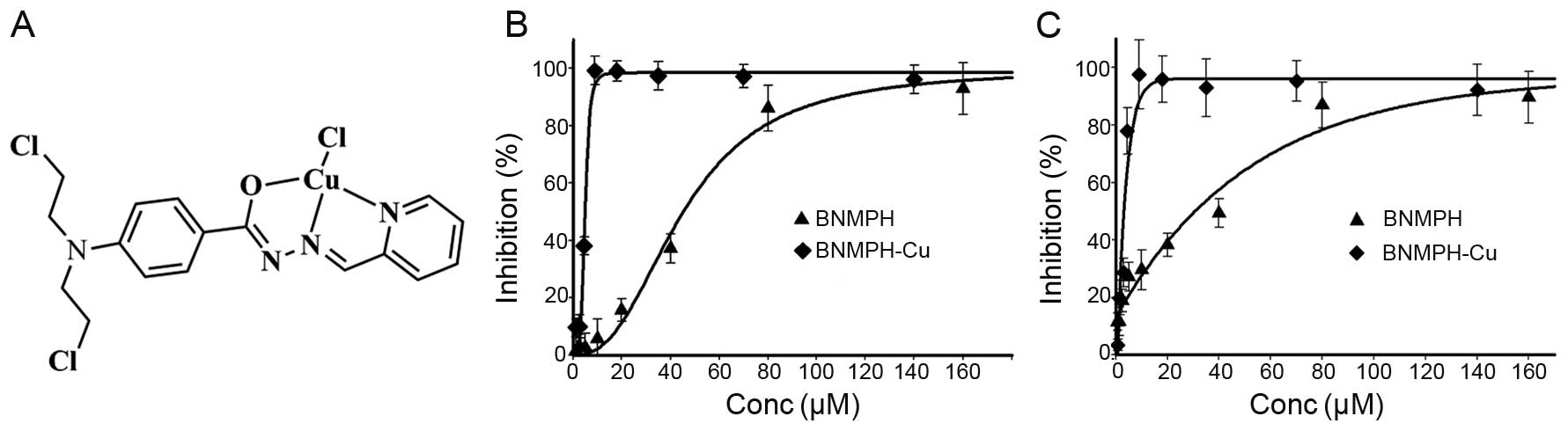
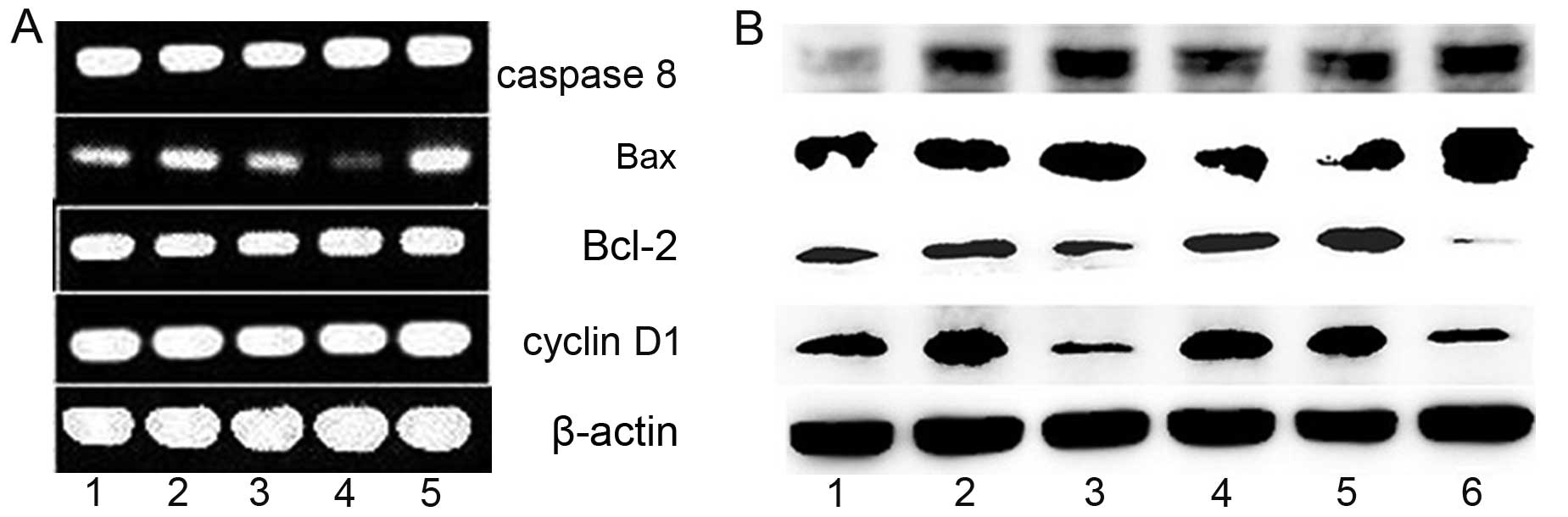
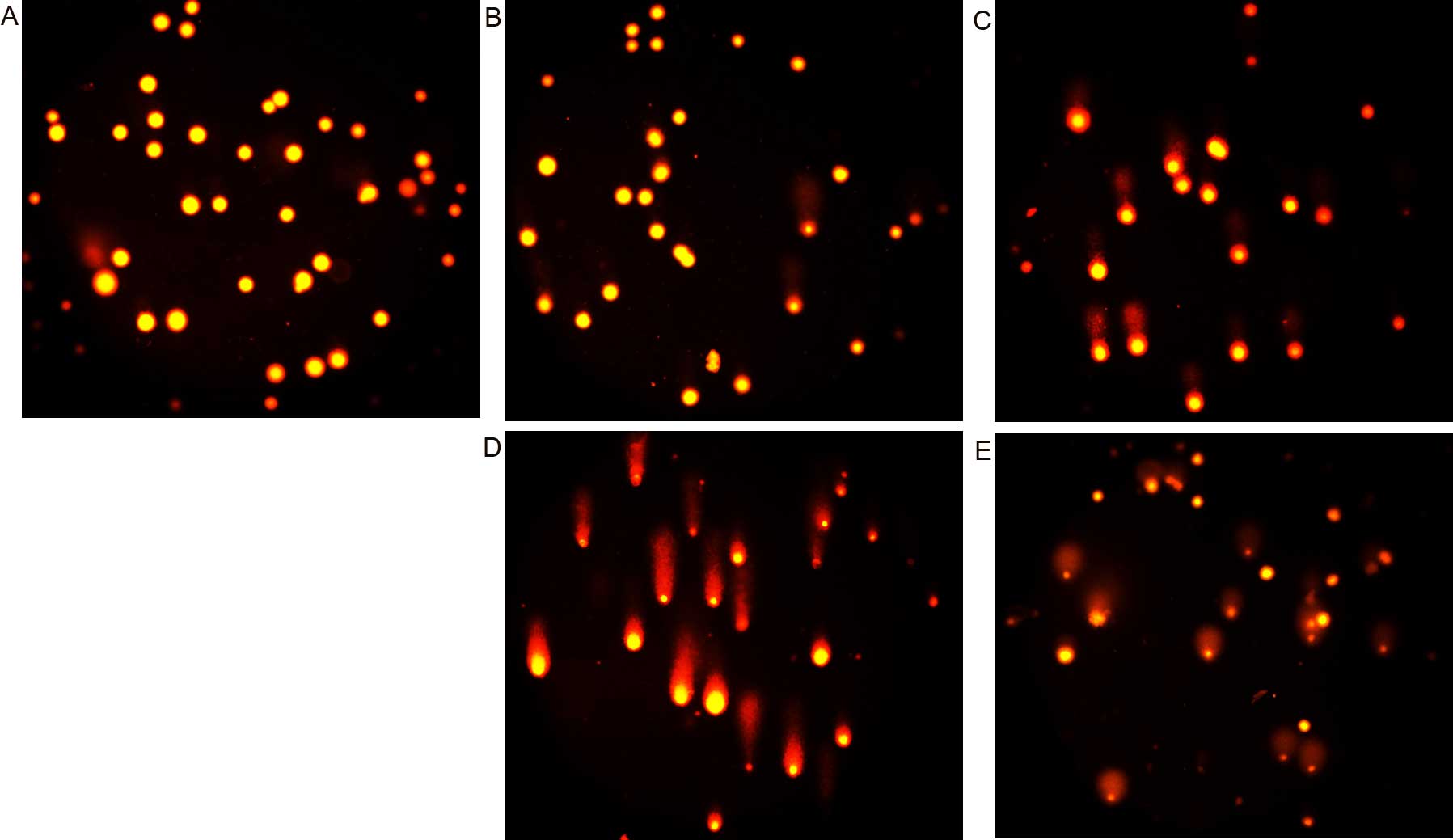
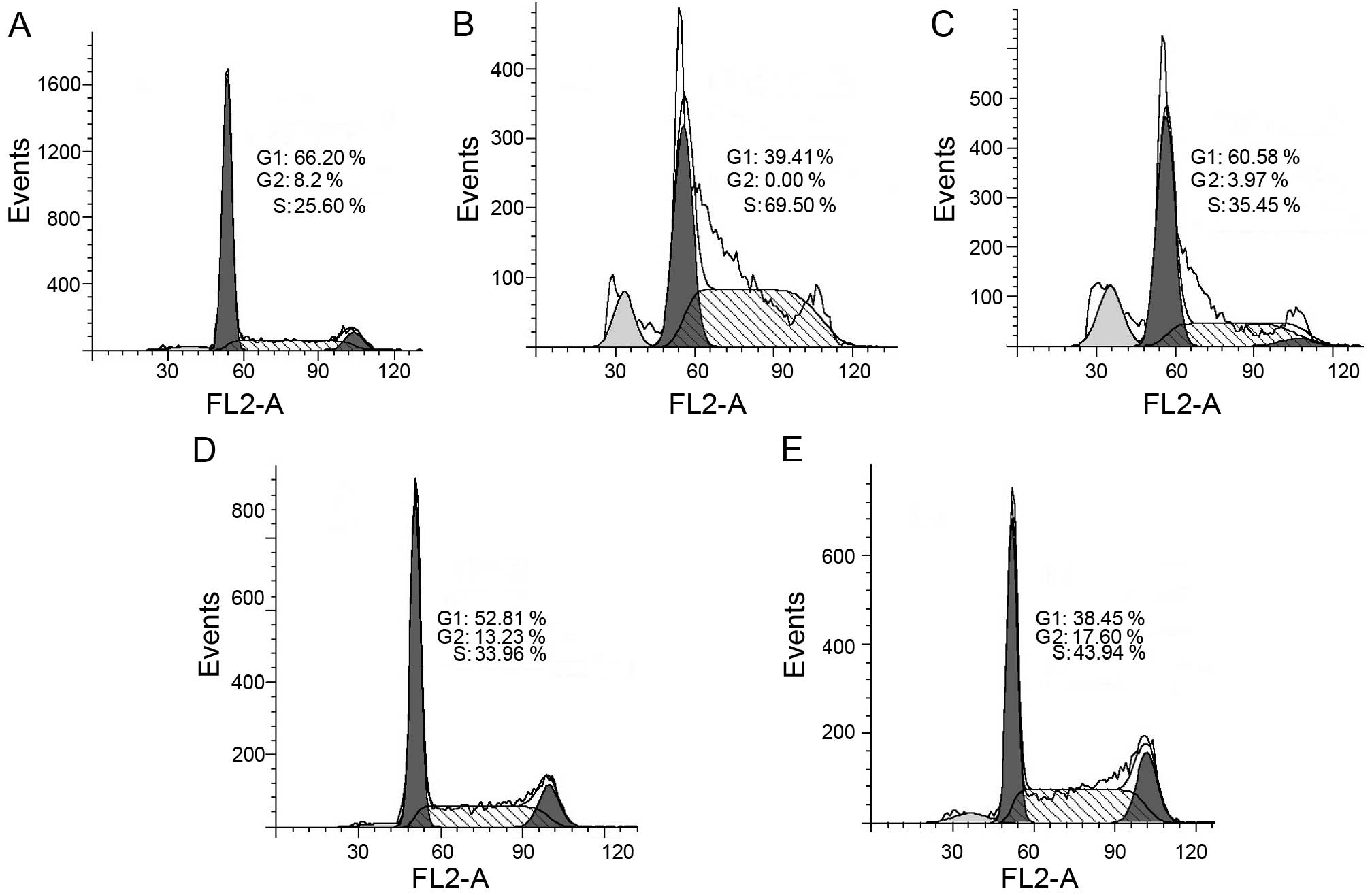
|
1
|
Chen Y and Hu L: Design of anticancer
prodrugs for reductive activation. Med Res Rev. 29:29–64. 2009.
View Article : Google Scholar
|
|
2
|
Marzano C, Pellei M, Tisato F and Santini
C: Copper complexes as anticancer agents. Anticancer Agents Med
Chem. 9:185–211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kratz F: Albumin as a drug carrier: Design
of prodrugs, drug conjugates and nanoparticles. J Control Release.
