Introduction
P2X receptors are ligand-gated ion channels that are
activated by extracellular adenosine triphosphate. To date, seven
functional mammalian P2X receptor subunits (P2X1-7) have been
identified that assemble as either homo- or heterotrimeric
receptors (1). Accumulating evidence
indicates that P2X receptors serve an important role in the
generation and transmission of pain and inflammation nociceptive
signals (1,2). In particular, the P2X3 homomeric and
P2X2/3 heteromeric receptors occur in a subset of putative
nociceptive sensory neurons (1,2) and the
expression of these receptors has been reported to increase in
several peripheral nociceptive conditions (1,2).
Furthermore, using selective antagonists, antisense
oligonucleotides and gene knock-out mice, several studies confirmed
that these receptors are closely associated with peripheral
nociceptive mechanisms (1,2).
Previous studies suggested that in addition to P2X3
and P2X2/3 receptors, other P2X receptors may be also involved in
the peripheral nociceptive mechanism. For instance, except for
P2X7, all other P2X receptor subunits are expressed in various
primary sensory neurons including the dorsal root ganglion (DRG)
and trigeminal ganglion neurons (1,2). However,
compared with P2X3 and P2X2/3 receptors, the functional role of
other P2X receptors in the peripheral nociceptive mechanism remains
largely unknown. Specifically, little information is available
about the regulation of the receptor expression in peripheral
nociceptive conditions.
The aim of the present study was to evaluate the
alteration of expression of the P2X1-6 receptor subunits in
retrograde Flurorogold (FG)-labeled L4+L5 DRG neurons following
unilateral chronic constriction injury (CCI) of the rat sciatic
nerve using immunohistochemistry combined with a retrograde
fluorescence-tracing method. The results of the present study
provide the first evidence regarding the regulation of P2X1-6
receptor expression in sensory neurons directly associated with
chronic nerve injury in rats.
Materials and methods
Animals and neuropathic pain
model
All animal experiments in the present study were
performed in accordance with the National Institutes of Health
Guide for the Care and Use of Laboratory Animals, with the approval
of the Animal Care and Use Committee of Jianghan University (Wuhan,
China). A total of 24 male Sprague-Dawley rats (250-270 g), 7 weeks
old, which were purchased from the Beijing Vital River Laboratory
Animal Technology Co., Ltd., (Beijing, China) were individually
housed in cages in a temperature and humidity (23±1˚C and 50-55%)
controlled room under a reversed 12-h light-dark cycle with food
and water freely available. The CCI model was produced as
previously described (3). Briefly,
twelve rats were anesthetized by injection of pentobarbital sodium
(25 mg/kg, i.p), following induction in sample bottles containing
cotton balls dipped in ether used as anesthetic jars for 2 min.
After the right common sciatic nerve was exposed, ~7 mm of the
nerve was freed from adhering tissue and four ligatures (4.0
chromic gut) were tied loosely with ~1-mm spacing proximal to the
sciatica's trifurcation. Twelve rats with the right sciatic nerve
exposed without a ligature served as sham controls.
Mechanical and thermal sensitivity
measurements
Mechanical allodynia and heat hyperalgesia were
determined as previously described (4,5). An
automated Dynamic Plantar Aesthesiometer (UGO Basile, Camerio,
Italy) was used to detect the paw mechanical withdrawal threshold
(MWT). Briefly, rats were placed on a wire mesh floor in clear
cylindrical plastic enclosures. Following 20 min of acclimation, a
von Frey filament was placed on the plantar surface of the right
hind paw and the force was increased gradually until a withdrawal
response was evoked, and the amount of force needed to cause the
withdrawal response was recorded. A maximum cut-off value of 50 g
was used. Each trial was repeated 3 times at ~5-min intervals and
the mean force producing withdrawal response was determined.
Thermal nociceptive responses were determined using a plantar test
instrument (Ugo Basile). The rats were acclimatized to the
apparatus that consisted of three individual perspex boxes on a
glass table. A mobile radiant heat source was located under the
table and focused onto the desired paw. The paw withdrawal latency
was recorded three times for the right hind paw and the average was
taken as the value. In order to prevent tissue damage, an automatic
cut-off at 30 sec was set.
