which occurred for the copper
complex was involved in Fenton-like redox cycling, which may have
contributed to its enhanced antitumor activity compared to
BNMPH.
Materials and methods
Materials
All reactants and solvents were AR grade. MTT,
ethidium bromide (EB), RPMI-1640 medium and agarose were purchased
from Sigma. LC3 antibody was obtained from Proteintech Group
(Wuhan, China); cyclin D1, caspase 8, β-actin, Bax and Bcl-2 were
purchased from Boster (Wuhan, China).
Preparation of benzaldehyde nitrogen
mustard-2-pyridine carboxyl acid hydrazone (BNMPH) and its copper
complex
BNMPH and its copper complex were prepared as
previously described (13). The
synthesized BNMPH was also characterized by NMR. 1HNMR:
11.85(s, H), 8.72(d, H, J=4Hz), 8.53(s, H), 8.14(d, H, J=8 Hz),
8.08(m, H), 7.67(m, H), 7.59(d, 2H, J=8 Hz), 6.87(d, 2H, J=8 Hz),
2.53(d, 2H, J=4Hz).
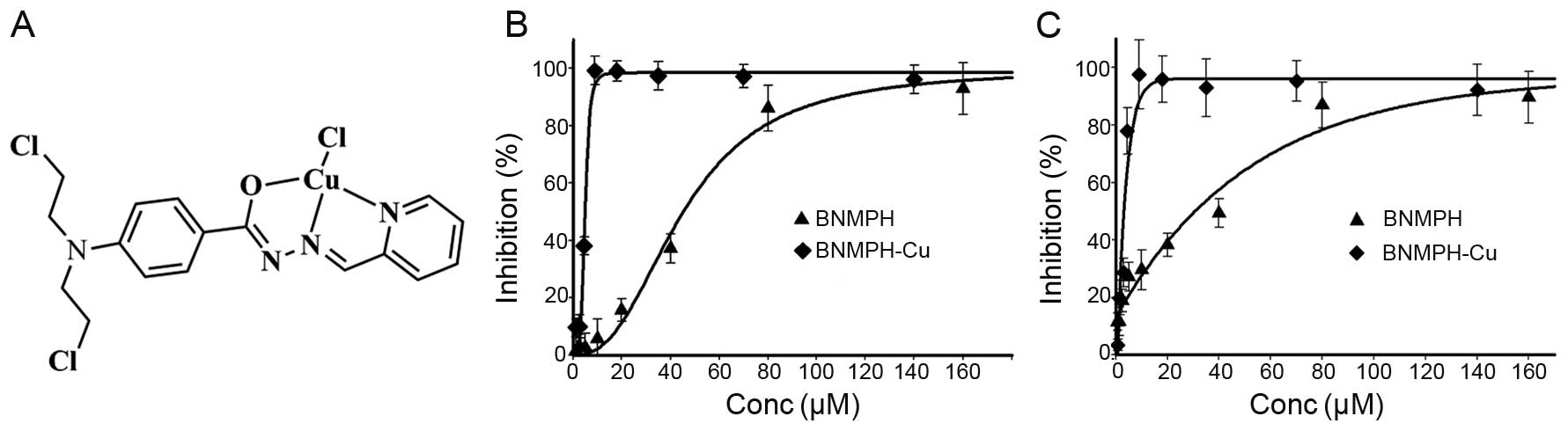
The structure of the BNMPH copper complex based on
previous characterization is shown in Fig. 1A.
Proliferation inhibition of BNMPH and its
copper complex
Stock solutions of the agents were prepared in
dimethyl-sulfoxide (DMSO). Human colorectal carcinoma cell line
(HCT-116) and liver carcinoma cells (HepG2) were cultured in
RPMI-1640 medium supplemented with 10% fetal calf serum (FCS) and
antibiotics. The cells in the exponential growth phase
(2×105/ml) were seeded equivalently into a 96-well plate
and various concentrations of the BNMPH-Cu complex (or BNMPH) were
added after the cells were adherent. Following a 48-h incubation at
37°C in a humidified atmosphere of 5% CO2, 10 µl
MTT solution (5 mg/ml) was added to each well, followed by further
incubation for 4 h. The cell culture was removed by aspiration, and
DMSO (100 µl/well) was added to dissolve the formazan
crystals. The measurement of absorbance of the solution in relation
to the number of live cells was performed on a microplate reader
(MK3; Thermo Scientific) at 570 nm. Percent growth inhibition was
defined as the percentage of absorbance decrease within the
appropriate absorbance in each cell line. The same assay was
performed in triplicate.
ROS detection
H2DCF-AM is converted to
dichlorofluorescein (DCF) according to a study by Jakubowski and
Bartosz (18). Briefly, 0.25 ml of
2 mM H2DCF-AM in absolute ethanol was added to 2.0 ml of
10 mM NaOH and allowed to stand at room temperature for 30 min. The
hydrolysate was then neutralized with 10 ml of 25 mM sodium
phosphate buffer (pH 7.2) and kept on ice for use. Reaction
mixtures contained 0.4 µM DCF in 50 mM sodium phosphate
buffer (pH 7.4) and eventual addition of 25 µl of 1 mM
NH4FeSO4 or 1 mM BNMPH in a total volume of
4.0 ml. After addition of 0.2 ml of 4 mM
H2O2, fluorescence was measured using an
FC-960 spectrofluorimeter (excitation at 488 and emission at 525
nm). The measurements were conducted at room temperature.
Measurement of intracellular ROS
production
The intracellular ROS production was measured
according to the manufacturer's recommendations (Beyotime
Biotechnology, Beijing, China). Approximately 1×106
HepG2 cells were collected and washed with phosphate-buffered
saline (PBS). The cell pellet was resuspended in DCFH-DA containing
serum-free culture medium and incubated for 30 min. The stained
cells were re-collected and washed with serum-free culture medium.
Then, 100 µl of the cells was transfered to PCR tubes and
the test compound was added. Following a 1-h incubation, the cell
suspension was used directly for ROS determination on an FC-960
spectrofluorimeter by excitation at 488 nm and emission at 525
nm.
