Introduction
Recent lifestyle changes involving prolonged
exposure to ultraviolet (UV) radiations (including increased
outdoor activity or use of tanning beds) and a history of excessive
UV exposure that is associated with aging have been advocated to
explain the increasing incidence of non-melanoma skin cancer (NMSC)
worldwide (1). The biological
effects of UV radiation are strictly correlated to their
wavelengths (1). Accordingly, the
UV spectrum is subdivided into three distinct wavelength regions:
UVA (320–400 nm), UVB (290–320 nm) and UVC (200–290 nm) (2,3).
Although ~95% of the UVB radiation emitted by the sun is absorbed
by the Earth’s ozone layer, animal models have shown that UVB
exposition is linked to a higher NMSC risk compared to UVA
(4). The latter phenomenon is
attributed to the fact that UVB is principally absorbed in the
epidermis, whereas UVA can reach the dermis (5). Additionally, the shorter wavelengths
of UVB have been found to produce more alterations in the main
biological macromolecules (DNA and proteins) compared to the longer
wavelengths of UVA (6). Notably,
UVB radiation, in contrast to UVA, is known to cause direct DNA
damage, mainly in the form of cyclobutane pyrimidine dimers (CPD)
and 8-hydroxy-2′-deoxyguanosine (8OHdG), which are the main genetic
markers for UVB-induced mutagenesis (7–10).
Although traditional physical and chemical
sunscreens remain the mainstay approach to reduce the adverse
oncogenic potential of UVB radiations, total protection against the
entire spectrum of molecular lesions associated with UVB exposure
cannot be ensured (11). Therefore,
the identification of novel strategies aimed at reducing
UVB-induced damage in human keratinocytes has been previously
studied (12–14). Several antioxidants and
cytoprotective compounds, including L-carnosine,
L-(+)-ergothioneine, L-ascorbic acid and DL-α-tocopherol, have been
shown to reduce UV-induced injury in experimental and animal models
(15–18). In contrast to the DNA alterations
that may lead to NMSC-related mutations, growing evidence indicates
that UVB radiation can induce protein carbonylation (PC), a major
form of oxidative protein damage that has been recently proposed as
a critical molecular signature of skin carcinogenesis (19). Notably, altered protein function
caused by PC may have a detrimental impact on cellular signaling
and activate the target genes that promote survival, progression
and metastasis (20).
Trehalose, a naturally occurring non-reducing
disaccharide consisting of two glucose units, is found in a large
variety of organisms, including bacteria, fungi and invertebrate
animals, where it may serve as a stress protectant or resilience
factor (21,22). Extremophile bacteria have the
ability to achieve a significant UV-radiation resistance via simple
non-enzymatic antioxidant mechanisms consisting of trehalose
complexed with manganese ions (22). Trehalose is known to act as a major
protein stabilizer and its topical application to the eye reduces
UVB-induced corneal damage (19).
However, the use of trehalose as a skin photoprotective agent is
limited due its low skin permeability, which makes the development
of topical formulations difficult. A previous study has
demonstrated that liposomes can markedly increase the intracellular
delivery of trehalose (23). Thus,
the aim of the present study was to assess the protective effects
of trehalose-loaded liposomes against UVB irradiation-induced
injury using the immortalized human keratinocyte cell line, HaCaT.
The effect of this compound was compared to other commonly used
photoprotective agents, including L-carnosine, L-(+)-ergothioneine,
L-ascorbic acid and DL-α-tocopherol.
Materials and methods
Materials
L-carnosine, L-(+)-ergothioneine, L-ascorbic acid
and DL-α-tocopherol were obtained from Sigma-Aldrich (St. Louis,
MO, USA) and prepared as fresh 100-μM stock solutions in
phosphate-buffered saline (adjusted to 7.4 pH), filter-sterilized
and stored at 4°C for ≤12 h.
Production of trehalose-loaded
liposomes
Trehalose was purchased from Hayashibara Co., Ltd.
(Okayama, Japan). Unilamellar negatively-charged
trehalose-containing liposomes were synthesized using an extrusion
procedure, as previously described (23). Briefly, the liposome-lipid bilayer
consisted of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC;
Avanti Polar Lipids, Alabaster, AL, USA), phosphatidylserine (PS;
Sigma-Aldrich) and cholesterol (Avanti Polar Lipids) in a 60:10:30
% mol ratio, resulting in a 25-mM final lipid solution. Following
lyophilization, the DPPC:PS:cholesterol lipid film was hydrated
with trehalose buffer [300 mM
α-d-glucopyranosyl-α-d-glucopyranoside, 10 mM HEPES (pH 7.4), 307
mOsm; Sigma-Aldrich] to produce negatively-charged liposomes.
