Introduction
Circulating tumor cells (CTCs) are currently
considered as the precursor of tumor metastases, which are closely
associated with the distant metastasis of human cancers (1). Currently, CTC detection is proposed as
a ‘liquid biopsy’ approach for real-time monitoring of tumor
progression (2). Given its easy
accessibility via blood draw, CTCs may represent an ideal source of
biomarkers for diagnosis and evaluation of the therapeutic effect
compared with various imaging approaches or more invasive
tissue-based biopsies. To date, numerous studies including ours
have observed that CTC counts were significantly associated with
multiple unfavourable clinicopathological features and dismal
prognosis (3–6). In addition, we found that CTC count
alone was not enough to demonstrate the important value of CTCs in
metastasis, nor can it clarify the clinical value of CTC detection
in cancer. Moreover, our research group as well as others
demonstrated that CTCs exhibited heterogeneous characteristics at
different time-points, which meant they can change in both terms of
number and phenotype during anticancer treatment (7–10).
Previously, great progress has been made in elucidating the
clinical significance of CTC counts and their dynamic change
(6,10), however, less attention is paid to
the phenotype change of CTCs during anticancer therapy. Thus, it is
critical to gain insight into the phenotypic characterization of
CTCs at different treatment points, which may contribute to the
further understanding of CTC-mediated metastasis and improve cancer
management.
The epithelial-mesenchymal transition (EMT) process,
which is known to increase cell motility and invasive ability, has
been identified as a crucial driver of cancer metastasis (11,12).
Currently, emerging and accumulating evidence has suggested that
EMT plays an important role in CTC-mediated metastasis (13), which also was demonstrated in our
recent studies (14–16). CTCs acquire mesenchymal traits
through EMT, thereby gaining survival advantages and improved
ability to metastasize (17).
Clinically, CTCs were reported to undergo EMT at different
time-points during the whole procedure of anticancer therapy,
including chemotherapy, radiotherapy or molecular targeted therapy
(18,19). Mesenchymal CTCs (MCTCs),
characterised by suppression of E-cadherin and upregulated
expression of mesenchymal markers (such as vimentin and
N-cadherin), were revealed to be significantly related with drug
resistance, cancer metastasis and dismal prognosis (9,18–20).
Despite this, the dynamic change and prognostic value of
MCTCs exhibited inconsistencies in various CTC detection
methods. Therefore, it is essential to further explore the clinical
value of the dynamic changes of MCTCs.
Previously, our group as well as others reported
numerous methods for CTC isolation and identification that achieved
efficient capture of CTCs based on their physicochemical features
or antigen expression (21–24). However, these antigen-dependent
approaches exhibited a narrow spectrum of CTC capture and failed to
isolate the EMT-CTC subpopulations (25,26).
Recently, our group and collaborators developed an optimized
one-stop CTC device-CTCBIOPSY® (Wuhan YZY Medical
Science and Technology Co., Ltd., Wuhan, China), which has been
demonstrated to automatically and specifically isolate and identify
CTCs in a label-free, quick and low-cost manner (27). Notably, this size-based method could
efficiently capture EMT-CTCs form the blood of patients (28), providing great advantage for dynamic
monitoring of the changes of MCTCs during anticancer
treatment. However, the clinical and prognostic value of the
dynamic changes of MCTCs based on this method in
colorectal cancer (CRC) still require further investigation.
In the present study, the CTCBIOPSY®
device was used with an immunocytochemistry assay to dynamically
monitor the changes of MCTCs before curative resection
and after adjuvant chemotherapy, and further analyzed the clinical
significance and prognostic value of the dynamic changes of
MCTCs in CRC patients.
Materials and methods
Study design and patient
recruitment
This study was designed as a prospective cohort
study at a single institute. From January 2014 to May 2015,
consecutive CRC patients with stage II–III, treated at Zhongnan
Hospital of Wuhan University were prospectively recruited in the
present study. The inclusion criteria were as follows: i) pathology
confirmed as CRC; ii) underwent radical surgery; iii) received
standard 6-cycle adjuvant chemotherapy (5-fluorouracil-based
combination chemotherapy); iv) adequate and available medical
records. The exclusion criteria were as follows: i) pre-op
chemotherapy or radiotherapy; ii) patients that did not require
post-operative adjuvant therapy; iii) lack of adequate CTC data;
iv) succumbed after/following 30 days of surgery. After screening
according to inclusion and exclusion criteria, 175 patients [114
males, 61 females; median age=62 (37–81) years] and 127 patients
[77 males, 50 females; median age=61 (37–81) years] were enrolled
for pre-MCTC and post-MCTC analyses,
respectively.
All clinicopathological data of included patients
were collected from the electronic medical record system. The
postoperative pathological stage of CRC was performed according to
the 7th edition of the AJCC/UICC tumor-node-metastasis (TNM)
staging manual (29). The present
study was approved by the Institutional Review Board (IRB) of
Zhongnan Hospital of Wuhan University in adherence with the
Declaration of Helsinki, and written informed consent was obtained
from all included patients.
CTC isolation and identification
A volume of 5 ml peripheral blood samples from all
included patients were collected in EDTA-containing tubes (BD
Biosciences) before surgery and after 6-cycle adjuvant
chemotherapy. CTCs were isolated by using our previously reported
CTCBIOPSY® device and stained by Wright's staining
(27). After isolation, based on
the criteria proposed by other researchers (28,30,31)
and reported by our previous study (27), all candidate CTCs were reviewed and
identified independently by two senior cyto-pathologists who were
blinded from the patients' information, and any discrepancies were
resolved by discussion or consulting a third senior pathologist.
The reference threshold for this device was 1 CTC, that is, 0 CTC
indicated CTC negative while ≥1 CTC indicated positive.
CTC immunocytochemical staining
To characterize the EMT traits of CTCs,
immunocytochemical staining was used as previously described
(32). The antibodies, including
anti-E-cadherin antibody (1:100; product code ab40772) and
anti-vimentin antibody (1:100; product code ab8978; both from
Abcam), were selected to respectively label either the epithelial
phenotype or mesenchymal phenotype according to the manufacturer's
instructions. The HCT116 and Panc-1 cell lines were used as
positive controls. As a negative control for immunocytochemistry
(ICC) to ensure the exclusion of cross-reactivity, the same cell
lines were used with omission of the primary antibody. Evaluation
of immunostaining was manually performed using the research system
fluorescence microscope (BX51; Olympus Corporation). Pre-treatment
mesenchymal CTCs (pre-MCTCs) were defined as CTCs that
were vimentin-positive prior to surgery. Post-treatment mesenchymal
CTCs (post-MCTCs) were defined as CTCs that were
vimentin-positive at the end of 6 cycles of adjuvant chemotherapy.
The reference threshold of MCTCs was 1 MCTC,
and 0 MCTC indicated negative while ≥1 MCTC
indicated positive.
