Introduction
Prostate cancer (PCa) is one of the most common
types of epithelial malignant tumor and its morbidity is the second
highest among males in USA (1).
Traditional treatment for metastatic PCa is androgen deprivation
therapy (ADT); however, the majority of patients will develop
castration-resistant PCa (CRPC) within 12–18 months (2). At present, few therapeutic strategies,
including docetaxel-based chemotherapy and new generation
anti-androgen therapy, are available for prolonging patient
survival. Therefore, it is necessary to identify novel drugs to
prevent or treat metastatic PCa.
Epithelial-mesenchymal transition (EMT) serves an
important role in cancer metastasis, which makes the cells more
motile and invasive (3,4). Additionally, metastasis is the main
cause of mortality in patients with PCa. The key characteristic of
EMT is the downregulation of epithelial markers and the
upregulation of mesenchymal markers (5). Several signaling pathways, including
the TGF-β, Wnt and Notch pathways, have been revealed to be
associated with these transitions (6–8).
Therefore, these pathways may be potential targets in the treatment
and prevention of metastatic prostate cancer.
Recently, numerous plant metabolites have exhibited
bioactivity (9–11), in which flavonoids have functions,
including anti-oxidative (12),
anti-angiocardiopathy (13),
anti-inflammatory (14) and
antitumor activities (15,16). Flavonoids are able to inhibit cell
proliferation, promote cell apoptosis and autophagy (17), and reverse drug resistance.
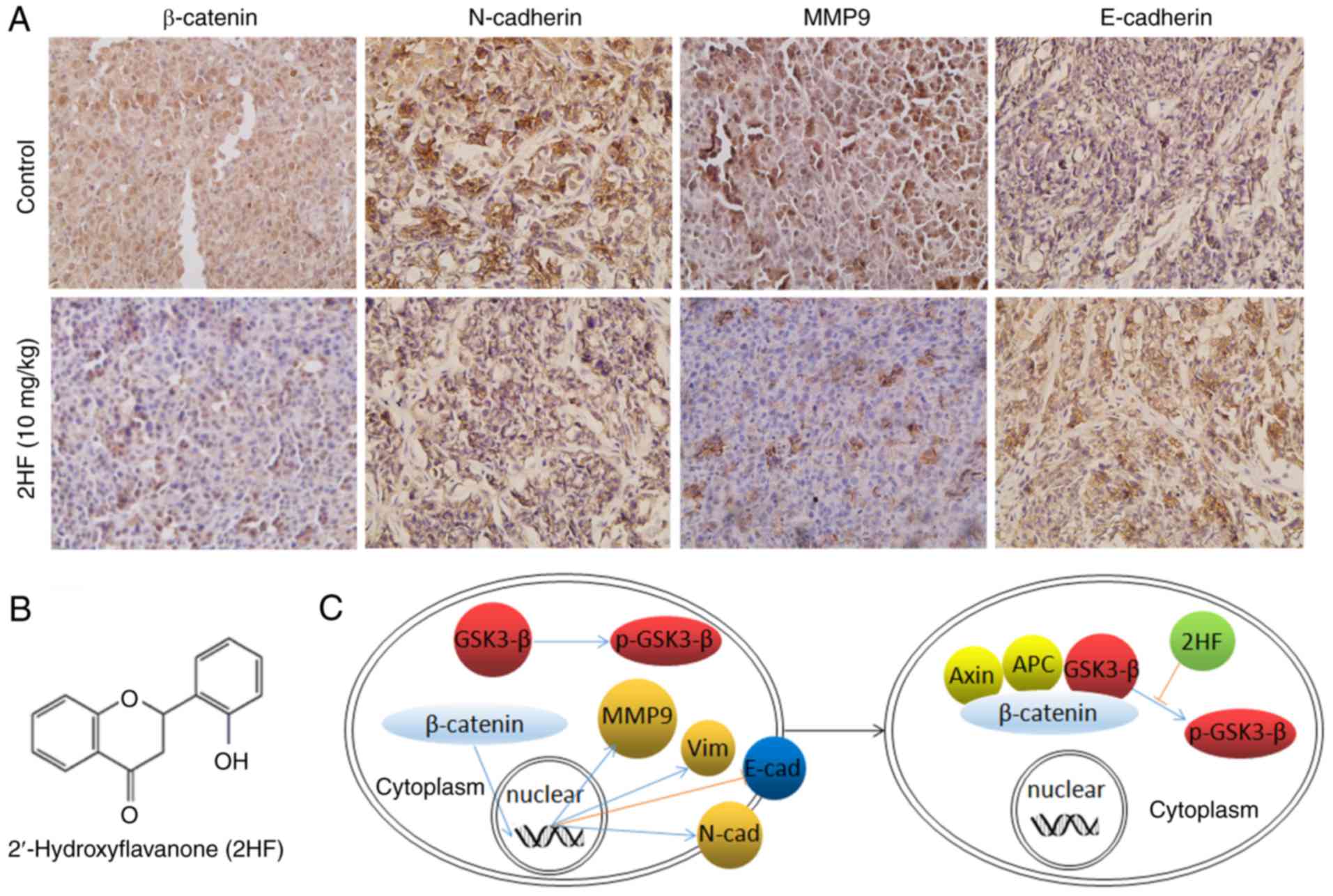
2′-Ηydroxyflavanone (2HF), an abundant natural flavonoid from
plants, has antitumor activity in different types of cancer,
including renal (18), lung
(19) and colon cancer (20), and osteosarcoma (21). Our previous study has also
demonstrated that 2HF could inhibit proliferation and induce
apoptosis through blocking the Akt/STAT3 signaling pathway in PCa
(17); however, the role of 2HF on
EMT, and cell migration and invasion remains unknown.