Liposomal trehalose was suspended in sterile 0.9% NaCl at 65°C to
yield a 100-μM stock solution.
HaCaT cell culture, pretreatment and
irradiation
HaCaT cells, an immortalized, non-tumorigenic human
keratinocyte cell line, were purchased from Cell Lines Service
(Eppelheim, Germany) and maintained in Dulbecco’s modified Eagle’s
medium (Invitrogen Life Technologies, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum and 1% antibiotics at
standard cell culture conditions (37°C, 5% CO2 in a
humidified incubator). The cells were cultured until 80% confluent
and pretreated with 100 μM of each stock solution
[trehalose-containing liposomes, L-carnosine, L-(+)-ergothioneine,
L-ascorbic acid and DL-α-tocopherol] for 24 h before UVB
irradiation. Exposure to UVB was performed using a Spectrolinker
XL-1500 UV crosslinker (Spectronics Corporation, Westbury, NY,
USA), which emits the majority of its energy within the UVB range
(280–320 nm), peaking at 312 nm. The intensity of UVB radiation was
measured using a phototherapy radiometer (International Light
Technologies, Newburyport, MA, USA). The cells were exposed to UVB
radiation at a dose of 20 mJ/cm2. Following UVB
radiation, cells were incubated in fresh medium in the absence of
any compounds until analysis. The cells that were exposed to UVB
radiation without any pretreatment were used as positive controls,
whereas the cells that received no pretreatment and were not
exposed to UVB irradiation served as negative controls.
Quantification of CPD, 8OHdG and PC in
HaCaT extracts
Approximately 3×106 cells were lysed
using 200 μl lysis buffer and were centrifuged at 13,000 × g for 10
min. PC in cell lysates was measured by OxiSelect™ Protein Carbonyl
ELISA kit (Cell Biolabs, Inc., San Diego, CA, USA) according to the
manufacturer’s instructions. For the CPD and 8OHdG measurements,
the samples were digested for 12 h at 60°C with proteinase K in 100
mmol/l Tris-HCl (pH 7.4), 150 mmol/l NaCl and 10 mmol/l EDTA (pH
8.0). Proteinase K was subsequently heat-inactivated at 95°C for 10
min and homogenates were extracted using the Puregene DNA isolation
kit (Gentra Systems, Inc., Minneapolis, MN, USA). The kit contains
two main reagents, which are the cell lysis and protein
precipitation solutions. Briefly, DNA was extracted from
homogenates using a lysis-buffer solution and treated with RNase A.
The kit removes proteins using a precipitation solution, followed
by 2-propanol to pellet the DNA. CPD and 8OHdG were measured in
duplicate and in a random order by specific ELISA kits [OxiSelect
Cellular UV-Induced DNA Damage ELISA kit (CPD), 96 assays (Cell
Biolabs, Inc.) and OxiSelect™ Oxidative DNA Damage ELISA kit (8OHdG
quantitation, Cell Biolabs, Inc.)] according to the manufacturer’s
instructions. The results of CPD, 8OHdG and PC measurements are
presented as arbitrary units (such as relative amount of absorbance
in ELISA relative to the untreated control, which was set as 1 by
convention). All the analyses were conducted whilst blinded to the
irradiation protocol.
Statistical analysis
All the data were from at least three independent
experiments and are expressed as mean ± standard deviation. The
comparisons of the biomarkers among the various experimental
conditions [positive controls (no pretreatment) or pretreatment
with trehalose-loaded liposomes, L-carnosine, L-(+)-ergothioneine,
L-ascorbic acid or DL-α-tocopherol] were performed using one-way
analysis of variance (ANOVA) to detect whether a group
(pretreatment) effect existed, followed by Tukey’s test for post
hoc pairwise comparisons. All the calculations were performed using
SPSS, version 17.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad
Prism, version 4.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Statistical analysis was two-tailed and P<0.05 was considered to
indicate a statistically significant difference.
