Introduction
The contrast-induced acute kidney injury (CI-AKI)
has become the third leading cause of hospital-acquired acute
kidney injury (AKI) (1).
Traditionally, AKI is defined by measuring an increase of the serum
creatinine concentration (Scr). Elevated levels of SCr following
intravascular injection of the contrast media indicates impairment
of renal function patients with CI-AKI (2,3). With the
increasing number of patients who received coronary intervention
procedure therapy, the morbidity and mortality rate of CI-AKI has
increased, particularly in those patients with diabetes mellitus
and chronic kidney disease. However, <30% of patients who
underwent percutaneous coronary intervention (PCI) had diabetes
mellitus, and suffered from a higher risk of CI-AKI than those
without diabetes mellitus. Therefore, it is crucial to make an
early diagnosis and prognosis of CI-AKI in patients with diabetes
mellitus. However, using the Scr levels as the AKI indicator has
numerous defects owing to that fact that it may be affected by a
number of non-renal factors, including ethnicity, age, basic
metabolism and nutrition. Only when the estimated glomerular
filtration rate (eGFR) level has decreased to 50% of the normal
level can the Scr level be increased (4,5).
A previous study has shown that KIM-1 is a
transmembrane glycoprotein located in the membrane of renal
proximal tubule epithelial cell. There was little expression in the
human normal renal tissue, but an increased expression evidently
occurred when the renal tissue suffered ischemia and hypoxia.
Furthermore, it was positively correlated with the severity of
renal injury (6). The expression
levels of KIM-1 in urine were consistent with the levels in renal
tissue. Currently, a large number of experimental evidence has
suggested that KIM-1 is not only a good biological indicator of
acute kidney injury, but also as a functional molecule to
participate in the process of renal tubular damage and repair
(7). The levels of KIM-1 in renal
tissue measured 2 h after injection of contrast agents evidently
increased, however, until 12 h after injection of contrast agents,
the injury of renal tubular epithelial cells was shown to occur in
the renal tissue biopsy, which has been shown in a previous rat
model of low permeability, contrast agent-induced acute injury of
renal tubular epithelial cell. The present study was designed to
confirm whether urinary KIM-1 is an earlier diagnosis and prognosis
indicator compared to Scr in CI-AKI patients with diabetes mellitus
who underwent PCI.
Patients and methods
Patient population
The study includes the general clinical data of 145
patients with diabetes mellitus who underwent PCI procedures at the
Department of Cardiology, Affiliated Hospital of Xuzhou Medical
College (Xuzhou, China) between March 1, 2013 and December 31,
2013. In total, 54 were female and 91 were male, and the average
age was 66.8±9.9 years. Exclusion criteria were: i) Severe hepatic
and renal dysfunction; ii) the use of drugs with renal injury
during the preoperative period; iii) severe heart failure or left
ventricular ejection fraction <35%; iv) tumors; v) acute or
chronic infectious diseases; vi) thyroid or adrenal dysfunction;
and vii) pregnant or breast-feeding women. The osmotic
concentration was 800 mOsm/kg. All the patients routinely received
anticoagulation, antiplatelet, antiangina and conventional
hydration therapy, as well as monitoring of blood pressure, lipids
and blood glucose.
The study was approved by the Ethics Committee of
the Affiliated Hospital of Xuzhou Medical College. All the patients
provided written informed consent for the procedure.
Laboratory assay
SCr and other corresponding indicators were
measured. Fasting blood specimens were collected prior to and 24
and 48 h after the procedure in the biochemical laboratory and were
subsequently analyzed using an Olympus AU2700 Automatic
Biochemistry Analyzer (Olympus, Center Valley, PA, USA) for
determination. Urine specimens were collected prior to and at 2, 6,
12, 24 and 48 h after the procedure and were immediately
centrifuged (1,409 × g for 20 min at 4°C). The superficial
fractions were collected and stored at −80°C. The levels of urinary
KIM-1 were measured using an ELISA kit purchased from R&D
Systems (Minneapolis, MN, USA). Renal function was calculated by
the eGFR using the Modification of Diet in Renal Disease formula
for Chinese patients (8): GFR
(ml/min/1.73 m2) = 175 × SCr (mg/dl)−1.1549 ×
age−0.2039 (x 0.79 if female). CI-AKI was diagnosed as
an increase of ≥0.5 mg/dl or ≥25% in SCr levels over the baseline
24–48 h after the intravascular injection of contrast medium,
without an alternative etiology (9).