In the present study, 2HF, which has a lower
cytotoxicity in cells, was used to treat metastatic PCa cells to
investigate its effects on EMT, and cell migration and invasion.
The results clearly demonstrated that 2HF could suppress EMT, and
cell migration and invasion in a dose- and time-dependent manner in
DU145 and PC-3 cell lines and a subcutaneous xenograft, in which
2HF could inhibit the activation of the Wnt/β-catenin signaling
pathway.
Materials and methods
Cell culture and treatment
PCa PC-3 and DU145 cell lines were purchased from
the American Type Culture Collection (Manassas, VA, USA) and
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at
37°C with 5% CO2. 2HF powder was obtained from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany) and dissolved in
dimethyl sulfoxide (DMSO). Cells were treated with 0, 5 or 10 µM
2HF in 1% FBS for 24 or 48 h at 37°C.
Plasmids and cell transfection
A constitutively active β-catenin (CA-β-catenin;
S37A) plasmid, at a concentration of 1 µg/ml, and its control
vector were obtained from Dr Zijie Sun (Stanford University) and
described in our previous study (22). For cell transfection,
3×105 cells were seeded into a 6-well plate the day
before transfection. Transfection was performed using X-tremeGENE
HP DNA Transfection Reagent (Roche Diagnostics, Basel,
Switzerland), according to the manufacturer's protocol. The cells
were used in subsequent experiments following transfection for 48
h.
MTT assay
A total of 2×103 cells in 200 µl complete
DMEM were seeded into each well of a 96-well plate. Different
concentrations of 2HF (0, 5, 10, 20 or 40 µM) were added into each
well on the second day. A total of 48 h later, 20 µl MTT
(Sigma-Aldrich; Merck KGaA) was added into cells and the cells were
incubated for another 4 h at 37°C with 5% CO2.
Subsequently, medium was removed and 150 µl DMSO was added into the
wells to dissolve the purple formazan. Following agitating for 10
min, the optical density value at 490 nm was measured by a
microplate autoreader (Bio-Tek Instruments, Inc., Winooski, VT,
USA). Independent experiments were repeated three times and all the
procedures were performed according to the manufacturer's
protocol.
Wound healing assay
2HF was added when the cells in the 6-well plate
reached 100% confluence. A total of 24 h later, artificial wounds
were made using a 200-µl pipette tip. Wounds were monitored by an
inverted microscope (magnification, ×200) after 12 and 24 h.
Transwell migration and invasion
assay
DU145 and PC-3 cells were harvested following 2HF
treatment for 24 h. For the migration assay, 3×104 cells
in 200 µl serum-free DMEM were seeded into the upper chamber (8-µM
pore polycarbonate membrane filters) of Transwell inserts without
Matrigel. For the invasion assay, 6×104 cells in 200 µl
serum-free medium were seeded into the upper chamber of Transwell
inserts coated with Matrigel (Sigma-Aldrich; Merck KGaA). The lower
chamber was filled with 800 µl medium containing 20% FBS. Following
incubation for 24 h, the Transwell inserts were fixed with 4%
paraformaldehyde for 15 min at room temperature and stained with
0.1% crystal violet for 15 min at room temperature. Next, the
number of cells was counted in three random fields using an
inverted light microscope (magnification, ×200).
Western blot analyses
Following treatment with 2HF for 48 h, cells were
washed with cold PBS 3 times. Next, the radioimmunoprecipitation
assay buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 0.1% SDS, 1% NP40
and 0.5% sodium deoxycholate) containing proteinase inhibitors (1%
inhibitors cocktail and 1 mM PMSF; Sigma-Aldrich; Merck KGaA) was
used to prepare cell lysates. Protein determination was performed
using abicinchoninic protein assay kit (Thermo Fisher Scientific,
Inc.). Subsequently, 10–20 µg proteins were separated on 10–12%
SDS-PAGE and transferred to nitrocellulose membranes. Following
blocking of the membrane in Tris-buffered saline with 0.1% Tween-20
and 5% skimmed milk for 1 hat room temperature, the membrane was
incubated with the following specific primary antibodies diluted in
5%BSA (dilution 1:1,000): Mouse β-catenin (cat. no. 610153; BD
Biosciences, Franklin Lakes, NJ, USA), mouse glycogen synthase
kinase-3β (GSK-3β; cat. no. sc-71186; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), rabbit phosphorylase-glycogen synthase
kinase-3β (p-GSK-3β; ser9; cat. no. 5558; Cell Signaling
Technology, Inc.), rabbit Vimentin (cat. no. 5741; Cell Signaling
Technology, Inc.), rabbit E-cadherin (cat. no. sc-21791; Santa Cruz
Biotechnology, Inc.), rabbit N-cadherin (cat. no. sc-59987; Santa
Cruz Biotechnology, Inc.), rabbit matrix metalloproteinase 9 (MMP9;
cat. no. sc-21733; Santa Cruz Biotechnology, Inc.) and GAPDH
(mouse; cat. no. KC-5G4; Kangchen BioTech Co., Ltd., Shanghai,
China) overnight at 4°C. Following washing with TBST, membranes
were incubated with horseradish peroxidase-conjugated secondary
antibodies: Goat anti-mouse IgG (cat. no. P/N 925-32210; LI-COR
Biosciences, Lincoln, NE, USA), goat anti-rabbit IgG (cat. no. P/N
925-32211; LI-COR Biosciences) for 1 h at room temperature. The
secondary antibodies were diluted in TBST (1:5,000). Following
washing with TBST, the membranes were visualized by an enhanced
chemiluminescence detection system (version 3.0; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). GAPDH was used as an
internal control. All the densitometric analysis was performed
using ImageJ software (version 1.48; National Institutes of Health,
Bethesda, MD, USA).
