Introduction
Esophageal squamous cell carcinoma (ESCC) is one of
the most fatal malignancies with a relatively high incidence
worldwide (1,2). Clinical evidence indicates that the
etiology of ESCC includes multiple factors containing both
environmental and genetic determinants (3). Although a high number of genetic and
epigenetic alterations has been reported in ESCC, molecular markers
for early diagnosis and prognosis remain to be discovered. The
majority of ESCC deaths are due to invasive disease; therefore,
identification of novel genes involved in the tumorigenesis and
development of ESCC could contribute to improving the outcome of
this disease.
Translesion DNA synthesis (TLS) is one type of DNA
damage tolerance mechanisms that allows continuing DNA synthesis
even in the presence of DNA damage (4,5).
Protein reversionless 3-like (Rev3L), the catalytic subunit of the
DNA polymerase (pol) ζ, is well known to participate in error-prone
TLS with less stringent and lower processivity (4). Rev3L maintains genomic integrity by
inserting a substitute nucleotide in the opposite DNA adducts,
which increases the mutation rate and contributes to carcinogenesis
(6). Rev3L gene
polymorphisms have been correlated with the risk of lung and breast
cancer (7,8). Pol ζ was reported to promote tumor
formation and is significantly associated with poor progression in
cervical cancer (9,10). Thus, Rev3L may play an important
role in carcinogenesis and tumor progression.
The REV3L gene appears to be ubiquitously
expressed in normal and tumor tissues, while its expression pattern
remains contentious in different cancer tissues (11,12).
REV3L expression was found to be downregulated in colon,
lung, gastric and renal cancer tissues as compared to adjacent
tissues (13), whereas it was found
to be upregulated in human glioma tissues (14). Our previous study indicated that the
mRNA level of Rev3L was significantly elevated in ESCC when
compared with the level in normal controls (15), yet its role in ESCC development is
unclear. In the present study, we analyzed the expression of REV3L
in ESCC and adjacent normal tissues, as well as its association
with clinicopathological parameters. Furthermore, we elucidated the
role of REV3L in ESCC progression and drug-resistance using
well-established ESCC cell lines.
Materials and methods
Tissue samples
Human ESCC and adjacent tissues used in the present
study were obtained from the Nanjing Medical University affiliated
Suzhou hospital (Jiangsu, China). These tissues were resected from
patients before chemotherapy and radiation therapy, and were
immediately frozen and stored at −80°C for reverse transcriptase
(RT)-PCR and real-time PCR analysis. All patients gave signed,
informed consent for their tissues to be used for scientific
research. The study was approved by the Institutional Ethics
Committee of Nanjing Medical University Affiliated Suzhou
Hospital.
Cell culture
Human esophageal cancer cell lines ECA-109 and TE-1
were obtained from the Shanghai Cell Bank (Shanghai, China). Cells
were cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) (both from HyClone)
in a humidified atmosphere, with 5% CO2 at 37°C.
Generation of stable cell lines
The shRNA construct against REV3L (shREV3L) and the
control plasmid (shNC) were purchased from Shanghai GenePharma
(Shanghai, China). The shREV3L construct or control vector was
transfected into ECA-109 and TE-1 cells using Lipofectamine 2000
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer's protocols. Stable clones were selected in medium
containing G418 (500 µg/ml) (Sigma-Aldrich, St. Louis, MO,
USA) for 4 weeks. Individual clones were isolated and expanded for
further characterization.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies) following the manufacturer's
instructions. For reverse transcription, 1.0 µg of
RNA/sample was reverse transcribed using an oligo(dT)12
primer and SuperScript II reverse transcriptase (Invitrogen Life
Technologies). The primers for REV3L and β-actin were as follows:
forward, 5′-CGCGTCAGTTGGGACTTAAG-3′ and reverse,
5′-ACTATCGCCAACCTCAATGC-3′ (REV3L); forward,
5′-AGCGAGCATCCCCCAAAGTT-3′ and reverse, 5′-GGGCACGAAGGCTCATCATT-3′
(β-actin). RT-PCR was conducted using the 2X Taq PCR Master Mix
(Takara Bio, Dalian, China) according to the manufacturer's
instructions. The PCR products were separated by electrophoresis on
1.5% agarose gels, and the quantification of each band was
performed using Quantity One software (Bio-Rad).
