Introduction
Breast cancer is one of the most common malignancies
and a leading cause of cancer-related mortality in women (1). Hormone receptor (HR), especially
estrogen receptor (ER), plays important roles in the development
and progression of breast cancer (2). There are different therapy choices in
clinic according to HR status of breast cancer. The HR-positive
sub-type which requires estrogen to grow potentially is susceptible
to endocrine therapy that blocks the receptors to improve the
prognosis (3,4), while the HR-negative sub-type, mostly
relys on traditional chemotherapy. For example, anthracycline and
taxanes based regimens are widely used as the first-line scheme
(5,6). Although HR-negative breast cancer is
sensitive to chemotherapy in initial treatment (7), tumor recurrence frequently occurs
(8). In fact, drug resistance is
believed to be one of the most common causes of tumor recurrence
and is associated with a poor outcome for HR-negative breast cancer
patients.
Women with recurrent or metastatic HR-positive
breast cancer are appropriate candidates for initial endocrine
therapy, and endocrine therapy may be active in patients with
negative HR examination, especially in soft tissue disease and/or
bone-dominant disease (9–11). Endocrine therapy is also associated
with relatively low toxicity. However, to date, endocrine therapy
and chemotherapy are recommended to be given sequentially, there is
little evidence supporting the combination of endocrine therapy and
chemotherapy as the ideal therapy strategy. Fulvestrant (ICI
182,780, Faslodex) is a new type of selective ER downregulator
(12–14). It binds, blocks and degrades ER,
then inhibits ER-mediated transcriptional activity. Considering
that fulvestrant is indicated for patients with disease progression
which may imply the development of aquired drug resistance, we
wonder whether fulvestrant could further enhance efficacy in
combination regimens. Several research groups have reported the
rationale and evidence for the efficacy of fulvestrant in
combination with other agents such as gefitinib and trastuzumab
(15,16).
In our previous studies, we found that the
combination of fulvestrant could markedly reverse the ER-mediated
resistance and sensitize ER-positive BCap37 cells which were
derived from stable transfection of an ER-α expression vector into
ER-negative BCap37 cells to antimicrotubule agents such as
paclitaxel and vinca alkaloids in vitro and in vivo
(17–19). So fulvestrant not only works as
endocrine therapy but can also sensitize the efficacy of
conventional chemotherapeutic drugs for ER-positive breast cancer.
More recently, we successfully established two independent novel
paclitaxel-resistant cell lines Bats-72 and Bads-200 from the same
parental BCap37 cell line, both of which were ER-negative and
showed cross-resistance to other anticancer drugs including
doxorubicin (20). Compared to
parental BCap37 cells, both Bads-200 and Bats-72 cells overexpress
P-glycoprotein (P-gp), which functions as an ATP-dependent efflux
pump with a variety of substrates and plays an important role in
mediating multidrug resistance (21–23).
Interestingly, we found that fulvestrant could significantly
reverse the resistance to paclitaxel in Bads-200 and Bats-72 cell
lines. In addition, we also found fulvestrant could enhance their
sensitivity to many other chemotherapy drugs including docetaxel,
vinorelbine and doxorubicin (24).
To further explore this interesting phenomenon, we
performed a series of experiments to investigate the combination
treatment of fulvestrant and doxorubcin in ER-negative breast
cancer cell lines Bads-200 and Bats-72 which may support the
feasibility of the combination of fulvestrant and chemotherapeutic
drugs for MDR breast cancer in the clinic.
Materials and methods
Cell lines and cell culture
The human breast cancer cell line BCap37, two MDR
cell lines Bads-200 and Bats-72, paclitaxel-selected derivative
obtained from parental BCap37 cell line (20), the human oral squamous carcinoma
cell line KB and and its vincristine-selected derivative KBv200
were cultured in RPMI-1640 medium with 10% fetal bovine serum and
1% penicillin/streptomycin. Bads-200 cells were maintained in
medium containing 200 nM paclitaxel (20), and KBv200 were grown in medium added
100 nM vincristine to keep their drug resistance characteristics
(25).
Drugs and treatments
Doxorubicin, fulvestrant, verapamil and tamoxifen
were purchased from Sigma (St. Louis, MO) for studies in
vitro. All chemicals were prepared according to the drug
specifications and diluted with culture medium to the desired
concentrations before use. All cells were cultured in drug-free
medium for more than 24 h before treatments. Then the cells were
treated with distinct dose of doxorubicin with or without 3-h
pretreatment of fulvestrant.
In vitro cytotoxicity assays
The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was used to measure the drug-induced cytotoxicity. Briefly,
104 cells/well were seeded and incubated overnight,
varying concentrations of designated drugs were added into each
well. At the end of drug exposure for 72 h, MTT solution was added
and the plates were further incubated for 3 h. Then, the medium was
removed and 200 µl of DMSO was added to dissolve the formazan
crystals, then individual wells were determined at 570 nm with a
microplate reader. The relative fraction of survival was calculated
by dividing the absorbance of treated wells by that of the
untreated control. Background absorbencies were subtracted,
IC50 values represent concentrations causing 50%
inhibition of cell growth.
Cell cycle analysis
Cell cycle distributions were assessed by flow
cytometric analysis. Cells were incubated in 6-well plates with
105 cells/well. After 48 h of drug treatment, Both
floating and adherent cells were collected and washed twice with
ice-cold PBS. Following fixation in 70% ethanol diluted in PBS, the
fixed cells were washed twice with PBS and treated with 100 µg/ml
RNase and 40 µg/ml propidium iodide at room temperature for 0.5–1.0
h in the dark. Cell cycle distribution and DNA content were tested
by a Coulter Epics V instrument (Beckman Coulter, Inc., Fullerton,
CA, USA) with an argon laser set to excite at 488 nm.
Intracellular doxorubicin distribution
and accumulation
Confocal cell images were determined to assess
intracelluar doxorubicin distribution and accumulation. Cells were
seeded on coverslips in 6-well tissue culture plates and incubated
for 48 h to grow as monolayers, then they were treated with or
without 5 µM fulvestrant for 2 h before exposure with 5 µM
doxorubicin. After washing twice with ice-cold PBS, 10 µg/ml
Hoechst-33342 was added into plates for nuclear staining, followed
by fixation with 4% paraformaldehyde solution. Finally, air-dried
coverslips were mounted on slides with glycerol-PBS (1:1) and
imaged using a confocal laser scanning microscope at 600 times
magnification.
Quantitation of doxobicin uptake and
efflux
Cells were plated into 6-well plates and allowed to
grow for 48 h. Then cells were exposed with doxorubicin in the
presence or absence of fulvestrant followed by incubation for 2 h.
To evaluate doxorubicin efflux, cells treated 2 h with the
combination of doxorubicin and fulvestrant were further incubated
in drug-free medium with or without fulvestrant for additional 2 h.