132:171–183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Firer MA and Gellerman G: Targeted drug
delivery for cancer therapy: The other side of antibodies. J
Hematol Oncol. 5:702012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Finlay GJ, Wilson WR and Baguley BC:
Chemoprotection by 9-aminoacridine derivatives against the
cytotoxicity of topoisomerase II-directed drugs. Eur J Cancer Clin
Oncol. 25:1695–1701. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marvania B, Lee PC, Chaniyara R, Dong H,
Suman S, Kakadiya R, Chou TC, Lee TC, Shah A and Su TL: Design,
synthesis and antitumor evaluation of phenyl N-mustard-quinazoline
conjugates. Bioorg Med Chem. 19:1987–1998. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abraham SA, Edwards K, Karlsson G,
MacIntosh S, Mayer LD, McKenzie C and Bally MB: Formation of
transition metal-doxorubicin complexes inside liposomes. Biochim
Biophys Acta Biomembr. 1565:41–54. 2002. View Article : Google Scholar
|
|
8
|
Cuin A, Massabni AC, Pereira GA, Leite CQ,
Pavan FR, Sesti-Costa R, Heinrich TA and Costa-Neto CM:
6-Mercaptopurine complexes with silver and gold ions:
Anti-tuberculosis and anti-cancer activities. Biomed Pharmacother.
65:334–338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Navarro M, Castro W, González S, Abad MJ
and Taylor P: Synthesis and anticancer activity of
gold(I)-chloroquine complexes. J Mex Chem Soc. 57:220–229.
2013.
|
|
10
|
Martínez A, Suárez J, Shand T, Magliozzo
RS and Sánchez-Delgado RA: Interactions of arene-Ru(II)-chloroquine
complexes of known antimalarial and antitumor activity with human
serum albumin (HSA) and transferrin. J Inorg Biochem. 105:39–45.
2011. View Article : Google Scholar
|
|
11
|
Li W, Jiang GB, Yao JH, Wang XZ, Wang J,
Han BJ, Xie YY, Lin GJ, Huang HL and Liu YJ: Ruthenium(II)
complexes: DNA-binding, cytotoxicity, apoptosis, cellular
localization, cell cycle arrest, reactive oxygen species,
mitochondrial membrane potential and western blot analysis. J
Photochem Photobiol B. 140:94–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li CZ, Wang LF, Meng XQ and Zhao H:
Synthesis, characterization and antitumor activity of benzaldehyde
nitrogen mustard picolinoyl hydrazone complexes. Transit Metab
Chem. 24:206–209. 1999. View Article : Google Scholar
|
|
13
|
Fu Y, Zhou SF, Liu YX, Yang YL, Sun XZ and
Li CZ: The cytotoxicity of benzaldehyde nitrogen mustard-2-pyridine
carboxylic acid hydrazone being, involved in topoisomerase IIα
inhibition. Biomed Res Int. 2004:5270422014.
|
|
14
|
Tan M, Zhu J, Pan Y, Chen Z, Liang H, Liu
H and Wang H: Synthesis, cytotoxic activity, and DNA binding
properties of copper (II) complexes with hesperetin, naringenin,
and apigenin. Bioinorg Chem Appl. 347872:3478722009.
|
|
15
|
Xu DF, Shen ZH, Shi Y, He Q and Xia QC:
Synthesis, characterization, crystal structure, and biological
activity of the copper complex. Russ J Coord Chem. 36:458–462.
2010. View Article : Google Scholar
|
|
16
|
Ruan BF, Liang YK, Liu WD, Wu JY and Tian
YP: Synthesis, characterization, and antitumor activities of two
copper(II) complexes with pyrazole derivatives. J Coord Chem.
65:2127–2134. 2012. View Article : Google Scholar
|
|
17
|
Hindo SS, Frezza M, Tomco D, Heeg MJ,
Hryhorczuk L, McGarvey BR, Dou QP and Verani CN: Metals in
anticancer therapy: Copper(II) complexes as inhibitors of the 20S
proteasome. Eur J Med Chem. 44:4353–4361. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jakubowski W and Bartosz G:
2,7-Dichlorofluorescin oxidation and reactive oxygen species: What
does it measure? Cell Biol Int. 24:757–760. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singh NP, McCoy MT, Tice RR and Schneider
EL: A simple technique for quantitation of low levels of DNA damage
in individual cells. Exp Cell Res. 175:184–191. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brodská B and Holoubek A: Generation of
reactive oxygen species during apoptosis induced by DNA-damaging
agents and/or histone deacetylase inhibitors. Oxid Med Cell Longev.
2011:2535292011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Johansson AC, Appelqvist H, Nilsson C,
Kågedal K, Roberg K and Ollinger K: Regulation of
apoptosis-associated lysosomal membrane permeabilization.
Apoptosis. 15:527–540. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rehman SU, Zubair H, Sarwar T, Husain MA,
Ishqi HM, Nehar S and Tabish M: Redox cycling of Cu(II) by
6-mercaptopurine leads to ROS generation and DNA breakage: Possible
mechanism of anticancer activity. Tumour Biol. 36:1237–1244. 2015.