Adjuvant treatment and surveillance
strategy
Adjuvant treatments [including chemotherapy
(5-fluorouracil-based combination chemotherapy) and radiotherapy]
and surveillance strategy were planned based on the guidelines that
were recommended by the National Comprehensive Cancer Network
(NCCN) for CRC clinical management. Follow-up data were available
from patient files or by telephone interview with the patients or
guardians. All patients were monitored either until June 1 2019 or
their death. Recurrence-free survival (RFS) was defined as the time
interval from the date of resection to the date of tumor recurrence
or distant metastasis. Overall survival (OS) was defined as the
time in months between the operation and death or last
follow-up.
Statistical analysis
All statistical analyses were performed with SPSS
22.0 (IBM Corp.). Continuous variables were presented as the median
with ranges (minimum and maximum) or the mean ± standard deviation
(mean ± SD), and compared using the Student's t-test. The
relationships between the status and dynamic changes of pre- and
post-MCTCs and clinicopathologic characteristics were
analyzed with the Pearson χ2-test or Fisher's exact
test. The cumulative probability and differences of RFS and OS
among groups were determined using Kaplan-Meier curves with
log-rank tests. Univariate and multivariate Cox regression analysis
were further performed to identify the prognostic factors of CRC
patients and estimate the corresponding hazard ratio (HR) with 95%
confidence intervals (CIs). For all tests, a two-sided P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics and cohort
design
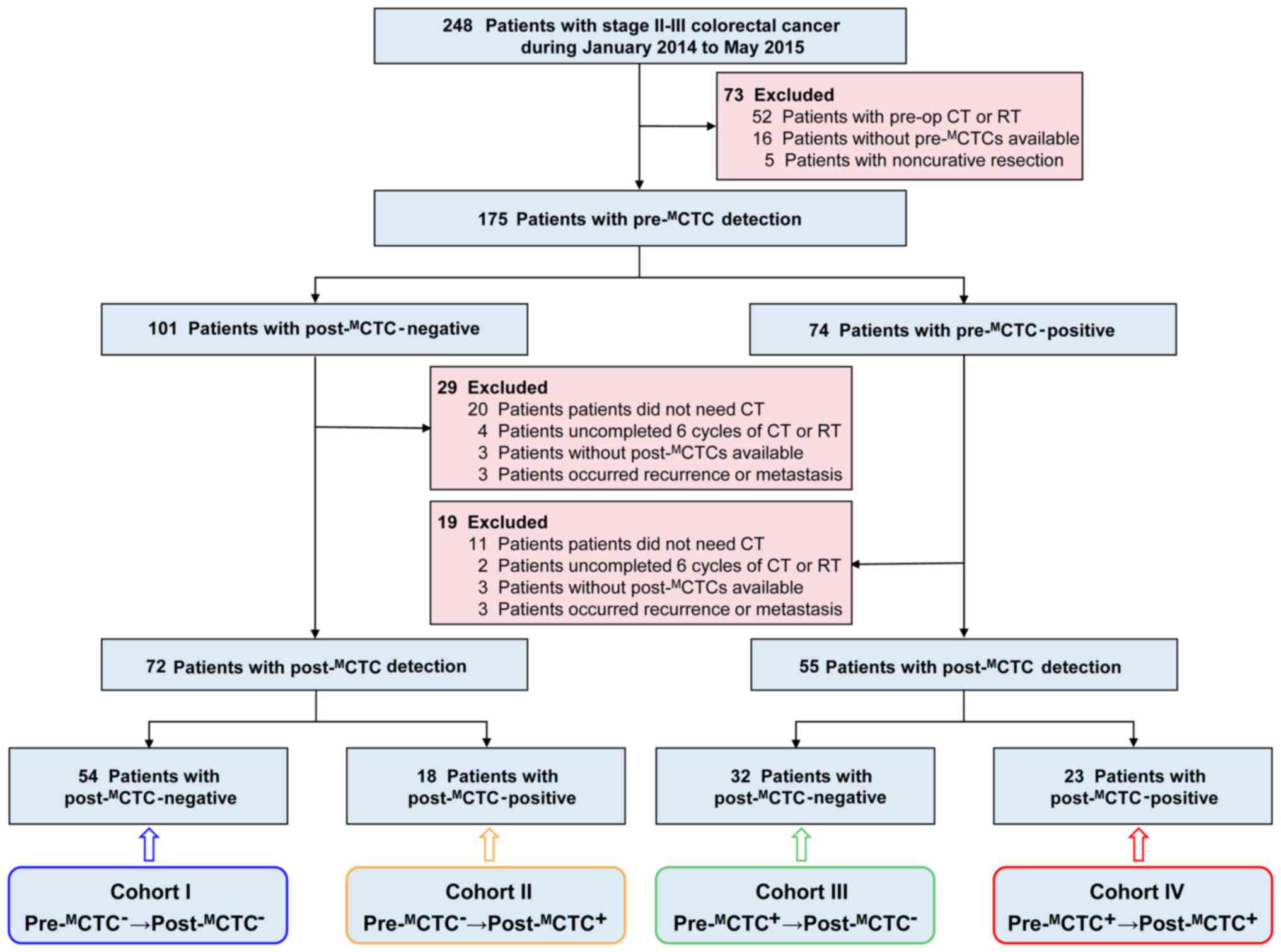
A total of 248 CRC patients were preliminarily
recruited into this prospective study (Fig. 1). After screening according to
inclusion and exclusion criteria, 175 patients [114 males, 61
females; median age=62 (37–81) years] and 127 patients [77 males,
50 females; median age=61 (37–81) years] were finally enrolled for
pre-MCTC and post-MCTC analyses,
respectively. Among the included patients for post-MCTC
analyses, there were 58 patients (45.7%) with colon cancer and 69
patients (54.3%) with rectum cancer; tumor grade with low and
middle and high (33) were 76
(59.8%) and 51 (40.2%), respectively; patients for stage II and III
were 39 (30.7%) and 88 (69.3%), respectively. The detailed
clinicopathologic characteristics of the included patients for
pre-MCTC and post-MCTC analyses were
respectively summarized in Tables
SI and I.
 | Table I.Relationships between the change of
pre- and post-MCTC status and clinicopathological characteristics
of CRC patients. |
Table I.
Relationships between the change of
pre- and post-MCTC status and clinicopathological characteristics
of CRC patients.