Immunofluorescence
Cells were fixed with 4% paraformaldehyde, washed
with phosphate-buffered saline (PBS) and permeabilized with 1%
Triton X-100 in PBS. Samples were then blocked with blocking
solution (PBS containing 10% BSA and 1% triton X-100) and incubated
overnight at 4°C with the REV3L antibody (1:1,000; Abnova, Taiwan,
China). After washing, the Rhodamine-labeled goat anti-rabbit
antibody (1:100; KPL, Gaithersburg, MD, USA) was added and
incubated for 1 h at room temperature. Nuclear counterstaining was
performed using 4′6-diamidino-2-phenylindole (DAPI)
(Sigma-Aldrich). Cells were visualized under a fluorescence
microscope (Eclipse 80i; Nikon, Tokyo, Japan).
Cell proliferation and cell viability
assays
Cell proliferation was measured using Cell Counting
Kit-8 (CCK-8) (Beyotime Biotechnology, China). A total of
2×103 cells/well were seeded in triplicate into 96-well
plates for 1–6 days. Then, 10 µl of CCK-8 solution was added
to each well, and incubated for 2 h at 37°C. The optical density
(OD) was measured at 450 nm with a microplate reader (Thermo,
Waltham, MA, USA). The viability index was calculated as the
experimental OD value/the control OD value. For 5-fluorouracil
(5-FU) treatment experiments, cells (5×103) were
initially plated in quadruplicate into 96-well plates. Twenty-four
hours later, the cells were treated with various concentrations of
5-FU for 48 h. Cell viability was measured as described above.
Three independent experiments were performed.
Cell invasion assay
The invasive potential of cells was evaluated using
24-well Transwell inserts (Costar, New York, NY, USA). The inserts
were pre-coated with 40 µl Matrigel (1:4 dilution; BD
Biosciences, San Jose, CA, USA). Then, 1×105 cells (200
µl) in serum-free medium were added to the upper chambers.
The lower chambers were filled with medium that contained 10% FBS.
After incubation for 24 h, the inserts were fixed with 3.7%
paraformaldehyde/PBS and stained with 2% crystal violet. The cells
remaining in the upper chambers were scraped off, and the invading
cells in the lower chambers were photographed under a
microscope.
Flow cytometric analysis of cell cycle
and apoptosis
A total of 5×105 cells/well were seeded
in triplicate into 6-well plates. Twenty-four hours later, the
cells were treated with 50 and 100 µM 5-FU for 24 or 48 h.
Cell cycle analysis was performed using the propidium iodide (PI)
single staining method. Cells were collected and fixed with 70%
ice-cold ethanol at 4°C overnight. Then, the cells were washed with
PBS and stained with PI (100 µg/ml) for 30 min in the dark
before analysis. The cell cycle profiles were assayed using a
FACScan flow cytometry, and data were analyzed using MultiCycle
software (both from BD Biosciences). Cell apoptosis was measured
using the PE Annexin V apoptosis detection kit (BD Biosciences).
After 48 h of culture, the cells were harvested and processed as
described in the Annexin V-PE apoptosis detection kit and analyzed
on a FACScan flow cytometry.
Western blot analysis
Western blotting was carried out on whole-cell
extracts which were lysed in RIPA lysis buffer (50 mM Tris-HCl, pH
7.4, 150 mM NaCl, 1% Triton X-100 and 1% sodium deoxycholate, 0.1%
SDS) that contained protease inhibitors for 20 min at 4°C. The
proteins were separated by 10% SDS-PAGE and transferred to PVDF
membranes (Millipore, Bedford, MA, USA). After blocking with 5%
non-fat milk in PBS-Tween-20 for 1 h at room temperature, the
membranes were incubated with primary antibodies targeting β-actin,
survivin, cyclin D1 and PARP (Abcam Inc., Cambridge, MA, USA).
After washing three times with TBST, the membranes were incubated
with a horseradish peroxidase (HRP)-conjugated anti-rabbit or
anti-mouse secondary antibody (Beyotime Biotechnology) for 2 h. The
proteins were visualized using enhanced chemiluminescence (ECL;
Beyotime, Nantong, China).