Dual-luciferase reporter assay
For the reporter gene assay, 5×104 cells
seeded into 24-well plates were transfected with 200 ng β-catenin
Firefly luciferase reporter gene constructs (TOP or FOP) and 1 ng
of the pRL-SV40 Renilla luciferase construct (as an internal
control) using X-tremeGENE HP DNA Transfection Reagent (Roche
Diagnostics, Basel, Switzerland) for 24 h, prior to being subjected
to 2HF treatment. Cell extracts were prepared 48 h after treatment,
and the luciferase activity was measured using the Dual-Luciferase
Reporter assay system (Promega Corporation, Madison, WI, USA)
according to the manufacturer's protocol. β-catenin transcriptional
activity was measured by the ratio of TOP and FOP luciferase
activity. Relative luciferase activity is represented as the mean ±
standard error of the mean of each sample after normalizing to the
control.
Immunohistochemical (IHC) staining and
evaluation
Xenograft tumor tissues and sections were collected
as described in our previous study (17). Xenograft tumor tissues were obtained
from the BALB/c-nu mice in our previous study. A total of 16 male
BALB/c-nu mice aged 3–4 weeks and weighing 15–20 g were purchased
from the Shanghai Laboratory Animal Center (SLAC, Shanghai, China).
The mice were housed at room temperature in a sterile room and fed
ad libitum with high pressure sterilized food and water
without any light/dark cycle (17).
Xenografts were harvested for staining, and IHC was performed using
a Dako Autostainer Plus system (Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA) as previously described (17), in which the fixative was processed
at room temperature (20–25°C) in 4% paraformaldehyde. The thickness
of each slide was 5 µm. Following deparaffinization, washing in
xylene, rehydration in a descending alcohol series, antigen
retrieval for 5 min in a pressure cooker at 120°C, and 10-min
endogenous enzyme activity blocking in 3% hydrogen
peroxide-methanol at room temperature. Following blocking of the
slides in 5% BSA for 30 min at room temperature, slides were
incubated with specific primary antibodies as described earlier at
4°C overnight. Following washing with PBS 3 times, slides were
incubated with a secondary antibody as described earlier for 30 min
at room temperature. Substrate hydrogen peroxide was used to detect
the signal and hematoxylin was used for counterstaining at room
temperature for 30 sec. Next, the slides were mounted in resin.
Images were captured using an inverted microscope (magnification,
×200).
Statistical analysis
All assays were repeated in triplicate in three
independent experiments, and quantitative data are presented as the
mean ± standard error of the mean. The statistical analyses were
conducted using one-way analysis of variance followed by