Retrograde Flurorogold (FG)-tracing of
DRG neurons
A total of 15 days following CCI, the rats were
anesthetized by injection of pentobarbital sodium (25 mg/kg, i.p).
The right common sciatic nerve was exposed and bisected completely.
Then, 2 µl 2% FG (Fluorochrome, LLC, Denver, CO, USA) was smeared
on the distal cuff of the ligature on the sciatic nerve. The fascia
and skin were then closed.
Tissue preparation and
immunohistochemistry staining
A total of 3 days after FG retrograde, rats were
anesthetized and then were systemically perfused intracardially
with 250 ml ice-cold normal saline followed by 250 ml 4%
paraformaldehyde in 0.01 M PBS (pH 7.4). The corresponding segments
(L4+L5) of DRG were carefully separated following fixation. After
paraffin embedding, DRG paraffin tissue blocks were cut into
4-µm-thick slices. The 4-µm serial sections were deparaffinized in
xylene, rehydrating in graded ethanol, rinsed in distilled water
and then pre-incubated with 3% hydrogen peroxide for 15 min to
inactivate endogenous peroxidase. Antigen retrieval slides were
incubated at 95˚C in 10 mM citric acid buffer (pH=6.0) in a
microwave oven (750 W) for 15 min. Following washing with PBS three
times, the preparations were preincubated with 10% normal goat
serum (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) for 40 min in a moisture chamber at 37˚C. The sections were
then incubated with rabbit anti-P2X1-6 (1:200; cat. nos. APR-022,
APR-025, APR-026, APR-024, APR-027 and APR-028; Alomone Labs, Inc.,
Jerusalem, Israel) overnight at 4˚C. After 3 rinses in PBS, the
sections were then incubated with fluorescent secondary antibody
(1:200; cat. no. ab150079; Abcam, Cambridge, UK) in the dark at
37˚C for 40 min. The prepared sections were given three times
washes again in PBS before mounted in mounting medium and then
cover slipped. After these steps, the sections were observed with
fluorescence microscopy. The negative controls were processed in
the same manner except that PBS was used instead of the primary
antibody.
Image analysis and quantification
Fluorescence images of DRG sections were acquired
with an OLYMPUS BX51 fluorescence microscope outfitted with the
relevant filter blocks, a Hamamatsu C5810 color CCD camera and its
proprietary Image Processor software v1.7 (Hamamatsu Photonic
System, Bridgewater, NJ, USA). Cell sizes were determined by the
previously described method (6). Cell
diameters <30 µm were classified as small-diameter neurons, cell
diameters from 30 to 50 µm were medium-diameter neurons and cell
diameters >50 µm were large-diameter neurons (6). The numbers of FG-labeled neurons and
FG/P2X1-6 double-tagged neurons for each animal were counted. This
procedure was performed in a blinded manner.
Statistical analysis
Mechanical and thermal sensitivity measurements were
repeated three times. All results were expressed as the mean ±
standard error of the mean. GraphPad Prism 5.0 (GraphPad Software,
Inc., La Jolla, CA, USA) was used for statistical analyses.
