Introduction
Glioblastoma (GBM) is the most common primary brain
tumor (1). According to the World
Health Organization classification, it is considered a grade IV
malignancy, and its standard treatment entails surgery,
radiotherapy and chemotherapy (2,3).
However, the diffusely infiltrative growth pattern of GBM typically
prevents total surgical resection, resulting in rapid tumor
recurrence (4). Despite
improvements in diagnostic and therapeutic strategies, the clinical
prognosis for patients with high-grade gliomas remains poor, with a
typical median survival of <16 months (2). Temozolomide, a chemotherapeutic agent
commonly used against gliomas, only extends patient survival by a
few months (2,5,6).
Therefore, intensive research is now focused on the development of
improved treatment strategies.
Tumor hypoxia is a pivotal factor involved in
promoting GBM progression and its marked resistance to radiation
and chemotherapy (7–9). An insufficient oxygen supply prompts a
number of adverse metabolic changes and intensifies angiogenesis
and apoptosis (10). These changes
are predominantly regulated by hypoxia-inducible factor-1 (HIF-1),
which comprises two subunits: α and β (8,11,12).
HIF-1α expression strongly depends on the cell oxygenation level
(13).
Hyperbaric oxygen (HBO) therapy improves neoplastic
tissue oxygenation and inhibits HIF-1α activity (14–16).
Theoretically, improved oxygenated tumor tissue may become more
susceptible to anticancer therapies. HBO is widely used as an
adjunctive treatment for various pathological states, including
inadequately healing wounds, decompression sickness and carbon
monoxide poisoning (17). Previous
studies have investigated its use in oncology in combination with
radiotherapy, chemotherapy, photodynamic therapy and surgery
(10,14,16,18).
Although some of these studies have reached the clinical trial
phase, HBO is not yet widely applied in cancer treatment.
Tumor progression is commonly accompanied with the
development of resistance mechanisms to medical treatment, which
suggests more effective chemotherapeutic agents for innovative
treatments are necessary (4).
Heterocyclic isothiourea derivatives, also called
pentabromobenzyl-isothioureas (ZKKs), are a novel promising group
of cytotoxic compounds. Their proapoptotic and cytotoxic properties
toward various tumor cells, including GBM, have been demonstrated
in vitro (19,20). ZKKs have a chemical structure
similar to casein kinase 2 (CK2) inhibitors, including
benzotriazoles (TBB) and benzimidazoles (TBI and DMAT) (21). However, ZKKs do not effectively
inhibit CK2 activity. Studies using a wide panel of protein kinases
have indicated that N,N′-dimethyl-S-(2,3,4,5,6-pentabromobenzyl)-
isothiouronium bromide (ZKK-3) specifically inhibits kinases other
than CK2, including protein kinase D1 (PKD1) (21,22).
Notably, PKD1 mediates the detoxification of mitochondrial reactive
oxygen and nitrogen species, protecting cells from oxidative stress
(23). Disruption of PKD1
expression can promote the development of numerous pathological
states, including neoplastic processes (24,25).
In the present study, the effects of various oxygen
conditions on the cytotoxic potential of ZKK-3 against the T98G GBM
cell line were examined. Cells were maintained under conditions of
normoxia, anoxia, hypoxia, hyperbaric oxygen (HBO),
hypoxia/hypoxia, and hypoxia/HBO, and ZKK-3 was applied at
concentrations of 10, 25 and 50 µM. The cell proliferation and
viability, and protein expression levels of HIF-1α, PKD1,
phosphorylated (p)PKD1 (Ser 916) and pPKD1 (Ser 744/748) kinases
were evaluated.
Materials and methods
Cell line
Experiments were conducted using the human GBM T98G
cell line (American Type Culture Collection, Manassas, VA, USA).
Cells were maintained at 37°C in an atmosphere containing 95%
absolute humidity and 95% air/5% CO2 in minimum
essential media (MEM; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) supplemented with 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 1%
penicillin/streptomycin solution (Gibco; Thermo Fisher Scientific,
Inc.) and 1% non-essential amino acid solution (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany).
Examined compound and oxygen
conditions
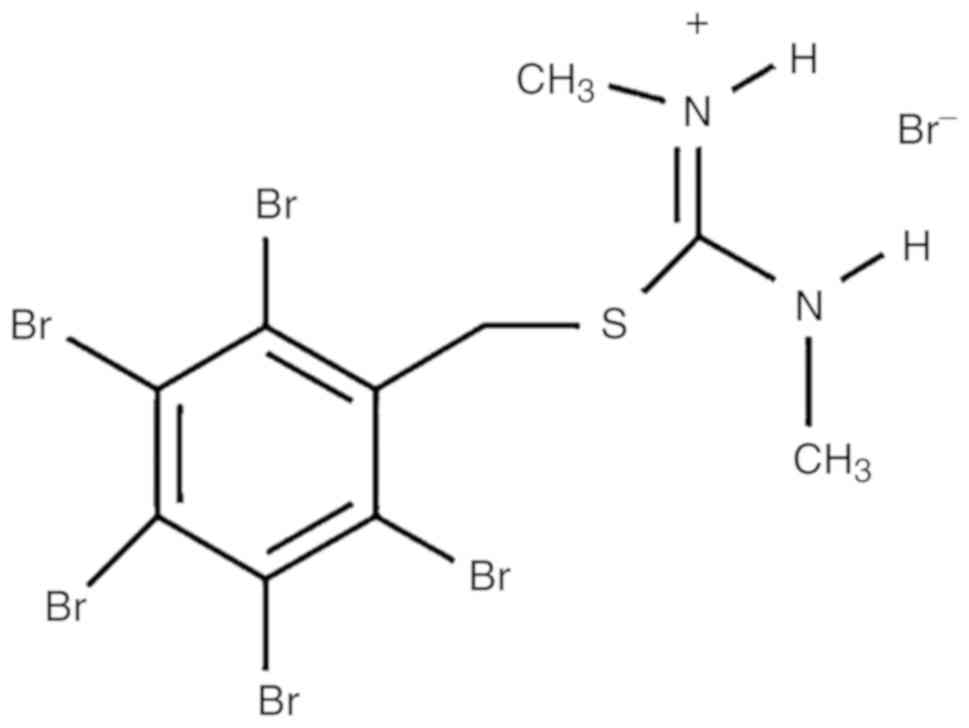
The modified isothiourea derivative ZKK-3 (Fig. 1) was synthesized by Professor
Zygmunt Kazimierczuk according to a previously described procedure
(20). The compound was dissolved
in dimethyl sulfoxide (DMSO; AppliChem GmbH, Darmstadt, Germany)
and added to the culture medium at concentrations of 10, 25 and 50
µM. Control cultures were grown in standard conditions with DMSO
but without ZKK-3 application (0 µM).
Cells were cultured under different gas mixtures
with varying oxygen contents as follows: Normoxia (21%
O2/5% CO2/74% N2 was applied for
24 h post-ZKK-3 treatment), anoxia (5% CO2/95%
N2 was applied for 24 h post-ZKK-3 treatment); hypoxia
(1% O2/5% CO2/94% N2 was applied
for 24 h post-ZKK-3 treatment); HBO (97.5%O2/2.5%
CO2 under pressure of 2 ATA was applied for 1 h
post-ZKK-3 treatment, which was followed by 23 h of normoxia);
hypoxia/hypoxia (double hypoxia; hypoxic gas (1% O2/5%
CO2/94% N2) was applied for 24 h prior to
ZKK-3 treatment, and then for an additional 24 h post-ZKK-3
treatment); and hypoxia/hyperbaric oxygen (hypoxia/HBO; hypoxia was
applied for 24 h prior to ZKK-3 treatment, and HBO was applied
post-ZKK-3 treatment). Anoxia and hypoxia experiments were
performed in a Modular Incubator Chamber (MIC-101;
Billups-Rothenberg, San Diego, CA, USA), whereas HBO experiments
were conducted using a hyperbaric chamber (own design).
Cell proliferation rate
assessment
T98G cells, seeded in dishes (6-cm diameter) at a
density 1.2×105 cells/dish, were incubated using a
HERAcell 150i CO2 Incubator (Thermo Fisher Scientific,
Inc.) for 24 h with ZKK-3 at concentrations of 10, 25 and 50 µM, at
37°C under various oxygen conditions, and the number of cells was
subsequently determined. For this process, the medium was removed,
and the cells were washed with phosphate-buffered saline (PBS),
trypsinized with 0.05% Trypsin-EDTA (both from Gibco; Thermo Fisher
Scientific, Inc.), and rotated for 10 min at 200 × g at 4°C
(Laboratory Centrifuge MPW-350R; MPW Med. Instruments, Warsaw,
Poland). Following this, the pellet was suspended in 1 ml MEM and 4
ml Coulter Isoton II Diluent (Beckman Coulter, Inc., Indianapolis,
IN, USA). The cell numbers were counted using a Multisizer 3 cell
counter (Beckman Coulter, Inc.). Control cells were grown under the
examined oxygen conditions without ZKK-3. These investigations
comprised at least six independent experiments with three
repetitions in each (n≥18). Pairwise comparisons of T98G
cell proliferation under different oxygen conditions were performed
as follows: Normoxia vs. anoxia, normoxia vs. hypoxia, normoxia vs.
HBO and hypoxia vs. HBO.
Cell viability assay
T98G cells, seeded in 96-well plates at a density
5×103 cells/well, were incubated for 24 and 48 h with
ZKK-3 at concentrations of 10, 25 and 50 µM at 37°C under various
oxygen conditions and the viability was subsequently determined. In
the 24 h examination, cells were treated with the CellTiter 96
AQueous One Solution Cell Proliferation Assay (Promega
Corporation, Madison, WI, USA) and incubated at 37°C for 3 h. The
absorbance was measured at 490 nm using spectrophotometer (Epoch
Microplate Reader; BioTek Instruments, Inc., Winooski, VT, USA). In
the 48 h test, following the first 24 h incubation, the culture
medium was replaced with fresh MEM containing ZKK-3, and cells were
again incubated for 24 h at 37°C under the examined oxygen
conditions. Following this, the viability was assessed using the
CellTiter 96 AQueous One Solution Cell Proliferation
Assay and spectrophotometer, as described above. Control groups
included cells sustained under the examined oxygen conditions
without ZKK-3. The study comprised at least five independent
experiments with 10 repetitions in each (n≥50). Pairwise
comparisons of the viability of T98G cells under different oxygen
conditions were performed as follows: Normoxia vs. anoxia, normoxia
vs. hypoxia, normoxia vs. HBO and hypoxia vs. HBO.
Determination of HIF-1α expression
level
T98G cells were cultured with the examined compound
under various oxygen conditions and lysed using the
radioimmunoprecipitation assay lysis buffer system (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) according to the protocol of
the manufacturer. HIF-1α expression was assessed by determining the
HIF-1α protein expression level in cell lysates using the HIF-1A
ELISA kit (cat. no. EHIF1A5; Thermo Fisher Scientific Inc.)
according to the protocol of the manufacturer. The total protein
level in the cell lysates was evaluated using the Pierce BCA
Protein Assay Kit (Thermo Fisher Scientific, Inc.) according to the
protocol of the manufacturer.
Determination of the expression levels
of PKD1 and its phosphorylated forms
The expression levels of PKD1 and its phosphorylated
forms were evaluated using western blot analysis. Cells were lysed
using the RIPA Lysis Buffer System (Santa Cruz Biotechnology, Inc.)
according to the protocol of the manufacturer, and the total
protein level in the cell lysates was evaluated using the Pierce
BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.) according to
the protocol of the manufacturer. Cell lysates, containing total
protein, were mixed with Laemmli 2X Concentrate Sample Buffer
(Sigma-Aldrich; Merck KGaA) and loaded (20 µg total protein per
lane) onto Mini-PROTEAN TGX Precast Gels (8–16%; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Following this, the
proteins were electrotransferred onto nitrocellulose membranes
(Bio-Rad Laboratories, Inc.). The membranes were washed in PBS,
blocked for 1 h in non-fat 5% milk (for PKD1) or 5% bovine serum
albumin (Sigma-Aldrich; Merck KGaA) for phosphorylated forms of
PKD1 at 4°C, and subsequently incubated for 15 h at 4°C with
primary antibodies against PKD/PKCµ (cat. no. 2052; 1:500 dilution;
polyclonal, rabbit; Cell Signaling Technology, Inc., Danvers, MA,
USA), Phospho-PKD/PKCµ (Ser 916) (cat. no. 2051; 1:1,000 dilution;
polyclonal, rabbit; Cell Signaling Technology, Inc., Danvers, MA,
USA), or Phospho-PKD/PKCµ (Ser 744/748) (cat. no. 2054; 1:1,000
dilution; polyclonal, rabbit; Cell Signaling Technology, Inc.).