Comet assay
The comet assay was adapted as previously described
(19). HepG2 cells were treated
with or without the investigated agents (40 and 80 µM for
BNMPH, or 2.5 and 5 µM for the BNMPH-Cu complex) with a 24-h
incubation in a humidified atmosphere of 5% CO2. The
cells were harvested by centrifugation after trypsinization and
then embedded in 0.5% low-melting-point agarose at a final
concentration of 1×104 cells/ml. A 20 µl aliquot
of this cellular suspension was then spread onto duplicate frosted
slides that had previously been covered with 1% normal melting
point agarose as a basal layer. Slides were allowed to solidify for
10 min at 4°C before being placed in lysis buffer for 1 h [2.5 M
NaCl, 0.1 M ethylene diamine tetraacetic acid (EDTA), 0.01 M Tris,
1% Triton X-100, 10% DMSO, pH 10.0]. After lysis, the slides were
transferred into alkaline buffer for 40 min (0.001 M EDTA, 0.3 M
NaOH, pH>13.0) to allow the DNA to unwind before migration at
0.66 V/cm and 300 mA for 30 min. All these steps were performed in
the dark. After neutralization in 0.4 M Tris-HCl pH 7.4, the slides
were stained with EB (20 µg/ml) and covered with a
coverslip. The images were captured using fluorescence
microscopy.
Gene regulation of BNMPH and its copper
complex
Total RNA was extracted from the cells treated with
the investigated agents for 24 h using TRIzol reagent (Sangon,
Shanghai, China) according to the manufacturer's protocol. Two
micrograms of total RNA was used for reverse transcription in a
total volume of 20 µl with the M-MLV reverse transcriptase
system (LifeFeng Biological Technology Corp., Shanghai, China). Two
microliters of cDNA was subsequently amplified in a total volume of
20 µl using the 2X Taq PCR kit (LifeFeng Biological
Technology Corp.) according to the conditions recommended by the
manufacturer. The sense and antisense primers (synthesized by
Shanghai Generay Bioengineering Co. Ltd., Shanghai, China) for
β-actin were 5′-ACACTGTGCCCATCTACGAGG-3′ and
5′-CGGACTCGTCATACTCCTGCT-3′ (615 bp) that were used as an internal
control. The sense and antisense primers for caspase 8 were
5′-AAGTTCCTGAGCCTGGACTACAT-3′ and 5′-ATTTGAGCCCTGCCTGGTGTCT-3′ (227
bp). The sense and antisense primers for bcl-2 were
5′-TTACCAAGCAGCCGAAGA-3′ and 5′-TCCCTCCTTTACATTCACAA-3′ (309 bp).
The sense and antisense primers for bax were 5′-TTTTGCTTCAGGGT
TTCATC-3′ and 5′-GGCCTTGAGCACCAGTTT-3′ (299 bp). The sense and
antisense primers for cyclin D1 were 5′-CTGGATGCTGGAGGTCTGCGAGGA-3′
and 5′-TGAACTTCACATCTGTGGCACAGA-3′ (400 bp), respectively. RT-PCR
was performed on a Nexus Gradient Mastercycler (Eppendorf). The
cycling conditions consisted of 94°C for 5 min, followed by 30
cycles of 94°C for 30 sec, 53–56°C for 30 sec and 72°C for 1 min,
and a final extension of 72°C for 10 min. PCR products were
separated on a 1.5% agarose gel and viewed by DNA green staining.
The data were acquired using Tocan 360 gel imager (version 3.2.1
software).
Western blotting was employed to assess the related
gene expression at the protein level. Briefly, 1×107
HepG2 cells treated with or without the agents were scraped off in
lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1.0% NP-40, 10%
glycerol and protease inhibitors) and subjected to sonication,
following spin down by centrifugation at 14,000 × g. The clear
supernatant was stored at −80°C. The protein concentration was
determined using a colorimetric Bio-Rad DC protein assay on a
microplate reader MK3 at 570 nm. Proteins (30 µg) were
separated on a 13% sodium dodecyl sulfate-polyacrylamide gel at 200
V for 1 h. Then, the separated proteins were subsequently
transferred onto a PVDF membrane at 60 V for 1 h. The membrane was
washed three times with tris-buffered saline (TBS) and was then
blocked for 2 h in TBS containing 0.1% Tween-20 and 5% non-fat
skimmed milk. The membrane was incubated at 4°C overnight with the
primary monoantibody used at a dilution of 1:300 in TBS plus 0.1%
Tween-20 (TBST). The membrane was washed several times with TBST
and was subsequently incubated with HRP-conjugated secondary
antibody (1:2,000 in TBST) for 1 h at room temperature. After
another wash of the membrane with TBST, the protein bands were
detected using a super sensitive ECL solution (Boster Biological
Technology Co. Ltd.), and visualized on a Tocan 360 gel imager.
Cell cycle analysis
HepG2 cells (1×105) were seeded in a
6-well plate and incubated for 24 h at 37°C (5% CO2).
The medium was replaced with fresh medium supplemented or not
(control) with the agents (40 and 80 µM for BNMPH, or 2.5
and 5 µM for the BNMPH-Cu complex). After 24 h of
incubation, the cells were harvested with trypsin, followed by
washing with PBS, fixed in 70% ethanol and stored at −20°C. The
cellular nuclear DNA was stained using propidium iodide (PI).
Briefly, after removing the 70% ethanol, the cells were washed with
PBS and then suspended in 0.5 ml PBS containing 50 µg/ml PI
and 100 µg/ml RNase. The cell suspension was incubated at
37°C for 30 min. DNA flow cytometry was performed in duplicate with
a FACSCalibur flow cytometer (Becton-Dickinson, USA). For each
sample, 10,000 events were collected, and fluorescent signal
intensity was recorded and analyzed by CellQuest and ModiFit
(Becton-Dickinson).
BNMPH and its copper complex induce
autophagy
Cells were seeded into a 24-well flask and treated
as described above for the cell viability assay. The cells were
treated with different concentrations of the agents (20 and 40
µM for BNMPH, or 2.5 and 5 µM for the copper complex)
for 24 h. For detection of the acidic cellular compartment,
acridine orange (or LysoTracker Red; Invitrogen) was used, which
emits bright red fluorescence in acidic vesicles but green
fluorescence in the cytoplasm and nucleus. After treatment of the
cells with the agent, acridine orange was then added at a final
concentration of 1 µg/ml (the concentration of LysoTracker
Red, as recommended) for a period of 15 min. Following PBS washing,
the fluorescent micrographs were captured using an inverted
fluorescence microscope.
Results
Cytotoxicity of BNMPH and its copper
complex
Previous studies have demonstrated that BNMPH
exhibits a proliferation inhibitory effect against several tumor
cell lines (12,13), and its copper complex exhibits much
stronger inhibitory activity (12).