After washed with ice-cold PBS, intracellular doxorubicin
fluorescent intensity (accumulation fluorescence) was determined
with Coulter Epics V instrument (Beckman Coulter, Inc.). The cells
were excited at 485 nm, and emission was collected at 530 nm for
doxorubicin.
ATPase activity assay
P-gp ATPase activity was measured with the Pgp-Glo
assay system according to the manufacturer's instructions (Promega,
Madison, WI, USA) to identify the impact of fulvestrant on P-gp
ATPase activity (26). Fulvestrant
(5 µM) or 5 µM doxorubicin were incubated with 5 mM MgATP and 25 µg
recombinant human P-gp membranes at 37°C for 40 min. Luminescence
was initiated by ATP detection buffer. After 20 min to develop
luminescent signals, the multiplate was read on a plate-reading
luminometer. The decreased luminescence reflects ATP
consumption.
Apoptosis assay
Cells were treated with doxorubicin and fulvestrant
alone or in combination for 48 h and the cell morphology was
identified using a light microscope at × 400 magnification. Annexin
V-FITC staining (Beyotime, Haimen, China) was used to detect cell
apoptosis according to the manufacturer's instructions (27). Briefly, after the designated
treatment and at the end of time-point, both detached and attached
cells were harvested and washed twice with ice-cold PBS. The
collected cells were then resuspended with Annexin V binding buffer
and incubated with 5 µl of fluorescein isothiocyanate (FITC)
Annexin V for 15 min at 4°C in the dark. The percentages of
apoptotic cells were determined by flow cytometry.
Western blotting
Cells were treated with doxorubicin and fulvestrant
alone or in combination for 24 h. Cellular protein was isolated
with a protein extraction buffer (Beyotime). Protein concentrations
were measured with the BCA protein assay kit (Pierce). Equal
amounts (40 µg/lane) of proteins were fractionated on 10–12%
SDS-PAGE gels and transferred to polyvinylidene difluoride
membranes. The membranes were incubated with the desired primary
antibodies, respectively. After washing with PBS containing 0.1%
(v/v) Tween-20, the membranes were incubated with anti-mouse or
anti-rabbit IgG coupled to HRP second antibodies for 2 h at room
temperature followed by enhanced chemiluminescent staining using
the ECL system. β-actin was used for normalization of protein
loading.
Statistical analysis
Data are presented as mean ± standard error (SE).
Student's t-test was used to determine the statistical difference
for two-group comparisons, and multiple-treatment groups were
analyzed by one-way ANOVA. Differences were considered
statistically significant at a level of P<0.05.
Results
Fulvestrant sensitizes
doxorubicin-induced cytotoxicity in ER-negative MDR cell lines
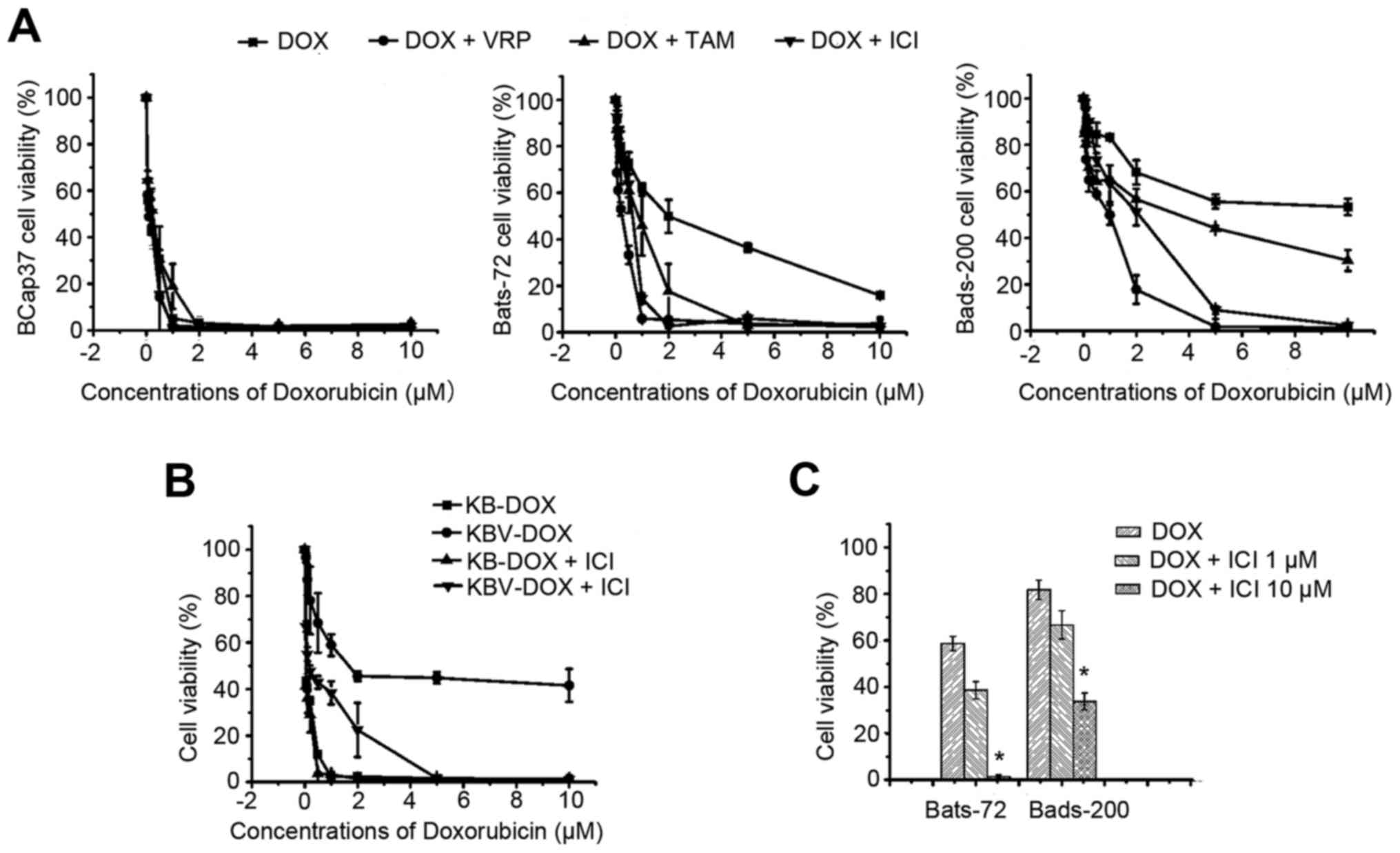
To evaluate the modulation activity of fulvestrant
to doxorubicin in ER-negative MDR cell lines, including Bats-72,
Bads-200 cell lines and KBv200 cell lines all with MDR phenotype as
a result of P-gp overexpression, we examined its intrinsic
cytotoxicity. The survival rates of the two MDR cell lines were
>90% after exposure to 1–10 µM fulvestrant for 72 h (data not
shown) and we chose 5 µM fulvestrant for the following test to
evaluate its reversal activity. The sensitivities of the two
different MDR cell lines treated with a series of concentrations of
doxorubicin in the absence or presence of 5 µM fulvestrant are
shown in Fig. 1A and B. The
IC50 values of 72-h doxorubicin exposure approximately
were 0.11±0.03, 1.91±0.17 and 10.97±3.86 µM respectively.