Results
Analysis of the UVB-induced biomarker
changes
Following UVB irradiation, the levels of CPD, 8OHdG
and PC were significantly increased (by 27.5-, 8.1- and 7.2-fold,
respectively; Table I) in
positive-control HaCaT cells compared to non-irradiated
negative-control cells (all P<0.001). To investigate whether
pretreatment with trehalose-loaded liposomes, L-carnosine,
L-(+)-ergothioneine, L-ascorbic acid and DL-α-tocopherol could
inhibit UVB-induced DNA and protein damage in cultured
keratinocytes, CPD, 8OHdG and PC were measured in HaCaT cells
pretreated with fresh 100-μM stock solutions for each compound
prior to exposure to UVB light.
 | Table ICyclobutane pyrimidine dimers (CPD),
8-hydroxy-2′-deoxyguanosine (8OHdG) and protein carbonylation (PC)
in human HaCaT cells under different experimental conditions. |
Table I
Cyclobutane pyrimidine dimers (CPD),
8-hydroxy-2′-deoxyguanosine (8OHdG) and protein carbonylation (PC)
in human HaCaT cells under different experimental conditions.
| Negative control | Positive control | Trehalose-loaded
liposomes | L-carnosine |
L-(+)-ergothioneine | L-ascorbic acid | DL-α-tocopherol |
|---|
| CPD | 1 | 27.5±0.6 | 6.1±0.3 | 12.1±1.3 | 8.4±1.2 | 15.3±1.4 | 12.6±0.5 |