To probe the underlying mechanism, more tumor cell lines were used
to investigate the proliferation inhibition of BNMPH and its copper
complex. The dose-response curves of BNMPH-Cu and BNMPH against
HepG2 and HCT-116 cell lines are depicted in Fig. 1B and C. As shown in Fig. 1B and C, BNMPH exhibited moderate
growth inhibition in the HepG2 (IC50, 31.6±1.7
µM) and HCT-116 cell lines (IC50, 44.1±1.5 μM);
however, the BNMPH-Cu complex exhibited a ~10-fold increase in
proliferation inhibition (IC50 at 3.5±0.8 µM for
HepG2, and 4.5±0.5 µM for HCT-116, respectively). The
significant increase was not fully understood based on a
synergistic effect between the ligand and copper for the copper ion
was not toxic at the investigated concentration, thus it was
important to probe the potent mechanism.
BNMPH and its copper complex induce ROS
generation
The aim of the introduction of a chelator into an
alkylating agent is to enhance ROS production. Thus, it was
necessary to determine whether the BNMPH-metal (iron and copper)
complex was redox active in Fenton-like reaction since the
cytotoxicity of many drugs is correlated with ROS production. As
shown in Fig. 2A, BNMPH-Fe
significantly promoted ROS generation based on the DCF fluorescence
intensity. Similarly the BNMPH-Cu complex exhibited the same
behavior in the Fenton-like reaction except it had weaker ability.
However, the ROS level was significantly increased when the
BNMPH-Cu was reduced by ascorbic acid (Vc) compared to that of the
copper ion, implying that BNMPH-Cu in a reduced environment was
more efficient at ROS generation. To further determine the
correlation between ROS and cytotoxicity in vivo, the HepG2
cells were treated with the agents and stained using DCFH-DA. As
shown in Fig. 2B, both BNMPH and
its copper complex induced ROS formation, indicating that ROS were
involved in the proliferation inhibitory activity of the agents. It
was noted that BNMPH-Cu had a much stronger ability to induce ROS,
while the ability of BNMPH was weaker in vivo. The
difference in ROS generation in vivo may suggest that both
agents used a different mode to generate ROS. The copper complex
may utilize redox cycling, while BNMPH may depend on the
availability of a labile iron pool or another pathway.
BNMPH and its copper complex induce cell
apoptosis
ROS play a crucial role in cell growth and
apoptosis. ROS generation induced by BNMPH and its copper complex
prompted us to investigate the underlying molecular mechanism.
Thus, RT-PCR was conducted to determine the changes in apoptotic
genes in the HepG2 cells following treatment with BNMPH or its
copper complex. As shown in Fig.
3A, the expression levels of caspase 8 and Bax were increased,
while Bcl-2 was not evident at the mRNA level. To further confirm
that the cytotoxic effects of the agents involved apoptosis,
western blotting was used to determine the changes in levels of the
corresponding proteins. As expected, an increase in caspase 8 and
Bax, and a decrease in Bcl-2 were observed in the cells following
exposure to the agents (Fig. 3B),
indicating that they exhibited cytotoxicity via apoptosis and
shared a similar pathway.