|
|
|
Pre-MCTCs→Post-MCTCs |
|
|
|---|
|
|
|
|
|
|
|---|
| Parameters | N (%) | N→N | N→P | P→N | P→P |
χ2-value | P-value |
|---|
| Sex |
|
|
|
|
| 0.339 | 0.953 |
|
Male | 77 (60.6) | 34 | 10 | 19 | 14 |
|
|
|
Female | 50 (39.4) | 20 | 8 | 13 | 9 |
|
|
| Age |
|
|
|
|
| 5.661 | 0.129 |
| <60
years | 48 (37.8) | 19 | 11 | 12 | 6 |
|
|
| ≥60
years | 79 (62.2) | 35 | 7 | 20 | 17 |
|
|
| Tumor location |
|
|
|
|
| 3.764 | 0.288 |
|
Colon | 58 (45.7) | 24 | 9 | 18 | 7 |
|
|
|
Rectal | 69 (54.3) | 30 | 9 | 14 | 16 |
|
|
| Tumor size |
|
|
|
|
| 4.740 | 0.192 |
| <5
cm | 47 (37.0) | 29 | 7 | 6 | 5 |
|
|
| ≥5
cm | 80 (63.0) | 51 | 11 | 26 | 18 |
|
|
| Tumor grade |
|
|
|
|
| 3.262 | 0.353 |
|
Low | 76 (59.8) | 29 | 12 | 18 | 17 |
|
|
| Middle
and High | 51 (40.2) | 25 | 6 | 14 | 6 |
|
|
| LVI |
|
|
|
|
| 22.737 |
<0.001a |
|
Absence | 82 (64.6) | 45 | 6 | 22 | 9 |
|
|
|
Presence | 45 (35.4) | 9 | 12 | 10 | 14 |
|
|
| PNI |
|
|
|
|
| 5.821 | 0.121 |
|
Absence | 86 (67.7) | 41 | 9 | 23 | 13 |
|
|
|
Presence | 41 (32.3) | 13 | 9 | 9 | 10 |
|
|
| Tumor invasion |
|
|
|
|
| 1.424 | 0.746 |
|
T1-2 | 22 (17.3) | 8 | 4 | 7 | 3 |
|
|
|
T3-4 | 105 (82.7) | 46 | 14 | 25 | 20 |
|
|
| LNM |
|
|
|
|
| 1.606 | 0.675 |
|
N0-1 | 41 (32.3) | 17 | 5 | 13 | 6 |
|
|
|
N2-3 | 86 (67.7) | 37 | 13 | 19 | 17 |
|
|
| TNM stage |
|
|
|
|
| 8.632 | 0.033a,b |
| II | 39 (30.7) | 16 | 3 | 16 | 4 |
|
|
|
III | 88 (69.3) | 38 | 15 | 16 | 19 |
|
|
| CEA level |
|
|
|
|
| 6.945 | 0.076 |
| <5
ng/ml | 87 (68.5) | 35 | 9 | 27 | 16 |
|
|
| ≥5
ng/ml | 40 (31.5) | 19 | 9 | 5 | 7 |
|
|
|
Overall | 175 (100.0) | 54 | 18 | 32 | 23 | – | – |
According to the pre- and post-MCTC
status, included CRC patients were grouped into four cohorts:
Cohort I, 54 patients whose pre- and post-MCTCs were
both negative
(pre-MCTC−→post-MCTC−);
cohort II, 18 patients with pre-MCTC-negative but post-
MCTC-positive
(pre-MCTC−→post-MCTC+);
cohort III, 32 patients with pre-MCTC-positive but
post-MCTC-negative
(pre-MCTC+→post-MCTC−);
cohort IV, 23 patients with pre- and post-MCTCs both
positive
(pre-MCTC+→post-MCTC+).
The detailed information of the aforementioned cohorts is outlined
in Fig. 1.
EMT characterization of CTCs from
patients with CRC
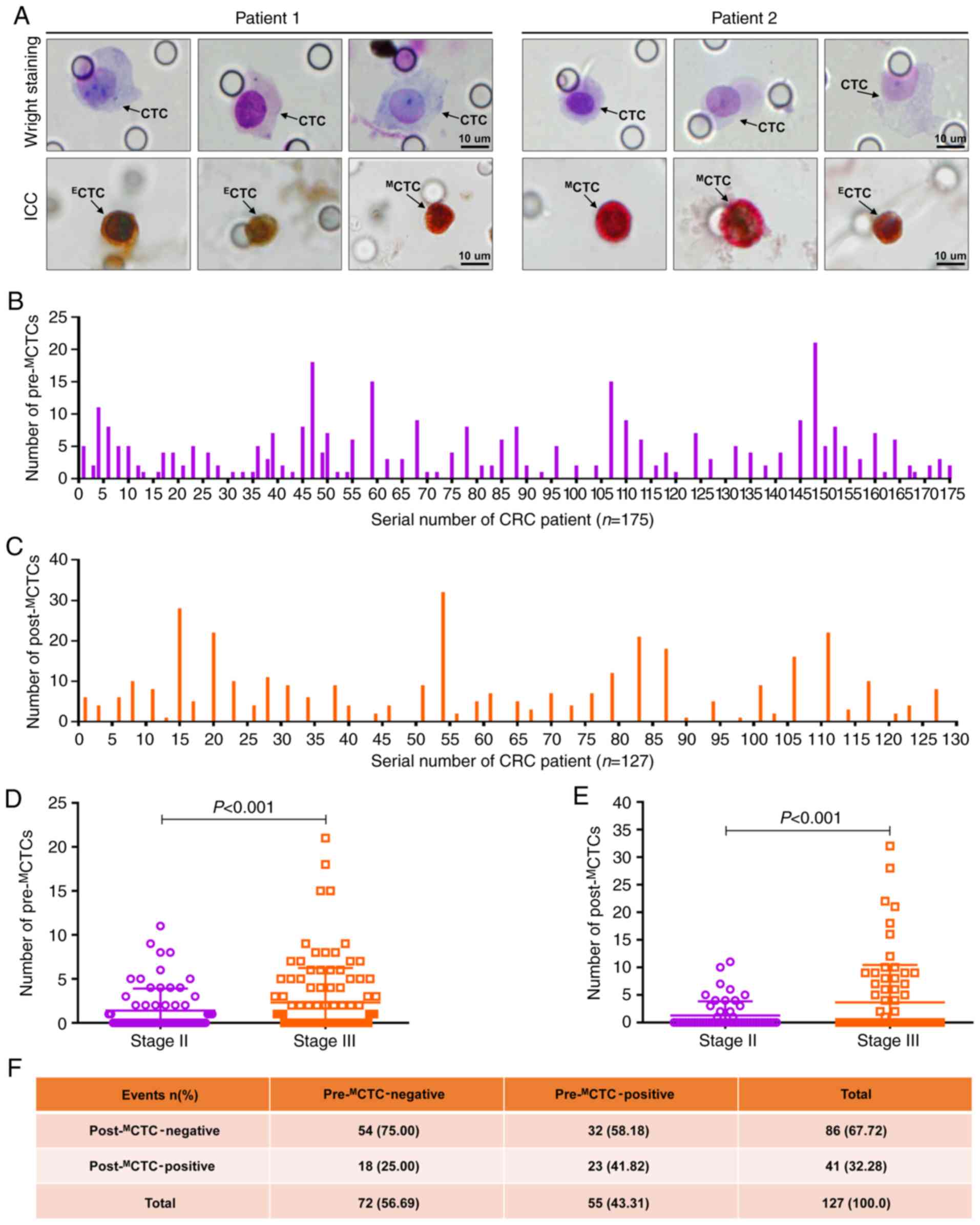
To characterize the EMT traits of CTCs, CTCs from
the peripheral blood of patients were isolated using the
CTCBIOPSY® device and stained by Wright's staining.
Then, candidate CTCs were identified by morphological analysis, and
cells with an abnormal morphology and an irregular nucleus, a
diameter >15 µm, a nucleus-to-cytoplasm ratio >0.8 and a
hyperchromatic nucleus and nonhomogeneous staining were considered
as CTCs. The representative CTC images from two included patients
are presented in Fig. 2A.