Statistical analysis
Continuous variables are expressed as the mean ±
standard deviation. The Student's t-test and one-way analysis of
variance were used for the comparison of continuous variables.
Categorical data are shown as absolute values and percentages. The
χ2 or the Fisher's exact test were used for the
comparison of categorical variables. The Pearson's correlation
analysis was used to evaluate the correlations. P<0.05 was
considered to indicate a statistically significant difference. All
the hypothesis testing was two-tailed. The SPSS version 16.0 (SPSS,
Inc., Chicago, IL, USA) package was used for all calculations.
Results
Differences in SCr and eGFR values
prior and subsequent to the procedure
The differences in SCr and eGFR values are shown in
Table I. Samples were collected from
all 145 patients with diabetes mellitus undergoing PCI. In total,
19 of 145 (13.1%) patients were diagnosed with CI-AKI. The 145
patients were categorized to a CI-AKI (19 patients) and non-CI-AKI
group (126 patients). No significant difference was shown in
gender, age, hemoglobin, body mass index, eGFR, SCr and the
incident rate of hypertension between the CI-AKI and non-CI-AKI
groups. The therapy and hydration volumes used were also not
statistically different during hospitalization. The peak level of
Scr in the CI-AKI group (92.32±15.05 µmol/l) was clearly higher
than that in the non-CI-AKI group (66.54±16.80 µmol/l) at 48 h
after the procedure (P<0.05). There was a significant difference
(P<0.05) in the level of eGFR at 24 and 48 h after the procedure
between the non-CI-AKI groups and CI-AKI groups.
 | Table I.Differences in the SCr and eGFR values
prior and subsequent to the procedure in the two groups. |
Table I.
Differences in the SCr and eGFR values
prior and subsequent to the procedure in the two groups.
| Groups | SCr, µmol/l | eGFR,
ml/min−1/1.73 m2 |
|---|
| No-CI-AKI |
|
|
|
Pre-procedure | 60.41±14.20 | 109.42±23.72 |
|
Post-procedure |
|
|
|
24 h | 66.87±16.01 | 105.86±25.58 |
|
48 h | 66.54±16.80 | 106.94±26.16 |
| CI-AKI |
|
|
|
Pre-procedure | 70.58±9.97 | 97.00±16.93 |
|
Post-procedure |
|
|
|
24 h | 74.05±12.58 |
88.48±16.54a |
|
48 h |
92.32±15.05a |
68.69±14.19a |
Differences in the level of urinary
KIM-1 prior and subsequent to the procedure
The differences in the level of urinary KIM-1 are
shown in Table II. The urinary KIM-1
levels were increased 2 h after the procedure in the non-CI-AKI
group, but no statistically significant difference was identified
(P>0.05). There was a significant difference (P<0.05) between
the urinary KIM-1 levels measured 2, 6, 12, 24 and 48 h after the
procedure and those before the procedure in the CI-AKI group, and
for the levels measured 6, 12, 24 and 48 h after the procedure in
non-CI-AKI groups. Furthermore, significant differences in the
urinary KIM-1 levels measured 6, 12, 24 and 48 h were identified
compared to those in the non-CI-AKI. The KIM-1 levels had not
returned to the normal level 48 h after the procedure.
 | Table II.Differences in the urinary KIM-1 prior
and subsequent to the procedure in the two groups. |
Table II.