| 8OHdG | 1 | 8.1±0.6 | 3.6±0.1 | 5.2±0.3 | 4.7±0.2 | 6.1±0.2 | 6.0±0.4 |
| PC | 1 | 7.2±0.2 | 2.1±0.1 | 4.7±0.1 | 4.3±0.2 | 5.5±0.3 | 4.9±0.2 |
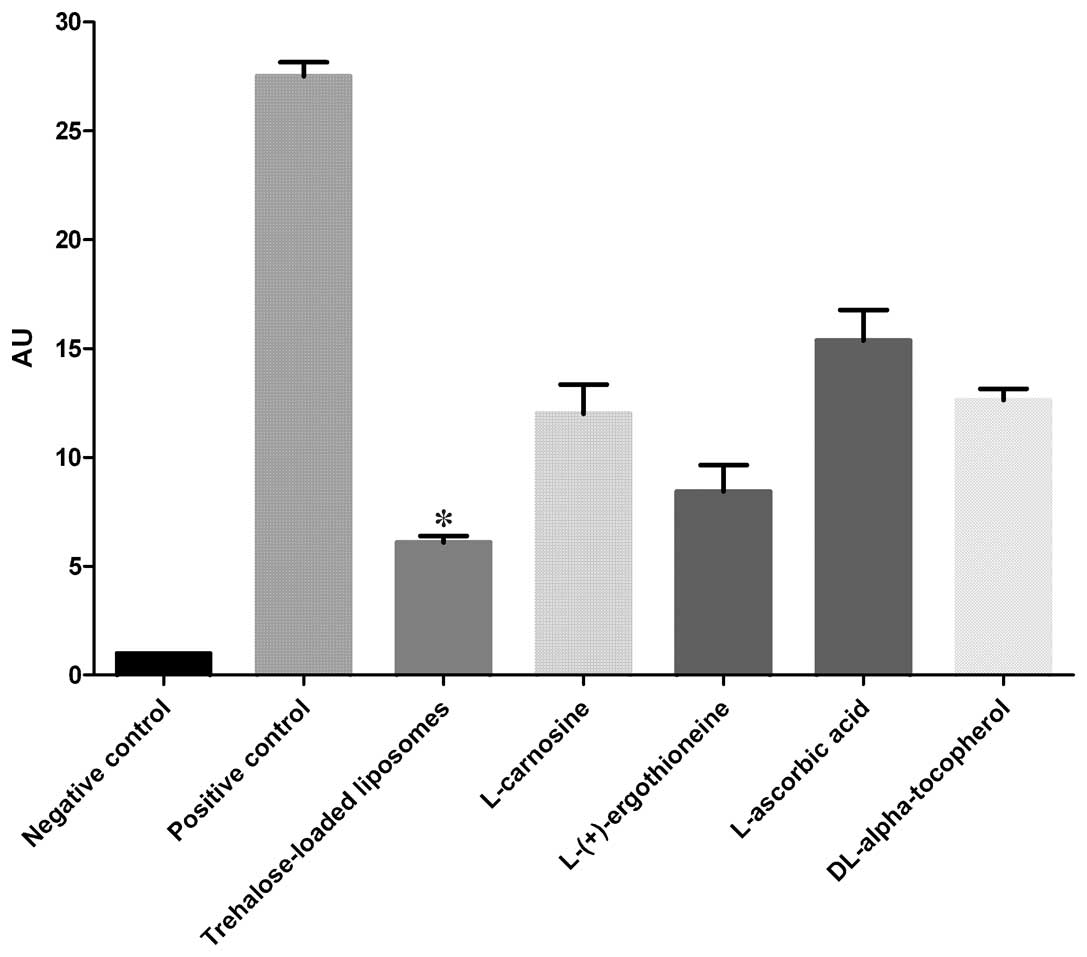
CPD
The protective effects of trehalose-loaded
liposomes, L-carnosine, L-(+)-ergothioneine, L-ascorbic acid and
DL-α-tocopherol against CPD formation in UVB-irradiated HaCaT cells
are shown in Fig. 1. The ANOVA
tests showed a significant pretreatment effect (P<0.001). In
post hoc analyses, all the tested compounds were able to reduce the
formation of CPD in UVB-irradiated cells compared to the positive
controls (all P<0.001). Post hoc analyses also revealed that
trehalose-loaded liposomes and L-(+)-ergothioneine were
significantly more effective in inhibiting CPD formation compared
to the other compounds (all P<0.001). Notably, trehalose-loaded
liposomes were significantly more effective than
L-(+)-ergothioneine (P<0.05; Fig.
1).
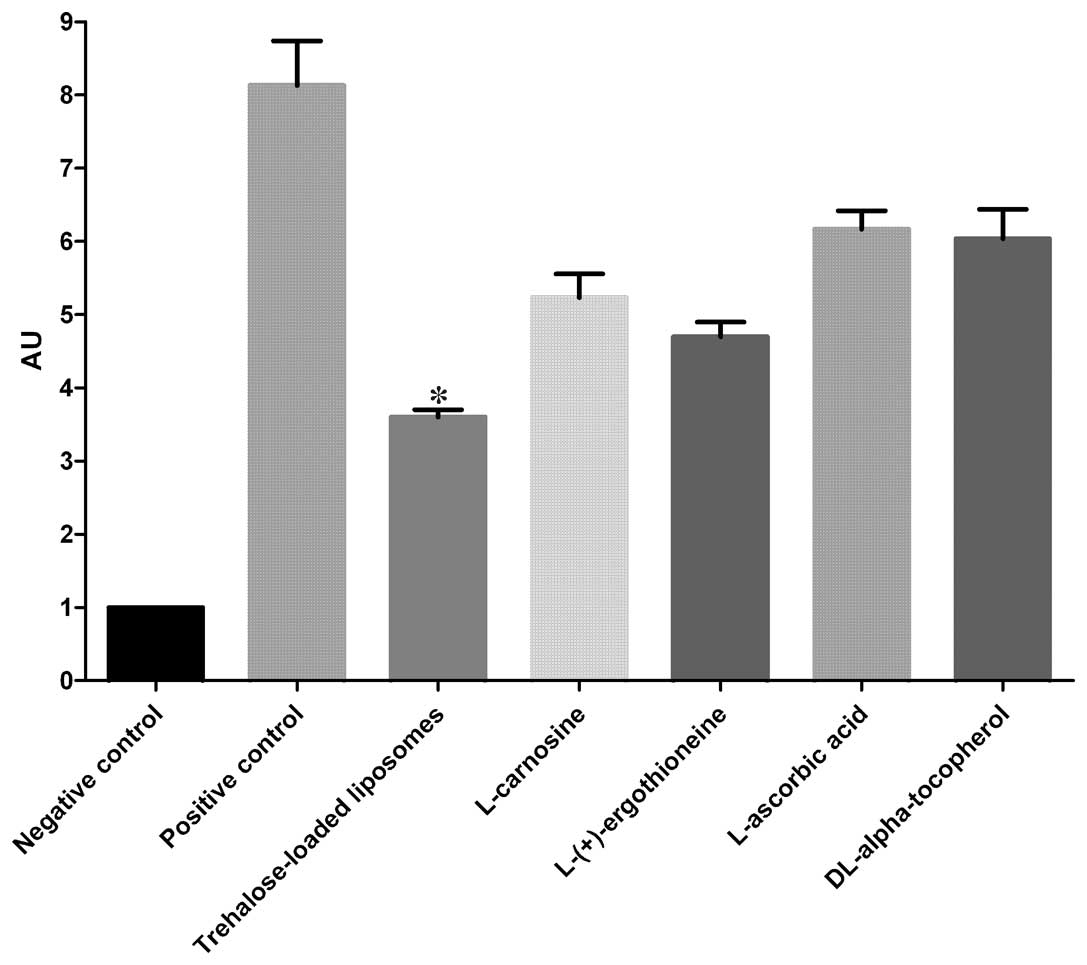
8OHdG
The effects of trehalose-loaded liposomes,
L-carnosine, L-(+)-ergothioneine, L-ascorbic acid and
DL-α-tocopherol in inhibiting 8OHdG formation in UVB-irradiated
HaCaT cells are shown in Fig. 2.