Following this, the membranes were washed three times in
Tris-buffered saline with Tween-20 washing buffer and then twice in
Tris-buffered saline washing buffer. The membranes were
subsequently incubated at 37°C for 2 h with secondary anti-rabbit
IgG, horseradish peroxidase (HRP)-linked antibody (cat. no. 7074;
1:1,000 dilution; polyclonal, goat anti-rabbit; Cell Signaling
Technology, Inc.). Protein bands were detected using Amersham ECL
Prime Western Blotting Detection Reagent (GE Healthcare Life
Sciences, Little Chalfont, UK), Carestream Medical X-ray film,
Carestream Dental X-ray Developer and Carestream Dental X-ray Fixer
(Carestream Dental; Carestream Health Inc., Rochester, NY, USA).
Monoclonal anti-β-actin clone AC-15 (cat. no. A5441; 1:20,000
dilution; mouse; Sigma-Aldrich; Merck KGaA) and secondary chicken
anti-mouse IgG-HRP (cat. no. sc-2954; 1:5,000 dilution; polyclonal;
Santa Cruz Biotechnology, Inc.) were used as loading controls. The
temperature and duration of incubation were the same as those
indicated for primary and secondary antibodies indicated above,
respectively. The grey value of the protein bands was determined
using ImageJ 1.50i software (National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
Statistical analysis of the data was performed using
one-way analysis of variance and the Tukeys post hoc test. Results
were expressed as the mean ± standard error. P<0.05 was
considered to indicate a statistically significant difference.
Results
Cell proliferation rate
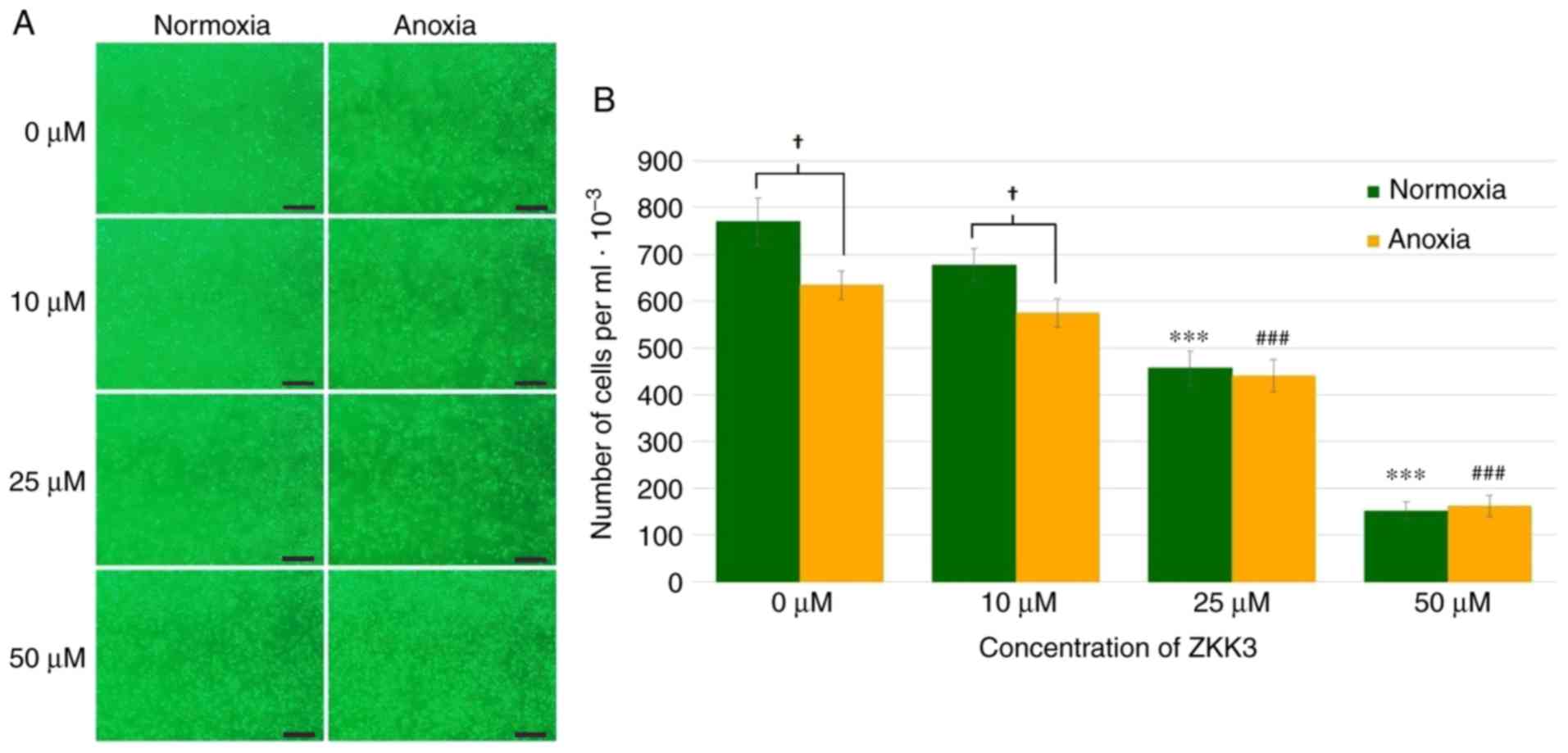
Application of 25 and 50 µM ZKK-3 resulted in a
markedly decreased numbers of cells compared with the controls
under normoxia and anoxia, respectively (Fig. 2A). In cells without ZKK-3 or those
treated with 10 µM ZKK-3, markedly reduced proliferation under
oxygen deprivation was indicated when compared with standard
conditions. Statistical analysis revealed that the application of
25 and 50 µM ZKK-3 significantly decreased cell proliferation by 41
and 80% under normoxia, and by 31 and 75% under anoxia,
respectively, compared with the controls (Fig. 2B). Furthermore, anoxia alone or
combined with 10 µM ZKK-3 caused the number of living cells to
significantly decrease by 18 and 15%, respectively, relative to
standard culture conditions. With 25 and 50 µM ZKK-3 treatment,
cell proliferation was similar under both oxygen conditions.
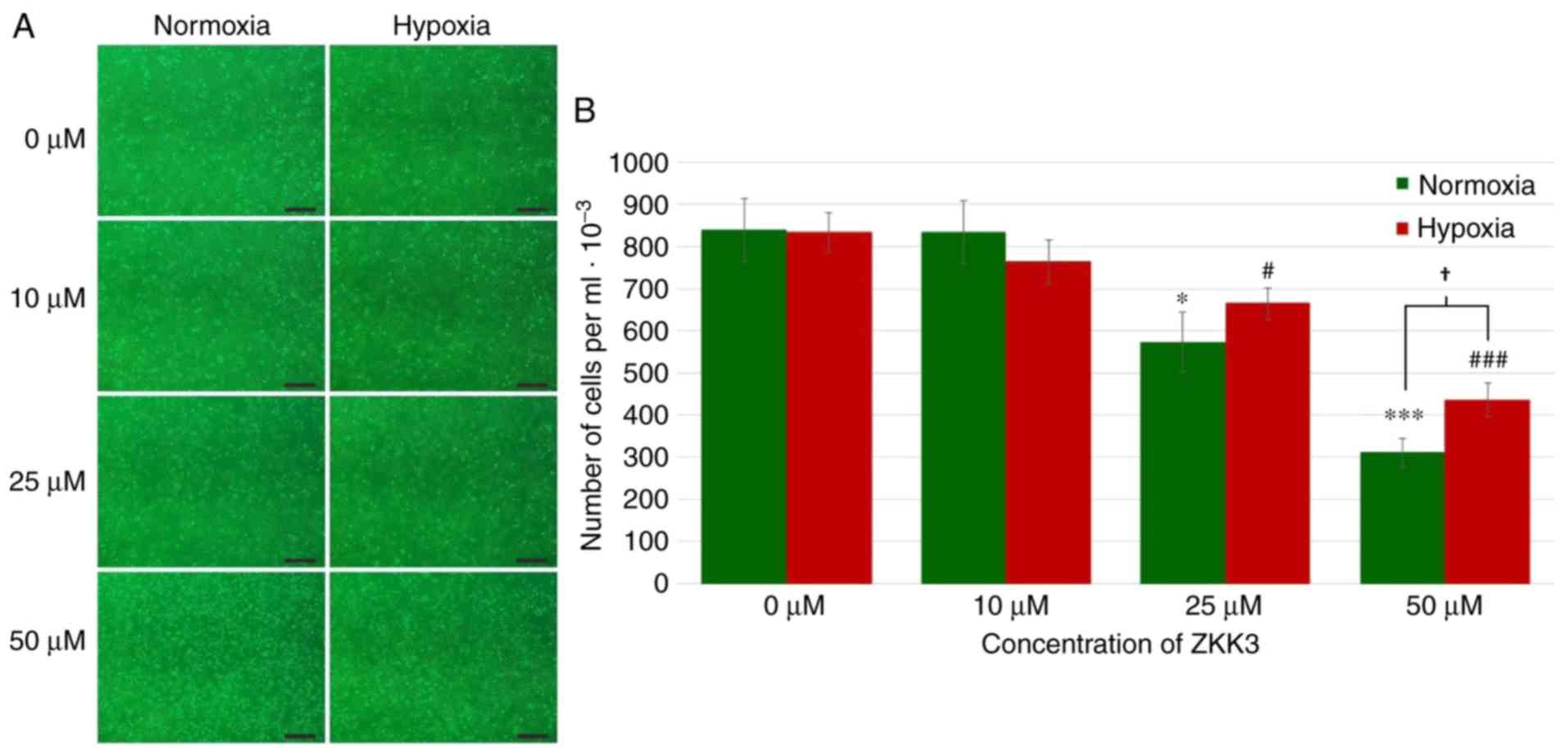
Under standard conditions, the number of cells
markedly decreased with increasing ZKK-3 concentrations (Fig. 3A). However, under hypoxia, cell
proliferation was not significantly changed under specific ZKK-3
concentrations. Statistical analysis revealed that the number of
T98G cells declined with increasing ZKK-3 concentrations under
normoxic and hypoxic conditions when compared with the controls.
Notably, this decrease was statistically significant for cells
treated with 25 and 50 µM ZKK-3 under normoxia (32 and 63%,
respectively) and hypoxia (20 and 48%, respectively) compared with
the controls. With 50 µM ZKK-3, there were 40% more cells under
hypoxia compared with cells under normoxia. With other ZKK-3
concentrations, no significant differences were observed between
the number of cells under hypoxia when compared with normoxia
(Fig. 3B).