Three-hour-pretreatment with fulvestrant, which alone had no effect
on cell viability, significantly sensitized Bats-72 and Bads-200 to
doxorubicin in a dose-dependent manner. The IC50 values
were decreased to 0.50±0.10 µM in Bats72 and 1.47±0.05 µM in
Bads200, respectively. For KB, KBv200 cells, similar results were
found, the IC50 value for KBv200 cells was decreased
from 2.30±0.9 to 0.20±0.03 µM after treated with doxorubicin alone
or in combination with fulvestrant for 72 h (Fig. 1B and Table I). Other doses of fulvestrant were
selected to further determine whether the reversal potency is
dose-dependent. As Fig. 1C shows,
10 µM fulvestrant produced a more significant reversal effect than
1 µM fulvestrant after Bats72 and Bads200 cells were cotreated with
1 µM doxorubicin, although 1 µM fulvestrant also can enhance
doxorubicin-induced cytotoxicity. These data indicated that
fulvestrant strongly sensitized doxorubicin-induced cytotoxicity in
MDR cell lines.
 | Table I.The reversal activities of fulvestrant
and other P-gp modulators. |
Table I.
The reversal activities of fulvestrant
and other P-gp modulators.
|
| IC50
(µm)b |
|---|
|
|
|
|---|
| Druga | BCap37 | Bats-72 | Bads-200 | KB | KBv200 |
|---|
| DOX | 0.11±0.03 | 1.91±0.17 | 10.97±3.86 | 0.05±0.01 | 2.30±0.91 |
| DOX+ICI | 0.13±0.04 | 0.50±0.10 |
1.47±0.05 | 0.05±0.01 | 0.20±0.03 |
| DOX+VRP | 0.09±0.03 | 0.17±0.08 |
1.20±0.01 | – | – |
| DOX+TAM | 0.15±0.02 | 0.86±0.13 |
4.26±0.70 | – | – |
The reversal activity of Fulvestrant was further
compared with the classic P-gp modulators' efficacies, like
verapamil and tamoxifen. First the cytotoxicities of verapamil and
tamoxifen alone were determined by MTT assay, their concentrations
at ≤10 µM exerted slight cytotoxicity on BCap37, Bats-72, Bads-200
cells, and the cell survival rates were >90% (data not shown).
Verapamil (5 µM) or tamoxifen were co-treated with serial
concentrations of doxorubicin to BCap37, Bats-72, Bads-200 cells,
respectively. As shown in Fig. 1A
and Table I, verapamil reduced the
IC50 values of doxorubicin approximately to 0.17±0.08
and 1.20±0.01 µM in Bats72 and Bads200 cells, respectively, and the
IC50 values of doxorubicin in the presence of tamoxifen
were 0.86±0.13 and 4.26±0.70 µM in Bats72 and Bads200 cells,
respectively. The trend of cell survival curves also showed that
the reversal potency of fulvestrant is similar to that of verapamil
and is more effective than that of tamoxifen when in combination
with doxorubicin at the same doses.
Fulvestrant potentiates
doxorubicin-induced apoptosis
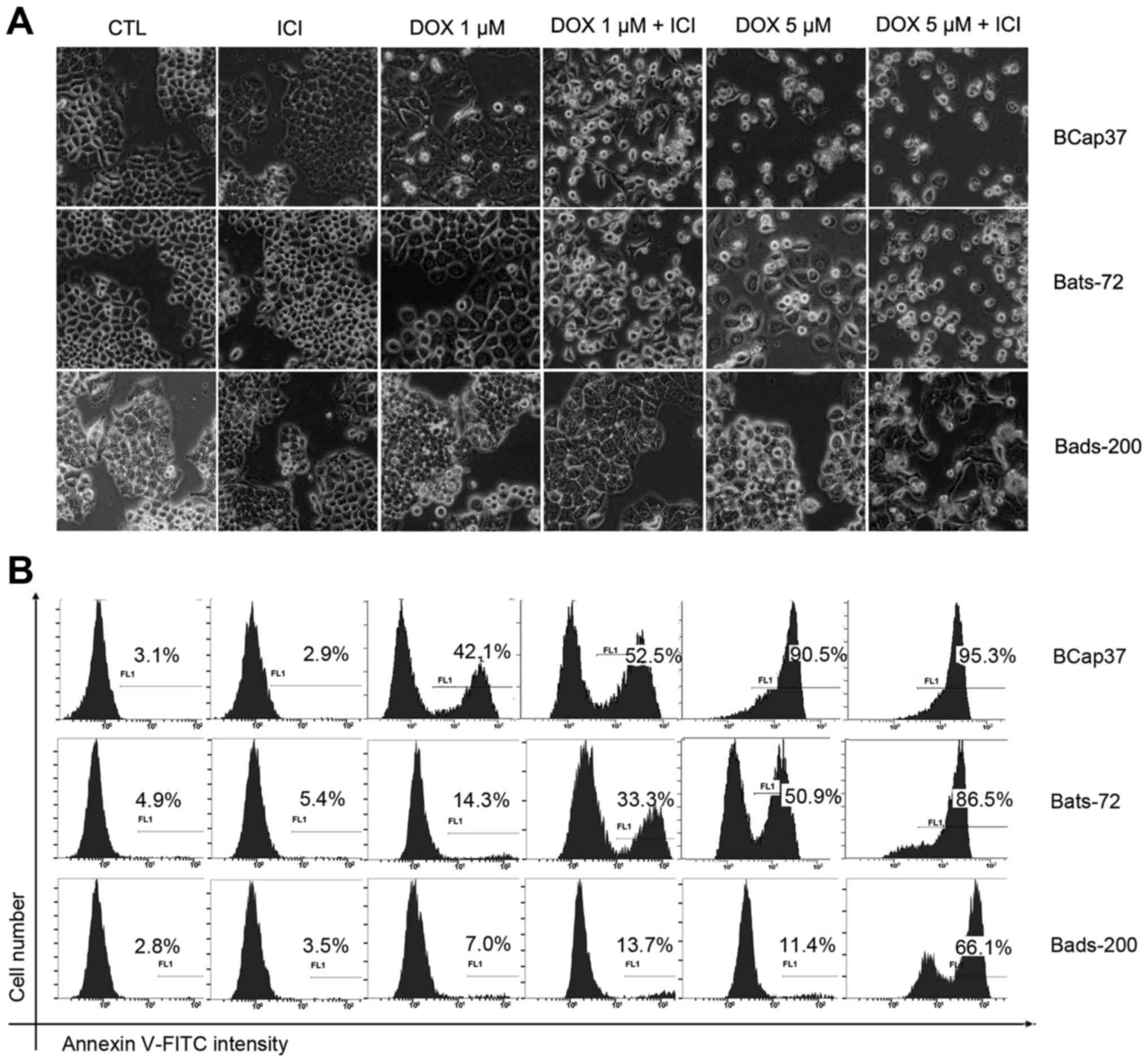
To further investigate whether fulvestrant
potentiates the cytotoxicity of doxorubicin to induce apoptosis,
morphologic analysis was done using a regular light microscope
after BCap37, Bats-72, Bads-200 cells treated with doxorubicin and
fulvestrant alone or in combination for 48 h. As depicted in