The ANOVA tests showed a significant pretreatment effect
(P<0.001). In post hoc analyses, all the tested compounds were
able to significantly reduce the formation of CPD in UVB-irradiated
cells compared to the positive controls (P<0.001). Notably,
trehalose-loaded liposomes were significantly more effective in
inhibiting 8OHdG formation compared to the other tested compounds
(all P<0.001; Fig. 2).
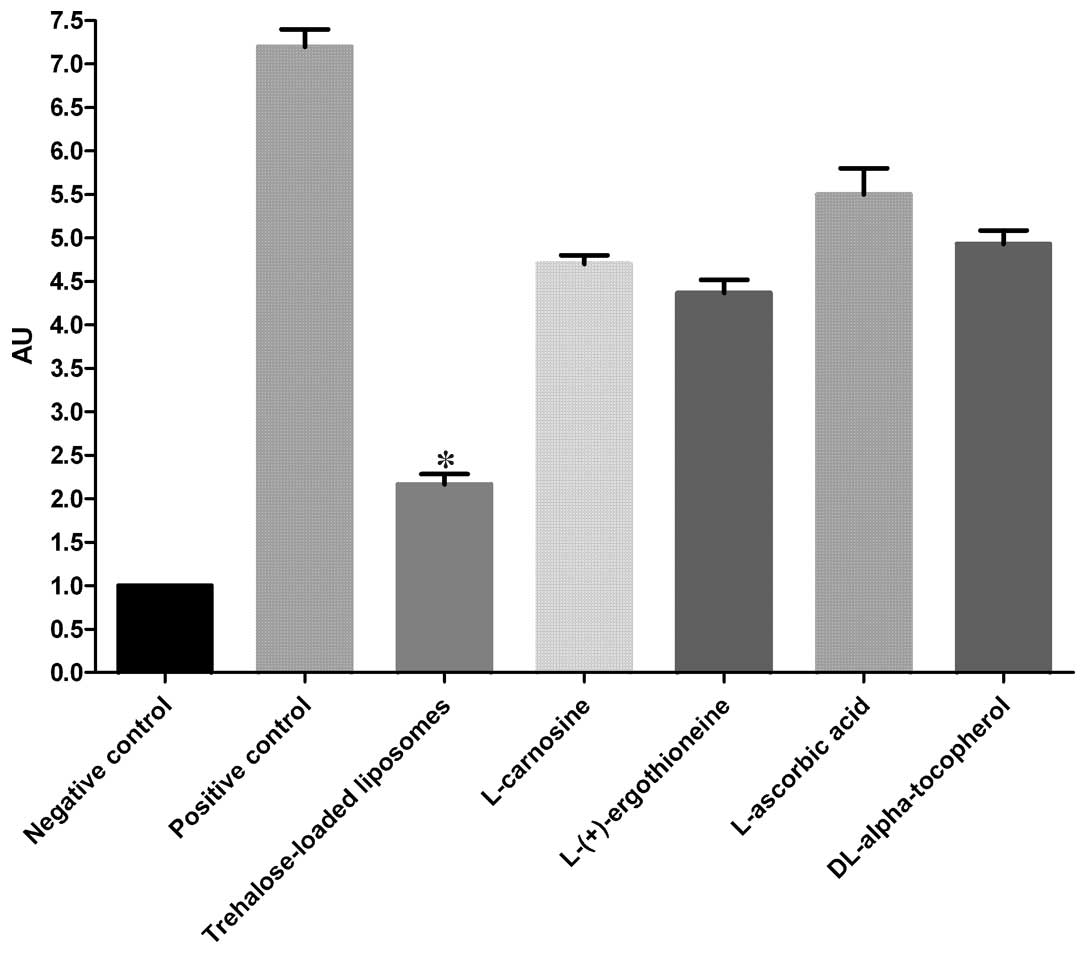
PC
The protective effects of trehalose-loaded
liposomes, L-carnosine, L-(+)-ergothioneine, L-ascorbic acid and
DL-α-tocopherol against the development of PC in UVB-irradiated
HaCaT cells are shown in Fig. 3.