Bonferroni's adjustment for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using SPSS 15.0 (SPSS Inc.,
Chicago, IL, USA).
Results
2HF inhibits EMT, and the cell
migration and invasion of PCa cells
Our previous study reported that higher
concentrations (20 and 40 µM) of 2HF could inhibit proliferation
and induce apoptosis of PCa cells (17), but the effects of lower
concentrations of 2HF on EMT, and cell migration and invasion in
PCa remains unknown. The present study used 5 and 10 µM 2HF with a
lower cytotoxicity to treat two metastatic PCa cell lines, DU145
and PC-3, respectively (Fig. 1A).
The results of the present study demonstrated that 2HF could
decrease the wound healing rate in a dose-dependent manner in DU145
and PC-3 cell lines (Fig. 1B). In
line with this, western blot analysis also demonstrated that the
expression of the epithelial marker E-cadherin increased but that
of the mesenchymal markers (N-cadherin, vimentin and MMP9)
decreased following 2HF treatment in DU145 and PC-3 cell lines
(Fig. 1C). Similarly, Transwell
migration and invasion assays demonstrated that 2HF could
significantly decrease cell migration and invasion in a
dose-dependent manner (Fig. 1D).
Therefore, as well as inhibiting proliferation and inducing
apoptosis, 2HF could also inhibit EMT, and cell migration and
invasion in PCa cells.
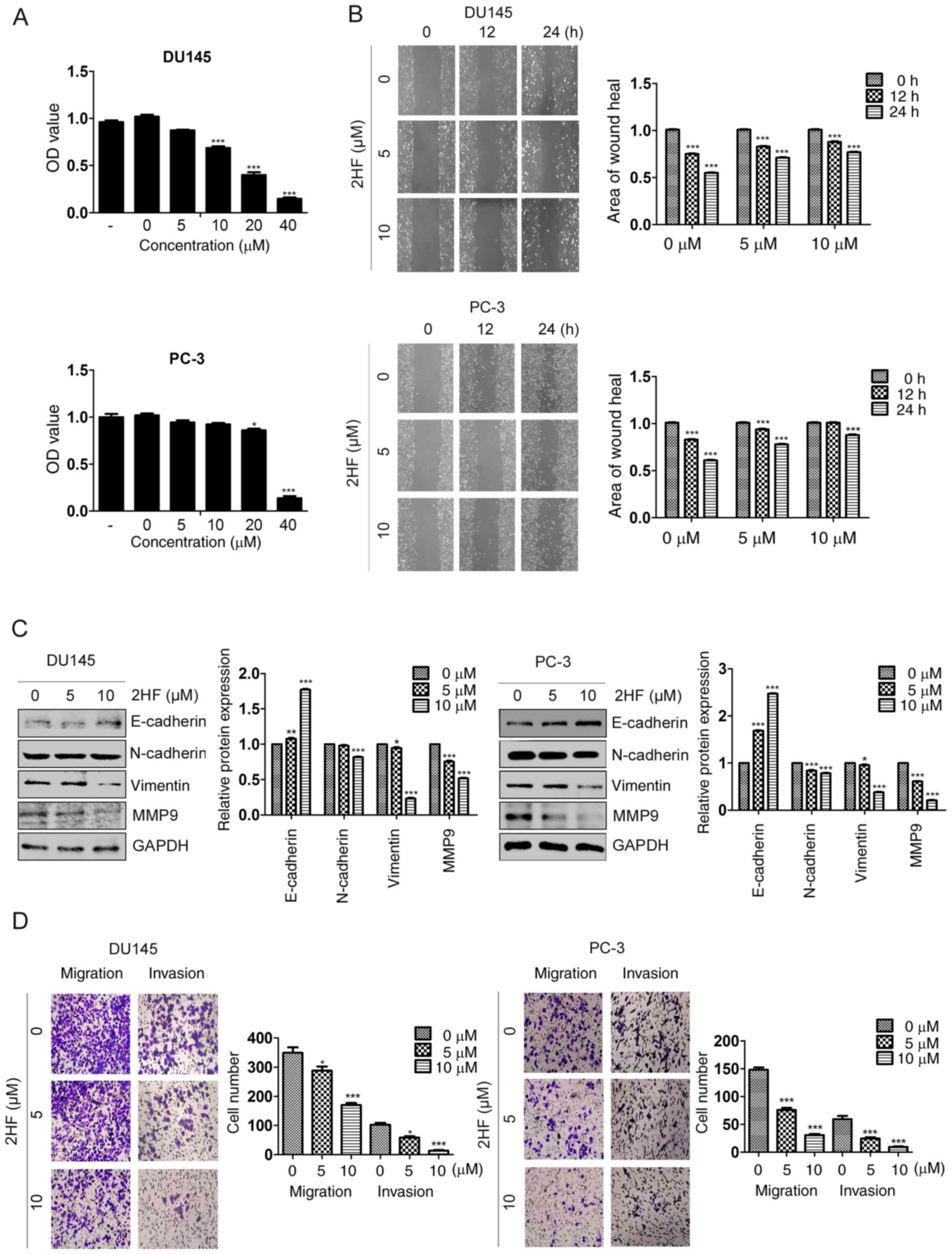 | Figure 1.2HF inhibit epithelial-mesenchymal
transition, and cell migration and invasion. (A) DU145 and PC-3
cells were treated with different concentrations of 2HF (0, 5, 10,
20 and 40 µM) for 48 h, prior to being subjected to an MTT assay to
measure its cytotoxicity to cells. *P<0.05, ***P<0.001. (B)
Representative pictures demonstrating the wound healing rate of
DU145 and PC-3 cells treated with different concentrations of 2HF.
Quantification analysis is shown. ***P<0.001. (C) E-cadherin,
N-cadherin, Vimentin and MMP9 proteins were detected by western
blot analysis in DU145 and PC-3 cells treated with different
concentrations of 2HF. GAPDH was used as a loading control and all
the experiments were repeated 3 times. Quantification analysis is
shown. *P<0.05, **P<0.01, ***P<0.001 vs. control. (D)
Representative pictures of Transwell migration and invasion assays
demonstrating the migration and invasion abilities of DU145 and
PC-3 cells treated with different concentrations of 2HF.
Quantification analysis is shown. *P<0.05, ***P<0.001 vs.
control. OD, optical density; MMP9, matrix metalloproteinase 9. |
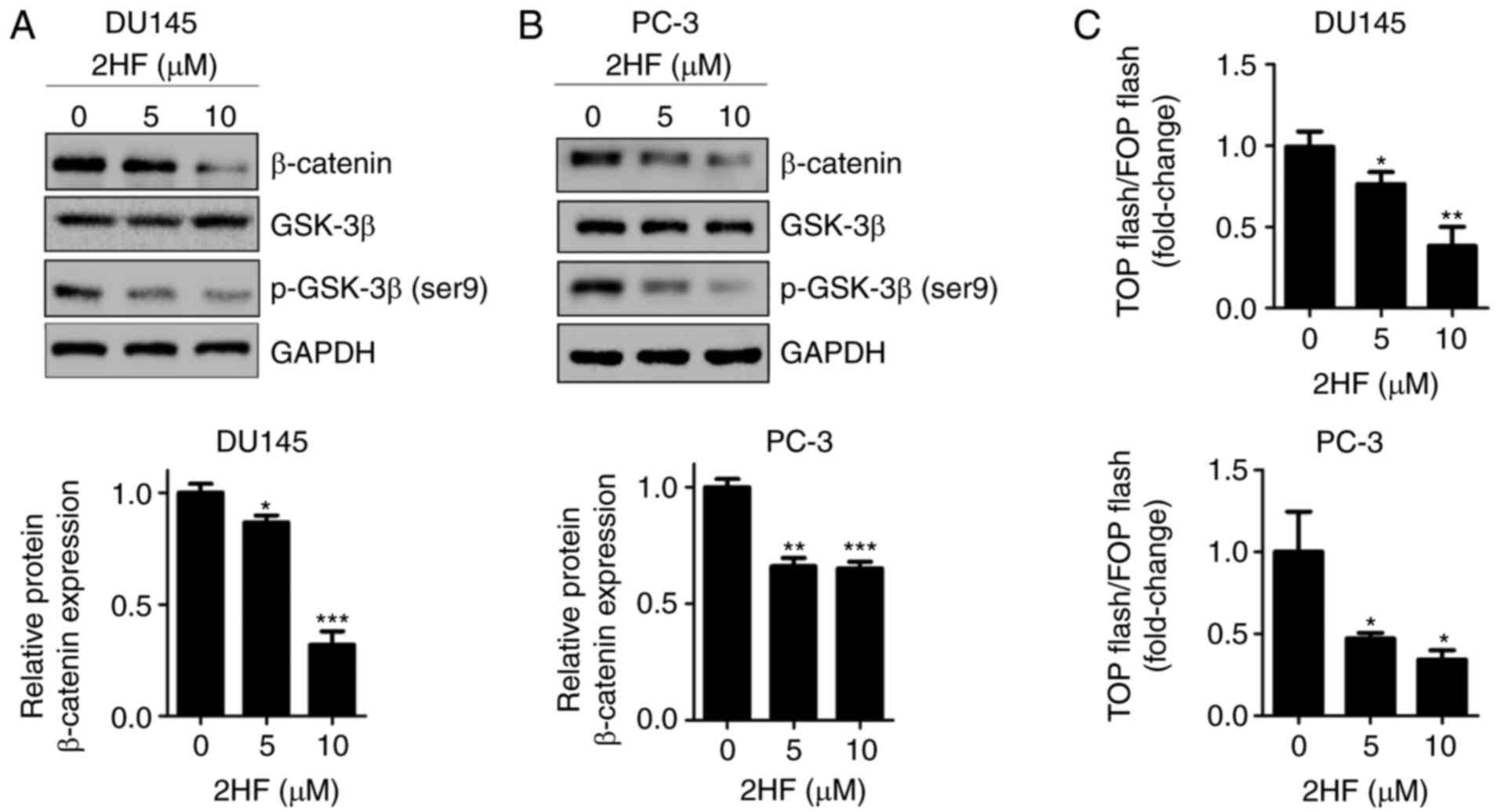
2HF suppresses GSK3β/β-catenin
signaling in PCa cells
The Wnt/β-catenin signaling pathway is crucial for
the regulation of cancer cell migration and invasion (23), in which the APC/Axin/GSK-3β complex
mediates β-catenin phosphorylation and ubiquitination, and then
degrades β-catenin to induce EMT (24,25).