Fig. 2, fulvestrant reinforced the
degree of doxorubicin-induced cell death in Bats-72 and Bads-200
cells, while treatment with fulvestrant alone showed no change on
cellular morphology. Quantification of apoptosis was determined by
Annexin V-FITC assay. 5 µM fulvestrant significantly increased the
percentage of Annexin V-positive cells after treated with 1 µM or 5
µM doxorubicin in Bats72 cells and Bads200 cells. Interestingly,
fulvestrant also increased apoptosis induced by 1 µM doxorubicin in
Bcap37 cells and obviously changed their cellular morphology, while
there was little difference between treatment with 5 µM doxorubicin
in the presence or absence of fulvestrant, both of which induced
>90% percentage of Bcap37 cells death, as a result that 5 µM
doxorubicin alone killed almost all the Bcap37 cells. The results
demonstrate that the apoptosis levels increased with higher
doxorubicin concentrations and fulvestrant potentiated
doxorubicin-induced apoptotic in the three cell lines.
Fulvestrant enhances
doxorubicin-induced G2/M arrest and upregulation of cyclin B1
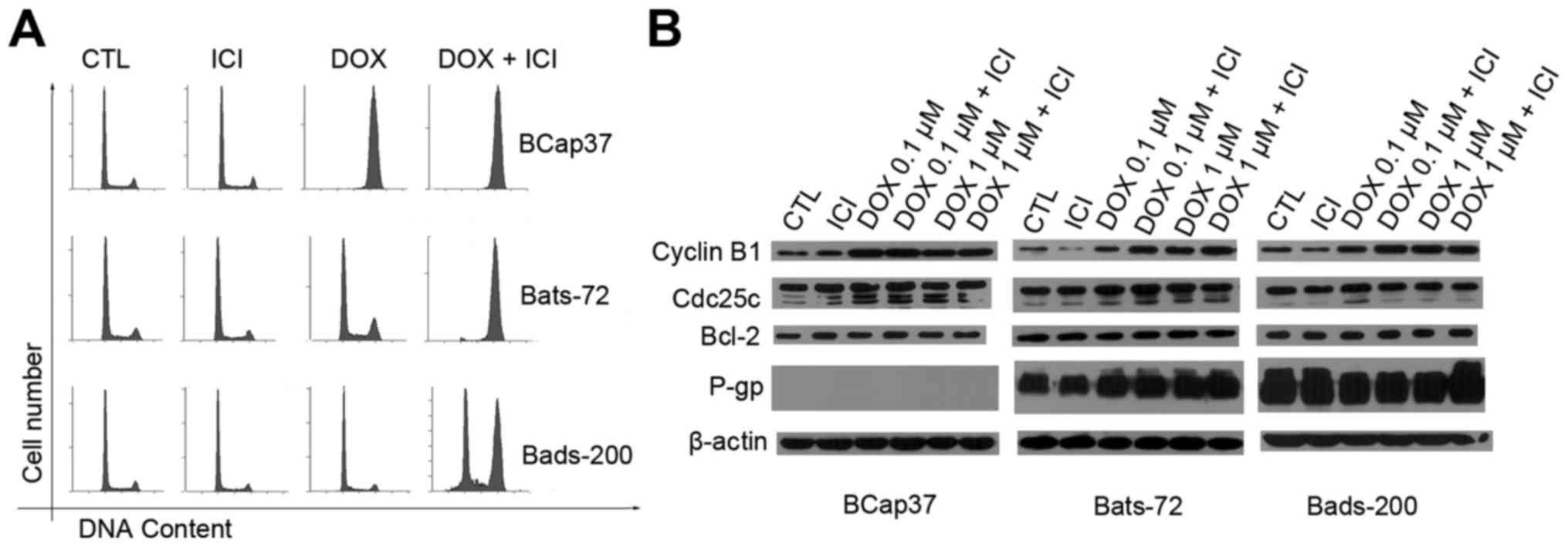
In previous studies, different levels of doxorubicin
dose produced different cell death pathways, and lower doses may
induce G2/M arrest (28,29). To investigate whether fulvestrant
also enhanced doxorubicin-induced G2/M arrest, cell cycle
distribution was analysed by flow cytometric assay (Fig. 3A). After 48-h exposure of 0.1 µM
doxorubicin with 3-h pretreatment of 5 µM fulvestrant, the
population of cells in G2/M phase in the Bats72 and Bads200
increased markedly compared with that of the doxorubicin treatment
alone. Conversely, fulvestrant showed little effect on the cell
cycle of BCap37 cells while the majority of BCap37 cells were
arrested in G2/M phases with or without fulvestrant.
In order to further elucidate that fulvestrant
increased doxorubicin-induced cell cycle arrest at G2/M phase, we
examined its modulation on protein levels associated with the G2/M
phase of the cell cycle. BCap37, Bats-72 and Bads-200 cells were
exposed to 0.1 and 1 µM doxorubicin for 24 h, and then the levels
of several protein were detected. Western blotting (Fig. 3B) revealed fulvestrant upregulated
cyclin B1 expression following co-treatment with 0.1 µM doxorubicin
in Bats-72 cells and both 0.1 and 1 µM doxorubicin in Bads-200
cells, while 0.1 µM doxorubicin resulted in the maximal expression
levels of cyclin B1 in BCap37 cells with or without fulvestrant.
Cyclin B1 expression seems cell cycle-dependent, the increase in
cyclin B1 protein levels was associated with the extent of
doxorubicin-induced G2/M arrest in all the three cell lines.
Another protein, the cdc25c which is an upstream regulator of
cyclin B1 showed no change in protein levels compared with various
treatment, further indicating that the doxorubicin-induced G2/M
arrest was more likely mediated by the level of cyclin B1.