The ANOVA tests showed a significant pretreatment effect
(P<0.001). In post hoc analyses, all the tested compounds were
able to significantly reduce PC in UVB-irradiated cells compared to
the positive controls (all P<0.001). In particular, the results
revealed that trehalose-loaded liposomes were significantly more
effective in inhibiting PC compared to the other tested compounds
(all P<0.001; Fig. 3).
Discussion
There is a high demand for novel and effective
strategies aimed at reducing photodamage and the increasing
problems associated with human skin cancer. UVB radiation is known
to play a major role in the pathogenesis of NMSC (4), owing to its ability to cause direct
and indirect DNA damage (mainly in the form of inducing CPD and
8OHdG) (7–10). In addition, growing evidence
indicates that UVB may induce widespread protein oxidation, which
can ultimately impair a number of intracellular enzymatic
activities (including endogenous DNA repair enzymes) (19,24).
Although trehalose has been shown to reduce UVB-induced corneal
damage when applied topically to the eye (19), its use as a skin photoprotective
agent has been limited by its poor permeability (25), which was improved in the present
study by loading liposomes. Notably, the main results of the study
indicate that, compared to other common photoprotective compounds,
trehalose-loaded liposomes showed the highest efficacy in reducing
the levels of the three UVB-associated markers following
experimental irradiation of HaCaT cells.
Several natural antioxidants have been tested for
their ability to prevent UVB-induced damage to biological
macromolecules (26). Among them,
L-ascorbic acid and DL-α-tocopherol have been extensively studied.
Specifically, L-ascorbic acid has been shown to reduce UV-induced
photodamage in a porcine skin model (27) and human cells (28). Notably, Lin et al have
developed a topical formulation consisting of 15% L-ascorbic acid
combined with 1% DL-α-tocopherol (17). When DL-α-tocopherol neutralizes
oxidative stress in lipids, its oxidation product can be
regenerated by L-ascorbic acid (28–36).
L-(+)-ergothioneine is a potent, natural sulfur-containing
antioxidant that can protect biological macromolecules against
copper-dependent oxidative damage (37) and enhance DNA repair in
UV-irradiated cells (38).
Similarly, L-carnosine, a naturally occurring histidine-containing
dipeptide with antioxidant properties, has been shown to provide
protection against UVB radiation, possibly via immunological
mechanisms (39).
The results of the present study clearly confirm
that L-carnosine, L-(+)-ergothioneine, L-ascorbic acid and
DL-α-tocopherol can significantly prevent experimentally-induced
photodamage. Notably, to the best of our knowledge, the findings
demonstrate for the first time that trehalose-loaded liposomes had
a significantly improved performance when compared to the other
common cytoprotective compounds in reducing UVB-induced molecular
damage in a human keratinocytes cell line. The noteworthy capacity
of trehalose-loaded liposomes in preventing the formation of
UVB-associated molecular signatures at the DNA (CPD, 8OHdG) and
protein levels (PC) may be due to the ability of liposomes to
effectively cross the keratinocyte membrane and increase
intracellular trehalose concentrations. In turn, intracellular
trehalose may decrease PC by stabilizing proteins and acting as a
molecular chaperone (21). In this
regard, it is noteworthy that extremophilic bacteria, which are
characterized by the ability to resist large amounts of UV
radiation, have high intracellular levels of trehalose combined
with manganese ions (19,22). The in vitro results of the
present study also showed that trehalose may reduce UVB-induced DNA
damage to a greater extent than other cytoprotective molecules.
These findings indicate the possibility that trehalose can exert a
strong influence on DNA repair by collectively preserving the
function of endogenous DNA repair enzymes and other molecular
chaperones. Thus, the results suggest that at least part of the
trehalose-mediated effect on DNA markers is mediated by a ‘proteome
effect’, in which trehalose protects a number of protein functions
simulaneously (including those of DNA repair enzymes) rather than
by a direct genoprotective effect (40). Further biochemical studies are
required to extensively test this hypothesis.