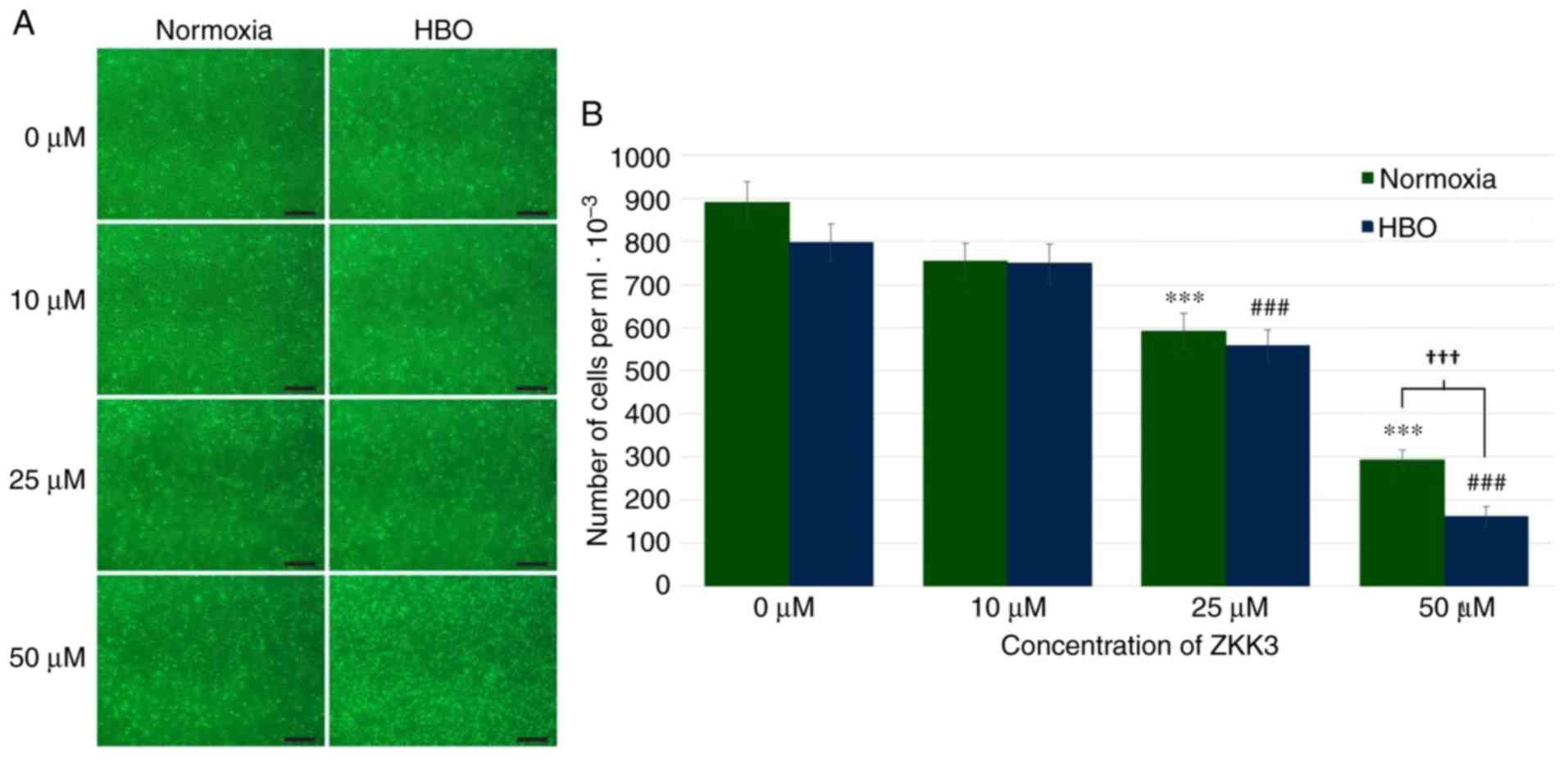
In control groups and groups treated with 10 µM
ZKK-3, similar numbers of GBM cells were observed under normoxia
and HBO conditions (Fig. 4A).
Incubation with 25 and 50 µM ZKK-3 resulted in diminished
proliferation compared with the respective controls, particularly
following exposure to HBO. Statistical analysis of the
proliferation assay results confirmed that application of 25 and 50
µM ZKK-3 combined with HBO significantly reduced the number of GBM
cells (Fig. 4B). Notably, under
normoxia and HBO, respectively, treatment with 25 µM ZKK-3
significantly reduced proliferation to 66 and 70% of control cells,
while 50 µM ZKK-3 significantly reduced proliferation to 33 and 20%
of control cells. A statistically significant 45% difference in the
extent of cell number reductions with 50 µM ZKK-3 was identified
between normoxia and HBO conditions.
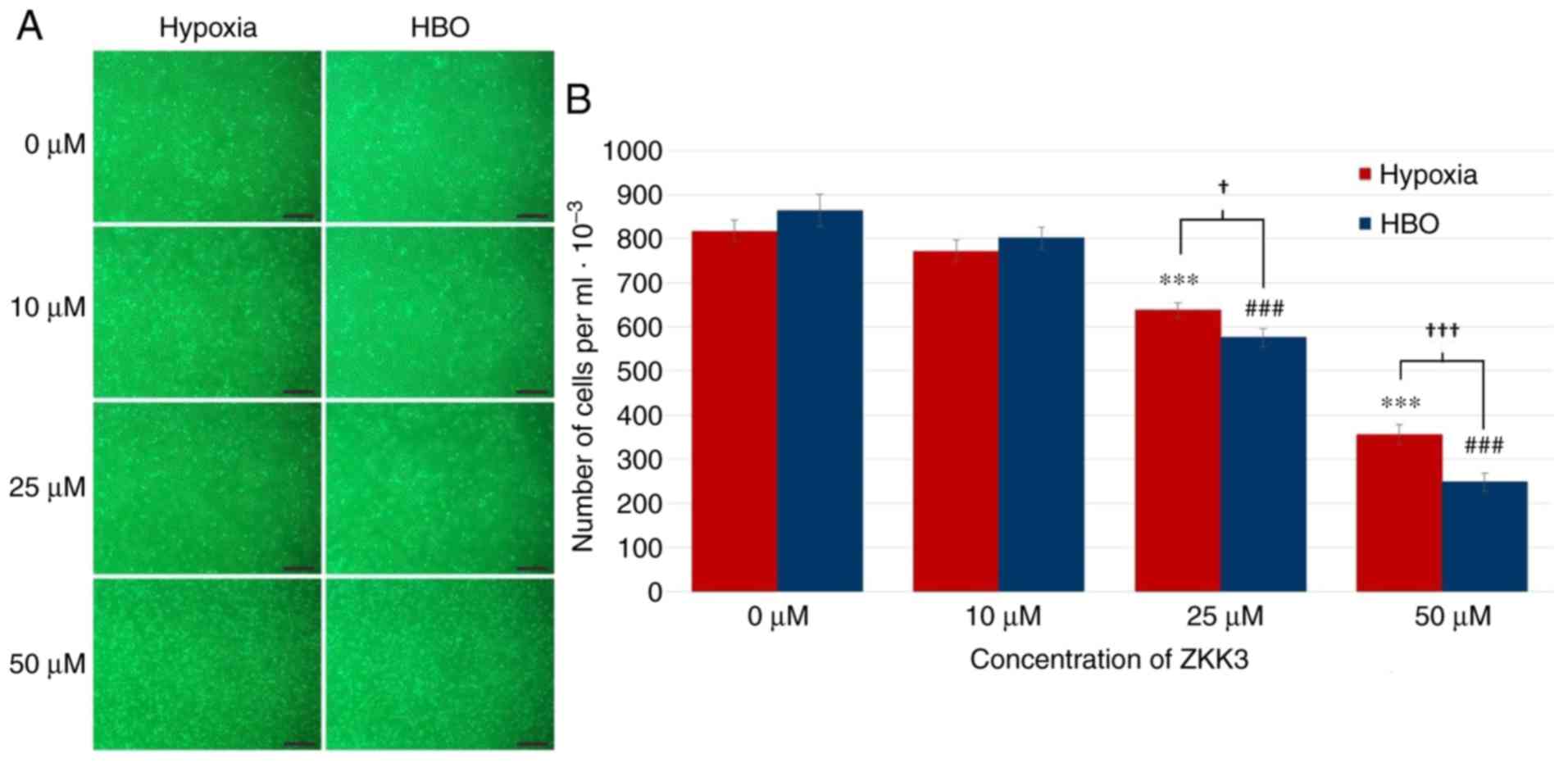
Following incubation with 25 and 50 µM ZKK-3, the
number of morphologically intact T98G cells was reduced under HBO
compared with hypoxia (Fig. 5A). In
control groups and cultures treated with 10 µM ZKK-3, cell numbers
were similar under hypoxic and HBO conditions. Statistical analysis
revealed that treatment with 25 and 50 µM ZKK-3 significantly
reduced the number of neoplastic cells to 78 and 43% of control
under hypoxia, and to 67 and 29% of control under HBO, respectively
(Fig. 5B). For these ZKK-3
concentrations, the decrease in cell number was 10 and 30% greater,
respectively, under HBO when compared with hypoxic conditions.
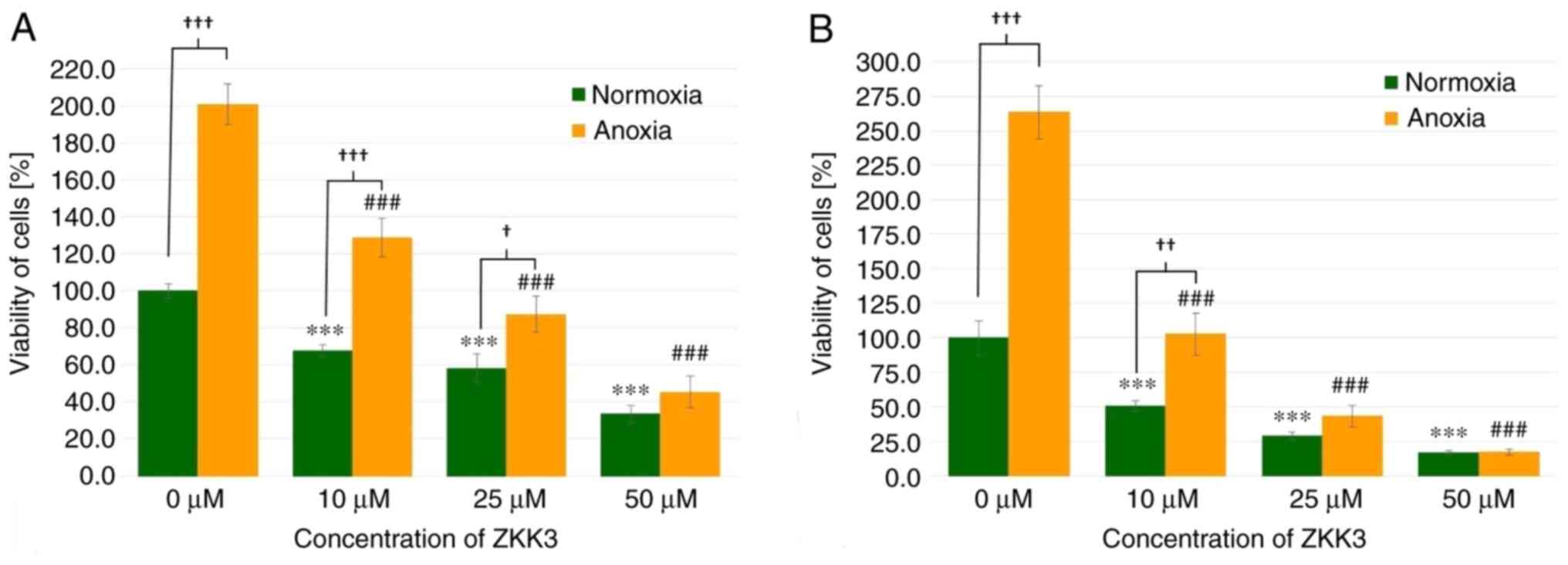
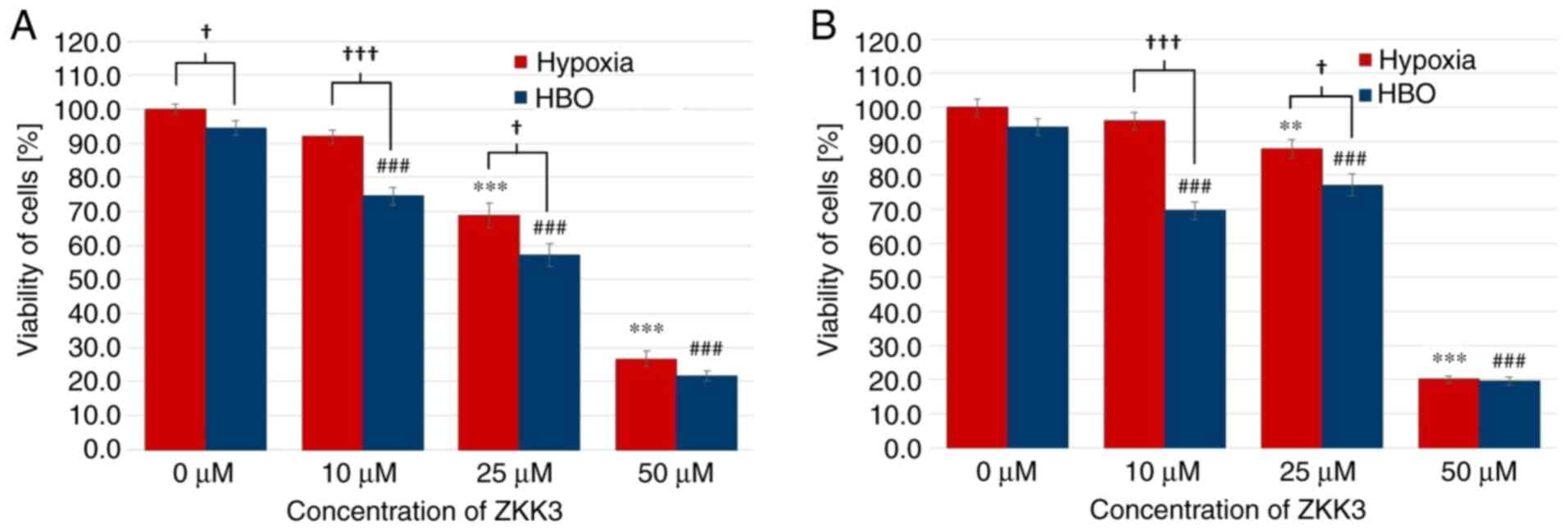
Cell viability
GBM cell viability was significantly diminished
following 24 h of incubation with 10, 25 and 50 µM of ZKK-3 under
normoxia (by 32, 42 and 67%, respectively) as well as under anoxia
(by 36, 57, and 77%, respectively) when compared with the controls
(Fig. 6A). The number of living
cells significantly differed between standard and anoxic conditions
in control groups (increased by 101%) and groups that were treated
with 10 or 25 µM ZKK-3 (increased by 90 and 50%, respectively). In
the 48 h experiment, 10, 25 and 50 µM ZKK-3 treatment resulted in
significantly decreased cell viability, with decreases of 49, 71
and 83% under normoxia, and 61, 83 and 93% under anoxia,
respectively, when compared with the controls (Fig. 6B). However, the number of living
cells was more than twice greater under anoxic conditions, alone or
with 10 µM ZKK-3 when compared with normoxia.