The present study revealed that the expression of β-catenin and
p-GSK-3β (Ser9) was downregulated following 2HF treatment in DU145
and PC-3 cells (Fig. 2A and B).
Since β-catenin is a transcriptional coactivator of TCF/LEF
transcription factors, TOP/FOP luciferase reporter assay was
applied to test the transactivation of β-catenin. The results
demonstrated that the transactivation of β-catenin was inhibited by
2HF treatment (Fig. 2C).
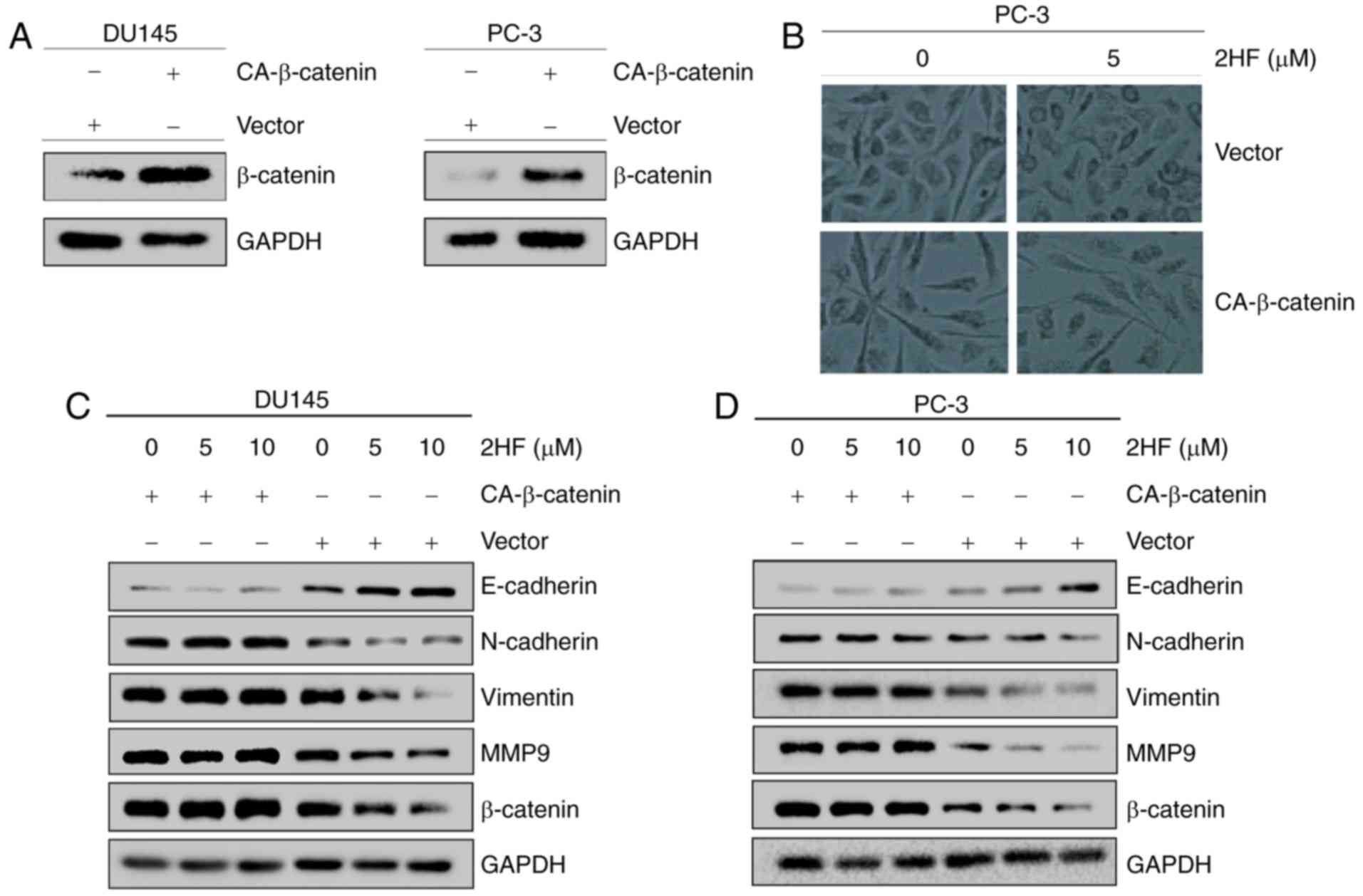
Overexpression of activated β-catenin
rescues the inhibition of EMT following 2HF treatment
To confirm the critical role of β-catenin in EMT,
activated CA-β-catenin was overexpressed in DU145 and PC-3 cells
(Fig. 3A), and the cells were
treated with 2HF. 2HF treatment changed the morphology of PC-3 from
spindle to cobble-stone, but overexpression of β-catenin reversed
it (Fig. 3B). Additionally, the
results demonstrated that overexpression of β-catenin could abolish
the change in EMT-associated makers (i.e., E-cadherin, N-cadherin,
vimentin and MMP9) in DU145 and PC-3 cells following 2HF treatment
(Fig. 3C and D). These results
demonstrated that 2HF reversed EMT via downregulation of β-catenin
in PCa cells.