Furthermore, identified CTCs were stained for E-cadherin and
vimentin by ICC to distinguish their EMT traits. As revealed in
Fig. 2A, CTCs with E-cadherin
expression were presented as brown and considered epithelial CTCs
(ECTCs), while CTCs with vimentin expression were
presented as red and considered MCTCs.
Overall, pre-MCTCs were detected from 74
patients with a positive rate of 42.29% (74/175) and an average
count of 2.17 (Fig. 2B), while
post-MCTCs were detected from 41 patients with a
positive rate of 32.28% (41/127) and an average count of 2.66
(Fig. 2C). In the stratified
analysis of TNM staging, both the number of pre-MCTCs
and post-MCTCs were significantly higher in stage III
patients than that in stage II patients (P<0.001, respectively;
Fig. 2D and E). Furthermore, the
dynamic changes of pre- and post-MCTCs under the
pressure of anticancer therapy were determined, and the results
revealed that 54 patients (75.00%) remained
post-MCTC-negative while 18 patients (25.00%) converted
to post-MCTC-positive among the 72 patients with
pre-MCTC-negative; by contrast, 32 patients (58.18%)
converted to post-MCTC-negative while 23 patients
(41.82%) remained post-MCTC-positive among the 55
patients with pre-MCTC-positive (Fig. 2F).
Association of MCTCs with
clinicopathological characteristics of CRC patients
The association of pre- and post-MCTC
status with clinicopathological characteristics of CRC patients are
presented in Table SI.
Pre-MCTC-positive was significantly correlated with
tumor grade (χ2=6.897, P=0.009), lymphovascular invasion
(LVI) (χ2=15.495, P<0.001), tumor invasion
(χ2=24.044, P<0.001) and lymph node metastasis (LNM)
(χ2=10.382, P=0.001), whereas no significant association
was found between pre-MCTCs and gender, age, tumor
location, tumor size, perineural invasion (PNI),
tumor-node-metastasis (TNM) stage and carcinoembryonic antigen
(CEA) level (P>0.05 for all). Post-MCTC-positive was
significantly associated with LVI (χ2=8.791, P=0.003)
and LNM (χ2=4.517, P=0.034), but not related to gender,
age, tumor location, tumor size, PNI, tumor invasion, TNM stage,
and serum CEA level (P>0.05 for all).
Furthermore, the clinical associations of the
dynamic changes of pre- and post-MCTCs in CRC patients
were explored, and the results revealed that both pre- and
post-MCTC-positive were significantly related with LVI
(χ2=22.737, P<0.001) and TNM stage
(χ2=8.632, P=0.033), but not statistically associated
with gender, age, tumor location, tumor size, tumor grade, PNI,
tumor invasion, LNM and serum CEA level (P>0.05 for all)
(Table I).
Prognostic value of
pre-MCTCs and post-MCTCs in patients with
CRC
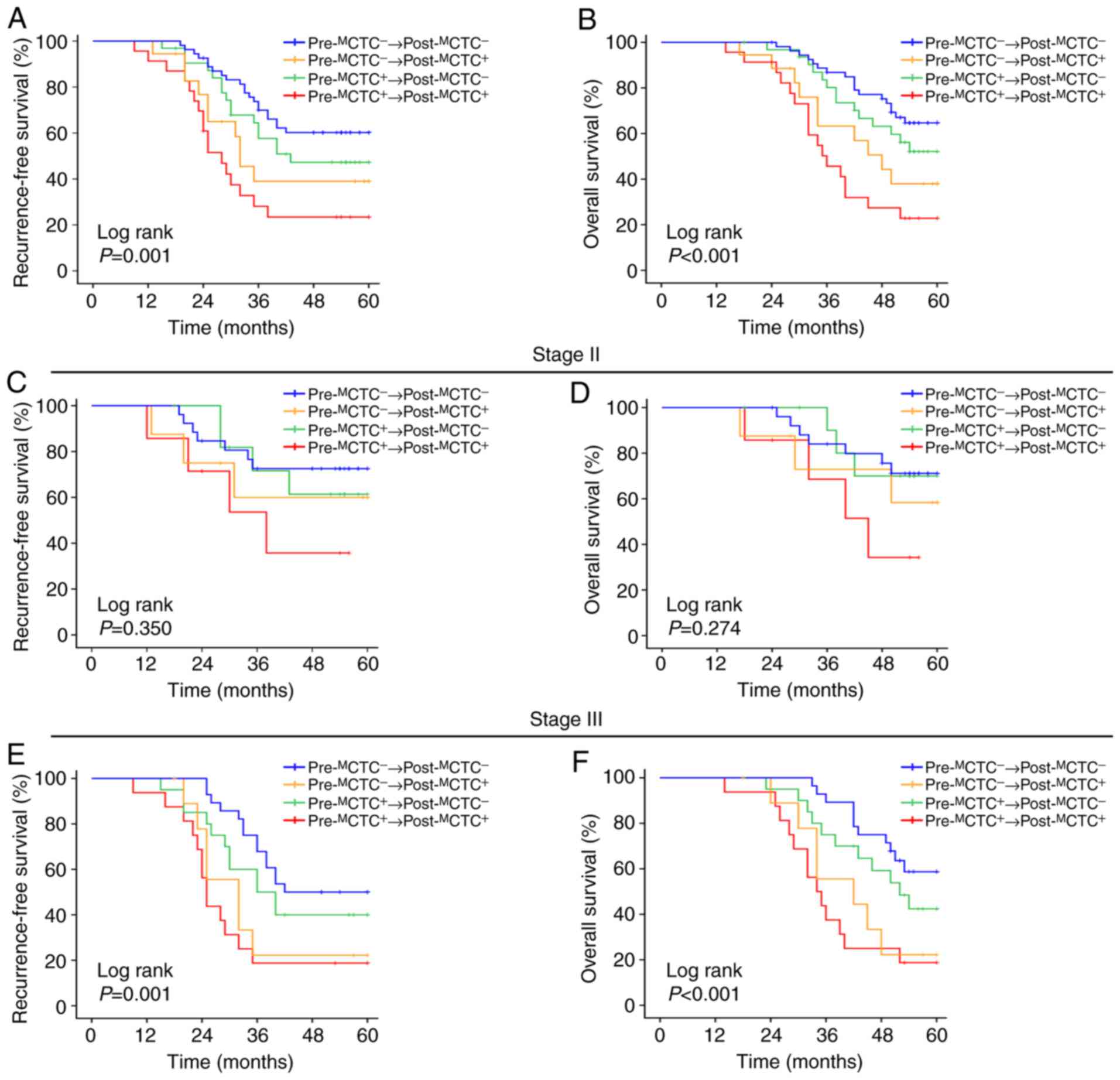
Moreover, the prognostic value of
pre-MCTCs and post-MCTCs in patients with CRC
was analyzed, and it was revealed that patients with
pre-MCTC-positive had a significantly unfavourable RFS
(P=0.002, Fig. 3A) and OS (P=0.005,
Fig. 3B) compared to pre-
MCTC-negative patients. In addition,
post-MCTC-positive patients also had a worse RFS
(P<0.001, Fig. 3C) and OS
(P<0.001, Fig. 3D) compared to
post-MCTC-negative ones.