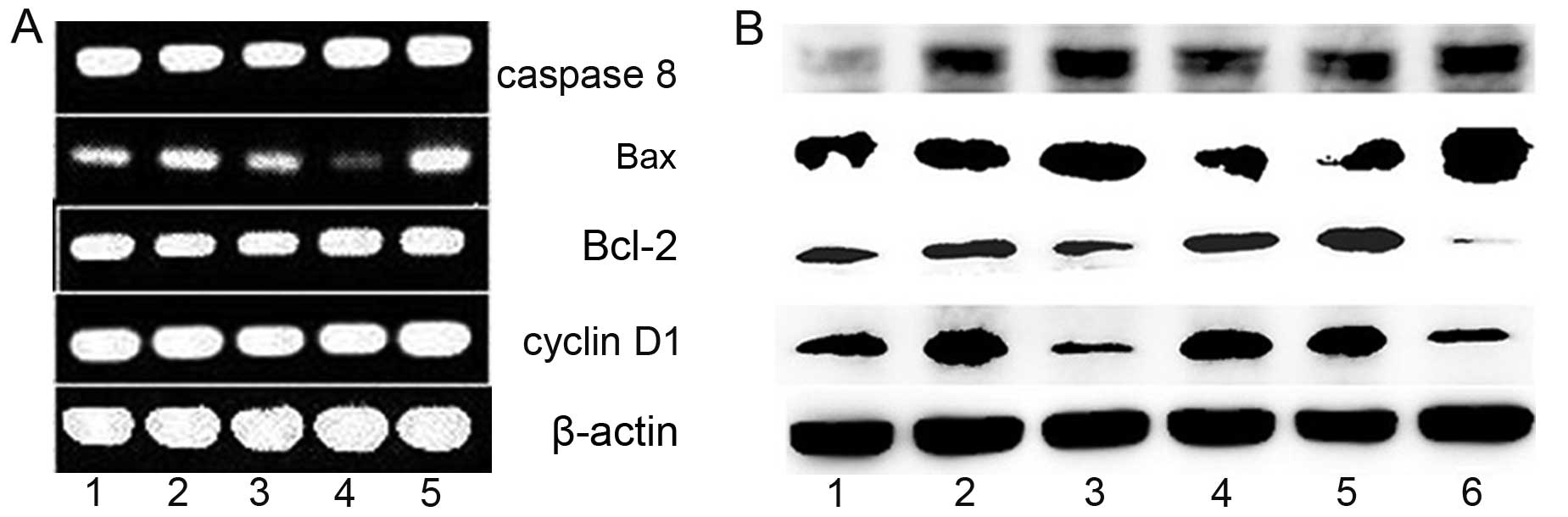 | Figure 3The effect of BNMPH on the regulation
of apoptosis-related genes in HepG1 cells in the absence or
presence of the agents after incubation for 24 h. (A) Gene
regulation at the mRNA level: lane 1, 40 µM BNMPH; lane 2,
20 µM BNMPH; lane 3, 0.75% DMSO; lane 4, 3 µM
BNMPH-Cu; lane 5, 4.5 µM BNMPH-Cu. (B) Gene regulation at
the protein level: lane 1, 0.5% DMSO; lane 2, 12.5 µM BNMPH;
lane 3, 25 µM BNMPH; lane 4, 0.75% DMSO; lane 5, 1.5
µM BNMPH-Cu; lane 6, 3 µM BNMPH-Cu. |
Cellular DNA fragmentation by BNMPH and
its copper complex
It is well documented that ROS cause genetic DNA
breakage of host cells and contribute to cytotoxicity. To further
support the effect of ROS on genetic DNA, the comet assay was
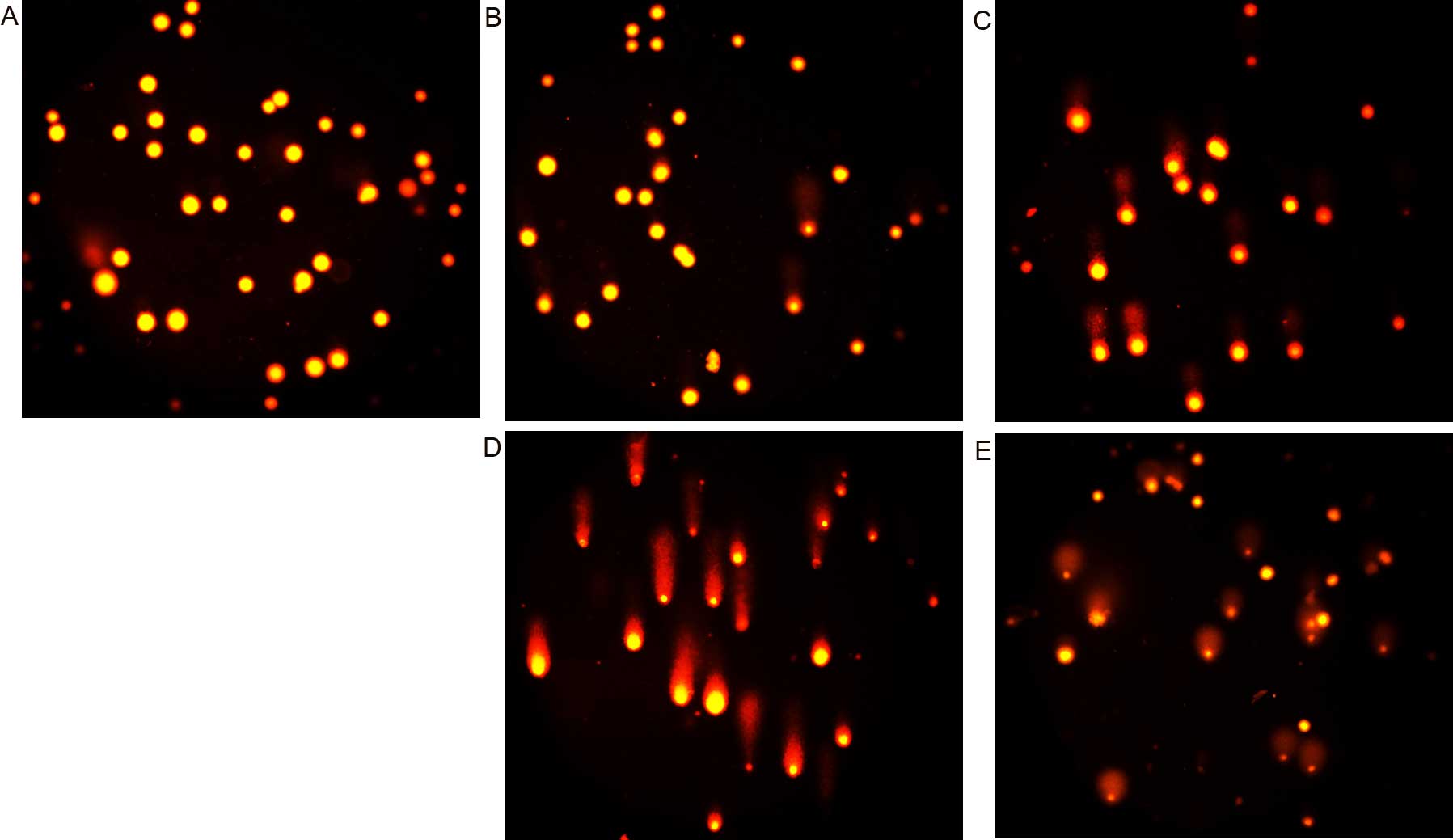
conducted. As shown in Fig. 4, the
comet tails were clearly observed following exposure to BNMPH
(Fig. 4B and C) or its copper
complex (Fig. 4D and E), indicating
that both agents caused cellular DNA fragmentation. However, it was
obvious that the copper complex caused DNA fragmentation more
rapidly as it required a much lower concentration compared to
BNMPH, which was consistent with the result from the ROS generation
in vivo (Fig. 2B).
BNMPH and its copper complex induce cell
cycle arrest at the S phase
It has been reported that ROS induce cell cycle
delay and arrest cell cycle progression at the G1/S boundary
(20). We, therefore, evaluated the
effect of BNMPH and its copper complex on the cell cycle
distribution using PI staining and flow cytometry. As shown in
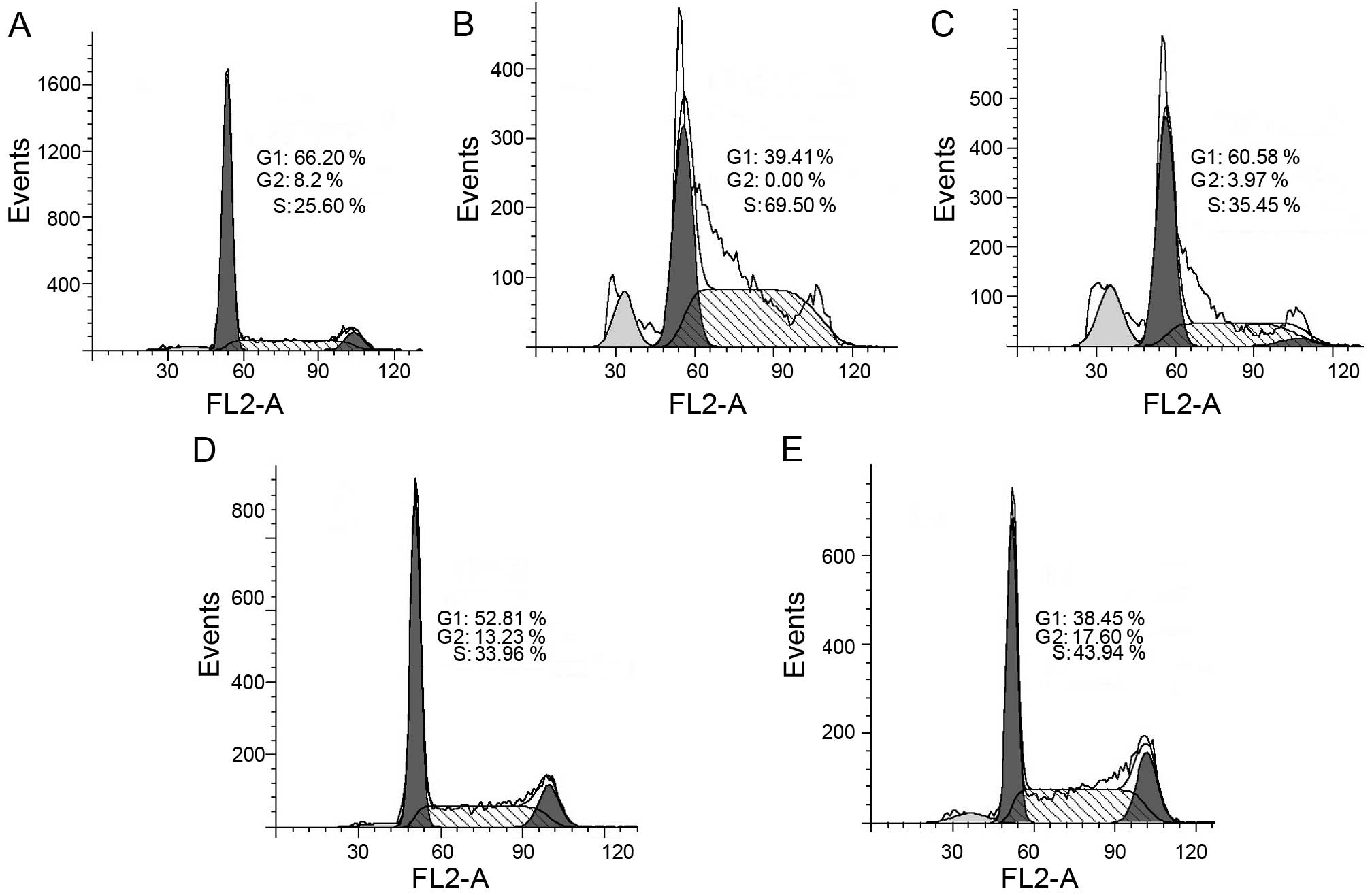
Fig. 5, BNMPH caused an
accumulation of cells in the S phase. The percentage of cells in
the S phase was significantly increased from 25.60% to 69.50 and
35.45% after treatment with 20 and 40 µM BNMPH, respectively
(Fig. 5B and C). Similarly, the
percentage of cells in the S phase was significantly increased from
25.60% to 33.96 and 43.94% after treatment with 1.5 and 3 µM
of the BNMPH-Cu complex (Fig. 5D and
E), indicating that BNMPH and its copper complex exerted a
similar effect on the cell cycle.
Change in lysosomal (autophagosome)
membrane permeability (LMP) in cells following exposure to BNMPH
and its copper complex
It has been demonstrated that caspase 8 plays a
significant role in the engagement of the lysosomal death pathway
and Bax is translocated from the cytosol to the lysosomal membrane,
indicating that these proteins regulate lysosomal membrane
integrity (21). Treatment with
BNMPH and its copper complex resulting in upregulation of caspase 8
and Bax (Fig. 3B) may also affect
LMP and autophagosome membrane permeability. To test this
hypothesis, LysoTracker Red, that accumulates within lysosomes, was
used to assess the LMP (22). As
shown in Fig. 6A–C, the red
fluorescence intensities of the HepG2 cells were significantly
increased after exposure to BNMPH and its copper complex,
indicating that the LMP was altered. Using a similar procedure, the
autophagosomes were stained by acridine orange (Fig. 6D–H). As expected, the red
fluorescence in acidic vacuoles was observed in the HepG2 cells