Additionally, we also detected the anti-apoptotic Bcl-2 protein
levels which remained constant before and after treatment.
Fulvestrant functions as a substrate
for transport by P-gp, without affecting its expression
It has been believed that the change of P-gp
expression or its function may influence the efficacy of
P-gp-mediated MDR, therefore the relationship between fulvestrant
and P-gp was studied. Western blotting (Fig. 3B) showed doxorubicin, fulvestrant
alone or in combination did not alter the expression of P-gp. To
further determine the interaction between fulvestrant and P-gp
which acts as an ATP-dependent efflux pump and relies on ATP
hydrolysis, the effect of fulvestrant on P-gp ATPase activity was
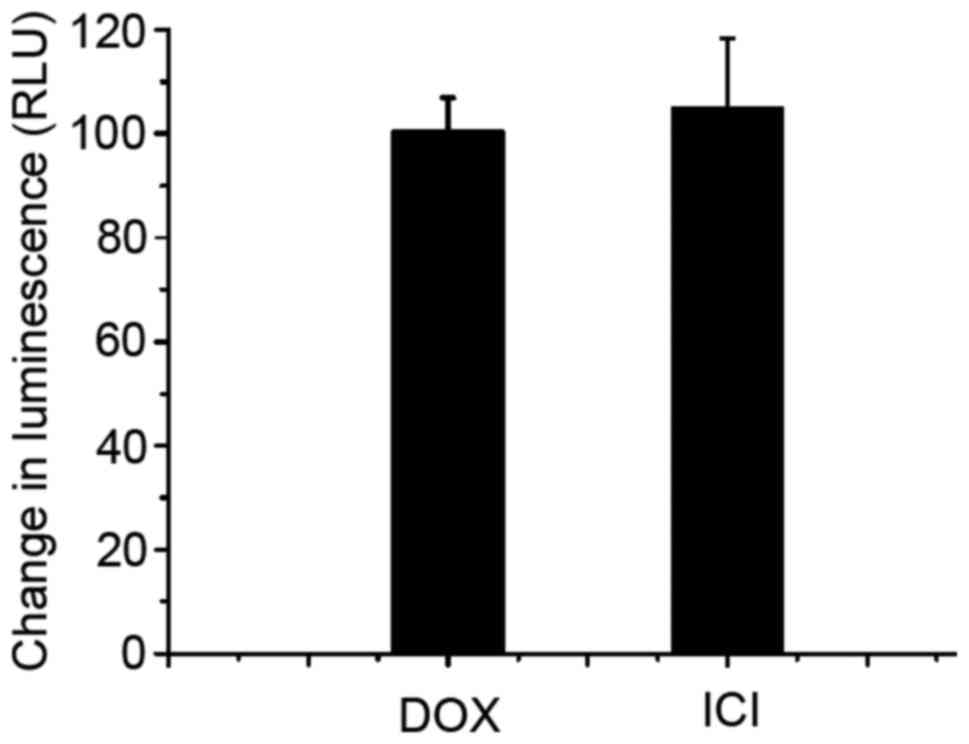
measured next. As Fig. 4 shows,
fulvestrant significantly stimulated the P-gp ATPase activity like
doxorubicin which means fulvestrant is a substrate for transport by
P-gp. Interestingly, the decrease in luminescence (the average
relative light units ∆RLU) of 5 µM fulvestrant-treated samples was
approximately 105.17±13.21, which is almost equal to that of 5 µM
doxorubicin-treated samples which was 100.73±6.18. These data
demonstrate fulvestrant reverses doxorubcin resistance as a
substrate of P-gp that inhibits its role of drug-efflux pump.
Fulvestrant alters intracellular
doxorubicin distribution, accumulation and retention
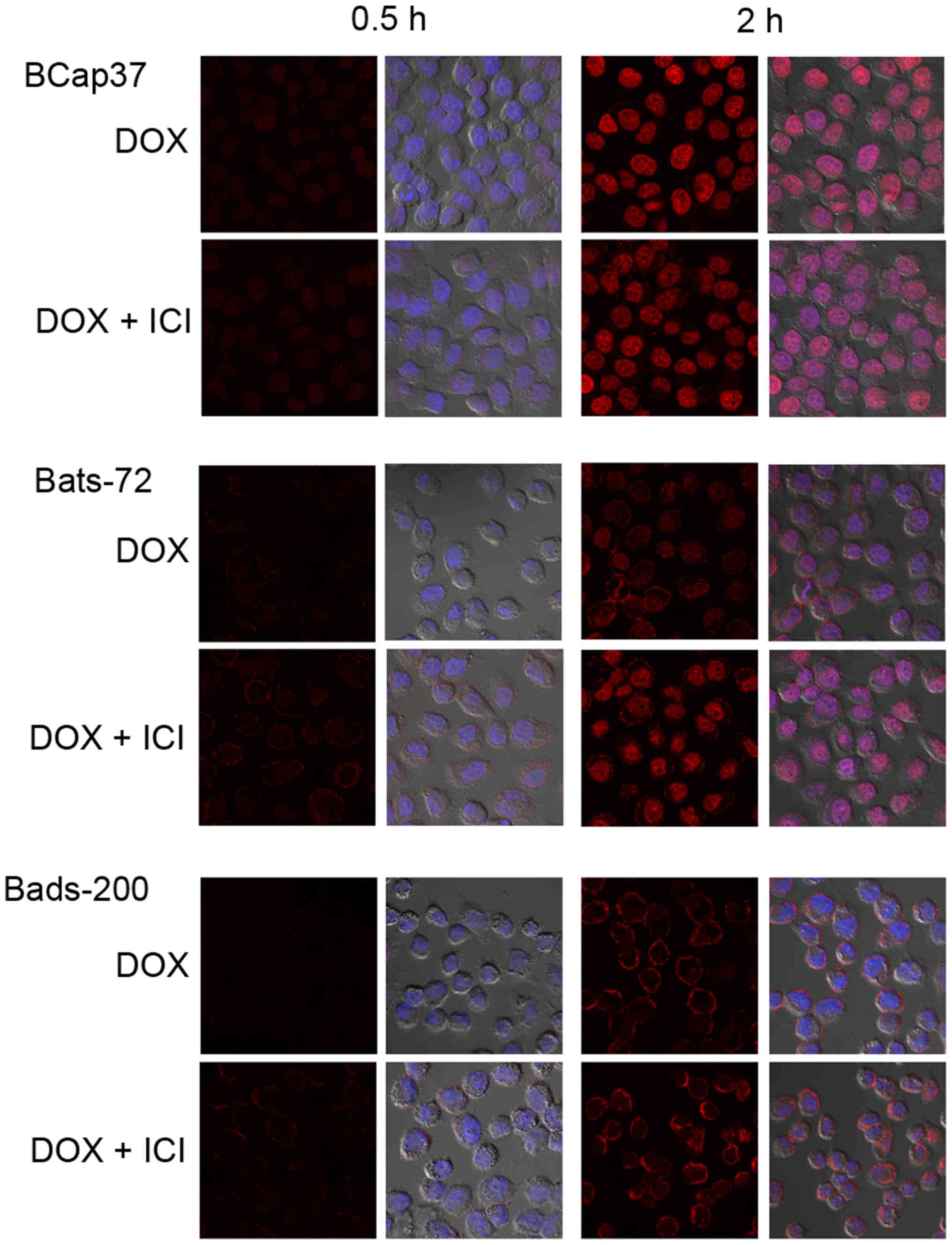
Using confocal fluorescent microscopy, we observed
the doxorubicin auto-fluorescent intensity to assess the
intracellular doxorubicin distribution and accumulation in BCap37,
Bats-72 and Bads-200 cells. Fig. 5
indicated that intracellular doxorubicin which increased in a
time-dependent manner were mostly accumulated in nuclei of the
parental BCap37 cells, but localized both in the nuclei and
cytoplasm of Bats-72 and Bads-200 cells. Co-treatment of
fulvestrant increased doxorubicin accumulation and relocalized it
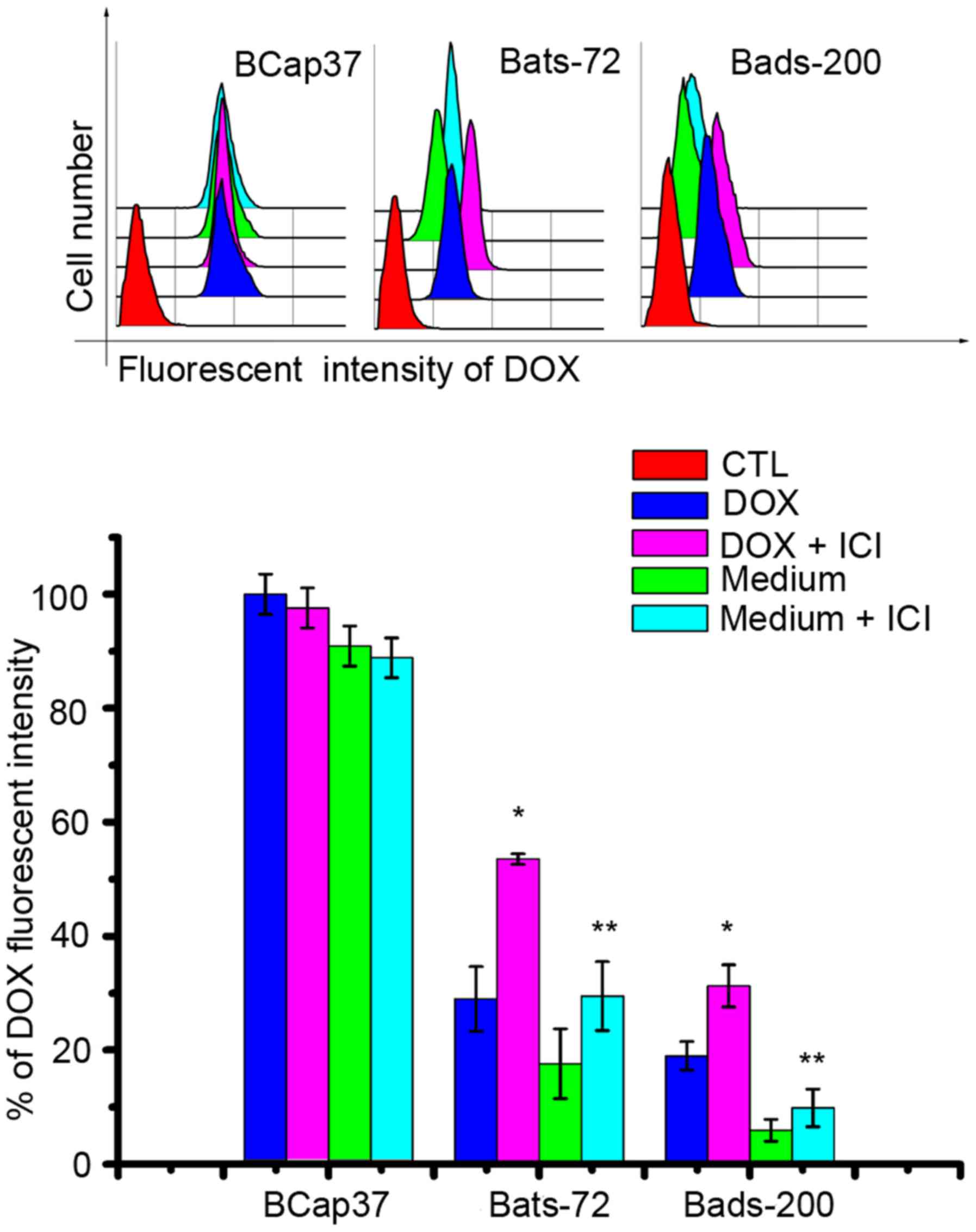
to the nuclei in Bats-72 and Bads-200 cells. Quantitation of