Statistical analysis
Results are expressed as means ± standard error of
the mean (SEM). Statistical analyses were performed using SPSS 17.0
software (IBM, Armonk, NY, USA). P-value <0.05 was considered to
indicate a atatistically significant result.
Results
Elevated REV3L mRNA expression level in
ESCC and its correlation with lymph node metastasis and clinical
stage
To investigate REV3L gene expression in ESCC and
adjacent tissues, the mRNA level of REV3L was analyzed by qRT-PCR
using 68 ESCC and 48 adjacent tissues. As shown in Fig. 1a, the expression of REV3L in the
ESCC tissues was significantly higher than that in the adjacent
tissues (P<0.05). To further evaluate the correlation of REV3L
mRNA expression and clinicopathological features, the
characteristics of 68 ESCC patients included in the present study
were analyzed (Table I). As shown
by Mann-Whitney U test, REV3L mRNA expression was positively
correlated with lymph node metastasis (Fig. 1B; P<0.05) and clinical stage (IIb
and III) (Fig. 1C; P<0.05). In
contrast, REV3L mRNA expression was not correlated with pT, gender,
age and histological grade among the groups of patients.
Collectively, these findings indicate that overexpression of REV3L
in ESCC is associated with lymph node metastasis and tumor
progression.
 | Table IRelationship between REV3L expression
and clinicopathological parameters of the esophageal squamous cell
carcinoma cases (n=68). |
Table I
Relationship between REV3L expression
and clinicopathological parameters of the esophageal squamous cell
carcinoma cases (n=68).
| Clinicopathological
parameters | Relative REV3L
expression
(relative to GAPDH) |
|---|
| Cases |
Median (Q3-Q1) | P-value |
|---|
| Age (years) | | | |
| <60 | 28 | 0.031 (6.499) | 0.151 |
| ≥60 | 40 | 0.120 (0.773) | |
| Gender | | | |
| Male | 50 | 0.094 (3.768) | 0.149 |
| Female | 18 | 0.030 (0.970) | |
| Histological
grade | | | |
| Poor | 9 | 0.016 (0.032) | 0.946 |
| Moderate | 33 | 0.080 (9.232) | |
| Well | 26 | 0.143 (2.389) | |
| pT | | | |
| pT1-2 | 25 | 0.077 (1.297) | 0.900 |
| pT3-4 | 43 | 0.080 (4.047) | |
| pN | | | |
| (−) | 28 | 0.031 (0.143) | 0.013 |
| (+) | 40 | 0.511 (8.480) | |
| Stage | | | |
| I–IIa | 26 | 0.031 (0.132) | 0.019 |
| IIb–III | 42 | 0.485 (8.147) | |
Establishment of REV3L-knockdown cell
lines
To study the potential role of REV3L in esophageal
cancer, stable cell lines with REV3L knockdown were established
using the ECA-109 and TE-1 cells. REV3L mRNA and protein expression
levels were analyzed by RT-PCR and immunofluorescence to verify the
successful knockdown of REV3L in these cells. The results revealed
that REV3L expression at the mRNA and protein levels was
significantly lower (P<0.05) in the cells stably transfected
with the shREV3L construct as compared to the cells transfected
with the control vector (shNC), indicating effective knockdown of
REV3L expression (Fig. 2A and
B).
Effect of REV3L knockdown on the
proliferation and invasion of esophageal cancer cells in
vitro
To examine whether the modulation of REV3L
expression affects the tumorigenic properties of the esophageal
cancer in vitro, we measured the abilities of cell
proliferation and invasion using CCK-8 assay and transwell
analysis, respectively. As shown in Fig. 3, the cell proliferation (Fig. 3A) and invasion (Fig. 3B) were both decreased in the
REV3L-knockdown cells (TE-1/shREV3L and ECA-109/shREV3L cells)
compared to the control cells (TE-1/shNC and ECA-109/shNC cells)
(P<0.05). These results suggest that REV3L plays an important
role in esophageal cancer progression.