Statistical significance of results was analyzed with Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Rat neuropathic pain model
assessment
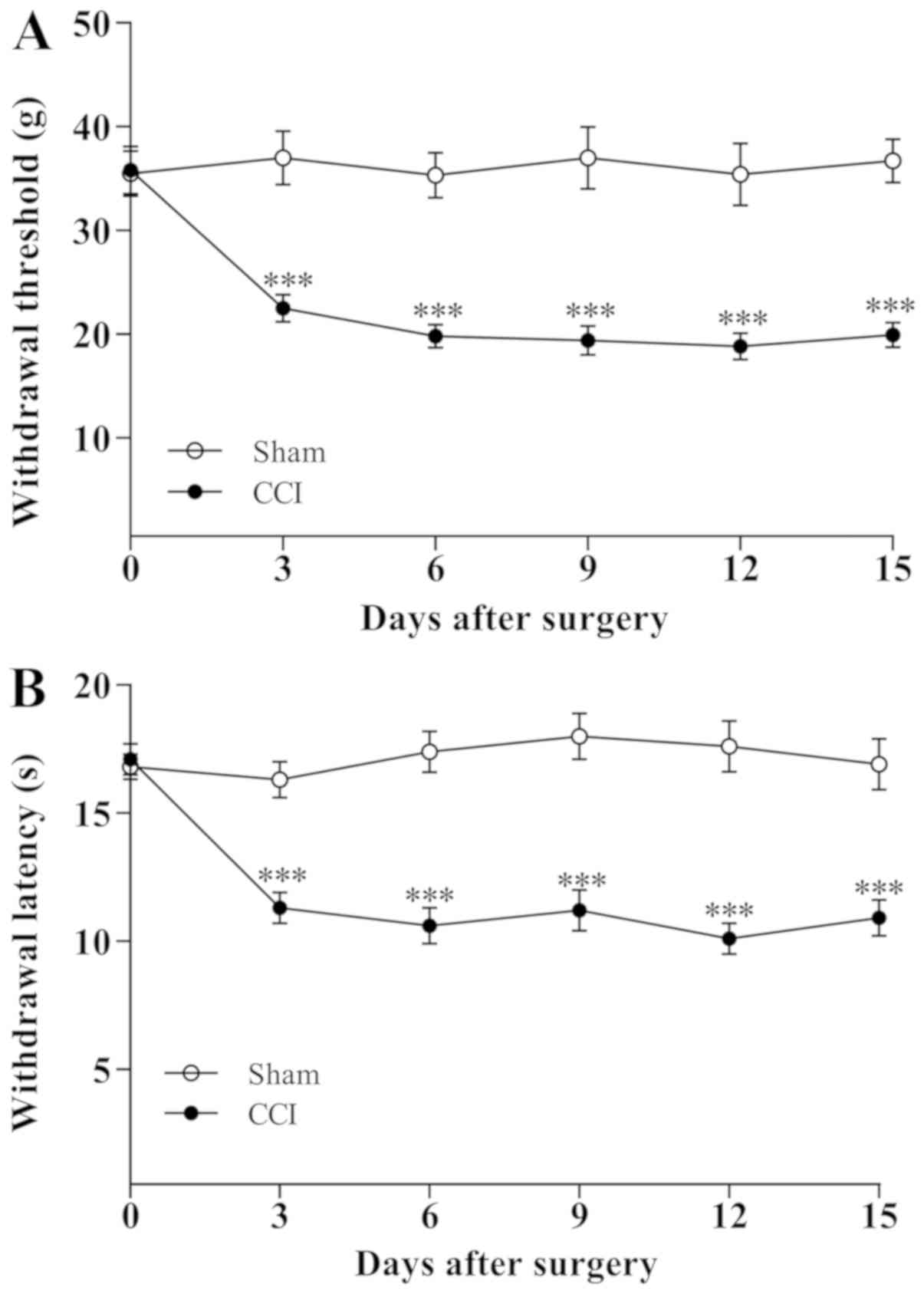
A total of 3 days following the sciatic nerve CCI
operation, the rats gradually exhibited the typical signs of
hyperalgesic responses including toe closing, foot eversion and
paw-licking. By contrast, the behavior of the sham-operated rats
did not obviously alter. The changes in ipsilateral MWT and thermal
withdrawal latency (TWL) are demonstrated in Fig. 1. The MWT and TWL values for rats in
CCI group significantly decreased (P<0.001) on day 3 following
CCI operation and further reduced on day 5 (P<0.001) compared
with the sham-operated rats, indicating that the mechanical
allodynia and thermal hyperalgesia were established on the third
day following CCI operation.
Retrograde FG-tracing of DRG
neurons
As presented in Fig.
2A, neurotracer FG-labeled neurons were identified in L4+L5 DRG
neurons in the sham and CCI groups. The average proportions of the
FG-labeled neurons were 44±7.6 and 55±6.2% of total L4+L5 DRG
neurons in the sham and CCI groups, respectively, and no
significant difference was detected in different sizes of neurons
between these two groups (Fig. 2B).