following treatment with the agents when compared to the control
(Fig. 6D), suggesting that the
agents may induce autophagy. To further determine the involvement
of autophagy, the microtuble-associated protein light chain 3
(LC3), an autophagosome marker, was detected by western blotting.
An increase in cleaved LC3-II and a decrease in LC3-I indicated
that autophagy occurred after exposure of the cells to the agents
(Fig. 6I). In view of the
involvement of apoptosis, autophagy may be an apoptosis-associated
phenomenen. The results demonstrated that BNMPH and its copper
complex had a similar action of mechanism. A difference was noted
only in the concentration applied; the copper complex appeared to
have a stronger effect than BNMPH in regards to the destruction of
LMP.
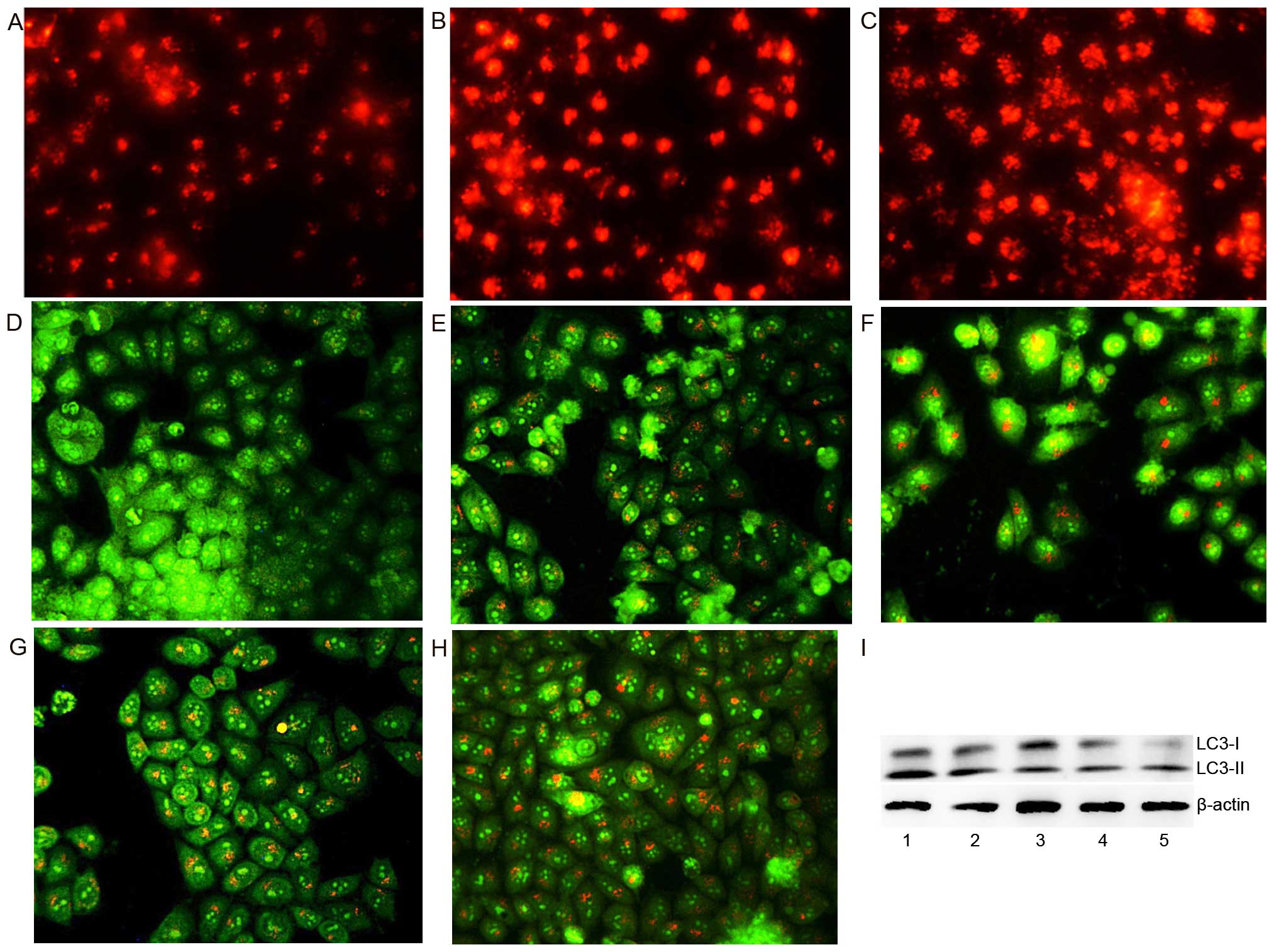 | Figure 6BNMPH and its copper complex induce
autophagy (or changes in LMP) in HepG2 cells. (A) LysoTracker Red
stained HepG2 cells (control), (B) 20 µM BNMPH, (C) 1.5
µM BNMPH-Cu, (D) control, (E) 20 µM BNMPH, (F) 40
µM BNMPH, (G) 1.5 µM BNMPH-Cu, (H) 3 µM
BNMPH-Cu. (I) Immunoblotting of molecular markers of autophagy in
the presence or absence of the investigated agents: lane 1, 20
µM BNMPH; lane 2, 40 µM BNMPH; lane 3, control; lane
4, 1.5 µM BNMPH-Cu; lane 5, 3 µM BNMPH-Cu for 24
h. |
Discussion
Alkylating agents are a class of antineoplastic
drugs. They substitute alkyl groups for hydrogen atoms on DNA,
resulting in the formation of crosslinks within the DNA chain
thereby resulting in cytotoxic, mutagenic and carcinogenic effects.
Caspase-dependent apoptosis, ROS generation and mitochondrial
damage are frequently observed concurrently in cells exposed to
anticancer drug treatment (20).
Copper complexes exhibiting enhanced cytotoxicity when compared to
the ligand alone in cancer cells have been observed in numerous
studies (23,24). The potent mechanisms proposed to
interpret their biological activities include via the lysosome
(25), proteasome (17) or inducing oxidative stress (22,26).
We introduced a chelation group into an alkylating agent, which may
chelate iron (or the copper ion) from a labile iron (copper) pool,
and the iron (copper) chelate may be redox active and participate
in the Fenton-like reaction to generate ROS, thus improving the
cytotoxic effect of the drugs. As expected, BNMPH exhibited
significant antitumor activity, and notably the formation of the
copper complex markedly enhanced its antitumor activity compared to
the ligand (Fig. 1B and C) as shown
in previous research (12).
However, the underlying mechanism has received limited attention.
As an extended study, we evaluated the effect of BNMPH on the
Fenton reaction, a well documented ROS generation system. We found
that both BNMPH-Fe and BNMPH-Cu complexes were redox active,
promoting ROS production in vitro. To support this finding,
we determined whether or not the cytotoxicity of these agents was
related to ROS. An assay in vivo was conducted and showed
that the copper complex was more efficient than BNMPH in ROS
generation (Fig. 2A and B), which
may be correlated with the enhanced cytotoxicity. Thus, we