To further identify the mechanisms by which the
silencing of REV3L inhibited esophageal cancer cell proliferation
and invasion, we analyzed the expression levels of cyclin D1 and
survivin proteins due to their established roles in cell
proliferation and invasion. Western blot analysis revealed that
REV3L knockdown significantly downregulated cyclin D1 and survivin
protein expression in the esophageal cancer cells (Fig. 3C).
Knockdown of REV3L enhances 5-FU-induced
cytotoxicity in the esophageal cancer cells
To study the possible role of REV3L in affecting the
sensitivity of esophageal cancer cells to 5-FU, several
concentrations of 5-FU were used to treat ECA-109 and TE-1 cells
for 48 h. The cell viability was determined by the CCK-8 assay. As
shown in Fig. 4, a dose-dependent
inhibition in cell growth was observed in the 5-FU-treated TE-1 and
ECA-109 cells, and the REV3L-knockdown cells were more sensitive to
the cytotoxic effect of 5-FU. The results from the cell cycle
analysis using PI staining showed that the number of cells in the
G1 phase in the ECA-109/shREV3L cells was significantly higher than
the number in the ECA-109/shNC cells after treatment with different
doses of 5-FU for 24 h (Fig. 5).
Together, these results indicate that REV3L plays a role in
esophageal cancer resistance to 5-FU.
REV3L plays a critical role in esophageal
cancer resistance to 5-FU-induced apoptosis
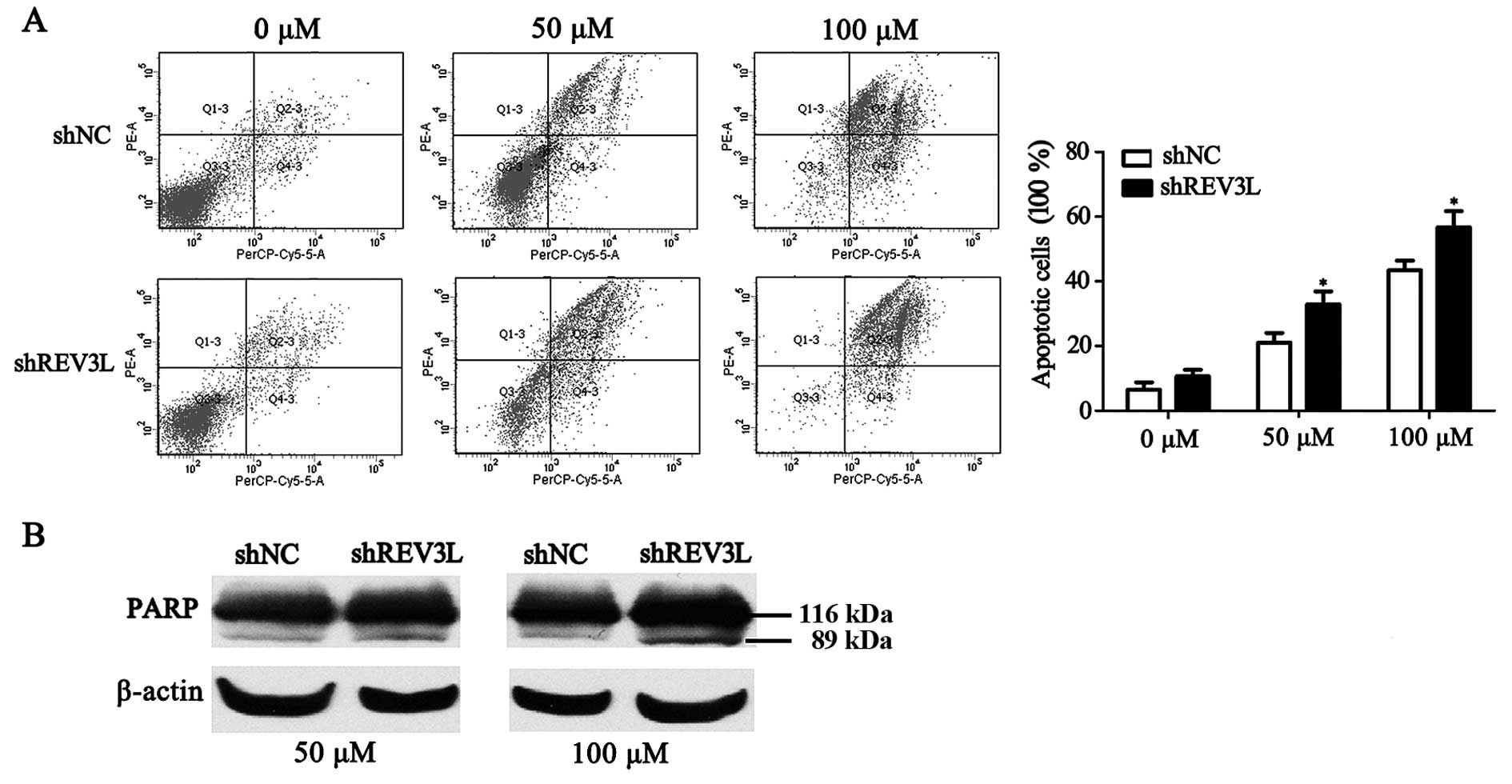
Next, we investigated whether the increased 5-FU
cytotoxicity observed in the REV3L-knockdown cells was related to
apoptosis. The extent of apoptosis was determined by measuring the
percentage of Annexin V stained cells, which detect both early- and
late-stage apoptosis. As shown in Fig.