The diameter of cells varied from 17 to 70 µm.
P2X1-6 receptor expression in
FG-labeled neurons of L4+L5 DRG
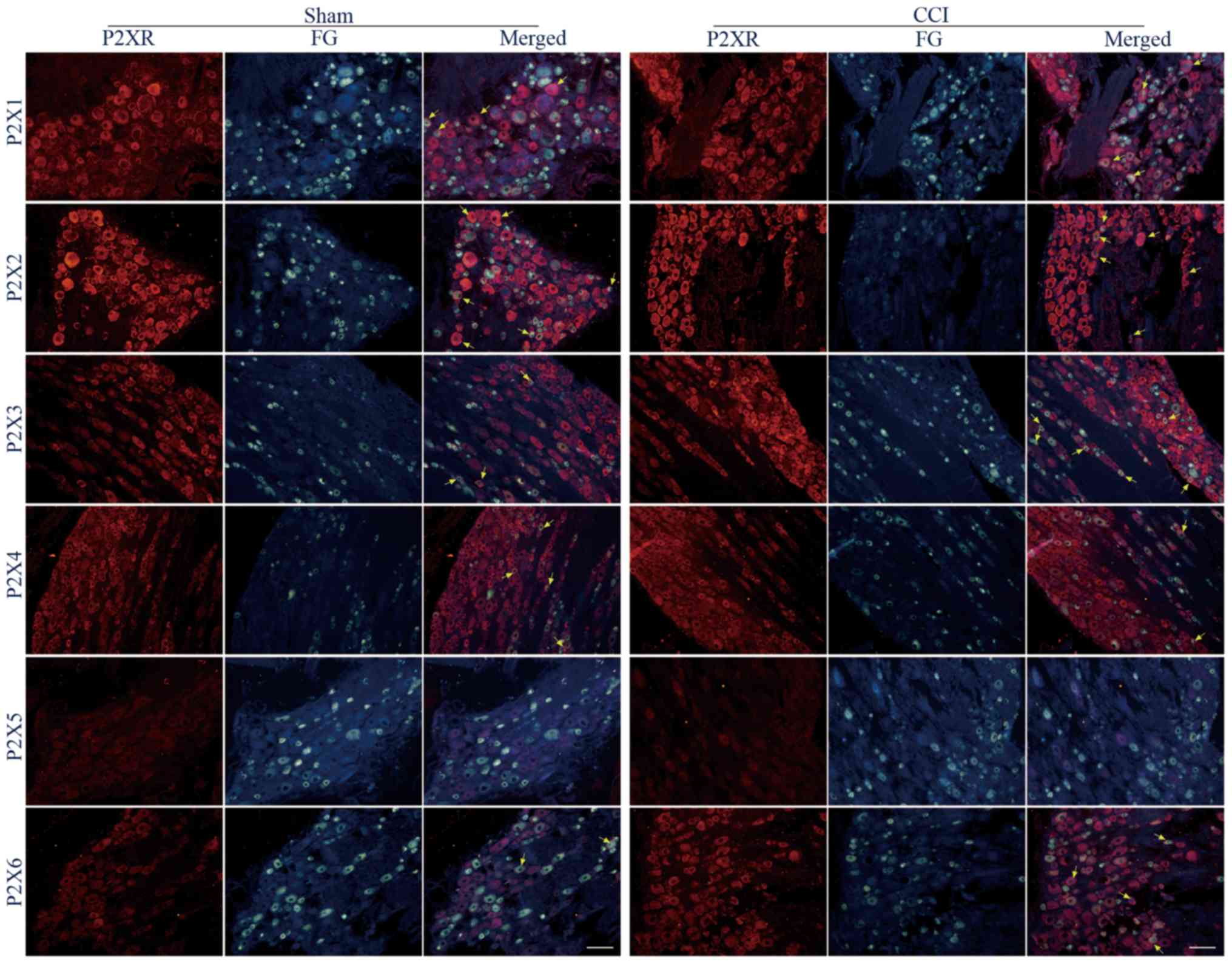
The protein expression of P2X1-6 receptor subtypes
in FG-labeled L4+L5 DRG neurons following sciatic nerve CCI were
compared. It was demonstrated that all P2X receptor proteins were
expressed in FG-labeled DRG neurons of the sham and CCI groups,
except the signal of P2X5 receptors was not detected just like a
previous study (Fig. 3) (7).