For the dynamic change of pre- and
post-MCTCs, survival was significantly different among
the four groups. Median RFS for patients with
pre-MCTC−→post-MCTC−,
pre-MCTC−→post-MCTC+,
pre-MCTC+→post-MCTC−
and
pre-MCTC+→post-MCTC+
were, respectively, 48.6 months, 39.2 months, 44.3 months and 32.8
months (P=0.001, Fig. 4A). The
median OS for these four groups was, respectively, 53.2 months,
44.9 months, 50.3 months and 39.0 months (P<0.001, Fig. 4B). In the TNM stage-stratification
analyses, the results revealed that the RFS and OS of the
aforementioned four groups were not significantly different in
patients with stage II (RFS: P=0.350, Fig. 4C; OS: P=0.274, Fig. 4D). For patients with stage III
disease, patients with
pre-MCTC+→post-MCTC+
had a significant shorter RFS and OS than the other three groups
(RFS: P=0.001, Fig. 4E; OS:
P<0.001, Fig. 4F).
Furthermore, univariate analyses indicated that poor
tumor grade (P=0.025), presence of LVI (P=0.001), presence of PNI
(P=0.034), deeper tumor invasion (P=0.027), more LNM (P=0.013),
higher TNM stage (P<0.001) and
pre-MCTC+→post-MCTC+
(P=0.001) were significantly associated with dismal RFS, while,
poor tumor grade (P=0.042), presence of LVI (P=0.006), presence of
PNI (P=0.022), deeper of tumor invasion (P=0.042), more LNM
(P=0.021), higher TNM stage (P<0.001) and
pre-MCTC+→post-MCTC+
(P<0.001) were significantly associated with unfavorable OS
(Table II). Multivariate Cox
regression model demonstrated that more LNM (HR: 1.257, 95%CI:
1.052–1.751, P=0.019), higher TNM stage (HR: 1.794, 95%CI:
1.113–3.124, P=0.012) and
pre-MCTC+→post-MCTC+
(HR: 1.302, 95%CI: 1.033–1.639, P=0.025) were the independent
prognostic factors for shorter RFS; more LNM (HR: 1.915, 95%CI:
1.004–3.652, P=0.049), higher TNM stage (HR: 1.491, 95%CI:
1.138–1.955, P=0.004) and
pre-MCTC+→post-MCTC+
(HR: 1.366, 95%CI: 1.070–1.742, P=0.012) were the independent
prognostic factors for shorter OS (Table III).
 | Table II.Univariate analyses of factors
associated with RFS and OS of CRC patients. |
Table II.
Univariate analyses of factors
associated with RFS and OS of CRC patients.
|
| RFS | OS |
|---|
|
|
|
|
|---|
| Factors | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex (male vs.
female) | 0.777 | 0.466–1.296 | 0.334 | 0.792 | 0.467–1.343 | 0.387 |
| Age (<60 years
vs. ≥60 years) | 1.453 | 0.881–3.409 | 0.686 | 1.361 | 0.809–2.288 | 0.245 |
| Tumor location
(colon vs. rectal) | 1.590 | 0.963–2.618 | 0.070 | 1.610 | 0.956–2.710 | 0.073 |
| Tumor size (<5
cm vs. ≥5 cm) | 1.450 | 0.772–2.748 | 0.251 | 1.063 | 0.637–1.773 | 0.817 |
| Tumor grade (poor
vs. moderate and well) | 0.465 | 0.245–0.917 | 0.025a | 0.531 | 0.272–0.976 | 0.042a |
| LVI (absence vs.
presence) | 3.001 | 1.582–5.684 | 0.001a | 2.065 | 1.236–3.450 | 0.006a |
| PNI (absence vs.
presence) | 2.213 | 1.065–4.598 | 0.034a | 2.073 | 1.114–3.876 | 0.022a |
| Tumor invasion
(T1-2 vs. T3-4) | 1.481 | 1.047–2.096 | 0.027a | 1.314 | 1.013–2.021 | 0.042a |
| LNM (N0-1 vs.
N2-3) | 1.498 | 1.201–1.869 | 0.013a | 1.606 | 1.376–2.020 | 0.021a |
| TNM stage (II vs.
III) | 2.725 | 1.577–4.710 |
<0.001a | 3.038 | 1.687–5.469 |
<0.001a |
| CEA (<5 ng/ml
vs. ≥5 ng/ml) | 1.344 | 0.818–2.208 | 0.243 | 1.235 | 0.734–2.075 | 0.427 |
| Change of
pre-MCTCs to post-MCTCs | 2.387 | 1.616–3.937 | 0.001a | 2.717 | 1.623–4.454 |
<0.001a |
 | Table III.Multivariate analyses of factors
associated with RFS and OS of CRC patients. |
Table III.
Multivariate analyses of factors
associated with RFS and OS of CRC patients.
|
| RFS | OS |
|---|
|
|
|
|
|---|
| Factors | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Tumor grade (low
vs. middle and high) | 1.274 | 0.931–1.729 | 0.126 | 1.224 | 0.825–1.806 | 0.324 |
| LVI (absence vs.
presence) | 1.382 | 0.789–2.422 | 0.258 | 1.377 | 0.762–2.490 | 0.289 |
| PNI (absence vs.
presence) | 0.617 | 0.358–1.065 | 0.083 | 1.406 | 0.803–1.418 | 0.233 |
| Tumor invasion
(T1-2 vs. T3-4) | 1.143 | 0.765–1.714 | 0.528 | 1.462 | 0.645–3.332 | 0.374 |
| LNM (N0-1 vs.
N2-3) | 1.257 | 1.052–1.751 | 0.019a | 1.915 | 1.004–3.652 | 0.049a |
| TNM stage (II vs.
III) | 1.794 | 1.113–3.124 | 0.012a | 1.491 | 1.138–1.955 | 0.004a |
| Change of
pre-MCTCs to post-MCTCs | 1.302 | 1.033–1.639 | 0.025a | 1.366 | 1.070–1.742 | 0.012a |
Discussion
In the present study, it was revealed that the
status of MCTCs remained dynamically changed under the
pressure of anticancer therapy, and these changes were
significantly correlated with multiple unfavourable
clinicopathological features and dismal prognosis of patients with
CRC. Furthermore, univariate and multivariate analyses demonstrated
that persistent positivity of MCTCs before and after
anticancer therapy was an independent risk factor affecting the RFS
and OS of CRC patients. To the best of our knowledge, the present
study is the first investigation of the potential prognostic value
of the dynamic change of MCTCs during anticancer therapy
in CRC.
Given that EMT and CTCs are the critical mediators
in tumor metastasis, analyzing EMT-CTCs has great potential for
cancer prognosis and progression monitoring (11,34,35).