doxorubicin uptake and efflux was measured by flow cytometric
analysis. As Fig. 6 illustrates,
the doxorubicin uptake was slower but efflux was faster in Bats-72
and Bads-200 cells than in BCap37 cells. Incubation with the
addition of fulvestrant increased doxorubicin uptake in Bats-72
(28.97±5.68 versus 53.52±0.94%, P<0.05) and Bads-200 cells
(18.99±2.49 versus 31.27±3.68%, P<0.05), and incubation with
doxorubicin-free medium for another 2 h after that co-treatment,
fulvestrant also can inhibited efflux and increased retention in
Bats-72 (17.60±6.14 versus 29.49±6.04%, P<0.05) and Bads-200
cells (5.94±1.93 versus 9.86±3.3%, P<0.05). These findings
suggested fulvestrant increased intracellular doxorubicin
accumulation and retention and relocalized it to the nuclei in
Bats-72 and Bads-200 cells, but had no significant influence in
parental BCap37 cells.
Discussion
By a series of cytotoxicity assays in vitro,
we found that fulvestrant significantly sensitized
doxorubicin-induced cytotoxicity in a dose-dependent manner in
ER-negative MDR cell lines including Bats-72, Bads-200 cell lines
and KBv200 cells. Direct comparison with known modulators further
elucidated that the reversal potency of fulvestrant is similar to
that of verapamil and more potent than that of tamoxifen when
administered at the same doses in vitro. In addition,
compared to other MDR modulators, another prominent feature of
fulvestrant is its safety. The concentration of fulvestrant
required to achieve a marked reversal has little cytotoxicity by
itself. Clinical observations further confirmed this conclusion,
fulvestrant which is a pure ER antagonist possesses no agonist
effects, while tamoxifen is thought to be a partial estrogen
agonist. Estrogen side effects may cause endometrial hyperplasia or
cancer, uterine sarcoma, and may increase the risk of deep vein
thrombosis and stroke (30–32). Considering the effective and
well-tolerated properties, fulvestrant is a promising modulator for
the treatment for P-gp-mediated drug resistance.