6A, the apoptotic rates in the ECA-109/shREV3L cells were
significantly higher than the rates in the ECA-109/shNC cells in
response to different doses of 5-FU for 48 h. We then performed
western blotting to analyze the cleavage of PARP protein, another
established marker of apoptosis. As shown in Fig. 6B, ECA-109 REV3L-knockdown cells
exhibited increased PARP cleavage compared with the control cells
after treatment with 50 or 100 µM 5-FU for 48 h.
Discussion
Pol ζ is an error-prone DNA polymerase involved in
TLS that is characterized by a less-stringent active site and a
lower processivity compared with the high-fidelity replicative DNA
polymerases (16). As the catalytic
subunit of the pol ζ, REV3L is thought to be one of the major
components of error-prone TLS and may play a significant role in
tumor mutagenicity, progression, cytotoxicity and chemoresistance
(17). In the present study, the
expression of REV3L was demonstrated to be significantly
upregulated and positively correlated with lymph node metastasis
and clinical stage in ESCC tissues. These findings indicate that
overexpression of Rev3L may accumulate genetic damages that are
involved in the tumorigenesis and progression of ESCC.
There have been various controversial studies
regarding the effect of REV3L on cancer cell growth. On the one
hand, REV3L was established to be necessary for proliferation of
mouse embryonic fibroblasts, and inhibition of REV3L expression
resulted in a pronounced growth arrest in Burkitt lymphoma, lung,
breast, mesothelioma and colon cancer cells (6,18). In
contrast, suppression of REV3L expression did not alter cell
growth/survival in HCT116, U2OS and HeLa cancer cell lines
(13,19). In the present study, we found that
downregulation of REV3L expression decreased cell growth, migratory
and invasive potential of ESCC cells. This favors the notion that
REV3L plays an important role in the tumorigenesis and progression
of ESCC.
To elucidate the molecular mechanisms whereby REV3L
modulates growth and invasion of esophageal cancer cells, we
examined the expression of cyclin D1 and survivin, key regulators
of cancer cell proliferation and therapeutic resistance. Cyclin D1,
a key cell cycle regulator for cancer cell growth, is frequently
overexpressed in a variety of human types of cancers including ESCC
(20). Elevated levels of cyclin D1
have been found to correlate with early cancer onset, tumor
progression, increased metastasis and reduced survival of cancer
patients (21–23). Similarly, survivin, a member of the
inhibitor of apoptosis protein family, functions to inhibit the
mitochondrial pathway of apoptosis (24). Overexpression of survivin in ESCC
was found to be associated with poor prognosis and resistance to
radio-chemotherapy (25). Our data
indicated that cyclin D1 and survivin were significantly decreased
in ESCC cell lines following REV3L knockdown. These findings
indicate that cyclin D1 and survivin are involved in the REV3L
actions in promoting esophageal cancer cell growth and
invasion.