To date, more attention has been paid in exploring the clinical
significance of CTC enumeration in CRC (6,7,10,36–38),
and few studies have focused on the molecular traits of CTCs and
its dynamic changes under the pressure of anticancer therapy. Zhang
et al detected the phenotype of CTCs in advanced CRC
patients during chemotherapy using a size-based platform combined
with immunofluorescence staining, and the results revealed that
patients with vimentin-positive CTCs had shorter progression-free
survival (PFS) and OS than patients with vimentin-negative CTCs
(28). In other types of solid
tumors, Qi et al used an advanced CanPatrol CTC-enrichment
technique and RNA in situ hybridization (RNA-ISH) to detect
CTCs undergoing EMT in patients with hepatocellular carcinoma, and
the results revealed that MCTCs positive before surgery
were significantly related to early recurrence, multi-intrahepatic
recurrence, and lung metastasis (9). Another group of researchers used the
same method to explore the dynamic changes of different phenotypic
CTCs in renal cell carcinoma, and revealed that the recurrence or
metastasis of RCC was uncorrelated with initial CTC counts, but
probably related with the variation trend of CTCs, especially
MCTCs (39). In
addition, Markiewicz et al used general breast cancer
markers to detect the EMT phenotype of CTCs, and revealed that
MCTCs were characterized by the most aggressive
phenotype, presence of LNM, larger tumor size and higher risk of
death (40). In our present study,
we used a size-based CTCBIOPSY® device combined with an
immunocytochemistry (ICC) assay to dynamically monitor the changes
of MCTCs before curative resection and after adjuvant
chemotherapy. The results revealed that the status of
MCTCs remained dynamically changed under the pressure of
anticancer therapy, and these dynamic changes were significantly
associated with presence of LVI, higher TNM stage, and dismal
prognosis. Notably, persistent positivity of MCTCs
before and after anticancer therapy was considered as an
independent risk factor affecting the RFS and OS of CRC patients.
These results were consistent with previous results (7,9),
indicating that the dynamic change of MCTCs during
anticancer therapy is a useful prognostic tool for solid cancers
and dynamically monitoring CTCs-EMT traits may provide more
detailed information for cancer management.
The EMT process, characterized by epithelial cell
loss of cell-cell junctions, acquisition of a migratory phenotype,
and increased cellular motility, has been demonstrated to play a
key role in tumor development (11,12).
Currently, emerging and accumulating evidence has demonstrated that
EMT is widely involved in CTC generation (20,35);
in addition, CTCs may also undergo EMT changes during anticancer
therapy to resist external intervention, thereby surviving in the
peripheral system and eventually forming metastatic tumors
(9,17,20,41).
The generation of CTCs requires several key steps, including
detachment from the tumor lesion, invasion of the basal membrane,
entry of vessels and survival in circulation. Specifically, EMT and
related regulatory networks play important roles in this process,
by increasing tumor cell invasiveness, promoting intravasation and
facilitating tumor cell survival to mediate CTC generation
(34,35). Theoretically, tumor lesions can
release thousands of CTCs into the bloodstream every day, but only
a few can survive in circulation because they may encounter strong
anoikis signals and anticancer therapy. Nevertheless, under these
stressful stimuli, CTCs could undergo EMT changes to form
MCTCs that facilitate their survival by avoiding
apoptosis, anoikis and senescence and promoting drug resistance
(42,43). Additionally, EMT-inducing
transcription factors (EMT-TFs), such as Snail, Slug, Twist and
SIP1, can protect CTCs from anoikis by interfering with the normal
apoptosis cascade, resisting senescence, and/or collaborating with
TrkB (44–47). These studies indicated that CTCs can
undergo EMT phenotype changes during treatment to cope with
external attacks including radiotherapy and chemotherapy to promote
tumor metastasis, thereby affecting patient prognosis.
Given the important role of CTCs in tumor
progression, great attempts have been made to develop reliable
methods for detecting these cells in the past few decades (48,49),
and numerous technologies based on the physical and biological
properties of CTCs have been designed to isolate them, including
cytometric methods (50), PCR-based
assays (51,52), antibody-dependent methods (53), and size-exclusion technologies
(54). Among these methods, the
CellSearch™ system (Veridex LLC) is the first and only method
approved by the US Food and Drug Administration (FDA) for clinical
application to detect CTCs in CRC (55). However, as an EpCAM-dependent
isolation technology, this approach may fail to detect the CTCs
undergoing EMT (e.g., MCTCs) (56). Recently, several non-EpCAM-based CTC
isolation platforms have been developed to analyse CTCs with EMT,
such as ISET® (Rarecells Diagnostics, Paris, France)
(57), CanPatrol™ (9,41) and
the Vitatex CAM platform (58). In
the present study, we used the self-developed CTCBIOPSY®
device combined with an ICC assay to detect MCTCs. As an
isolation by size of epithelial tumor cell assay,
CTCBIOPSY® exhibited excellent performance in capturing
the CTCs of patients (27).
Previously, this size-based method has been demonstrated to
efficiently capture EMT-CTCs form the blood samples of patients
(28). Combined with our present
results, we have reason to believe that CTCBIOPSY®-based
EMT-CTC detection can provide a potential biomarker for prognosis
assessment of CRC. Although, further studies are still required to
evaluate the practical impact of EMT-CTC count on treatment
strategy optimization.
There are several limitations in the present study.
First, the limited number of patients included and the use of
samples from a single center reduce the validity of this
prospective cohort study. Second, since we did not evaluate the
tumor load of patients during the process of conducting this study,
the relationship between MCTC count and tumor load was
not further analyzed. In fact, tumor load is an important indicator
reflecting the malignant degree of a tumor, and its degree is
closely related to the generation of CTCs. Therefore, evaluating
the relationship between MCTCs and tumor load can
provide important information for us to further understand the
clinical significance of MCTCs in CRC. In the future
studies, this will be focused on in depth.
In conclusion, the present study demonstrated that
the count and status of MCTCs remained dynamically
changed under the pressure of anticancer therapy, and these changes
were significantly associated with multiple unfavourable
clinicopathological parameters and dismal prognosis of patients
with CRC. Further univariate and multivariate analyses demonstrated
that persistent positivity of MCTCs before and after
anticancer therapy was an independent risk factor affecting the OS
and RFS of CRC patients. Collectively, these findings highlight the
importance of the dynamic monitoring of MCTC change in
the prognosis assessment, providing additional insights into the
clinical significance of CTCs in CRC.
Supplementary Material
Supporting Data
Acknowledgements
The authors appreciate Wuhan YZY Medical Science
and Technology Co., Ltd. for providing equipment and excellent
technical support in CTC detection.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81572874, 81702411,
and 81872376) and the Health Commission of Hubei Province
Scientific Research Project (grant no. WJ2019H012).
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
DDS, SYW and BX conceived and designed the study.