Cumulative evidence suggests that resistance to cell
death programs and cell cycle arrest also contributes to the
development of MDR (33–35). Our data indicate that the extent of
doxorubicin-induced apoptosis and G2/M arrest is closely related to
the potency of drug resistance in BCap37, Bats-72, Bads-200 cell
lines. Fulvestrant significantly potentiated doxorubicin-induced
apoptosis and G2/M arrest in Bats-72 and Bads-200 cell lines, while
it alone did not induce apoptosis or change cell cycle progression,
probably representing fulvestrant restored sensitivity to
doxorubicin in Bats-72 and Bads-200 cell lines partially through
regulation of cell death and cell cycle pathways. The western blot
analyses further indicated that the doxorubicin-induced G2/M arrest
was more likely mediated by the level of cyclin B1, and fulvestrant
enhanced doxorubicin-induced G2/M arrest through upregulation of
cyclin B1 expression. It is well known that alterations of the
levels of Bcl-2 family proteins play an active role in apoptotic
pathways (36,37). It seemed that the levels of the
major anti-apoptotic protein Bcl-2 which remained stable with or
without doxorubicin did not associate with the sensitivity to
doxorubicin-induced apoptosis.
The interaction between fulvestrant and P-gp was
further investigated. The ATPase assay showed fulvestrant could
stimulate the ATPase activity of P-gp, which means that it acts as
a substrate of P-gp to inhibit its function of drug-efflux possibly
by competitively binding to P-gp. Western blotting further
indicated that fulvestrant did not alter P-gp expression.
Therefore, fulvestrant could modulate P-gp mediated resistance
mainly by inhibiting its function, and not by inhibiting its
expression. On the other hand, fulvestrant increased intracellular
doxorubicin accumulation and retention in Bats-72 and Bads-200
cells, but had no significant influence in parental BCap37 cells
that lack P-gp, also implied that the reversal of drug resistance
by fulvestrant was probably attributable to the inhibition of
P-gp-mediated drug transport. Interestingly, we observed that
Bats-72 and Bads-200 cells altered intracellular doxorubicin
distribution and accumulation compared with parental BCap37 cells.
Confocal cell images displayed that intracellular doxorubicin was
mostly concentrated in nuclei of the parental BCap37 cells, but
localized both in the nuclei and cytoplasm of Bats-72 and Bads-200
cells, especially for Bads-200 cells, the majority of doxorubicin
was still in the cytoplasm. Those results suggested that
doxorubicin could not easily get access to nuclear targets of MDR
cells. Fulvestrant not only restored doxorubicin accumulation but
also tried to relocalize it to the nuclei in Bats-72 and Bads-200
cells. The mechanism of doxorubicin activity is thought to interact
with DNA by intercalation (38–40),
so fulvestrant increased the amount of doxorubicin accessed to
nuclear targets in MDR cells, which maybe another potential reason
related to its reversal potency to P-gp mediated doxorubicin
resistance.
In conclusion, we have shown that fulvestrant
significantly reverses P-gp mediated resistance to doxorubcin in
vitro. The results suggest that fulvestrant not only takes part
in ER-mediated pathway for breast cancer therapy, but also has an
important role in the reversal of drug resistance when combined
with chemotherapy agents independent of ER expressing. This study
may provide useful clues for understanding the novel anticancer
mechanism of fulvestrant and supporting the clinical application of
fulvestrant when added to chemotherapy regimens for the treatment
of metastatic and progressive breast cancer, however, further
research is still needed to determine the ideal combination therapy
strategy.
Acknowledgements
This study was supported by grants LQ14H160004 from
Zhejiang Provincial Natural Science Foundation of China and
NSFC-81302288 from National Natural Science Foundation of
China.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McGuire WL, Horwitz KB, Pearson OH and
Segaloff A: Current status of estrogen and progesterone receptors
in breast cancer. Cancer. 39:(Suppl). S2934–S2947. 1977. View Article : Google Scholar
|
|
3
|
Dickson RB and Lippman ME: Estrogenic
regulation of growth and polypeptide growth factor secretion in
human breast carcinoma. Endocr Rev. 8:29–43. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuukasjärvi T, Kononen J, Helin H, Holli K
and Isola J: Loss of estrogen receptor in recurrent breast cancer
is associated with poor response to endocrine therapy. J Clin
Oncol. 14:2584–2589. 1996.PubMed/NCBI
|
|
5
|
Lück HJ and Roché H: Weekly paclitaxel: An
effective and well-tolerated treatment in patients with advanced
breast cancer. Crit Rev Oncol Hematol. 44:(Suppl). S15–S30. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sledge GW, Neuberg D, Bernardo P, Ingle
JN, Martino S, Rowinsky EK and Wood WC: Phase III trial of
doxorubicin, paclitaxel, and the combination of doxorubicin and
paclitaxel as front-line chemotherapy for metastatic breast cancer:
An intergroup trial (E1193). J Clin Oncol. 21:588–592. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Andre F and Pusztai L: Molecular
classification of breast cancer: Implications for selection of
adjuvant chemotherapy. Nat Clin Pract Oncol. 3:621–632. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Buzdar A, Jonat W, Howell A, Jones SE,
Blomqvist C, Vogel CL, Eiermann W, Wolter JM, Azab M, Webster A, et
al: Arimidex Study Group: Anastrozole, a potent and selective
aromatase inhibitor, versus megestrol acetate in postmenopausal
women with advanced breast cancer: Results of overview analysis of
two phase III trials. J Clin Oncol. 14:2000–2011. 1996.PubMed/NCBI
|
|
10
|
Dombernowsky P, Smith I, Falkson G,
Leonard R, Panasci L, Bellmunt J, Bezwoda W, Gardin G, Gudgeon A,
Morgan M, et al: Letrozole, a new oral aromatase inhibitor for
advanced breast cancer: Double-blind randomized trial showing a
dose effect and improved efficacy and tolerability compared with
megestrol acetate. J Clin Oncol. 16:453–461. 1998.PubMed/NCBI
|
|
11
|
Lønning PE, Bajetta E, Murray R,
Tubiana-Hulin M, Eisenberg PD, Mickiewicz E, Celio L, Pitt P, Mita
M, Aaronson NK, et al: Activity of exemestane in metastatic breast
cancer after failure of nonsteroidal aromatase inhibitors: A phase
II trial. J Clin Oncol. 18:2234–2244. 2000.PubMed/NCBI
|
|
12
|