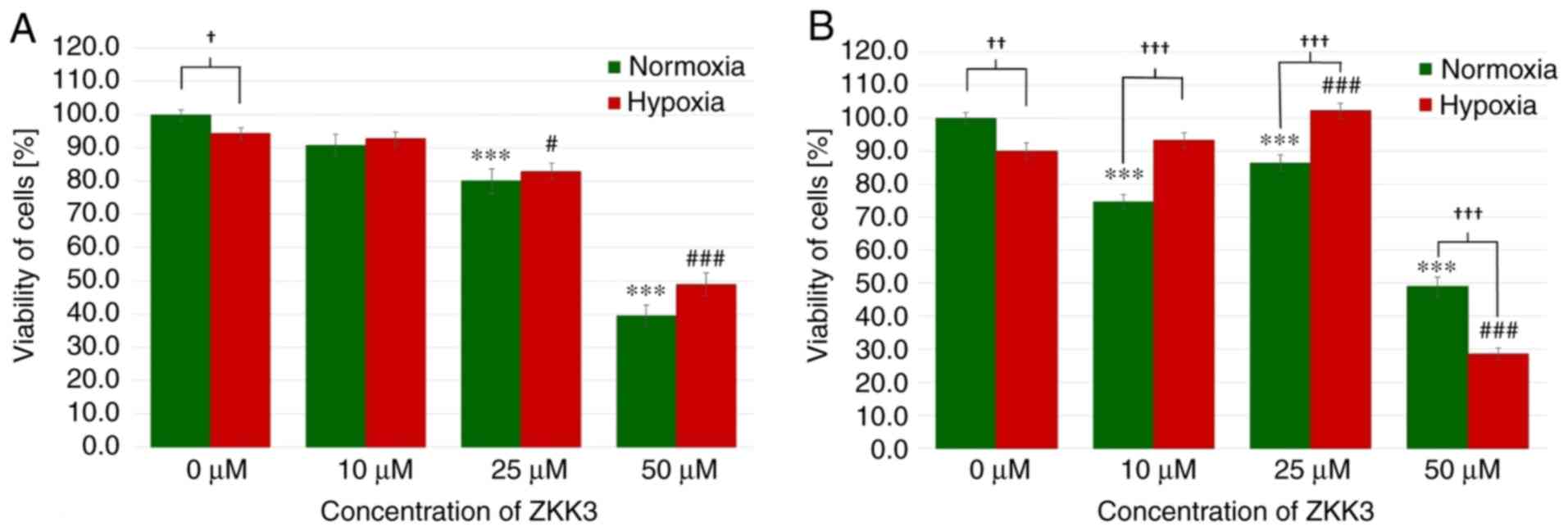
Following an incubation of 24 h under normoxic and
hypoxic conditions, the number of T98G cells significantly
decreased with 25 and 50 µM ZKK-3 treatment (Fig. 7A). With 25 and 50 µM ZKK-3
treatment, cell numbers were significantly reduced to 80 and 40% of
vehicle control under standard conditions, and to 88 and 52% of
vehicle control under hypoxia, respectively. In control groups,
cell viability was significantly reduced under hypoxia compared
with normoxia, whereas no significant differences were observed in
cells with ZKK-3 supplementation under hypoxia compared with
normoxia. Following 48 h of incubation, the cell viability under
normoxia was significantly diminished following treatment with 10,
25 and 50 µM ZKK-3 (by 25, 13 and 51%, respectively) when compared
with the control, whereas cell viability under hypoxia was
significantly decreased only when cells were treated with 50 µM
ZKK-3 (by 68%) when compared with the control (Fig. 7B). Notably, under hypoxia the cell
viability was significantly increased at 25 µM ZKK-3 when compared
with the respective control. Compared with normoxic conditions, the
number of cells under hypoxia was significantly reduced by 10%
without ZKK-3 application, and 41% reduced with application of 50
µM ZKK-3. However, the number of cells under hypoxia was
significantly elevated following application of 10 and 25 µM ZKK-3
when compared with normoxia conditions.
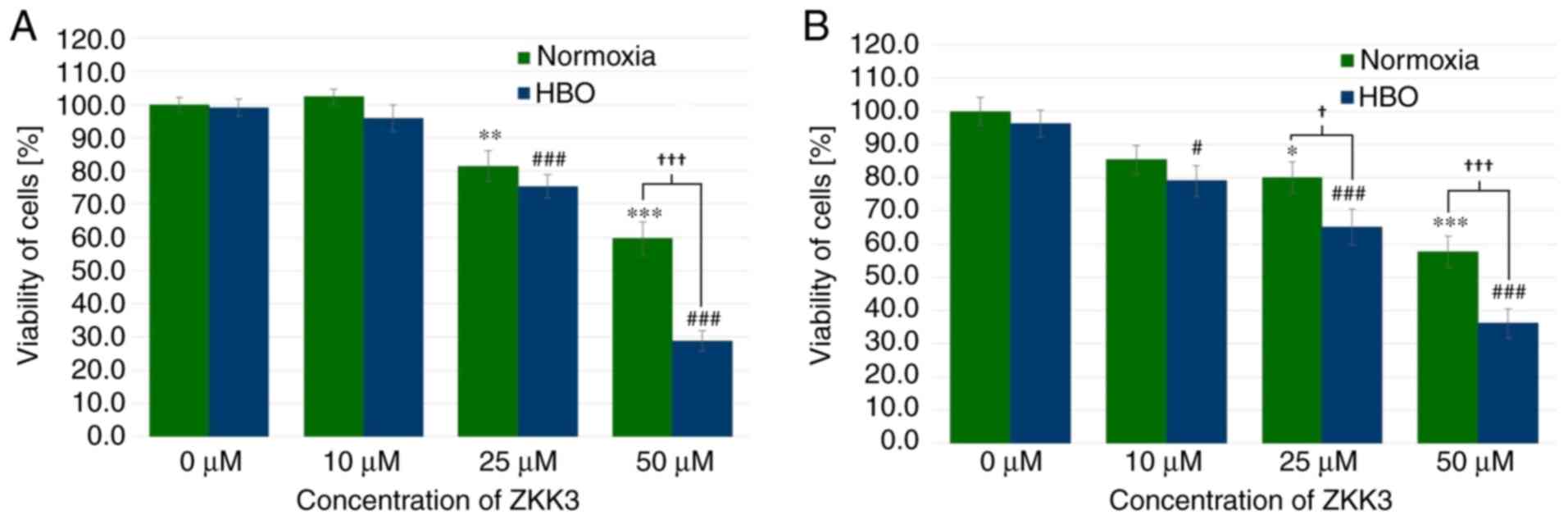
Under normoxic and HBO conditions at 24 h, GBM cell
viability was significantly decreased with 25 µM ZKK-3 treatment
(by 19 and 24%, respectively), as well as 50 µM ZKK-3 treatment (40
and 71%, respectively) compared with the controls (Fig. 8A). Furthermore, a statistically
significant difference of 52% was observed between the decrease of
the number of cells in normoxic compared with HBO conditions when
using the highest ZKK-3 concentration. Extending the ZKK-3 exposure
time to 48 h resulted in a significant reduction of cell viability
to 80 and 58% of control under normoxia with 25 and 50 µM ZKK-3,
respectively, and to 82, 68 and 37% of control under HBO with 10,
25, and 50 µM, respectively (Fig.
8B). The number of living cells was significantly reduced by 19
and 37% with 25 and 50 µM ZKK-3, respectively, under HBO compared
with normoxic conditions.
In the 24 h experiment, T98G cell viability was
significantly decreased with 25 and 50 µM ZKK-3 under hypoxia
(decreases of 31 and 73%, respectively), and with 10, 25 and 50 µM
ZKK-3 under HBO (decreases of 21, 40 and 77%, respectively) when
compared with the controls. In the HBO group, the number of living
cells was lower than that in the hypoxia group, with decreases of
19% in the control groups, and of 17 and 19% when cells were
treated with 10 and 25 µM ZKK-3, respectively. Similar alterations
were observed in 48 h incubations with ZKK-3 (Fig. 9B). Under hypoxia, cell viability
significantly decreased with 25 and 50 µM ZKK-3 treatment (by 12
and 80%, respectively) compared with the control. By contrast,
under HBO conditions, the living cell number was significantly
diminished following the application of 10, 25 and 50 µM ZKK-3 (by
26, 18 and 79%, respectively) compared with the control. Compared
with hypoxia, cell viability under HBO conditions was diminished by
28 and 12% with 10 and 25 µM ZKK-3.