View Article : Google Scholar
|
|
23
|
Yang Y, Huang T, Zhou S, Fu Y, Liu Y, Yuan
Y, Zhang Q, Li S and Li C: Antitumor activity of a
2-pyridinecarboxaldehyde 2-pyridinecarboxylic acid hydrazone copper
complex and the related mechanism. Oncol Rep. 34:1311–1318.
2015.PubMed/NCBI
|
|
24
|
Fu Y, Yang Y, Zhou S, Liu Y, Yuan Y, Li S,
Li C and Li CZ: Ciprofloxacin containing Mannich base and its
copper complex induce antitumor activity via different mechanism of
action. Int J Oncol. 45:2092–2100. 2014.PubMed/NCBI
|
|
25
|
Lovejoy DB, Jansson PJ, Brunk UT, Wong J,
Ponka P and Richardson DR: Antitumor activity of metal-chelating
compound Dp44mT is mediated by formation of a redox-active copper
complex that accumulates in lysosomes. Cancer Res. 71:5871–5880.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fatfat M, Merhi RA, Rahal O, Stoyanovsky
DA, Zaki A, Haidar H, Kagan VE, Gali-Muhtasib H and Machaca K:
Copper chelation selectively kills colon cancer cells through redox
cycling and generation of reactive oxygen species. BMC Cancer.
14:5272014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fussell KC, Udasin RG, Gray JP, Mishin V,
Smith PJ, Heck DE and Laskin JD: Redox cycling and increased oxygen
utilization contribute to diquat-induced oxidative stress and
cytotoxicity in Chinese hamster ovary cells overexpressing
NADPH-cytochrome P450 reductase. Free Radic Biol Med. 50:874–882.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guerriero JL, Ditsworth D, Catanzaro JM,
Sabino G, Furie MB, Kew RR, Crawford HC and Zong WX: DNA alkylating
therapy induces tumor regression through an HMGB1-mediated
activation of innate immunity. J Immunol. 186:3517–3526. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moungjaroen J, Nimmannit U, Callery PS,
Wang L, Azad N, Lipipun V, Chanvorachote P and Rojanasakul Y:
Reactive oxygen species mediate caspase activation and apoptosis
induced by lipoic acid in human lung epithelial cancer cells
through Bcl-2 down-regulation. J Pharmacol Exp Ther. 319:1062–1069.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chatterjee S, Kundu S, Sengupta S and
Bhattacharyya A: Divergence to apoptosis from ROS induced cell
cycle arrest: Effect of cadmium. Mutat Res. 663:22–31. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kumar R, Dwivedi PD, Dhawan A, Das M and
Ansari KM: Citrinin-generated reactive oxygen species cause cell
cycle arrest leading to apoptosis via the intrinsic mitochondrial
pathway in mouse skin. Toxicol Sci. 122:557–566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiong Y, Zhang H and Beach D: D type
cyclins associate with multiple protein kinases and the DNA
replication and repair factor PCNA. Cell. 71:505–514. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dronkert ML and Kanaar R: Repair of DNA
interstrand cross-links. Mutat Res. 486:217–247. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ben-Yehoyada M, Wang LC, Kozekov ID, Rizzo
CJ, Gottesman ME and Gautier J: Checkpoint signaling from a single
DNA interstrand crosslink. Mol Cell. 35:704–715. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou BB and Elledge SJ: The DNA damage
response: Putting checkpoints in perspective. Nature. 408:433–439.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Osawa T, Davies D and Hartley JA:
Mechanism of cell death resulting from DNA interstrand
cross-linking in mammalian cells. Cell Death Dis. 2:e1872011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Masta A, Gray PJ and Phillips DR: Nitrogen
mustard inhibits transcription and translation in a cell free
system. Nucleic Acids Res. 23:3508–3515. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen Y, Azad MB and Gibson SB: Methods for
detecting autophagy and determining autophagy-induced cell death.
Can J Physiol Pharmacol. 88:285–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Paglin S, Hollister T, Delohery T, Hackett
N, McMahill M, Sphicas E, Domingo D and Yahalom J: A novel response
of cancer cells to radiation involves autophagy and formation of
acidic vesicles. Cancer Res. 61:439–444. 2001.PubMed/NCBI
|
|
41
|
Li L, Ishdorj G and Gibson SB: Reactive
oxygen species regulation of autophagy in cancer: Implications for
cancer treatment. Free Radic Biol Med. 53:1399–1410. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kanematsu S, Uehara N, Miki H, Yoshizawa
K, Kawanaka A, Yuri T and Tsubura A: Autophagy inhibition enhances
sulforaphane-induced apoptosis in human breast cancer cells.
Anticancer Res. 30:3381–3390. 2010.PubMed/NCBI
|