Vergote I and Robertson JF: Fulvestrant is
an effective and well-tolerated endocrine therapy for
postmenopausal women with advanced breast cancer: Results from
clinical trials. Br J Cancer. 90:(Suppl 1). S11–S14. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dowsett M, Nicholson RI and Pietras RJ:
Biological characteristics of the pure antiestrogen fulvestrant:
Overcoming endocrine resistance. Breast Cancer Res Treat. 93:(Suppl
1). S11–S18. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Osborne CK, Wakeling A and Nicholson RI:
Fulvestrant: An oestrogen receptor antagonist with a novel
mechanism of action. Br J Cancer. 90:(Suppl 1). S2–S6. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Howell A: The future of fulvestrant
(‘Faslodex’). Cancer Treat Rev. 31:(Suppl 2). S26–S33. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen H, Liu J, Wang R, Qian X, Xu R, Xu T,
Li Q, Wang L, Shi Z, Zheng J, et al: Fulvestrant increases
gefitinib sensitivity in non-small cell lung cancer cells by
upregulating let-7c expression. Biomed Pharmacother. 68:307–313.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sui M, Huang Y, Park BH, Davidson NE and
Fan W: Estrogen receptor alpha mediates breast cancer cell
resistance to paclitaxel through inhibition of apoptotic cell
death. Cancer Res. 67:5337–5344. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sui M, Jiang D, Hinsch C and Fan W:
Fulvestrant (ICI 182,780) sensitizes breast cancer cells expressing
estrogen receptor alpha to vinblastine and vinorelbine. Breast
Cancer Res Treat. 121:335–345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang J, Sui M and Fan W: Estrogen
receptor α attenuates therapeutic efficacy of paclitaxel on breast
xenograft tumors. Breast Cancer Res Treat. 134:969–980. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang D, Sui M, Zhong W, Huang Y and Fan
W: Different administration strategies with paclitaxel induce
distinct phenotypes of multidrug resistance in breast cancer cells.
Cancer Lett. 335:404–411. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roepe PD: The P-glycoprotein efflux pump:
How does it transport drugs? J Membr Biol. 166:71–73. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sauna ZE, Kim IW and Ambudkar SV: Genomics
and the mechanism of P-glycoprotein (ABCB1). J Bioenerg Biomembr.
39:481–487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE
and Gottesman MM: P-glycoprotein: from genomics tomechanism.
Oncogene. 22:7468–7485. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang D, Huang Y, Han N, Xu M, Xu L, Zhou
L, Wang S and Fan W: Fulvestrant, a selective estrogen receptor
down-regulator, sensitizes estrogen receptor negative breast tumors
to chemotherapy. Cancer Lett. 346:292–299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu X, Sui M and Fan W: In vitro and in
vivo characterizations of tetrandrine on the reversal of
P-glycoprotein-mediated drug resistance to paclitaxel. Anticancer
Res 25B. 1953–1962. 2005.
|
|
26
|
Duan Z, Choy E and Hornicek FJ: NSC23925,
identified in a high-throughput cell-based screen, reverses
multidrug resistance. PLoS One. 4:e74152009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ren D, Zhu Q, Li J, Ha T, Wang X and Li Y:
Overexpression of angiopoietin-1 reduces doxorubicin-induced
apoptosis in cardiomyocytes. J Biomed Res. 26:432–438. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rebbaa A, Zheng X, Chou PM and Mirkin BL:
Caspase inhibition switches doxorubicin-induced apoptosis to
senescence. Oncogene. 22:2805–2811. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zuryń A, Litwiniec A, Gackowska L, Pawlik
A, Grzanka AA and Grzanka A: Expression of cyclin A, B1 and D1
after induction of cell cycle arrest in the Jurkat cell line
exposed to doxorubicin. Cell Biol Int. 36:1129–1135. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu R, Hilakivi-Clarke L and Clarke R:
Molecular mechanisms of tamoxifen-associated endometrial cancer
(Review). Oncol Lett. 9:1495–1501. 2015.PubMed/NCBI
|
|
31
|
Min CR, Kim MJ, Park YJ, Kim HR, Lee SY,
Chung KH and Oh SM: Estrogenic effects and their action mechanism
of the major active components of party pill drugs. Toxicol Lett.
214:339–347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iqbal J, Ginsburg OM, Wijeratne TD, Howell
A, Evans G, Sestak I and Narod SA: Endometrial cancer and venous
thromboembolism in women under age 50 who take tamoxifen for
prevention of breast cancer: A systematic review. Cancer Treat Rev.
38:318–328. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feng R and Dong L: Knockdown of
microRNA-127 reverses adriamycin resistance via cell cycle arrest
and apoptosis sensitization in adriamycin-resistant human glioma
cells. Int J Clin Exp Pathol. 8:6107–6116. 2015.PubMed/NCBI
|
|
34
|
Efferth T, Fabry U and Osieka R: Apoptosis
and resistance to daunorubicin in human leukemic cells. Leukemia.
11:1180–1186. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
De U, Chun P, Choi WS, Lee BM, Kim ND,
Moon HR, Jung JH and Kim HS: A novel anthracene derivative, MHY412,
induces apoptosis in doxorubicin-resistant MCF-7/Adr human breast
cancer cells through cell cycle arrest and downregulation of
P-glycoprotein expression. Int J Oncol. 44:167–176. 2014.PubMed/NCBI
|
|
36
|
Kelly PN and Strasser A: The role of Bcl-2
and its pro-survival relatives in tumourigenesis and cancer
therapy. Cell Death Differ. 18:1414–1424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Llambi F and Green DR: Apoptosis and
oncogenesis: Give and take in the BCL-2 family. Curr Opin Genet
Dev. 21:12–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bodley A, Liu LF, Israel M, Seshadri R,
Koseki Y, Giuliani FC, Kirschenbaum S, Silber R and Potmesil M: DNA
topoisomerase II-mediated interaction of doxorubicin and
daunorubicin congeners with DNA. Cancer Res. 49:5969–5978.
1989.PubMed/NCBI
|
|
39
|
Cirilli M, Bachechi F, Ughetto G, Colonna
FP and Capobianco ML: Interactions between morpholinyl
anthracyclines and DNA. The crystal structure of a morpholino
doxorubicin bound to d(CGTACG). J Mol Biol. 230:878–889. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kellogg GE, Scarsdale JN and Fornari FA
Jr: Identification and hydropathic characterization of structural
features affecting sequence specificity for doxorubicin
intercalation into DNA double-stranded polynucleotides. Nucleic
Acids Res. 26:4721–4732. 1998. View Article : Google Scholar : PubMed/NCBI
|