In conclusion, the present study demonstrates that
trehalose-loaded liposomes have a marked ability in reducing the
levels of UVB-associated molecular signatures following
experimental irradiation of human HaCaT cells. Therefore, topical
formulations containing trehalose-loaded liposomes may
significantly reduce UVB-induced molecular damage to DNA and
proteins, providing an effect beyond that achieved with other
common antioxidants. Notably, these findings indicate the
importance of careful selection of the ingredients when formulating
novel topical products aimed at reducing the problems associated
with photodamage and NMSC.
References
|
1
|
D’Orazio J, Jarrett S, Amaro-Ortiz A and
Scott T: UV radiation and the skin. Int J Mol Sci. 14:12222–12248.
2013.
|
|
2
|
Mabruk MJ, Toh LK, Murphy M, Leader M, Kay
E and Murphy GM: Investigation of the effect of UV irradiation on
DNA damage: comparison between skin cancer patients and normal
volunteers. J Cutan Pathol. 36:760–765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen AC, Halliday GM and Damian DL:
Non-melanoma skin cancer: carcinogenesis and chemoprevention.
Pathology. 45:331–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marks R: Photoprotection and prevention of
melanoma. Eur J Dermatol. 9:406–412. 1999.PubMed/NCBI
|
|
5
|
Krutmann J and Schroeder P: Role of
mitochondria in photoaging of human skin: the defective powerhouse
model. J Investig Dermatol Symp Proc. 14:44–49. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Besaratinia A, Yoon JI, Schroeder C,
Bradforth SE, Cockburn M and Pfeifer GP: Wavelength dependence of
ultraviolet radiation-induced DNA damage as determined by laser
irradiation suggests that cyclobutane pyrimidine dimers are the
principal DNA lesions produced by terrestrial sunlight. FASEB J.
25:3079–3091. 2011. View Article : Google Scholar
|
|
7
|
Ichihashi M, Ueda M, Budiyanto A, et al:
UV-induced skin damage. Toxicology. 189:21–39. 2003. View Article : Google Scholar
|
|
8
|
Svobodova A, Walterova D and Vostalova J:
Ultraviolet light induced alteration to the skin. Biomed Pap Med
Fac Univ Palacky Olomouc Czech Repub. 150:25–38. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kumar A, Pant MC, Singh HS and Khandelwal
S: Assessment of the redox profile and oxidative DNA damage
(8-OHdG) in squamous cell carcinoma of head and neck. J Cancer Res
Ther. 8:254–259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suzuki YJ, Carini M and Butterfield DA:
Protein carbonylation. Antioxid Redox Signal. 12:323–325. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bernerd F, Vioux C, Lejeune F and
Asselineau D: The sun protection factor (SPF) inadequately defines
broad spectrum photoprotection: demonstration using skin
reconstructed in vitro exposed to UVA, UVB or UV-solar simulated
radiation. Eur J Dermatol. 13:242–249. 2003.
|
|
12
|
Baliga MS and Katiyar SK: Chemoprevention
of photocarcinogenesis by selected dietary botanicals. Photochem
Photobiol Sci. 5:243–253. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yaar M and Gilchrest BA: Photoageing:
mechanism, prevention and therapy. Br J Dermatol. 157:874–887.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Afaq F and Mukhtar H: Botanical
antioxidants in the prevention of photocarcinogenesis and
photoaging. Exp Dermatol. 15:678–684. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jurkiewicz BA, Bissett DL and Buettner GR:
Effect of topically applied tocopherol on ultraviolet
radiation-mediated free radical damage in skin. J Invest Dermatol.
104:484–488. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheah IK and Halliwell B: Ergothioneine;
antioxidant potential, physiological function and role in disease.
Biochim Biophys Acta. 1822.784–793. 2012.PubMed/NCBI
|
|
17
|
Lin JY, Selim MA, Shea CR, et al: UV
photoprotection by combination topical antioxidants vitamin C and
vitamin E. J Am Acad Dermatol. 48:866–874. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paul BD and Snyder SH: The unusual amino
acid L-ergothioneine is a physiologic cytoprotectant. Cell Death
Differ. 17:1134–1140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Emanuele E, Spencer JM and Braun M: From
DNA repair to proteome protection: new molecular insights for
preventing non-melanoma skin cancers and skin aging. J Drugs
Dermatol. 13:274–281. 2014.PubMed/NCBI
|
|
20
|
Wondrak GT: Redox-directed cancer
therapeutics: molecular mechanisms and opportunities. Antioxid
Redox Signal. 11:3013–3069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jain NK and Roy I: Effect of trehalose on
protein structure. Protein Sci. 18:24–36. 2009.PubMed/NCBI
|
|
22
|
Webb KM and DiRuggiero J: Role of
Mn2+and compatible solutes in the radiation resistance
of thermophilic bacteria and archaea. Archaea.