speculated that the proliferation inhibitory effect of both BNMPH
and its copper complex is closely related to their capacity for ROS
induction except that DNA alkylation and the cytotoxicity of the
BNMPH-Cu complex involved redox cycling, while the weaker activity
of BNMPH may reflect its weaker binding ability of capturing iron
from the labile iron pool. It has been demonstrated that ROS induce
many biological effects including lipid oxidation and DNA
fragmentation, which contribute to cytotoxicity (27). To find the correlation between ROS
and DNA cleavage, a comet assay was also conducted. The results
indicated that BNMPH-induced cellular DNA breakage required a
higher dose, while its copper complex required a much lower dose
(Fig. 4A–E), which was consistent
with the ROS assay in vivo and correlated positively with
their cytotoxicity.
Apoptosis (programmed cell death) is a cascade of
events leading to the upregulation of caspases through intrinsic
and extrinsic pathways. Induction of apoptosis by ROS has been
previously demonstrated (28,29).
In the present study, both BNMPH and its copper complex induced
massive ROS formation which indicated the antitumor activity of the
agents may be via the apoptosis pathway. Therefore, the expression
levels of apoptosis-related genes were evaluated. As expected,
expression levels of caspase 8 and Bax were upregulated, while the
expression level of Bcl-2 was downregulated in a dose-dependent
manner (Fig. 3A and B), as
demonstrated previously in many studies (26,29),
supporting that ROS mediate caspase activation and apoptosis
(30). The cell cycle checkpoint is
an important target for various drugs, and ROS-induced cell cycle
arrest has been previously demonstrated (31,32),
BNMPH and its copper complex may have similar action. Flow
cytometry was used to investigate their effect on the cell cycle of
HepG2 cells. Both agents induced cell cycle arrest in the S phase
(Fig. 5A–E), indicating they
exhibited a similar action on the cell cycle. To understand the
above phenomenon, the gene expression of cyclin D1, an S phase cell
cycle gate keeper protein was evaluated, and downregulation of
cyclin D1 was observed (Fig. 3B).
It has been demonstrated that cyclin D1 must be suppressed in favor
of entry to the S phase for it has the capacity to inhibit DNA
synthesis by virtue of its ability to bind the critical regulator
of DNA synthesis, PCNA (33). Both
BNMPH and its copper complex downregulated cyclin D1 expression in
a dose-dependent manner, indicating that they share a similar
mechanism by which to disturb cell progression. Although the
decrease of cyclin D1 favored entry of the cell to the S phase, the
delay during the DNA synthesis may have another reason. It is well
known that DNA alkylating agents, in principle, can alkylate DNA
via interstrand or intrastrand crosslinking, leading to DNA
lesions. BNMPH (or its copper complex) tethers an aromatic mustard
that alkylates cellular DNA. The ICL can lead to a stalled
replication fork in the S phase (34), and likely activate the cell cycle
checkpoint and then arrest cells at the late S to G2 (35) to repair the DNA damage (36,37).