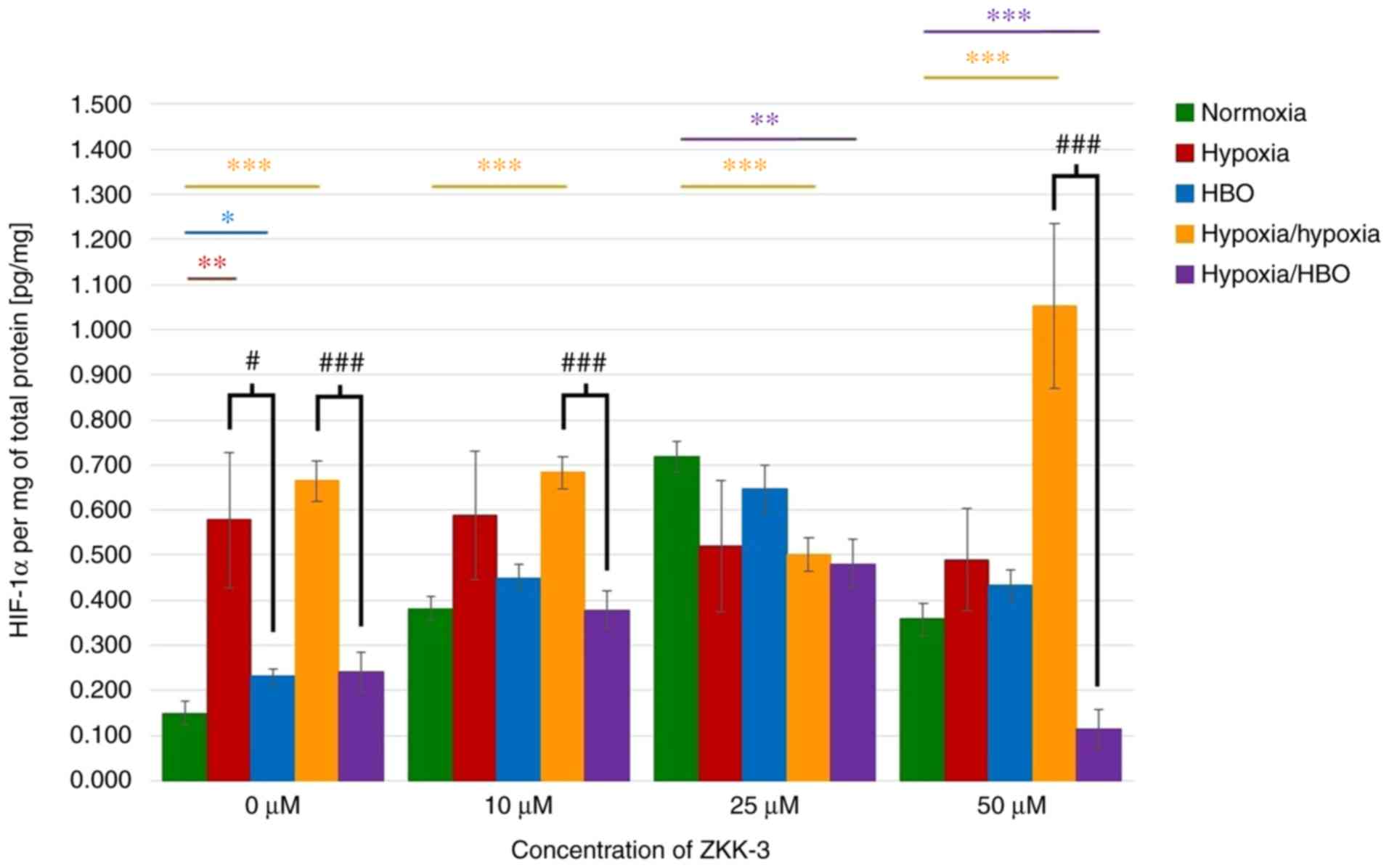
Expression levels of HIF-1α
HIF-1α expression levels in lysates of cells
cultured without ZKK-3 were analyzed. It was identified that the
HIF protein expression levels were significantly increased under
hypoxia (287%), HBO (56%), and hypoxia/hypoxia (346%) compared with
cells under normoxia (Fig. 10).
Under hypoxia/hypoxia, protein expression was also increased with
the addition of 10 and 50 µM ZKK-3 (by 78 and 194%, respectively)
compared with the respective normoxia groups. Furthermore,
significantly decreased HIF-1α expression was observed in cells
exposed to hypoxia/HBO with 25 and 50 µM ZKK-3 (by 33 and 68%,
respectively) when compared with the respective normoxia groups.
Significantly decreased levels of HIF-1α were also indicated for
cells under hypoxia/hypoxia at 25 µM ZKK-3 when compared with
normoxia. HBO alone caused the protein expression level to
significantly decrease by 60% relative to hypoxia without ZKK-3
treatment; however, decreases observed with ZKK-3 treatment were
not statistically significant. Notably, HIF-1α expression was
significantly reduced under hypoxia/HBO compared with
hypoxia/hypoxia in control groups (64%) and cells treated with 10
and 50 µM ZKK-3 (45 and 89%, respectively).
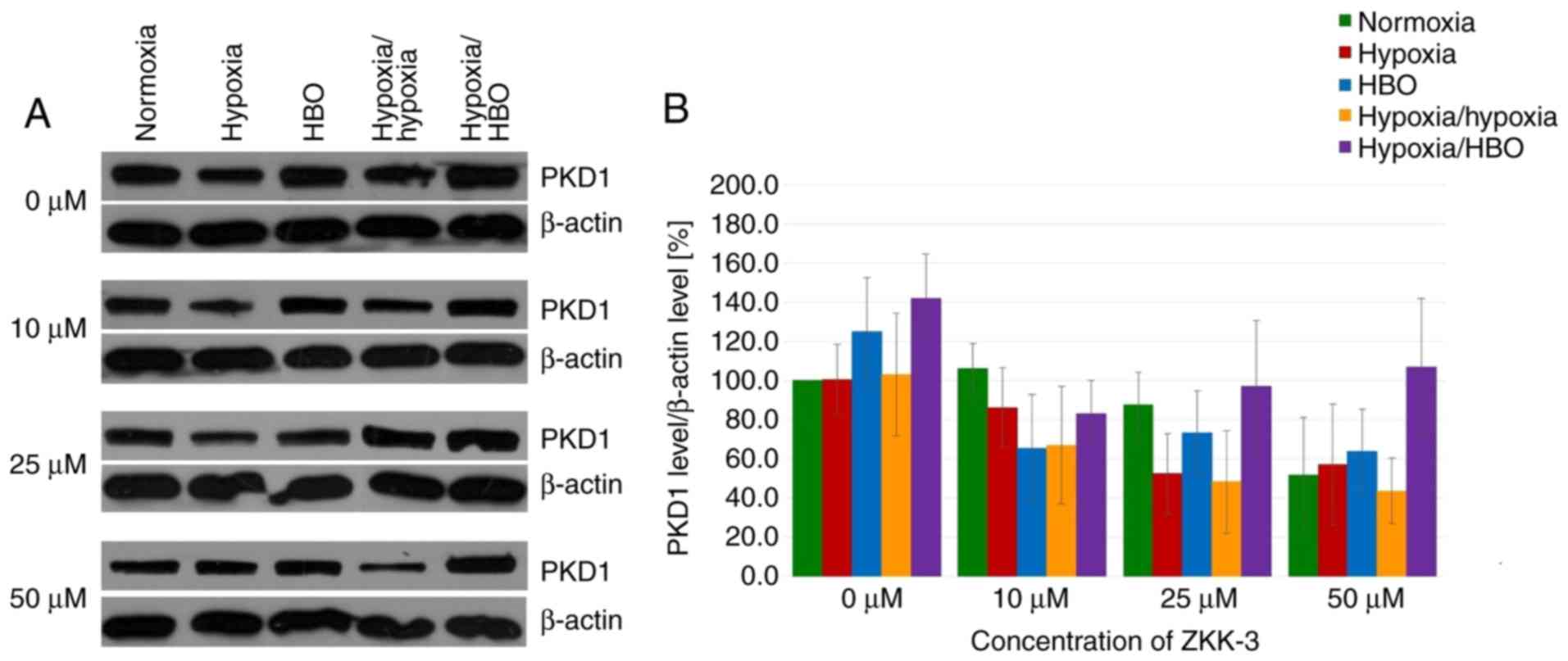
Expression levels of PKD1 and its
phosphorylated forms
In GBM cells under short-term and long-term hypoxia,
PKD1 expression levels were similar or marginally reduced compared
with cells under normoxia in control and ZKK-3-treated groups
(Fig. 11A). However, PKD1
expression levels were increased in cells under HBO and hypoxia/HBO
conditions compared with normoxia when 0 and 50 µM ZKK-3 was
applied. ZKK-3 at 25 and 50 µM seemed to decrease PKD1 levels under
all examined oxygen conditions, compared with the controls.
However, differences in total PKD1 expression levels in T98G cells
under different oxygen conditions and ZKK-3 treatments were not
statistically significant (Fig.
11B).
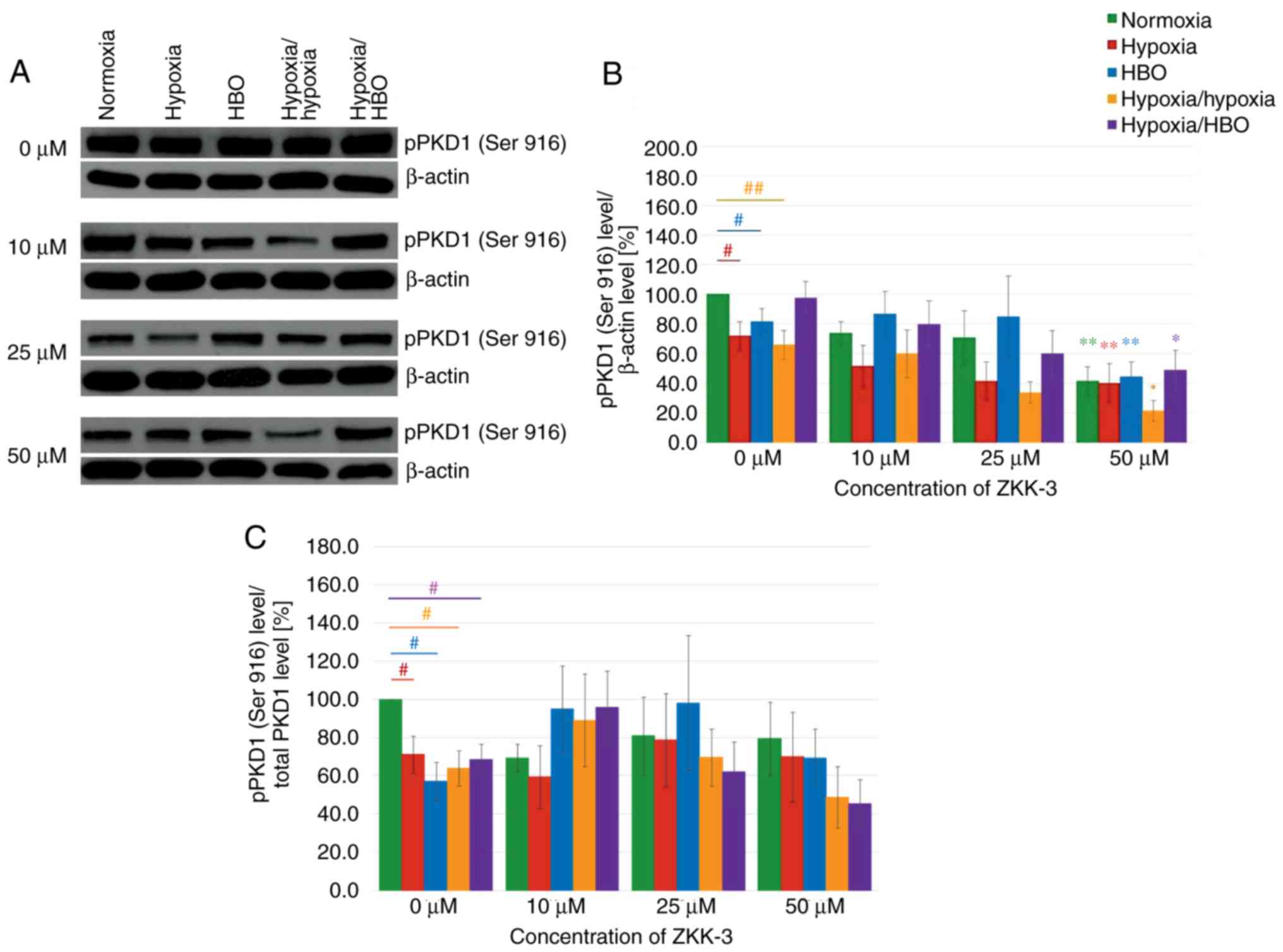
Increasing ZKK-3 concentrations were associated with
decreasing pPKD1 (Ser 916) expression levels, regardless of the
oxygen conditions (Fig. 12A). In
control groups (0 µM ZKK-3), compared with the normoxia group,
there were significant reductions of pPKD1 (Ser 916) expression
levels in cells under hypoxia, HBO and hypoxia/hypoxia (Fig. 12B). Following the addition of 50 µM
ZKK-3, pPKD1 (Ser 916) expression levels were reduced under
hypoxia-hypoxia and elevated under hypoxia/HBO when compared with
normoxia. Notably, there were no significant differences between
cells cultured under hypoxia and HBO conditions. However, treatment
with 50 µM ZKK-3 yielded a statistically significant decrease in
pPKD1 (Ser 916) expression under all examined oxygen conditions
compared with the respective controls: 59% for normoxia, 44% for
hypoxia, 45% for HBO, 68% for hypoxia/hypoxia and 50% for
hypoxia/HBO. However, the phospho/total ratio of pPKD1 demonstrated
a significant reduction of expression in the control groups (0 µM
ZKK-3). In addition, pPKD1 expression was decreased under
hypoxia/hypoxia and hypoxia/HBO at 50 µM ZKK-3 (Fig. 12C).