DDS, CGY and SH conducted the experiments. DDS and CGY provided the
study materials and assembled the patient samples. DDS collected
and assembled the data. DDS and CGY prepared the figures and
tables. DDS and CGY analyzed and interpreted the data. DDS and CGY
wrote the manuscript. SYW and BX revised the manuscript. All of the
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board (IRB) of Zhongnan Hospital of Wuhan University in
adherence with the Declaration of Helsinki, and written informed
consent was obtained from all included patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CTCs
|
circulating tumor cells
|
|
CRC
|
colorectal cancer
|
|
MCTCs
|
mesenchymal circulating tumor
cells
|
|
EMT
|
epithelial-mesenchymal transition
|
|
ICC
|
immunocytochemistry
|
|
LVI
|
lymphovascular invasion
|
|
PNI
|
perineural invasion
|
|
LNM
|
lymph node metastasis
|
|
TNM
|
tumor-node-metastasis
|
|
CEA
|
carcinoembryonic antigen
|
|
RNA-ISH
|
RNA in situ hybridization
|
|
FDA
|
US Food and Drug Administration
|
|
IRB
|
Institutional Review Board
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
|
RFS
|
recurrence-free survival
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
References
|
1
|
Massague J and Obenauf AC: Metastatic
colonization by circulating tumor cells. Nature. 529:298–306. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siravegna G, Marsoni S, Siena S and
Bardelli A: Integrating liquid biopsies into the management of
cancer. Nat Rev Clin Oncol. 14:531–548. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cohen SJ, Punt CJ, Iannotti N, Saidman BH,
Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et
al: Relationship of circulating tumor cells to tumor response,
progression-free survival, and overall survival in patients with
metastatic colorectal cancer. J Clin Oncol. 26:3213–3221. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Bono JS, Scher HI, Montgomery RB,
Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ
and Raghavan D: Circulating tumor cells predict survival benefit
from treatment in metastatic castration-resistant prostate cancer.
Clin Cancer Res. 14:6302–6309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hayes DF, Cristofanilli M, Budd GT, Ellis
MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV and
Terstappen LW: Circulating tumor cells at each follow-up time point
during therapy of metastatic breast cancer patients predict
progression-free and overall survival. Clin Cancer Res.
12:4218–4224. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang C, Wei C, Wang S, Han S, Shi D, Zhang
C, Lin X, Dou R and Xiong B: Combined features based on
preoperative controlling nutritional status score and circulating
tumor cell status predict prognosis for colorectal cancer patients
treated with curative resection. Int J Biol Sci. 15:1325–1335.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pernot S, Badoual C, Terme M, Castan F,
Cazes A, Bouche O, Bennouna J, Francois E, Ghiringhelli F, De La
Fouchardiere C, et al: Dynamic evaluation of circulating tumor
cells in patients with advanced gastric and oesogastric junction
adenocarcinoma: Prognostic value and early assessment of
therapeutic effects. Eur J Cancer. 79:15–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang S, Wu T, Peng X, Liu J, Liu F, Wu S,
Liu S, Dong Y, Xie S and Ma S: Mesenchymal phenotype of circulating
tumor cells is associated with distant metastasis in breast cancer
patients. Cancer Manag Res. 9:691–700. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qi LN, Xiang BD, Wu FX, Ye JZ, Zhong JH,
Wang YY, Chen YY, Chen ZS, Ma L, Chen J, et al: Circulating tumor
cells undergoing EMT provide a metric for diagnosis and prognosis
of patients with hepatocellular carcinoma. Cancer Res.
78:4731–4744. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang C, Shi D, Wang S, Wei C, Zhang C and
Xiong B: Prognostic value of pre- and post-operative circulating
tumor cells detection in colorectal cancer patients treated with
curative resection: A prospective cohort study based on ISET
device. Cancer Manag Res. 10:4135–4144. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brabletz T, Kalluri R, Nieto MA and
Weinberg RA: EMT in cancer. Nat Rev Cancer. 18:128–134. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chaffer CL, San Juan BP, Lim E and
Weinberg RA: EMT, cell plasticity and metastasis. Cancer Metastasis
Rev. 35:645–654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu M, Bardia A, Wittner BS, Stott SL, Smas
ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al:
Circulating breast tumor cells exhibit dynamic changes in
epithelial and mesenchymal composition. Science. 339:580–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin X, Wang S, Sun M, Zhang C, Wei C, Yang
C, Dou R, Liu Q and Xiong B: miR-195-5p/NOTCH2-mediated EMT
modulates IL-4 secretion in colorectal cancer to affect M2-like TAM
polarization. J Hematol Oncol. 12:202019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei C, Yang C, Wang S, Shi D, Zhang C, Lin
X, Liu Q, Dou R and Xiong B: Crosstalk between cancer cells and
tumor associated macrophages is required for mesenchymal
circulating tumor cell-mediated colorectal cancer metastasis. Mol
Cancer. 18:642019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang C, Wei C, Wang S, Shi D, Zhang C, Lin
X, Dou R and Xiong B: Elevated CD163+/CD68+
ratio at tumor invasive front is closely associated with aggressive
phenotype and poor prognosis in colorectal cancer. Int J Biol Sci.
15:984–998. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Micalizzi DS, Haber DA and Maheswaran S:
Cancer metastasis through the prism of epithelial-to-mesenchymal
transition in circulating tumor cells. Mol Oncol. 11:770–780. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Li S, Li W, Yang R, Zhang X, Ye Y,
Yu J, Ye L and Tang W: Circulating tumor cells undergoing EMT are
poorly correlated with clinical stages or predictive of recurrence
in hepatocellular carcinoma. Sci Rep. 9:70842019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Zhang X, Ge S, Gao J, Gong J, Lu M,
Zhang Q, Cao Y, Wang DD, Lin PP and Shen L: Clinical significance
of phenotyping and karyotyping of circulating tumor cells in
patients with advanced gastric cancer. Oncotarget. 5:6594–6602.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morrow CJ, Trapani F, Metcalf RL,
Bertolini G, Hodgkinson CL, Khandelwal G, Kelly P, Galvin M, Carter
L, Simpson KL, et al: Tumorigenic non-small-cell lung cancer
mesenchymal circulating tumor cells: A clinical case study. Ann
Oncol. 27:1155–1160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen Q, Xu L, Zhao L, Wu D, Fan Y, Zhou Y,
Ouyang WH, Xu X, Zhang Z, Song M, et al: Specific capture and
release of circulating tumor cells using aptamer-modified
nanosubstrates. Adv Mater. 25:2368–2373. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng B, He Z, Zhao L, Fang Y, Chen Y, He
R, Chen F, Song H, Deng Y, Zhao X and Xiong B: Transparent,
biocompatible nanostructured surfaces for cancer cell capture and
culture. Int J Nanomedicine. 9:2569–2580. 2014.PubMed/NCBI
|
|
23
|
Wang S, Zhang C, Wang G, Cheng B, Wang Y,
Chen F, Chen Y, Feng M and Xiong B: Aptamer-mediated
transparent-biocompatible nanostructured surfaces for
hepotocellular circulating tumor cells enrichment. Theranostics.
6:1877–1886. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang C, Zhang N, Wang S, Shi D, Zhang C,
Liu K and Xiong B: Wedge-shaped microfluidic chip for circulating
tumor cells isolation and its clinical significance in gastric
cancer. J Transl Med. 16:1392018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Austin RG, Huang TJ, Wu M, Armstrong AJ
and Zhang T: Clinical utility of non-EpCAM based circulating tumor
cell assays. Adv Drug Deliv Rev. 125:132–142. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen Z, Wu A and Chen X: Current detection
technologies for circulating tumor cells. Chem Soc Rev.