Masta et al demonstrated that nitrogen mustard can inhibit
transcription and translation in a cell-free system (38). The inhibition by BNMPH and its
copper complex as determine by PCR in our study was also observed,
indicating that they may inhibit DNA polymerase (data not shown).
Those factors could affect the cell process and induce S phase
arrest.
Autophagy is an evolutionarily conserved catabolic
process (also called type II programmed cell death), that functions
in degrading damaged proteins and/or organelles and recycling the
materials to maintain the quality of cellular components (39). One characteristic of autophagy is
the formation of acidic vesicular organelles (AVOs) (40). ROS-triggered autophagy has been
widely investigated (41). We
speculated that the proliferation inhibition of BNMPH or its copper
complex may involve autophagy. This was preliminarily confirmed by
evaluation of the AVOs in HepG2 cells after treatment with the
agents using acridine orange staining. Granular red fluorescence in
AVOs was formed in autophagosomes (Fig.
6D–H). Further supportive evidence that autophagy was induced
was carried out by the immunofluorescence detection of LC3, a
marker of autophagy. A decrease in LC3-I and an increase in LC3-II
were clearly observed (Fig. 6I).
Once autophagosomes are formed, subsequently, they fuse with
lysosomes to form autolysosomes in which the incorporated
organelles are degraded (42). The
lysosome formation after exposure of the agents determined by
LysoTracker Red (Fig. 6A–C) was
correlated with autophagosome formation and both had a similar
trend, supporting the notion that the agents induced autophagy.
However, whether or not the induced autophagy was due to the
cytotoxicity of the agents or a host cellular response needs to be
determined, which may require subsequent study.
In conclusion, BNMPH and its copper complex
suppressed the growth of HepG2 and HCT-116 cell lines. The possible
molecular mechanisms involved were caspase-dependent apoptosis,
cell cycle arrest and autophagy, which are related to their ability
to generate ROS. The excellent antitumor activity of BNMPH-Cu
stemmed from its ability for redox-cycling to generate massive ROS.
The introduction of copper into BNMPH (chelation) did not change
the mechanism of action.
Acknowledgments
The present study was supported by grants awarded by
the Natural Science Foundation of China (no. 21571153), the Henan
Science and Technology Agency (nos. 114300510012 and 152300410118),
Xinxiang Medical University (nos. 505026 and YJSCX20447Y), and the
Plan of Health Scientific and Technological Innovation Talents of
Henan Province (no. 2109901) to S.L..
References
|
1
|
Chen Y and Hu L: Design of anticancer
prodrugs for reductive activation. Med Res Rev. 29:29–64. 2009.
View Article : Google Scholar
|
|
2
|
Marzano C, Pellei M, Tisato F and Santini
C: Copper complexes as anticancer agents. Anticancer Agents Med
Chem. 9:185–211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kratz F: Albumin as a drug carrier: Design
of prodrugs, drug conjugates and nanoparticles. J Control Release.
132:171–183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Firer MA and Gellerman G: Targeted drug
delivery for cancer therapy: The other side of antibodies. J
Hematol Oncol. 5:702012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Finlay GJ, Wilson WR and Baguley BC:
Chemoprotection by 9-aminoacridine derivatives against the
cytotoxicity of topoisomerase II-directed drugs. Eur J Cancer Clin
Oncol. 25:1695–1701. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marvania B, Lee PC, Chaniyara R, Dong H,
Suman S, Kakadiya R, Chou TC, Lee TC, Shah A and Su TL: Design,
synthesis and antitumor evaluation of phenyl N-mustard-quinazoline
conjugates. Bioorg Med Chem. 19:1987–1998. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abraham SA, Edwards K, Karlsson G,
MacIntosh S, Mayer LD, McKenzie C and Bally MB: Formation of
transition metal-doxorubicin complexes inside liposomes. Biochim
Biophys Acta Biomembr. 1565:41–54. 2002. View Article : Google Scholar
|
|
8
|
Cuin A, Massabni AC, Pereira GA, Leite CQ,
Pavan FR, Sesti-Costa R, Heinrich TA and Costa-Neto CM:
6-Mercaptopurine complexes with silver and gold ions:
Anti-tuberculosis and anti-cancer activities. Biomed Pharmacother.
65:334–338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Navarro M, Castro W, González S, Abad MJ
and Taylor P: Synthesis and anticancer activity of
gold(I)-chloroquine complexes. J Mex Chem Soc. 57:220–229.
2013.
|
|
10
|
Martínez A, Suárez J, Shand T, Magliozzo
RS and Sánchez-Delgado RA: Interactions of arene-Ru(II)-chloroquine
complexes of known antimalarial and antitumor activity with human
serum albumin (HSA) and transferrin. J Inorg Biochem. 105:39–45.
2011. View Article : Google Scholar
|
|
11
|
Li W, Jiang GB, Yao JH, Wang XZ, Wang J,
Han BJ, Xie YY, Lin GJ, Huang HL and Liu YJ: Ruthenium(II)
complexes: DNA-binding, cytotoxicity, apoptosis, cellular
localization, cell cycle arrest, reactive oxygen species,
mitochondrial membrane potential and western blot analysis. J
Photochem Photobiol B. 140:94–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li CZ, Wang LF, Meng XQ and Zhao H:
Synthesis, characterization and antitumor activity of benzaldehyde
nitrogen mustard picolinoyl hydrazone complexes. Transit Metab
Chem. 24:206–209. 1999. View Article : Google Scholar
|
|
13
|
Fu Y, Zhou SF, Liu YX, Yang YL, Sun XZ and
Li CZ: The cytotoxicity of benzaldehyde nitrogen mustard-2-pyridine
carboxylic acid hydrazone being, involved in topoisomerase IIα
inhibition. Biomed Res Int. 2004:5270422014.
|
|
14
|
Tan M, Zhu J, Pan Y, Chen Z, Liang H, Liu
H and Wang H: Synthesis, cytotoxic activity, and DNA binding
properties of copper (II) complexes with hesperetin, naringenin,
and apigenin. Bioinorg Chem Appl. 347872:3478722009.
|
|
15
|
Xu DF, Shen ZH, Shi Y, He Q and Xia QC:
Synthesis, characterization, crystal structure, and biological
activity of the copper complex. Russ J Coord Chem. 36:458–462.