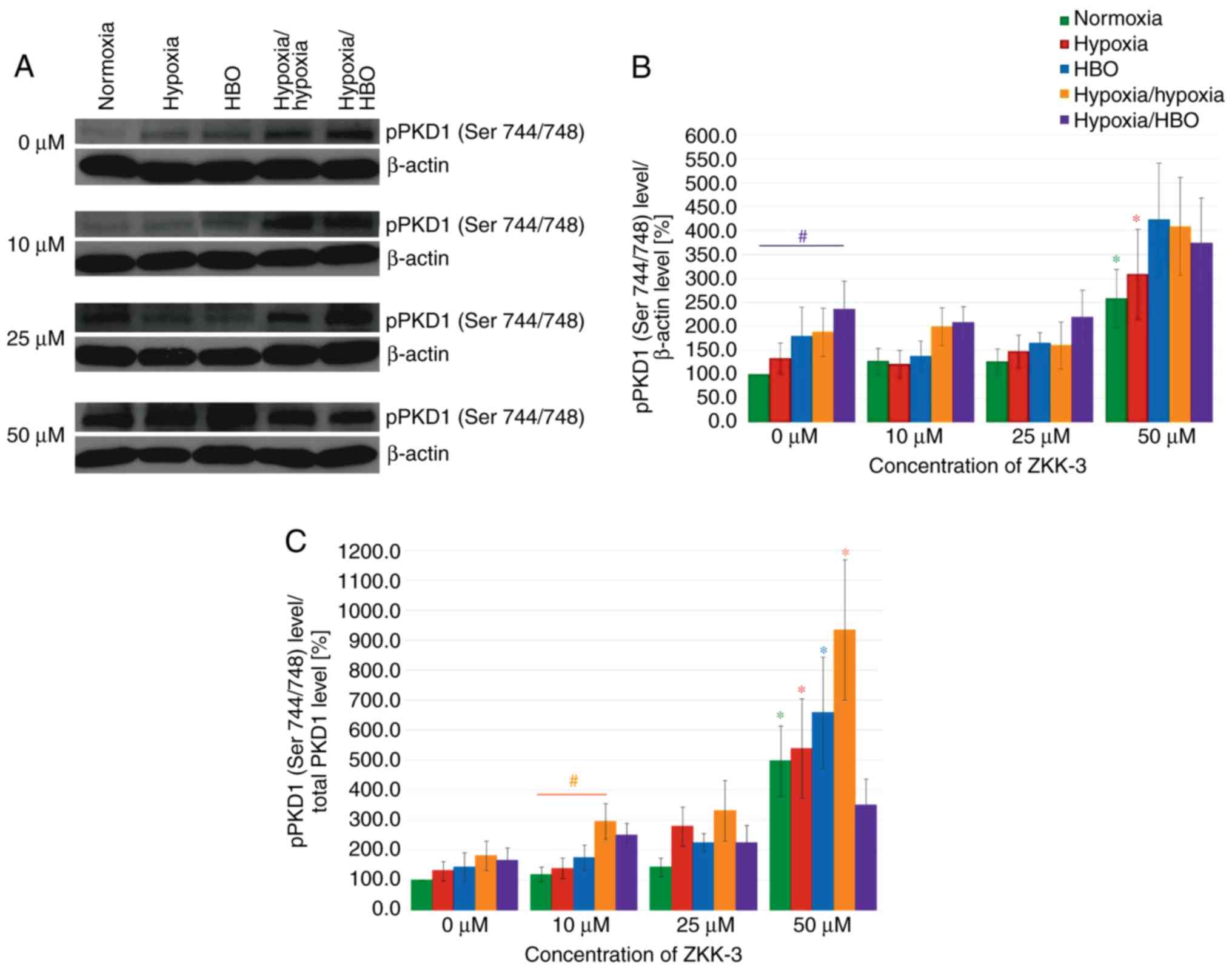
Western blot analysis revealed that hypoxia and HBO
conditions resulted in significantly increased pPKD1 (Ser 744/748)
expression levels compared with normoxia, in both control and
ZKK-3-treated T98G cells (Fig.
13A). pPKD1 (Ser 744/748) expression level seemed similar
compared with controls (0 µM ZKK-3) following treatment with lower
ZKK-3 concentrations under all oxygen conditions; however, 50 µM
ZKK-3 led to marked elevation irrespective of oxygen conditions.
Statistical analysis revealed that the pPKD1 (Ser 744/748)
expression level increased under the influence of oxygen pressure
changes compared with normoxia; however, the change was only
significant under hypoxia/HBO in control groups, which indicated a
nearly 2.5-fold rise (Fig. 13B).
No significant differences were observed between cells precultured
under the same oxygen conditions with different ZKK-3 treatment,
with the exception of cells treated with 50 µM ZKK-3, which
resulted in statistically significant increase of pPKD1 (Ser
744/748) expression level under normoxia (158%) and hypoxia (133%)
compared with the respective controls. This was confirmed when
analyzing the phospho/total ratio of pPKD1. The treatment of 50 µM
ZKK-3 was associated with significant increase of pPKD1 (Ser
744/748) expression in all oxygen conditions except hypoxia/HBO
compared with the controls (Fig.
13C).
Discussion
The medical treatment of patients with GBM remains
challenging as multiple treatment approaches do not provide
satisfactory therapeutic effects (2,5,6).
Therefore, intensive research is focused on developing novel
treatment strategies. S-benzylisothiourea derivatives have been
investigated, with in vitro results validating their
proapoptotic and cytotoxic properties against various neoplastic
cells, including several types of leukemia (20,21),
prostate adenocarcinoma (22) and
glioma (19,26). Furthermore, compounds from this
group have demonstrated greater cytotoxicity against human
glioblastoma cells in vitro compared with the clinically
used compound temozolomide (26).
However, ZKKs (including ZKK-3) also demonstrated cytotoxic
activity against normal human astrocytes in vitro (26), and this effect should therefore be
taken into account when considering anticancer therapy. The
cytotoxic effect of ZKKs towards normal glial elements of brain
tissue could be eliminated by modern methods of precise and direct
delivery of compounds to the tumor tissue. Notably, the present
results demonstrated that treatment with ZKK-3 dose dependently
resulted in the reduction of T98G proliferation and viability.
Numerous studies have revealed that poor tumor
tissue oxygenation is a pivotal factor in the development of
malignancies, including gliomas, and can foster radiotherapy and
chemotherapy resistance (7,8,27). The
present results demonstrated that anoxia resulted in significantly
reduced cell growth compared with normoxia. Under anaerobic
conditions, GBM cell proliferation declined following ZKK-3
administration; however, this decrease was not as severe when
compared with standard conditions. Notably, cell viability was
significantly increased in control and experimental groups under
anoxia compared with normoxia. Under anoxic conditions, significant
cytotoxic effects were only observed with higher ZKK-3 doses or
prolonged exposure. These findings may indicate that, despite the
impact of anoxia on cell proliferation, the cell viability can be
ameliorated by low oxygen conditions, possibly due to activation of
HIF-1α adaptive responses. Independent of these discrepancies, the
present results suggest that oxygen deprivation impaired the
antitumor properties of ZKK-3. However, relevant data from the
literature are somewhat contradictory. Liang (28) also reported that GBM cells are less
sensitive to several chemotherapeutics under anaerobic conditions.
By contrast, Papandreou et al (29) revealed decreased viability and
higher apoptotic potential of various tumor cell lines under
anoxia.
In the present study, hypoxia alone caused a slight
reduction of T98G cell line viability, but did not change GBM cell
proliferation relative to normoxia. A statistically significant
reduction of the number of GBM cells was observed following
treatment with 25 and 50 µM ZKK-3, regardless of the oxygen
conditions. However, following incubation with 50 µM ZKK-3, cell
proliferation was significantly higher under low oxygen
concentration compared with standard conditions. Furthermore, under
hypoxia, only high-dose ZKK-3 (50 µM) significantly reduced T98G
cell viability compared with normoxia after 2 days of exposure.
After 48 h of incubation with 10 and 25 µM ZKK-3, cell viability
was higher under low-oxygen conditions compared with that under
normoxia. The present results support the conclusion that prolonged
exposure to hypoxia significantly reduced the sensitivity of
neoplastic cells to ZKK-3 within a certain concentration range.
Following long-term incubation with 50 µM ZKK-3, inhibition of its
cytotoxic effects under hypoxia was no longer indicated.
Notably, Osawa et al (30) did not identify altered T98G GBM cell
growth following exposure to hypoxia conditions. Furthermore,
proliferation of spheroid-building cells is reportedly similar
between low-oxygen environments and normoxia (31). Studies conducted using a human
prostate cancer cell line suggest that hypoxia significantly
reduces cell viability and migration (32). However, the authors observed that
oxygen deficiency also promoted the chemotherapy resistance of
tumor cells. This association between the hypoxic tumor environment
and drug resistance has also been documented in other malignancies
in vitro (33,34). Overall, these studies suggest that
hypoxia can either impair or promote the antitumor properties of
chemotherapeutics, depending on the type of cytotoxic compound and
the tumor cell line (33,35). Previous results have also
demonstrated the reduction of GBM cell sensitivity to radiotherapy
in a low-oxygen environment (36).
It has been postulated that HBO may be a promising
method of improving tissue oxygenation (14–16);
however, some reports suggest that aggressive HBO treatment,
different from our protocol, may promote tumor progression
(37,38). The present results suggested that
HBO applied alone did not significantly alter the number and
viability of the GBM cell line relative to standard or
oxygen-deficiency conditions. However, combined application of
ZKK-3 and HBO resulted in significantly reduced proliferation and
viability of T98G cells compared with cells under normoxia and
hypoxia. It is noteworthy that hypoxia corresponds to the
physiological conditions of tumor tissue in vivo. Notably,
cells demonstrated higher sensitivity to ZKK-3 under HBO compared
with hypoxia, irrespective of the duration of exposure. Under the
influence of HBO, a significantly decreased number of living GBM
cells was observed, even at lower ZKK-3 concentrations and after
reduced exposure time to ZKK-3/HBO.
Data from the literature suggest that HBO
administration can significantly improve the effectiveness of
standard antitumor procedures, including chemotherapy and
radiotherapy (16,39–41).
Results indicate that HBO can enhance the cytotoxic effect of
various chemotherapeutic agents towards various types of tumors
in vivo and in vitro (42–45).
Such combined therapy seems to be particularly promising in
gliomas. Sun et al (46)
demonstrated that maintaining a GBM cell line under hyperoxic
conditions, even under normobaric pressure, increases its
sensitivity to the commonly used compound temozolomide. HBO used as
an adjuvant to temozolomide significantly inhibits GBM cell growth
in vitro and increases apoptosis (47). HBO-induced improvement of the
antiproliferative properties of temozolomide has also been observed
in GBM in vivo (48).
Similar results are reported following treatment with combined HBO
and nimustine in a mouse model of glioma (49). Clinical trials of glioma treatment
using HBO combined with radiotherapy and chemotherapy have also
produced encouraging results, including regression of tumor growth
and prolonged patient survival (50–54).
HIF-1 is crucial in regulating tumor cell
adaptations to hypoxic conditions, which further contributes to
their treatment resistance (8,11,12).
Zhou et al (55) examined
HIF expression in tumor cells of various origins in vitro
under different oxygen conditions. The study concluded that reduced
oxygen partial pressure led to increased HIF levels in all examined
cell lines. Other investigators have also reported enhanced
expression levels of HIF-1α and its downstream genes (vascular
endothelial growth factor, glucose transporter 1, glucose
transporter 3 and pyruvate dehydrogenase kinase 1) under hypoxia
compared with standard conditions (33,56).
Furthermore, Liu et al (57)
identified that higher HIF-1α levels promoted the development of
drug resistance and survival in cancer cell lines. Additionally,
HIF-1α reportedly leads to increased levels of multidrug resistance
protein 1 and antiapoptotic genes (B-cell lymphoma-2), along with
decreased expression of the proapoptotic B-cell lymphoma-2
associated X protein. Furthermore, survivin, an inhibitor of
apoptosis, is expressed in the majority of cancer cells and may
contribute to the anticancer therapy resistance (58,59).