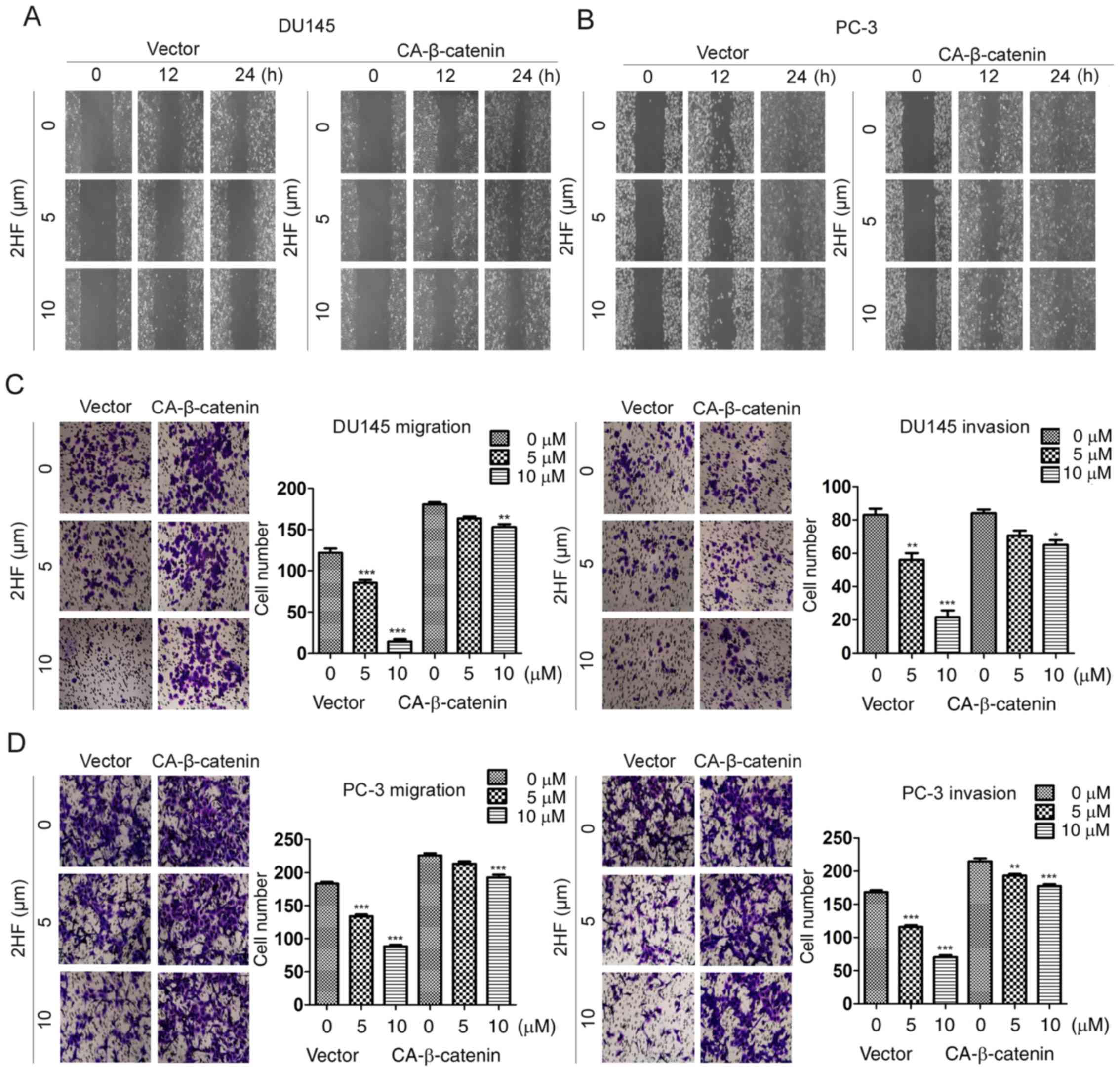
β-catenin reverses the suppression of
cell migration and invasion induced by 2HF treatment in PCa
cells
To further validate the function of β-catenin in
cell migration and invasion, CA-β-catenin-overexpressing DU145 and
PC-3 cell lines were generated and wound healing and Transwell
assays were applied. It was revealed that overexpression of
CA-β-catenin could abolish the suppression of the wound healing
rate following treatment of DU145 or PC-3 cells with 5 or 10 µM 2HF
(Fig. 4A and B). Consistently, the
Transwell assay also revealed that overexpression of β-catenin
could recover the inhibition of cell migration and invasion
following 2HF treatment (Fig. 4C and
D). Therefore, these data supported β-catenin as a critical
factor involved in the regulation of cell migration and invasion in
PCa.