5-Fluorouracil (5-FU), a pyrimidine analogue of a
broad-spectrum anticancer drug, is widely prescribed for ESCC
patients (26). The anticancer
activity of 5-FU is through the inhibition of thymidylate synthase
(TS) and incorporation of its metabolites into RNA and DNA
molecules (27), which result in
failed DNA synthesis leading to cell apoptosis (28). Thus, suppression of DNA replication
signaling is a potential approach to increase 5-FU sensitivity.
REV3L has been shown to play a critical role in preventing
cisplatin cytotoxicity (9,14), yet its role in 5-FU-resistance has
not been previously reported. In the present study, we found that
inhibition of REV3L expression increased the sensitivity of
esophageal cancer cells to 5-FU by inducing g1 phase arrest and
apoptosis. These results indicate that inhibition of REV3L is
likely a new strategy to overcome 5-FU-resistance in esophageal
cancer.
In summary, the present study demonstrated that
REV3L expression is significantly upregulated in ESCC tissues
compared with adjacent tissues, and is positively correlated with
lymph node metastasis and clinical stage. Inhibition of REV3L
expression reduces ESCC cell proliferation and invasion at least in
part through suppression of cyclin D1 and survivin expression.
Furthermore, we demonstrated that REV3L functions to confer
chemoresistance to 5-FU treatment via regulation of the cell cycle
and apoptosis. These findings illustrate a role of REV3L in human
ESCC progression and chemoresistance, and provide a potential new
diagnostic marker and therapeutic target for ESCC.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (81372433 and 81472919), the
natural Science Foundation of Jiangsu Province of China
(BK20131149), and the Suzhou Administration of Science and
Technology (SYS201360, SYS201254 and SYS201571).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Talukdar FR, Ghosh SK, Laskar RS and
Mondal R: Epigenetic, genetic and environmental interactions in
esophageal squamous cell carcinoma from northeast India. PLoS One.
8:e609962013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Friedberg EC: A history of the DNA repair
and mutagenesis field: I. The discovery of enzymatic
photoreactivation. DNA Repair. 33:35–42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gan GN, Wittschieben JP, Wittschieben BO
and Wood RD: DNA polymerase zeta (pol ζ) in higher eukaryotes. Cell
Res. 18:174–183. 2008. View Article : Google Scholar
|
|
6
|
Knobel PA, Kotov IN, Felley-Bosco E,
Stahel RA and Marti TM: Inhibition of REV3 expression induces
persistent DNA damage and growth arrest in cancer cells. Neoplasia.
13:961–970. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang S, Chen H, Zhao X, Cao J, Tong J, Lu
J, Wu W, Shen H, Wei Q and Lu D: REV3L 3′UTR 460 T>C
polymorphism in microRNA target sites contributes to lung cancer
susceptibility. Oncogene. 32:242–250. 2013. View Article : Google Scholar
|
|
8
|
Varadi V, Bevier M, Grzybowska E,
Johansson R, Enquist K, Henriksson R, Butkiewicz D, Pamula-Pilat J,
Tecza K, Hemminki K, et al: Genetic variation in genes encoding for
polymerase ζ subunits associates with breast cancer risk, tumour
characteristics and survival. Breast Cancer Res Treat. 129:235–245.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang L, Shi T, Liu F, Ren C, Wang Z, Li Y,
Tu X, Yang G and Cheng X: REV3L, a promising target in regulating
the chemosensitivity of cervical cancer cells. PLoS One.
10:e01203342015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi TY, Yang L, Yang G, Tu XY, Wu X, Cheng
X and Wei Q: DNA polymerase ζ as a potential biomarker of
chemoradiation resistance and poor prognosis for cervical cancer.
Med Oncol. 30:5002013. View Article : Google Scholar
|
|
11
|
Kawamura K, O-Wang J, Bahar R, Koshikawa
N, Shishikura T, Nakagawara A, Sakiyama S, Kajiwara K, Kimura M and
Tagawa M: The error-prone DNA polymerase ζ catalytic subunit (Rev3)
gene is ubiquitously expressed in normal and malignant human
tissues. Int J Oncol. 18:97–103. 2001.
|
|
12
|
Xiao W, Lechler T, Chow BL, Fontanie T,
Agustus M, Carter KC and Wei YF: Identification, chromosomal
mapping and tissue-specific expression of hREV3 encoding a putative
human DNA polymerase ζ. Carcinogenesis. 19:945–949. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brondello JM, Pillaire MJ, Rodriguez C,
Gourraud PA, Selves J, Cazaux C and Piette J: Novel evidences for a
tumor suppressor role of Rev3, the catalytic subunit of Pol ζ.