Differences in the urinary KIM-1 prior
and subsequent to the procedure in the two groups.
|
| Urinary KIM-1,
pg/ml |
|---|
|
|---|
| Procedure | Non-CI-AKI | CI-AKI |
|---|
| Pre |
3,863.07±1,081.56 | 3,515.98±945.58 |
| Post |
|
|
|
2 |
4,037.53±1,148.11 |
4,015.84±855.96b |
|
6 |
4,370.46±1,278.46a |
5,095.32±1,325.09b,c |
| 12 |
4,619.10±1,379.54b |
5,982.04±1,506.62b,d |
| 24 |
4,858.56±1,490.54b |
6,984.20±1,441.87b,d |
| 48 |
4,663.49±1,399.67b |
6,078.81±1,519.56b,d |
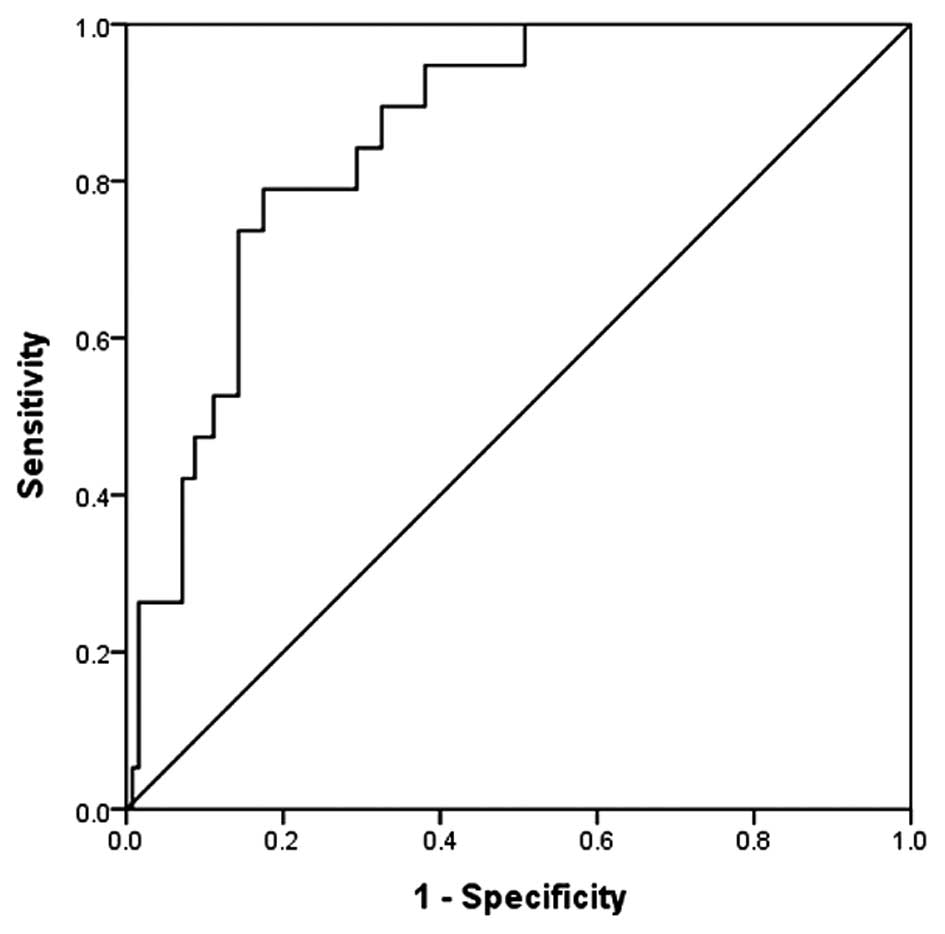
Receiver operating characteristic
(ROC) curve analysis
The ROC curve for the level of urinary KIM-1
measured 24 h after the procedure is shown in Fig. 1. The area under the ROC curve of KIM-1
24 h after the procedure was 0.865 and the 95% confidence interval
of the corresponding area was 0.782–0.929. When the pivotal point
of diagnosis of CI-AKI was 6,327.755 pg/ml, the specificity was
85.7% and the sensitivity was 73.7%.