2HF inhibits the expression of
β-catenin and EMT in vivo
To verify the in vitro results suggesting
that 2HF could inhibit EMT via β-catenin, IHC staining was used to
detect E-cadherin, N-cadherin, β-catenin and MMP9 expression in the
PC-3 ×enograft tissues with or without 2HF treatment. The results
demonstrated that 2HF treatment could suppress the expression of
N-cadherin, β-catenin and MMP9, but enhance the expression of
E-cadherin in vivo (Fig. 5),
which supported the in vitro results.
Discussion
Metastatic PCa has been a challenge for patients and
urologists for a long time. Although several drugs, including
docetaxel, abiraterone and enzalutamide, have been successful in
the treatment of PCa, drug resistance eventually occurs. Therefore,
it is necessary to identify novel drugs for metastatic PCa.
Flavonoids have a number of functions to target different types of
cancer, including PCa. Our previous study revealed that 2HF, one of
the natural flavonoids, could inhibit cell proliferation and induce
apoptosis in metastatic PCa cells via suppression of the Akt/STAT3
signaling pathway (17). The
present study further demonstrated that 2HF without any obvious
cytotoxicity, could inhibit EMT, and the cell migration and
invasion of metastatic PCa cells through suppression of the
Wnt/β-catenin signaling pathway.
2HF has been revealed to be a potential therapeutic
drug for the treatment of PCa. For example, 2HF could be a
selective inhibitor of AKR1C3, which is a critical aldo-keto
reductase that translates Δ4-androstene-3,17-dione into
testosterone (26), and 2HF could
also inhibit the transcriptional activity of the androgen receptor
(AR) (27). Based on this study,
2HF may serve as a promising drug for PCa therapy, which may not
only inhibit androgen-AR signaling and induce PCa cell death, but
also prevent cell migration and invasion during PCa metastasis. In
addition, there are several nanodiamonds combined with plant
compounds to improve their anticancer activity (28,29).
The biotechnological application of nanomaterials associated with
2HF is another potential treatment strategy for PCa.
The Wnt/β-catenin signaling pathway serves a crucial
role in cancer progression, including EMT induction (30). GSK-3β and β-catenin are two major
elements in the Wnt signaling pathway (31). When Wnt signals are inactivated,
GSK-3β can form a complex with Axin and APC, which then
phosphorylates and degrades β-catenin (23). When Wnt signals are activated,
GSK-3β is phosphorylated and inactivated, then β-catenin cannot be
degraded or transported into the nucleus. Following combination
with TCF/LEF, β-catenin will bind to the promoter region and
regulate the transcription of different target genes (32). The present study demonstrated that
2HF could inhibit the phosphorylation of GSK-3β and decrease the
expression of β-catenin in metastatic PCa cells, which then
inhibits EMT, and cell migration and invasion in vitro and
in vivo. However, the present study failed to demonstrate
the dose-dependent effect of 2HF in vivo. However, Singhal
et al (33) demonstrated
that the effects of 2HF on tumor growth and angiogenesis, in which
inhibited mesenchymal markers in breast cancer, were dose-dependent
in vivo.
In conclusion, to the best of our knowledge, the
present study was the first to demonstrate that 2HF with no clear
cytotoxicity, could inhibit EMT, and cell migration and invasion of
metastatic PCa cells via suppression of the Wnt/β-catenin signaling
pathway. The results of the present study, together with those of
our previous study and other studies, indicated that 2HF may serve
as a potential natural drug for the treatment of PCa.
Acknowledgements
The authors would like to thank Professor Jer-Tsong
Hsieh (University of Texas Southwestern Medical Center, Dallas, TX,
USA) for providing helpful discussion.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81202014
and 81130041), the ‘New-Star’ Young Scientists Program of Shaanxi
Province (grant no. 2017KJXX-35) and the Fundamental Research Funds
for the Central Universities (grant no. XJJ2017QNGZ).
Availability of data and materials
All data generated and/or analyzed during the
current study are included in this published article.
Authors' contributions
SW, JH, KH, YY and YG performed the experiments and
wrote the manuscript. ZN and XW provided certain reagents and
technical assistance. DH and KW designed this study and revised the
manuscript.
Ethics approval and consent to
participate
All the animal experiments were approved by the
Ethical Committee of Xi'an Jiaotong University (Xi'an, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lassi K and Dawson NA: Emerging therapies
in castrate-resistant prostate cancer. Curr Opin Oncol. 21:260–265.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McConkey DJ, Choi W, Marquis L, Martin F,
Williams MB, Shah J, Svatek R, Das A, Adam L, Kamat A, et al: Role
of epithelial-to-mesenchymal transition (EMT) in drug sensitivity
and metastasis in bladder cancer. Cancer Metastasis Rev.