The role of survivin in glioma progression was evaluated and its
association with poorer prognosis was suggested (60). As inhibition of ionizing radiation
resistance of human T98 GBM cells after survivin gene silencing has
been documented (61), the
involvement of survivin on the effect of ZKKs or ZKK-3 should be
clarified in the future. Notably, hypoxic conditions promote
greater HIF-1α expression in GBM (36,62).
Furthermore, numerous studies suggest elevated HIF activity in GBM
under normoxic conditions, which is further enhanced under hypoxia
(8,55,63).
Efforts have been made to reduce HIF-1α expression
using specific inhibitors or small interfering RNA silencing
(64,65). Unfortunately, few studies have
examined the influence of HBO on these protein expression levels in
tumor cells. Notably, some data indicate that oxygenation may help
decrease HIF levels (49,66). However, other reports have
demonstrated that HBO exerts no effect or even promotes HIF-1α
expression (37,67,68).
Thus, the issue remains controversial. The present results
indicated that the HIF-1α protein expression level in T98G cells
was dependent on the oxygen conditions. Under hypoxia and no ZKK-3
treatment, HIF-1α expression was significantly elevated compared
with normoxia. A prolonged duration of oxygen deficiency
(preincubation under hypoxia prior to ZKK-3 administration, i.e.,
hypoxia/hypoxia) resulted in an even greater increase of HIF-1α
expression. Notably, HIF-1α expression under hypoxia/HBO was
significantly suppressed relative to hypoxia/hypoxia without ZKK-3
and with 10 and 50 µM ZKK-3 treatment. The elevated HIF-1α protein
level under hypoxia and its reduction under HBO suggest that HBO
may reduce treatment resistance of neoplastic cells.
Abnormal PKD1 activity serves important roles in
various malignancies (69).
Therefore, numerous studies have been undertaken to develop
effective suppressors of this kinase in various tumor cells
(70–72). Unfortunately, the developed PKD1
inhibitors exhibit poor selectivity. Koronkiewicz et al
(21,22) reported that 10 µM ZKK-3 may inhibit
PKD1 expression by ~70% in human cell lines of prostate
adenocarcinoma and acute myelogenous leukemia. However, their
reports do not provide detailed information regarding the duration
of incubation with ZKK-3, or whether the results are associated
with the total kinase level or only to the level of its
non-phosphorylated form. Other research performed in the T98G cell
line indicates that a 48-h incubation with 10 µM ZKK-3 does not
alter PKD1 expression, but significantly decreases the expression
level of pPKD1 (Ser 916) (26). The
present results indicated that ZKK-3 did not significantly change
the expression of total PKD1 in GBM cells in vitro. However,
24 h incubation with 50 µM ZKK-3 resulted in a statistically
significant decrease of the phosphorylated form of pPKD1 (Ser 916)
and increased the level of pPKD1 (Ser 744/748) compared with the
respective controls. Therefore, it seems possible that ZKK-3
prevented PKD1 phosphorylation at Ser 916, resulting in a reduction
of pPKD1 (Ser 916) and accumulation of pPKD1 (Ser 744/748).
Generally, the expression levels of PKD1 and its
phosphorylated forms were not significantly different in T98G cells
under different oxygen conditions, including those under HBO, in
the control and in ZKK-3-treated groups. This suggests that
modulation of PKD1 activity may not be a leading mechanism
underlying the cytotoxic activity of ZKK-3 combined with HBO.
However, it is noteworthy that hypoxia/HBO tended to reduce the
increase of the level of pPKD1 (Ser 744/748) normalized to total
PKD1 (pPKD1/total PKD1 ratio) at 50 µM ZKK-3. Previous studies
investigating the influence of the oxygenation state of cells on
PKD1 expression have produced ambiguous results. Some indicate that
hypoxia conditions result in PKD1 phosphorylation and activation
(73–75), while others report no significant
changes in the expression of this kinase (73,76).
In conclusion, application of HBO significantly
reduced the proliferation of malignant GBM cells in vitro,
and increased their sensitivity to the isothiourea derivative
ZKK-3. The beneficial cytotoxic effects of ZKK-3/HBO could be
achieved using lower concentrations of ZKK-3 and reduced exposure
time. The decreased level of HIF-1α protein expression under HBO
suggests that HBO improved cell oxygenation, and thus may help
reduce tumor cell resistance to cytostatic compounds. Although
ZKK-3 exhibited inhibitory properties against pPKD1 (Ser 916)
kinase, various oxygen conditions, including hyperbaric
oxygenation, did not influence the expression of PKD1 or its
phosphorylated forms. Overall, the present findings suggest that
the combination of ZKK-3 and HBO may be a promising therapeutic
approach for the treatment of brain tumors with the highest grade
of histological malignancy. Further studies are required to
investigate this possibility.
Acknowledgements
Not applicable.
Funding
The research was supported by the Leading National
Research Centre-Mossakowski Medical Research Centre (KNOW-MMRC)
project and partially by Foundation for the Development of
Diagnostic and Therapy.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KZ performed the experiments, analyzed the
experiment data and wrote the manuscript. RPO made contribution to
conception of the study and assisted in the western blot analysis.
EM made contributions to the conception and design of the study and
interpreted the data. RPO and EM revised the manuscript. All
authors read and approved the final submission.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GBM
|
glioblastoma
|
|
HBO
|
hyperbaric oxygen therapy
|
|
HIF-1α
|
hypoxia-inducible factor-1α
|
|
PKD1
|
protein kinase D1
|
|
T98G
|
human glioblastoma cell line
|
|
ZKKs
|
pentabromobenzyl-isothioureas
|
|
ZKK-3
|
N,N′-dimethyl-S-(2,3,4,5,6-
pentabromobenzyl)-isothiouronium bromide
|
References
|
1
|
Batash R, Asna N, Schaffer P, Francis N
and Schaffer M: Glioblastoma multiforme, diagnosis and treatment;
recent literature review. Curr Med Chem. 24:3002–3009. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davis ME: Glioblastoma: Overview of
disease and treatment. Clin J Oncol Nurs. 20 (Suppl 5):S2–S8. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanif F, Muzaffar K, Perveen K, Malhi SM
and Simjee ShU: Glioblastoma multiforme: A review of its
epidemiology and pathogenesis through clinical presentation and
treatment. Asian Pac J Cancer Prev. 18:3–9. 2017.PubMed/NCBI
|
|
4
|
Liao W, Fan S, Zheng Y, Liao S, Xiong Y,
Li Y and Liu J: Recent advances on glioblastoma multiforme and
nano-drug carriers: A review. Curr Med Chem. 2018.
|
|
5
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF,
de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee SY: Temozolomide resistance in
glioblastoma multiforme. Genes Dis. 3:198–210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Höckel M and Vaupel P: Tumor hypoxia:
Definitions and current clinical, biologic, and molecular aspects.
J Natl Cancer Inst. 93:266–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jensen RL: Brain tumor hypoxia:
Tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a
therapeutic target. J Neurooncol. 92:317–335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rockwell S, Dobrucki IT, Kim EY, Marrison
ST and Vu VT: Hypoxia and radiation therapy: Past history, ongoing
research, and future promise. Curr Mol Med. 9:442–458. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Daruwalla J and Christophi C: Hyperbaric
oxygen therapy for malignancy: A review. World J Surg.
30:2112–2131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vaupel P: The role of hypoxia-induced
factors in tumor progression. Oncologist. 9 (Suppl 5):S10–S17.
2004. View Article : Google Scholar
|
|
12
|
Yang L, Lin C, Wang L, Guo H and Wang X:
Hypoxia and hypoxia-inducible factors in glioblastoma multiforme
progression and therapeutic implications. Exp Cell Res.
318:2417–2426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ostrowski RP and Zhang JH: The insights
into molecular pathways of hypoxia-inducible factor in the brain. J
Neurosci Res. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Al-Waili NS, Butler GJ, Beale J, Hamilton
RW, Lee BY and Lucas P: Hyperbaric oxygen and malignancies: A
potential role in radiotherapy, chemotherapy, tumor surgery and
phototherapy. Med Sci Monit. 11:RA279–289. 2005.PubMed/NCBI
|
|
15
|
Moen I and Stuhr LE: Hyperbaric oxygen
therapy and cancer-a review. Target Oncol. 7:233–242. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stepien K, Ostrowski RP and Matyja E:
Hyperbaric oxygen as an adjunctive therapy in treatment of
malignancies, including brain tumours. Med Oncol. 33:1012016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gill AL and Bell CN: Hyperbaric oxygen:
Its uses, mechanisms of action and outcomes. QJM. 97:385–395. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mayer R, Hamilton-Farrell MR, van der
Kleij AJ, Schmutz J, Granström G, Sicko Z, Melamed Y, Carl UM,
Hartmann KA, Jansen EC, et al: Hyperbaric oxygen and radiotherapy.
Strahlenther Onkol. 181:113–123. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaminska B, Ellert-Miklaszewska A, Oberbek
A, Wisniewski P, Kaza B, Makowska M, Bretner M and Kazimierczuk Z:
Efficacy and mechanism of antitumor action of new potential CK2
inhibitors toward glioblastoma cells. Int J Oncol. 35:1091–1100.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koronkiewicz M, Chilmonczyk Z and
Kazimierczuk Z: Proapoptotic effects of novel
pentabromobenzylisothioureas in human leukemia cell lines. Med Chem
Res. 21:3111–3118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koronkiewicz M, Chilmonczyk Z and
Kazimierczuk Z: Synergistic anti-leukemic effects of CK2 inhibitors
and pentabromobenzylisothioureas in vitro. Anticancer Res.
33:4891–4899. 2013.PubMed/NCBI
|
|
22
|
Koronkiewicz M, Kazimierczuk Z, Szarpak K
and Chilmonczyk Z: Proapoptotic effects of new
pentabromobenzylisothiouronium salts in a human prostate
adenocarcinoma cell line. Acta Pol Pharm. 69:1325–1333.
2012.PubMed/NCBI
|
|
23
|
Sundram V, Chauhan SC and Jaggi M:
Emerging roles of protein kinase D1 in cancer. Mol Cancer Res.
9:985–996. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eiseler T, Doppler H, Yan IK, Goodison S
and Storz P: Protein kinase D1 regulates matrix metalloproteinase
expression and inhibits breast cancer cell invasion. Breast Cancer
Res. 11:R132009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wille C, Seufferlein T and Eiseler T:
Protein Kinase D family kinases: Roads start to segregate.
Bioarchitecture. 4:111–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pucko E, Matyja E, Koronkiewicz M,
Ostrowski RP and Kazimierczuk Z: Potent antitumour effects of novel
pentabromobenzylisothioureas studied on human glial-derived tumour
cell lines. Anticancer Res. 38:2691–2705. 2018.PubMed/NCBI
|
|
27
|
Muz B, de la Puente P, Azab F and Azab AK:
The role of hypoxia in cancer progression, angiogenesis,
metastasis, and resistance to therapy. Hypoxia (Auckl). 3:83–92.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang BC: Effects of hypoxia on drug
resistance phenotype and genotype in human glioma cell lines. J
Neurooncol. 29:149–155. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Papandreou I, Krishna C, Kaper F, Cai D,
Giaccia AJ and Denko NC: Anoxia is necessary for tumor cell
toxicity caused by a low-oxygen environment. Cancer Res.