In retrograde FG-labeled L4+L5 DRG neurons, the
percentages of P2X1-immunoreactive (IR) neurons were 41.5±8.2 and
45.2±7.4% between the sham and CCI groups, and these values were
not significantly different (P<0.05). In the small-, medium- and
large-diameter FG-labeled L4+L5 DRG neurons, the percentages of
P2X1-IR neurons were 10.5±2.9 and 13.0±4.5% (P<0.05), 20.7±5.5
and 20.4±5.7% (P<0.05), 10.4±4.1 and 11.9±2.9% (P<0.05)
between the sham and CCI groups (Fig.
3; Table I).
 | Table ITable I. The percentage (%) of
Fluorogold-labeled L4+5 dorsal root ganglion neurons with P2X 1, 2,
3, 4, 6-immunoreactive positive staining between sham (n=6) and CCI
(n=7) groups. |
Table I
Table I. The percentage (%) of
Fluorogold-labeled L4+5 dorsal root ganglion neurons with P2X 1, 2,
3, 4, 6-immunoreactive positive staining between sham (n=6) and CCI
(n=7) groups.
| | | Cell size |
|---|
| Receptor | All size | Small | Medium | Large |
|---|
| P2X1 | | | | |
|
Sham | 41.5±8.2 | 10.5±2.9 | 20.7±5.5 | 10.4±4.1 |
|
CCI | 45.2±7.4 | 13.0±4.5 | 20.4±5.7 | 11.9±2.9 |
| P2X2 | | | | |
|
Sham | 58.1±6.2 | 21.5±3.5 | 20.5±2.3 | 15.5±5.1 |
|
CCI | 69.1±3.5 | 29.3±5.8 | 26.8±2.7 | 13.9±4.2 |
| P2X3 | | | | |
|
Sham | 28.5±3.4 | 9.6±2.3 | 15.5±2.3 | 3.5±0.9 |
|
CCI | 51.6±4.1b | 19.3±3.6a | 25.7±3.3a | 6.6±1.1a |
| P2X4 | | | | |
|
Sham | 45.0±3.7 | 18.7±4.1 | 20.1±2.4 | 6.2±1.7 |
|
CCI | 29.4±3.3a | 12.0±3.4 | 12.1±2.6a | 5.3±1.5 |
| P2X6 | | | | |
|
Sham | 22.6±3.3 | 7.1±1.9 | 11.8±2.6 | 3.7±0.6 |
|
CCI | 41.8±2.2b | 13.9±3.3 | 18.1±3.2 | 9.8±2.5 |
In retrograde FG-labeled L4+L5 DRG neurons, the
percentages of P2X2-IR neurons were 58.1±6.2 and 69.1±3.5% between
the sham and CCI groups, and these values were not significantly
different (P>0.05). In the small-, medium- and large-diameter
FG-labeled L4+L5 DRG neurons, the percentages of P2X2-IR neurons
were 21.5±3.5 and 29.3±5.8% (P<0.05), 20.5±2.3 and 26.8±2.7%
(P<0.05), 15.5±5.1 and 13.9±4.2% (P<0.05), which were
significantly different between the sham and CCI groups. (Fig. 3; Table
I).
In retrograde FG labeled L4+L5 DRG neurons, the
percentage of P2X3-IR neurons in the CCI group significantly
increased compared with the in sham group (51.6±4.1 vs. 28.5±3.4%,
P<0.01). In small-, medium-, large-diameter FG-labeled L4+L5 DRG
neurons, the percentages of P2X3-IR neurons in CCI group
significantly increased compared with the sham group (19.3±3.6 vs.