28:335–344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Katsuno Y, Lamouille S and Derynck R:
TGF-β signaling and epithelial-mesenchymal transition in cancer
progression. Curr Opin Oncol. 25:76–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Timmerman LA, Grego-Bessa J, Raya A,
Bertrán E, Pérez-Pomares JM, Díez J, Aranda S, Palomo S, McCormick
F, Izpisúa-Belmonte JC, et al: Notch promotes
epithelial-mesenchymal transition during cardiac development and
oncogenic transformation. Genes Dev. 18:99–115. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou BP, Deng J, Xia W, Xu J, Li YM,
Gunduz M and Hung MC: Dual regulation of Snail by
GSK-3beta-mediated phosphorylation in control of
epithelial-mesenchymal transition. Nat Cell Biol. 6:931–940. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Impei S, Gismondi A, Canuti L and Canini
A: Metabolic and biological profile of autochthonous Vitis
vinifera L. ecotypes. Food Funct. 6:1526–1538. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kinghorn AD, Su BN, Jang DS, Chang LC, Lee
D, Gu JQ, Carcache-Blanco EJ, Pawlus AD, Lee SK, Park EJ, et al:
Natural inhibitors of carcinogenesis. Planta Med. 70:691–705. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh B, Bhat TK and Singh B: Potential
therapeutic applications of some antinutritional plant secondary
metabolites. J Agric Food Chem. 51:5579–5597. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Masuda T, Miura Y, Inai M and Masuda A:
Enhancing effect of a cysteinyl thiol on the antioxidant activity
of flavonoids and identification of the antioxidative thiol adducts
of myricetin. Biosci Biotechnol Biochem. 77:1753–1758. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuhlmann CR, Schaefer CA, Kosok C,
Abdallah Y, Walther S, Lüdders DW, Neumann T, Tillmanns H, Schäfer
C, Piper HM, et al: Quercetin-induced induction of the NO/cGMP
pathway depends on Ca2+-activated K+
channel-induced hyperpolarization-mediated Ca2+-entry
into cultured human endothelial cells. Planta Med. 71:520–524.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chou TC: Anti-inflammatory and analgesic
effects of paeonol in carrageenan-evoked thermal hyperalgesia. Br J
Pharmacol. 139:1146–1152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin Z and MacDonald RS: Soy isoflavones
increase latency of spontaneous mammary tumors in mice. J Nutr.
132:3186–3190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piret JP, Mottet D, Raes M and Michiels C:
CoCl2, a chemical inducer of hypoxia-inducible factor-1,
and hypoxia reduce apoptotic cell death in hepatoma cell line
HepG2. Ann NY Acad Sci. 973:443–447. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu K, Ning Z, Zhou J, Wang B, Fan J, Zhu
J, Gao Y, Wang X, Hsieh JT and He D: 2′-Hydroxyflavanone inhibits
prostate tumor growth through inactivation of AKT/STAT3 signaling
and induction of cell apoptosis. Oncol Rep. 32:131–138. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singhal SS, Singhal J, Figarola JL, Riggs
A, Horne D and Awasthi S: 2′-Hydroxyflavanone: A promising molecule
for kidney cancer prevention. Biochem Pharmacol. 96:151–158. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hsiao YC, Kuo WH, Chen PN, Chang HR, Lin
TH, Yang WE, Hsieh YS and Chu SC: Flavanone and 2′-OH flavanone
inhibit metastasis of lung cancer cells via down-regulation of
proteinases activities and MAPK pathway. Chem Biol Interact.
167:193–206. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shin SY, Kim JH, Lee JH, Lim Y and Lee YH:
2′-Hydroxyflavanone induces apoptosis through Egr-1 involving
expression of Bax, p21, and NAG-1 in colon cancer cells. Mol Nutr
Food Res. 56:761–774. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu KH, Chen PN, Lue KH, Lai MT, Lin MS,
Hsieh YS and Chu SC: 2′-hydroxyflavanone induces apoptosis of human
osteosarcoma 143 B cells by activating the extrinsic TRAIL- and
intrinsic mitochondria-mediated pathways. Nutr Cancer. 66:625–635.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen W, Zhou J, Wu K, Huang J, Ding Y, Yun
EJ, Wang B, Ding C, Hernandez E, Santoyo J, et al: Targeting
XBP1-mediated β-catenin expression associated with bladder cancer
with newly synthetic Oridonin analogues. Oncotarget. 7:56842–56854.
2016.PubMed/NCBI
|
|
23
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu C, Zhuang Y, Jiang S, Liu S, Zhou J, Wu
J, Teng Y, Xia B, Wang R and Zou X: Interaction between
Wnt/beta-catenin pathway and microRNAs regulates
epithelial-mesenchymal transition in gastric cancer (Review). Int J
Oncol. 48:2236–2246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Faux MC, Coates JL, Kershaw NJ, Layton MJ
and Burgess AW: Independent interactions of phosphorylated
β-catenin with E-cadherin at cell-cell contacts and APC at cell
protrusions. PLoS One. 5:e141272010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Adeniji AO, Chen M and Penning TM: AKR1C3
as a target in castrate resistant prostate cancer. J Steroid
Biochem Mol Biol. 137:136–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ofude M, Mizokami A, Kumaki M, Izumi K,
Konaka H, Kadono Y, Kitagawa Y, Shin M, Zhang J, Keller ET, et al:
Repression of cell proliferation and androgen receptor activity in
prostate cancer cells by 2′-hydroxyflavanone. Anticancer Res.
33:4453–4461. 2013.PubMed/NCBI
|
|
28
|
Gismondi A, Nanni V, Reina G, Orlanducci
S, Terranova ML and Canini A: Nanodiamonds coupled with
5,7-dimethoxycoumarin, a plant bioactive metabolite, interfere with
the mitotic process in B16F10 cells altering the actin
organization. Int J Nanomedicine. 11:557–574. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu MX, Wang M and Yang WW: Gold-quercetin
nanoparticles prevent metabolic endotoxemia-induced kidney injury
by regulating TLR4/NF-kappaB signaling and Nrf2 pathway in high fat
diet fed mice. Int J Nanomedicine. 12:327–345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang J, Tian XJ and Xing J: Signal
transduction pathways of EMT induced by TGF-β, SHH, and WNT and
their crosstalks. J Clin Med. 5:E412016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Angers S and Moon RT: Proximal events in
Wnt signal transduction. Nat Rev Mol Cell Biol. 10:468–477. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Singhal J, Nagaprashantha L, Chikara S,
Awasthi S, Horne D and Singhal SS: 2′-Hydroxyflavanone: A novel
strategy for targeting breast cancer. Oncotarget. 8:75025–75037.
2017. View Article : Google Scholar : PubMed/NCBI
|