Oncogene. 27:6093–6101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang H, Zhang SY, Wang S, Lu J, Wu W, Weng
L, Chen D, Zhang Y, Lu Z, Yang J, et al: REV3L confers
chemoresistance to cisplatin in human gliomas: The potential of its
RNAi for synergistic therapy. Neuro Oncol. 11:790–802. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou J, Zhang S, Xie L, Liu P, Xie F, Wu
J, Cao J and Ding WQ: Overexpression of DNA polymerase iota (Polι)
in esophageal squamous cell carcinoma. Cancer Sci. 103:1574–1579.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Waters LS, Minesinger BK, Wiltrout ME,
D'Souza S, Woodruff RV and Walker GC: Eukaryotic translesion
polymerases and their roles and regulation in DNA damage tolerance.
Microbiol Mol Biol Rev. 73:134–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gibbs PE, McGregor WG, Maher VM, Nisson P
and Lawrence CW: A human homolog of the Saccharomyces cerevisiae
REV3 gene, which encodes the catalytic subunit of DNA polymerase ζ.
Proc Natl Acad Sci USA. 95:6876–6880. 1998. View Article : Google Scholar
|
|
18
|
Gueranger Q, Stary A, Aoufouchi S, Faili
A, Sarasin A, Reynaud CA and Weill JC: Role of DNA polymerases η, ι
and ζ in UV resistance and UV-induced mutagenesis in a human cell
line. DNA Repair. 7:1551–1562. 2008. View Article : Google Scholar
|
|
19
|
Hicks JK, Chute CL, Paulsen MT, Ragland
RL, Howlett NG, Guéranger Q, Glover TW and Canman CE: Differential
roles for DNA polymerases eta, zeta, and REV1 in lesion bypass of
intrastrand versus interstrand DNA cross-links. Mol Cell Biol.
30:1217–1230. 2010. View Article : Google Scholar :
|
|
20
|
Sarbia M, Stahl M, Fink U, Heep H,
Dutkowski P, Willers R, Seeber S and Gabbert HE: Prognostic
significance of cyclin D1 in esophageal squamous cell carcinoma
patients treated with surgery alone or combined therapy modalities.
Int J Cancer. 84:86–91. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Diehl JA: Cycling to cancer with cyclin
D1. Cancer Biol Ther. 1:226–231. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jares P, Colomer D and Campo E: Genetic
and molecular pathogenesis of mantle cell lymphoma: Perspectives
for new targeted therapeutics. Nat Rev Cancer. 7:750–762. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thomas GR, Nadiminti H and Regalado J:
Molecular predictors of clinical outcome in patients with head and
neck squamous cell carcinoma. Int J Exp Pathol. 86:347–363. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Altieri DC: Survivin, cancer networks and
pathway-directed drug discovery. Nat Rev Cancer. 8:61–70. 2008.
View Article : Google Scholar
|
|
25
|
Kato J, Kuwabara Y, Mitani M, Shinoda N,
Sato A, Toyama T, Mitsui A, Nishiwaki T, Moriyama S, Kudo J, et al:
Expression of survivin in esophageal cancer: Correlation with the
prognosis and response to chemotherapy. Int J Cancer. 95:92–95.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang L, Feng J, Chen X, Guo W, Du Y, Wang
Y, Zang W, Zhang S and Zhao G: Myricetin enhance chemosensitivity
of 5-fluorouracil on esophageal carcinoma in vitro and in vivo.
Cancer Cell Int. 14:712014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pinedo HM and Peters GF: Fluorouracil:
Biochemistry and pharmacology. J Clin Oncol. 6:1653–1664.
1988.PubMed/NCBI
|
|
28
|
Bijnsdorp IV, Peters GJ, Temmink OH,
Fukushima M and Kruyt FA: Differential activation of cell death and
autophagy results in an increased cytotoxic potential for
trifluorothymidine compared to 5-fluorouracil in colon cancer
cells. Int J Cancer. 126:2457–2468. 2010.
|