Correlation between SCr and urinary
KIM-1 concentration
Bivariate analysis showed that the level of urinary
KIM-1 measured prior to and 24–48 h after the procedure positively
correlated with SCr at the identical time points. Preoperative SCr
and preoperative urinary KIM-1 (r=0.322, P<0.01), postoperative
24 h SCr and postprocedure 24 h urine KIM-1 (r=0.317, P<0.01),
and postoperative 48 h SCr and postprocedure 48 h urinary KIM-1
(r=0.453, P<0.01) showed positive correlations.
Discussion
With the development of interventional diagnosis and
treatment technology in recent years, an increasing number of
patients with coronary disease prefer accepting interventional
treatment owing to its numerous advantages, such as simple surgery,
less pain, low risk and fast postoperative recovery rather than
surgical therapy. Approximately 30% of patients with coronary
disease have suffered from diabetes mellitus, and they may have
severe conditions predisposing them to CI-AKI (10). CI-AKI has become one of the three major
reasons of deteriorating the prognosis of the patients after PCI
(11). Due to this, CI-AKI has
received extensive attention from medical experts. However, there
is a lack of effective measures to control and prevent CI-AKI at
present. Therefore, early diagnosis and treatment of CI-AKI has
played a crucial role in patients with diabetes mellitus undergoing
PCI. Traditionally, it is defined by measuring the elevation of the
Scr concentration, but is currently considered a poor indicator of
acute renal dysfunction (12). Only
when the eGFR decreases to <50% of the normal rate is the SCr
level likely to increase. Therefore, the early diagnosis of CI-AKI
is critical for prevention (13).
It is generally accepted that the pivotal reason for
causing CI-AKI is renal medulla hypoxic (14), which is initiated by three different
ways: Contrast agent-induced hemodynamic changes, oxygen free
radical damage and a direct toxic effect to renal tubular disease
(15). KIM-1 is a transmembrane
glycoprotein located in the membrane of renal proximal tubule
epithelial cells. There is little expression in the human normal
renal tissue, but an increased expression evidently occurred when
the renal tissue was injured (6). A
previous study has shown that KIM-1 may participate in the process
of renal tubular damage and repair, which has a protective effect
on acute kidney injury and an adverse effect on chronic kidney
disease (7). The expression levels of
KIM-1 in urine were consistent with the levels in renal tissue
(16,17). As previously described, urinary KIM-1
is regarded as an early biomarker of acute renal tubular injury
owing to its high sensitivity and specificity for predicting
ischemic renal injury, and it also has an ability to distinguish
between acute ischemic renal injury and chronic kidney
insufficiency (11). Using urinary
KIM-1 to detect various factors causing renal injury was much
earlier than using other traditional biomarkers, such as Scr, urea
nitrogen, urine sugar and urine protein. Ichimura et al
(18) showed that the expression
levels of KIM-1 following renal injury clearly increased, and it
occurred earlier than the levels of Scr. The present study
confirmed that urinary KIM-1 could be considered as an improved
early diagnostic indicator for CI-AKI.
The result of the correlation analysis has shown
positive correlations between the urinary KIM-1 concentration and
SCr levels measured pre- and postprocedure in the present study. In
the CI-AKI group, the preoperative value was statistically
significantly different compared to the level of Scr 48 h after the
procedure, the eGFR value was significantly different at 24 h after
the procedure, while the urinary KIM-1 level differed 2 h after the
procedure. In summary, the urinary KIM-1 level rises earlier than
SCr in CI-AKI, which also agreed with the results of the study by
Ichimura et al (18). The only
effective prevention measure for CI-AKI is universally acknowledged
as hydration therapy (19).
Urinary KIM-1 can be measured using specific ELISAs,
which are reliable, fast and economical, to test urine specimens
from patients. This is a non-invasive and convenient technique,
which therefore can be extended clinically. In addition,
postoperative urinary KIM-1 offers an early prediction of the
emergence of CI-AKI, and may provide early intervention measures to
benefit patients. Therefore, KIM-1 was a better indicator for the
early prediction of CI-AKI.