65:3171–3178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Osawa T, Tsuchida R, Muramatsu M, Yuasa Y
and Shibuya M: Human glioblastoma cells exposed to long-term
hypoxia and nutrient starvation stimulated induction of secondary
T-cell leukemia in mice. Blood Cancer J. 1:e62011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Riffle S, Pandey RN, Albert M and Hegde
RS: Linking hypoxia, DNA damage and proliferation in multicellular
tumor spheroids. BMC Cancer. 17:3382017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mamede AC, Abrantes AM, Pedrosa L,
Casalta-Lopes JE, Pires AS, Teixo RJ, Gonçalves AC,
Sarmento-Ribeiro AB, Maia CJ and Botelho MF: Beyond the limits of
oxygen: Effects of hypoxia in a hormone-independent prostate cancer
cell line. ISRN Oncol. 2013:9182072013.PubMed/NCBI
|
|
33
|
Yao K, Gietema JA, Shida S, Selvakumaran
M, Fonrose X, Haas NB, Testa J and O'Dwyer PJ: In vitro
hypoxia-conditioned colon cancer cell lines derived from HCT116 and
HT29 exhibit altered apoptosis susceptibility and a more angiogenic
profile in vivo. Br J Cancer. 93:1356–1363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qian J, Shen S, Chen W and Chen N:
Propofol reversed Hypoxia-induced docetaxel resistance in prostate
cancer cells by preventing epithelial-mesenchymal transition by
inhibiting Hypoxia-lnducible factor lα. Biomed Res Int.
2018:41742322018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Strese S, Fryknäs M, Larsson R and Gullbo
J: Effects of hypoxia on human cancer cell line chemosensitivity.
BMC Cancer. 13:3312013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hsieh CH, Lee CH, Liang JA, Yu CY and Shyu
WC: Cycling hypoxia increases U87 glioma cell radioresistance via
ROS induced higher and long-term HIF-1 signal transduction
activity. Oncol Rep. 24:1629–1636. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ding JB, Chen JR, Xu HZ and Qin ZY: Effect
of hyperbaric oxygen on the growth of intracranial glioma in rats.
Chin Med J (Engl). 128:3197–3203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang YG, Zhan YP, Pan SY, Wang HD, Zhang
DX, Gao K, Qi XL and Yu CJ: Hyperbaric oxygen promotes malignant
glioma cell growth and inhibits cell apoptosis. Oncol Lett.
10:189–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kohshi K, Kinoshita Y, Terashima H, Konda
N, Yokota A and Soejima T: Radiotherapy after hyperbaric
oxygenation for malignant gliomas: A pilot study. J Cancer Res Clin
Oncol. 122:676–678. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kohshi K, Kinoshita Y, Imada H, Kunugita
N, Abe H, Terashima H, Tokui N and Uemura S: Effects of
radiotherapy after hyperbaric oxygenation on malignant gliomas. Br
J Cancer. 80:236–241. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kohshi K, Beppu T, Tanaka K, Ogawa K,
Inoue O, Kukita I and Clarke RE: Potential roles of hyperbaric
oxygenation in the treatments of brain tumors. Undersea Hyperb Med.
40:351–362. 2013.PubMed/NCBI
|
|
42
|
Stuhr LE, Iversen VV, Straume O, Maehle BO
and Reed RK: Hyperbaric oxygen alone or combined with 5-FU
attenuates growth of DMBA-induced rat mammary tumors. Cancer Lett.
210:35–40. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Granowitz EV, Tonomura N, Benson RM, Katz
DM, Band V, Makari-Judson GP and Osborne BA: Hyperbaric oxygen
inhibits benign and malignant human mammary epithelial cell
proliferation. Anticancer Res. 25:3833–3842. 2005.PubMed/NCBI
|
|
44
|
Kawasoe Y, Yokouchi M, Ueno Y, Iwaya H,
Yoshida H and Komiya S: Hyperbaric oxygen as a chemotherapy
adjuvant in the treatment of osteosarcoma. Oncol Rep. 22:1045–1050.
2009.PubMed/NCBI
|
|
45
|
Ohgami Y, Elstad CA, Chung E, Shirachi DY,
Quock RM and Lai HC: Effect of hyperbaric oxygen on the anticancer
effect of artemisinin on molt-4 human leukemia cells. Anticancer
Res. 30:4467–4470. 2010.PubMed/NCBI
|
|
46
|
Sun S, Lee D, Lee NP, Pu JK, Wong ST, Lui
WM, Fung CF and Leung GK: Hyperoxia resensitizes chemoresistant
human glioblastoma cells to temozolomide. J Neurooncol.
109:467–475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lu XY, Cao K, Li QY, Yuan ZC and Lu PS:
The synergistic therapeutic effect of temozolomide and hyperbaric
oxygen on glioma U251 cell lines is accompanied by alterations in
vascular endothelial growth factor and multidrug
resistance-associated protein-1 levels. J Int Med Res. 40:995–1004.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dagistan Y, Karaca I, Bozkurt ER, Ozar E,
Yagmurlu K, Toklu A and Bilir A: Combination hyperbaric oxygen and
temozolomide therapy in C6 rat glioma model. Acta Cir Bras.
27:383–387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lu Z, Ma J, Liu B, Dai C, Xie T, Ma X, Li
M, Dong J, Lan Q and Huang Q: Hyperbaric oxygen therapy sensitizes
nimustine treatment for glioma in mice. Cancer Med. 5:3147–3155.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Beppu T, Kamada K, Nakamura R, Oikawa H,
Takeda M, Fukuda T, Arai H, Ogasawara K and Ogawa A: A phase II
study of radiotherapy after hyperbaric oxygenation combined with
interferon-beta and nimustine hydrochloride to treat supratentorial
malignant gliomas. J Neurooncol. 61:161–170. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ogawa K, Yoshii Y, Inoue O, Toita T, Saito
A, Kakinohana Y, Adachi G, Ishikawa Y, Kin S and Murayama S:
Prospective trial of radiotherapy after hyperbaric oxygenation with
chemotherapy for high-grade gliomas. Radiother Oncol. 67:63–67.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ogawa K, Yoshii Y, Inoue O, Toita T, Saito
A, Kakinohana Y, Adachi G, Iraha S, Tamaki W, Sugimoto K, et al:
Phase II trial of radiotherapy after hyperbaric oxygenation with
chemotherapy for high-grade gliomas. Br J Cancer. 95:862–868. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ogawa K, Ishiuchi S, Inoue O, Yoshii Y,
Saito A, Watanabe T, Iraha S, Toita T, Kakinohana Y, Ariga T, et
al: Phase II trial of radiotherapy after hyperbaric oxygenation
with multiagent chemotherapy (procarbazine, nimustine, and
vincristine) for high-grade gliomas: Long-term results. Int J
Radiat Oncol Biol Phys. 82:732–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yahara K, Ohguri T, Udono H, Yamamoto J,
Tomura K, Onoda T, Imada H, Nishizawa S and Korogi Y: Radiotherapy
using IMRT boosts after hyperbaric oxygen therapy with chemotherapy
for glioblastoma. J Radiat Res. 58:351–356. 2017.PubMed/NCBI
|
|
55
|
Zhou W, Dosey TL, Biechele T, Moon RT,
Horwitz MS and Ruohola-Baker H: Assessment of hypoxia inducible
factor levels in cancer cell lines upon hypoxic induction using a
novel reporter construct. PLoS One. 6:e274602011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jones RB, Dorsett KA, Hjelmeland AB and
Bellis SL: The ST6Gal-I sialyltransferase protects tumor cells
against hypoxia by enhancing HIF-1α signaling. J Biol Chem.
293:5659–5667. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu L, Ning X, Sun L, Zhang H, Shi Y, Guo
C, Han S, Liu J, Sun S and Han Z: Hypoxia-inducible factor-1 alpha
contributes to hypoxia-induced chemoresistance in gastric cancer.
Cancer Sci. 99:121–128. 2008.PubMed/NCBI
|
|
58
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tamm I, Wang Y, Sausville E, Scudiero DA,
Vigna N, Oltersdorf T and Reed JC: IAP-family protein Survivin
inhibits caspase activity and apoptosis induced by Fas (CD95), Bax,
caspases, and anticancer drugs. Cancer Res. 58:5315–5320.
1998.PubMed/NCBI
|
|
60
|
Zhang SF, Zhang CW, Song YL, Zhang J and
Xu JG: Prognostic role of survivin in patients with glioma.
Medicine (Baltimore). 97:e05712018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li JC, Han Y, Zhou D, Zhou Y, Ye M, Wang H
and Du Z: Downregulation of Survivin gene expression affects
ionizing radiation resistance of human T98 glioma cells. Cell Mol
Neurobiol. 38:861–868. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Joseph JV, Conroy S, Pavlov K, Sontakke P,
Tomar T, Eggens-Meijer E, Balasubramaniyan V, Wagemakers M, den
Dunnen WF and Kruyt FA: Hypoxia enhances migration and invasion in
glioblastoma by promoting a mesenchymal shift mediated by the
HIF1α-ZEB1 axis. Cancer Lett. 359:107–116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Mendichovszky I and Jackson A: Imaging
hypoxia in gliomas. Br J Radiol 84 Spec No. 2:S145–S158. 2011.
View Article : Google Scholar
|
|
64
|
Rapisarda A, Uranchimeg B, Scudiero DA,
Selby M, Sausville EA, Shoemaker RH and Melillo G: Identification
of small molecule inhibitors of hypoxia-inducible factor 1
transcriptional activation pathway. Cancer Res. 62:4316–4324.
2002.PubMed/NCBI
|
|
65
|
Kummar S, Raffeld M, Juwara L, Horneffer
Y, Strassberger A, Allen D, Steinberg SM, Rapisarda A, Spencer SD,
Figg WD, et al: Multihistology, target-driven pilot trial of oral
topotecan as an inhibitor of hypoxia-inducible factor-1α in
advanced solid tumors. Clin Cancer Res. 17:5123–5131. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Stuhr LE, Raa A, Oyan AM, Kalland KH,
Sakariassen PO, Petersen K, Bjerkvig R and Reed RK: Hyperoxia
retards growth and induces apoptosis, changes in vascular density
and gene expression in transplanted gliomas in nude rats. J
Neurooncol. 85:191–202. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Selvendiran K, Kuppusamy ML, Ahmed S,
Bratasz A, Meenakshisundaram G, Rivera BK, Khan M and Kuppusamy P:
Oxygenation inhibits ovarian tumor growth by downregulating STAT3
and cyclin-D1 expressions. Cancer Biol Ther. 10:386–390. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Terraneo L, Virgili E, Caretti A,
Bianciardi P and Samaja M: In vivo hyperoxia induces
hypoxia-inducible factor-1alpha overexpression in LNCaP tumors
without affecting the tumor growth rate. Int J Biochem Cell Biol.
51:65–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Storz P: Mitochondrial ROS-radical
detoxification, mediated by protein kinase D. Trends Cell Biol.
17:13–18. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
La Valle CR, George KM, Sharlow ER, Lazo
JS, Wipf P and Wang QJ: Protein kinase D as a potential new target
for cancer therapy. Biochim Biophys Acta. 1806:183–192.
2010.PubMed/NCBI
|
|
71
|
Azoitei N, Kleger A, Schoo N, Thal DR,
Brunner C, Pusapati GV, Filatova A, Genze F, Möller P, Acker T, et
al: Protein kinase D2 is a novel regulator of glioblastoma growth
and tumor formation. Neuro Oncol. 13:710–724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bernhart E, Damm S, Wintersperger A,
DeVaney T, Zimmer A, Raynham T, Ireson C and Sattler W: Protein
kinase D2 regulates migration and invasion of U87MG glioblastoma
cells in vitro. Exp Cell Res. 319:2037–2048. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Fleegal MA, Hom S, Borg LK and Davis TP:
Activation of PKC modulates blood-brain barrier endothelial cell
permeability changes induced by hypoxia and posthypoxic
reoxygenation. Am J Physiol Heart Circ Physiol. 289:H2012–H2019.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Son JH, Cho YC, Sung IY, Kim IR, Park BS
and Kim YD: Melatonin promotes osteoblast differentiation and
mineralization of MC3T3-E1 cells under hypoxic conditions through
activation of PKD/p38 pathways. J Pineal Res. 57:385–392. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ha WH, Seong HS, Choi NR, Park BS and Kim
YD: Recombinant human bone morphogenic protein-2 induces the
differentiation and mineralization of osteoblastic cells under
hypoxic conditions via activation of protein kinase D and p38
mitogen-activated protein kinase signaling pathways. Tissue Eng
Regen Med. 14:433–441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Gozal E, Roussel AL, Holt GA, Gozal L,
Gozal YM, Torres JE and Gozal D: Protein kinase C modulation of
ventilatory response to hypoxia in nucleus tractus solitarii of
conscious rats. J Appl Physiol (1985). 84:1982–1990. 1998.
View Article : Google Scholar : PubMed/NCBI
|