2012:8457562012.PubMed/NCBI
|
|
23
|
Ulrich AS: Biophysical aspects of using
liposomes as delivery vehicles. Biosci Rep. 22:129–150. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maalouf S, El-Sabban M, Darwiche N and
Gali-Muhtasib H: Protective effect of vitamin E on ultraviolet B
light-induced damage in keratinocytes. Mol Carcinog. 34:121–130.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kayasuga-Kariya Y, Iwanaga S, Fujisawa A,
et al: Dermal cell damage induced by topical application of
non-steroidal anti-inflammatory drugs is suppressed by trehalose
co-lyophilization in ex vivo analysis. J Vet Med Sci. 75:1619–1622.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei H, Ca Q, Rahn R, Zhang X, Wang Y and
Lebwohl M: DNA structural integrity and base composition affect
ultraviolet light-induced oxidative DNA damage. Biochemistry.
37:6485–6490. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Darr D, Combs S, Dunston S, Manning T and
Pinnell S: Topical vitamin C protects porcine skin from ultraviolet
radiation-induced damage. Br J Dermatol. 127:247–253. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Placzek M, Gaube S, Kerkmann U, et al:
Ultraviolet B-induced DNA damage in human epidermis is modified by
the antioxidants ascorbic acid and D-alpha-tocopherol. J Invest
Dermatol. 124:304–307. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Njus D and Kelley PM: Vitamins C and E
donate single hydrogen atoms in vivo. FEBS Lett. 284:147–151. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shindo Y, Witt E, Han D, et al: Recovery
of antioxidants and reduction in lipid hydroperoxides in murine
epidermis and dermis after acute ultraviolet radiation exposure.
Photodermatol Photoimmunol Photomed. 10:183–191. 1994.PubMed/NCBI
|
|
31
|
Steenvoorden DP and van Henegouwen GM: The
use of endogenous antioxidants to improve photoprotection. J
Photochem Photobiol B. 41:1–10. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin FH, Lin JY, Gupta RD, et al: Ferulic
acid stabilizes a solution of vitamins C and E and doubles its
photoprotection of skin. J Invest Dermatol. 125:826–832. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Murray JC, Burch JA, Streilein RD,
Iannacchione MA, Hall RP and Pinnell SR: A topical antioxidant
solution containing vitamins C and E stabilized by ferulic acid
provides protection for human skin against damage caused by
ultraviolet irradiation. J Am Acad Dermatol. 59:418–425. 2008.
View Article : Google Scholar
|
|
34
|
Césarini JP, Michel L, Maurette JM,
Adhoute H and Béjot M: Immediate effects of UV radiation on the
skin: modification by an antioxidant complex containing
carotenoids. Photodermatol Photoimmunol Photomed. 19:182–189.
2003.PubMed/NCBI
|
|
35
|
Ichihashi M, Funasaka Y, Ohashi A, et al:
The inhibitory effect of DL-alpha-tocopheryl ferulate in lecithin
on melanogenesis. Anticancer Res. 19:3769–3774. 1999.PubMed/NCBI
|
|
36
|
McVean M and Liebler DC: Prevention of DNA
photodamage by vitamin E compounds and sunscreens: roles of
ultraviolet absorbance and cellular uptake. Mol Carcinog.
24:169–176. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhu BZ, Mao L, Fan RM, et al:
Ergothioneine prevents copper-induced oxidative damage to DNA and
protein by forming a redox-inactive ergothioneine-copper complex.
Chem Res Toxicol. 24:30–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Markova NG, Karaman-Jurukovska N, Dong KK,
Damaghi N, Smiles KA and Yarosh DB: Skin cells and tissue are
capable of using L-ergothioneine as an integral component of their
antioxidant defense system. Free Radic Biol Med. 46:1168–1176.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Reeve VE, Bosnic M and Rozinova E:
Carnosine (beta-alanylhistidine) protects from the suppression of
contact hypersensitivity by ultraviolet B (280–320 nm) radiation or
by cis urocanic acid. Immunology. 78:99–104. 1993.PubMed/NCBI
|
|
40
|
Emanuele E: Challenging the central dogma
of skin photobiology: are proteins more important than DNA? J
Invest Dermatol. 134:2052–2053. 2014. View Article : Google Scholar : PubMed/NCBI
|