46:2038–2056. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen F, Wang S, Fang Y, Zheng L, Zhi X,
Cheng B, Chen Y, Zhang C, Shi D, Song H, et al: Feasibility of a
novel one-stop ISET device to capture CTCs and its clinical
application. Oncotarget. 8:3029–3041. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang D, Zhao L, Zhou P, Ma H, Huang F,
Jin M, Dai X, Zheng X, Huang S and Zhang T: Circulating tumor
microemboli (CTM) and vimentin+ circulating tumor cells (CTCs)
detected by a size-based platform predict worse prognosis in
advanced colorectal cancer patients during chemotherapy. Cancer
Cell Int. 17:62017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vona G, Sabile A, Louha M, Sitruk V,
Romana S, Schütze K, Capron F, Franco D, Pazzagli M, Vekemans M, et
al: Isolation by size of epithelial tumor cells: A new method for
the immunomorphological and molecular characterization of
circulatingtumor cells. Am J Pathol. 156:57–63. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hofman VJ, Ilie MI, Bonnetaud C, Selva E,
Long E, Molina T, Vignaud JM, Fléjou JF, Lantuejoul S, Piaton E, et
al: Cytopathologic detection of circulating tumor cells using the
isolation by size of epithelial tumor cell method: Promises and
pitfalls. Am J Clin Pathol. 135:146–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hou JM, Krebs M, Ward T, Sloane R, Priest
L, Hughes A, Clack G, Ranson M, Blackhall F and Dive C: Circulating
tumor cells as a window on metastasis biology in lung cancer. Am J
Pathol. 178:989–996. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Deevi RK, Javadi A, McClements J,
Vohhodina J, Savage K, Loughrey MB, Evergren E and Campbell FC:
Protein kinase C zeta suppresses low- or high-grade colorectal
cancer (CRC) phenotypes by interphase centrosome anchoring. J
Pathol. 244:445–459. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: Emt: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jie XX, Zhang XY and Xu CJ:
Epithelial-to-mesenchymal transition, circulating tumor cells and
cancer metastasis: Mechanisms and clinical applications.
Oncotarget. 8:81558–81571. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Krebs MG, Renehan AG, Backen A, Gollins S,
Chau I, Hasan J, Valle JW, Morris K, Beech J, Ashcroft L, et al:
Circulating tumor cell enumeration in a phase II trial of a
four-drug regimen in advanced colorectal cancer. Clin Colorectal
Cancer. 14:115–122.e1-e2. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsai WS, Chen JS, Shao HJ, Wu JC, Lai JM,
Lu SH, Hung TF, Chiu YC, You JF, Hsieh PS, et al: Circulating tumor
cell count correlates with colorectal neoplasm progression and is a
prognostic marker for distant metastasis in non-metastatic
patients. Sci Rep. 6:245172016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rahbari NN, Aigner M, Thorlund K, Mollberg
N, Motschall E, Jensen K, Diener MK, Büchler MW, Koch M and Weitz
J: Meta-analysis shows that detection of circulating tumor cells
indicates poor prognosis in patients with colorectal cancer.
Gastroenterology. 138:1714–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang ZL, Zhang P, Li HC, Yang XJ, Zhang
YP, Li ZL, Xue L, Xue YQ, Li HL, Chen Q and Chong T: Dynamic
changes of different phenotypic and genetic circulating tumor cells
as a biomarker for evaluating the prognosis of RCC. Cancer Biol
Ther. 20:505–512. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Markiewicz A, Topa J, Nagel A, Skokowski
J, Seroczynska B, Stokowy T, Welnicka-Jaskiewicz M and Zaczek AJ:
Spectrum of Epithelial-mesenchymal transition phenotypes in
circulating tumor cells from early breast cancer patients. Cancers
(Basel). 11(pii): E592019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xu L, Mao X, Guo T, Chan PY, Shaw G, Hines
J, Stankiewicz E, Wang Y, Oliver RTD, Ahmad AS, et al: The Novel
Association of circulating tumor cells and circulating
megakaryocytes with prostate cancer prognosis. Clin Cancer Res.
23:5112–5122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cao Z, Livas T and Kyprianou N: Anoikis
and EMT: Lethal ‘Liaisons’ during cancer progression. Crit Rev
Oncog. 21:155–168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Frisch SM, Schaller M and Cieply B:
Mechanisms that link the oncogenic epithelial-mesenchymal
transition to suppression of anoikis. J Cell Sci. 126:21–29. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sayan AE, Griffiths TR, Pal R, Browne GJ,
Ruddick A, Yagci T, Edwards R, Mayer NJ, Qazi H, Goyal S, et al:
SIP1 protein protects cells from DNA damage-induced apoptosis and
has independent prognostic value in bladder cancer. Proc Natl Acad
Sci USA. 106:14884–14889. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Smit MA and Peeper DS: Zeb1 is required
for TrkB-induced epithelial-mesenchymal transition, anoikis
resistance and metastasis. Oncogene. 30:3735–3744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sharma S, Zhuang R, Long M, Pavlovic M,
Kang Y, Ilyas A and Asghar W: Circulating tumor cell isolation,
culture, and downstream molecular analysis. Biotechnol Adv.
36:1063–1078. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen L, Bode AM and Dong Z: Circulating
tumor cells: Moving biological insights into detection.
Theranostics. 7:2606–2619. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gross HJ, Verwer B, Houck D, Hoffman RA
and Recktenwald D: Model study detecting breast cancer cells in
peripheral blood mononuclear cells at frequencies as low as 10(−7).
Proc Natl Acad Sci USA. 92:537–541. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang C, Zou K, Zheng L and Xiong B:
Prognostic and clinicopathological significance of circulating
tumor cells detected by RT-PCR in non-metastatic colorectal cancer:
A meta-analysis and systematic review. BMC Cancer. 17:7252017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ghossein RA, Bhattacharya S and Rosai J:
Molecular detection of micrometastases and circulating tumor cells
in solid tumors. Clin Cancer Res. 5:1950–1960. 1999.PubMed/NCBI
|
|
53
|
Shahneh FZ: Sensitive antibody-based CTCs
detection from peripheral blood. Hum Antibodies. 22:51–54. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hao SJ, Wan Y, Xia YQ, Zou X and Zheng SY:
Size-based separation methods of circulating tumor cells. Adv Drug
Deliv Rev. 125:3–20. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Riethdorf S, O'Flaherty L, Hille C and
Pantel K: Clinical applications of the CellSearch platform in
cancer patients. Adv Drug Deliv Rev. 125:102–121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Andree KC, van Dalum G and Terstappen LW:
Challenges in circulating tumor cell detection by the CellSearch
system. Mol Oncol. 10:395–407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lecharpentier A, Vielh P, Perez-Moreno P,
Planchard D, Soria JC and Farace F: Detection of circulating tumor
cells with a hybrid (epithelial/mesenchymal) phenotype in patients
with metastatic non-small cell lung cancer. Br J Cancer.
105:1338–1341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Friedlander TW, Ngo VT, Dong H,
Premasekharan G, Weinberg V, Doty S, Zhao Q, Gilbert EG, Ryan CJ,
Chen WT and Paris PL: Detection and characterization of invasive
circulating tumor cells derived from men with metastatic
castration-resistant prostate cancer. Int J Cancer. 134:2284–2293.
2014. View Article : Google Scholar : PubMed/NCBI
|