2010. View Article : Google Scholar
|
|
16
|
Ruan BF, Liang YK, Liu WD, Wu JY and Tian
YP: Synthesis, characterization, and antitumor activities of two
copper(II) complexes with pyrazole derivatives. J Coord Chem.
65:2127–2134. 2012. View Article : Google Scholar
|
|
17
|
Hindo SS, Frezza M, Tomco D, Heeg MJ,
Hryhorczuk L, McGarvey BR, Dou QP and Verani CN: Metals in
anticancer therapy: Copper(II) complexes as inhibitors of the 20S
proteasome. Eur J Med Chem. 44:4353–4361. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jakubowski W and Bartosz G:
2,7-Dichlorofluorescin oxidation and reactive oxygen species: What
does it measure? Cell Biol Int. 24:757–760. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singh NP, McCoy MT, Tice RR and Schneider
EL: A simple technique for quantitation of low levels of DNA damage
in individual cells. Exp Cell Res. 175:184–191. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brodská B and Holoubek A: Generation of
reactive oxygen species during apoptosis induced by DNA-damaging
agents and/or histone deacetylase inhibitors. Oxid Med Cell Longev.
2011:2535292011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Johansson AC, Appelqvist H, Nilsson C,
Kågedal K, Roberg K and Ollinger K: Regulation of
apoptosis-associated lysosomal membrane permeabilization.
Apoptosis. 15:527–540. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rehman SU, Zubair H, Sarwar T, Husain MA,
Ishqi HM, Nehar S and Tabish M: Redox cycling of Cu(II) by
6-mercaptopurine leads to ROS generation and DNA breakage: Possible
mechanism of anticancer activity. Tumour Biol. 36:1237–1244. 2015.
View Article : Google Scholar
|
|
23
|
Yang Y, Huang T, Zhou S, Fu Y, Liu Y, Yuan
Y, Zhang Q, Li S and Li C: Antitumor activity of a
2-pyridinecarboxaldehyde 2-pyridinecarboxylic acid hydrazone copper
complex and the related mechanism. Oncol Rep. 34:1311–1318.
2015.PubMed/NCBI
|
|
24
|
Fu Y, Yang Y, Zhou S, Liu Y, Yuan Y, Li S,
Li C and Li CZ: Ciprofloxacin containing Mannich base and its
copper complex induce antitumor activity via different mechanism of
action. Int J Oncol. 45:2092–2100. 2014.PubMed/NCBI
|
|
25
|
Lovejoy DB, Jansson PJ, Brunk UT, Wong J,
Ponka P and Richardson DR: Antitumor activity of metal-chelating
compound Dp44mT is mediated by formation of a redox-active copper
complex that accumulates in lysosomes. Cancer Res. 71:5871–5880.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fatfat M, Merhi RA, Rahal O, Stoyanovsky
DA, Zaki A, Haidar H, Kagan VE, Gali-Muhtasib H and Machaca K:
Copper chelation selectively kills colon cancer cells through redox
cycling and generation of reactive oxygen species. BMC Cancer.
14:5272014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fussell KC, Udasin RG, Gray JP, Mishin V,
Smith PJ, Heck DE and Laskin JD: Redox cycling and increased oxygen
utilization contribute to diquat-induced oxidative stress and
cytotoxicity in Chinese hamster ovary cells overexpressing
NADPH-cytochrome P450 reductase. Free Radic Biol Med. 50:874–882.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guerriero JL, Ditsworth D, Catanzaro JM,
Sabino G, Furie MB, Kew RR, Crawford HC and Zong WX: DNA alkylating
therapy induces tumor regression through an HMGB1-mediated
activation of innate immunity. J Immunol. 186:3517–3526. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moungjaroen J, Nimmannit U, Callery PS,
Wang L, Azad N, Lipipun V, Chanvorachote P and Rojanasakul Y:
Reactive oxygen species mediate caspase activation and apoptosis
induced by lipoic acid in human lung epithelial cancer cells
through Bcl-2 down-regulation. J Pharmacol Exp Ther. 319:1062–1069.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chatterjee S, Kundu S, Sengupta S and
Bhattacharyya A: Divergence to apoptosis from ROS induced cell
cycle arrest: Effect of cadmium. Mutat Res. 663:22–31. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kumar R, Dwivedi PD, Dhawan A, Das M and
Ansari KM: Citrinin-generated reactive oxygen species cause cell
cycle arrest leading to apoptosis via the intrinsic mitochondrial
pathway in mouse skin. Toxicol Sci. 122:557–566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiong Y, Zhang H and Beach D: D type
cyclins associate with multiple protein kinases and the DNA
replication and repair factor PCNA. Cell. 71:505–514. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dronkert ML and Kanaar R: Repair of DNA
interstrand cross-links. Mutat Res. 486:217–247. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ben-Yehoyada M, Wang LC, Kozekov ID, Rizzo
CJ, Gottesman ME and Gautier J: Checkpoint signaling from a single
DNA interstrand crosslink. Mol Cell. 35:704–715. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou BB and Elledge SJ: The DNA damage
response: Putting checkpoints in perspective. Nature. 408:433–439.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Osawa T, Davies D and Hartley JA:
Mechanism of cell death resulting from DNA interstrand
cross-linking in mammalian cells. Cell Death Dis. 2:e1872011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Masta A, Gray PJ and Phillips DR: Nitrogen
mustard inhibits transcription and translation in a cell free
system. Nucleic Acids Res. 23:3508–3515. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen Y, Azad MB and Gibson SB: Methods for
detecting autophagy and determining autophagy-induced cell death.
Can J Physiol Pharmacol. 88:285–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Paglin S, Hollister T, Delohery T, Hackett
N, McMahill M, Sphicas E, Domingo D and Yahalom J: A novel response
of cancer cells to radiation involves autophagy and formation of
acidic vesicles. Cancer Res. 61:439–444. 2001.PubMed/NCBI
|
|
41
|
Li L, Ishdorj G and Gibson SB: Reactive
oxygen species regulation of autophagy in cancer: Implications for
cancer treatment. Free Radic Biol Med. 53:1399–1410. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kanematsu S, Uehara N, Miki H, Yoshizawa
K, Kawanaka A, Yuri T and Tsubura A: Autophagy inhibition enhances
sulforaphane-induced apoptosis in human breast cancer cells.
Anticancer Res. 30:3381–3390. 2010.PubMed/NCBI
|