The small sample size and a variety of confounding
factors are the limitations of the present study. Further studies
that are characterized by larger sample sizes, with a controlled
group of patients without diabetes mellitus and identify and
exclude potential factors that may also cause increases in KIM-1
levels are required to confirm the findings of the present
study.
References
|
1
|
Caixeta A and Mehran R.: Evidence-based
management of patients undergoing PCI: contrast-induced acute
kidney injury. Catheter Cardiovasc Interv. 75 (Suppl 1):S15–S20.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parfrey P: The clinical epidemiology of
contrast-induced nephropathy. Cardiovasc Intervent Radiol. 28
(Suppl 2):S3–S11. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mehran R and Nikolsky E: Contrast-induced
nephropathy: definition, epidemiology, and patients at risk. Kidney
Int. Suppl (100):S11–S15. 2006. View Article : Google Scholar
|
|
4
|
Odutayo A and Cherney D: Cystatin C and
acute changes in glomerular filtration rate. Clin Nephrol.
78:64–75. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mehran R: Contrast-induced nephropathy
remains a serious complication of PCI. J Interv Cardiol.
20:236–240. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tonomura Y, Tsuchiya N, Torii M and Uehara
T: Evaluation of the usefulness of urinary biomarkers for
nephrotoxicity in rats. Toxicology. 273:53–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huo W, Zhang K, Nie Z, Li Q and Jin F:
Kidney injury molecule-1 (KIM-1): A novel kidney-specific injury
molecule playing potential double-edged functions in kidney injury.
Transplant Rev (Orlando). 24:143–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Malhis M, Al-Bitar S and Al-Deen Zaiat K:
The role of theophylline in prevention of radiocontrast
media-induced nephropathy. Saudi J Kidney Dis Transpl. 21:276–283.
2010.PubMed/NCBI
|
|
9
|
McCullough PA and Soman SS:
Contrast-induced nephropathy. Crit Care Clin. 21:261–280. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shaw JE, Sicree RA and Zimmet PZ: Global
estimates of the prevalence of diabetes for 2010 and 2030. Diabetes
Res Clin Pract. 87:4–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han W K, Bailly V, Abichandani R, Thadhani
R and Bonventre JV: Kidney Injury Molecule-1 (KIM-1): A novel
biomarker for human renal proximal tubule injury. Kidney Int.
62:237–244. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Parikh CR, Abraham E, Ancukiewicz M and
Edelstein CL: Urine IL-18 is an early diagnostic marker for acute
kidney injury and predicts mortality in the intensive care unit. J
Am Soc Nephrol. 16:3046–3052. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Melnikov VY, Ecder T, Fantuzzi G, et al:
Impaired IL-18 processing protects caspase-1-deficient mice from
ischemic acute renal failure. J Clin Invest. 107:1145–1152. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heyman S N, Rosen S and Rosenberger C:
Renal parenchymal hypoxia, hypoxia adaptation, and the pathogenesis
of radiocontrast nephropathy. Clin J Am Soc Nephrol. 3:288–296.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Katzberg RW: Contrast medium-induced
nephrotoxicity: Which pathway? Radiology. 235:752–755. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bailly V, Zhang Z, Meier W, et al:
Shedding of kidney injury molecule-1, a putative adhesion protein
involved in renal regeneration. J Biol Chem. 277:39739–39748. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vaidya VS, Ramirez V, Ichimura T, et al:
Urinary kidney injury molecule-1: A sensitive quantitative
biomarker for early detection of kidney tubular injury. Am J
Physiol Renal Physiol. 290:F517–F529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ichimura T, Bonventre J V, Bailly V, et
al: Kidney injury molecule-1 (KIM-1), a putative epithelial cell
adhesion molecule containing a novel immunoglobulin domain, is
up-regulated in renal cells after injury. J Biol Chem.
273:4135–4142. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thomsen HS: How to avoid CIN: guidelines
from the European Society of Urogenital Radiology. Nephrol Dial
Transplant. 20 (Suppl 1):i18–i22. 2005. View Article : Google Scholar : PubMed/NCBI
|