9.6±2.3%, P<0.05; 25.7±3.3 vs. 15.5±2.3%, P<0.05; 6.6±1.1 vs.
3.5±0.9%, P<0.05, respectively; Fig.
3; Table I).
In retrograde FG-labeled L4+L5 DRG neurons, the
percentage of P2X4-IR neurons in CCI group significantly decreased
compared with the sham group (29.4±3.3 vs. 45.0±3.7%, P<0.05).
In small- and large-diameter FG-labeled L4+L5 DRG neurons, the
percentages of P2X4-IR neurons in CCI group were not significantly
different compared with the sham group (12.0±3.4 vs. 18.7±4.1%,
P<0.05; 5.3±1.5 vs. 6.2±1.7%, P<0.05). However, in
medium-diameter FG-labeled L4+L5 DRG neurons, the percentage of
P2X4-IR neurons in CCI group significantly decreased compared with
the sham group (12.1±2.6 vs. 20.1±2.4%, P<0.05; Fig. 3; Table
I).
In retrograde FG-labeled L4+L5 DRG neurons, the
percentage of P2X6-IR neurons in CCI group significantly increased
compared with the sham group (41.8±2.2 vs. 22.6±3.3%, P<0.01).
In small- and medium-diameter FG labeled L4+L5 DRG neurons, the
percentages of P2X6-IR neurons were not significantly different
from the sham group (13.9±3.3 vs. 7.1±1.9%, P>0.05; 18.1±3.2 vs.
11.8±2.6% P<0.05). However, in large-diameter FG-labeled L4+L5
DRG neurons, the percentage of P2X4-IR neurons in CCI group
significantly increased compared to that in the sham group (9.8±2.5
vs. 3.7±0.6%, P<0.01; Fig. 3;
Table I).
Discussion
Out of the seven cloned functional mammalian P2X
receptor subunits, a growing body of evidence suggests that P2X3
and P2X2/3 receptors serve important roles in the generation and
transduction of sensory nociceptive signals. For instance, it has
been reported that antagonist A-317491 selective for P2X3 and
P2X2/3 subunit-containing channels could reduce persistent, chronic
neuropathic and inflammatory pain in rats (8-10). In
addition, studies using P2X3-selective antisense (11-13)
or small interfering RNA (14), as
well as P2X3-deficient mice (15,16) or
P2X2/3 double knockout mice (17)
revealed comparable results. However, the underlying cellular and
molecular mechanism of the involvement of P2X3 and P2X2/3 receptors
in the generation and transduction of nociceptive signals has not
been established.
Previous studies revealed that the P2X3 and P2X2/3
receptors are widely expressed in peripheral sensory neurons,
especially in a subset of putative nociceptive sensory neurons
(1,2).
Notably, variable or conflicting experimental results have been
reported regarding the alteration of expression of P2X3 receptors
in different nociceptive conditions. For example,
immunohistochemical studies indicate that the P2X3 receptor
expression is markedly increased in DRG neurons following sciatic
nerve CCI in rats (18,19). Similarly, P2X3 receptor upregulation
has been reported in rat trigeminal primary sensory neurons
following inferior alveolar nerve injury (20). By contrast, a significant reduction in
P2X3 immunoreactivity was observed in DRG neurons following
peripheral axotomy (21) and spinal
nerve ligation (22) in rats. In
addition, it has been reported that P2X3 receptor expression was
not altered in rat DRG neurons following spinal nerve ligation
(23) and in trigeminal ganglion
neurons by lingual nerve injury in ferrets (22). Although the reason for these
discrepancies remains unknown, several factors could be involved
including animal species (7), animal
models used to produce nerve injury and dynamic regulation of P2X
receptor expression (22).
Similar to P2X3 and P2X2/3 receptors, in situ
hybridization and immunohistochemical studies revealed that other
P2X receptor subunits were widely expressed in sensory neurons
(1,2),
therefore raising the possibility that these P2X receptors may be
also involved in nociceptive sensation. However, compared to P2X3
and P2X2/3 receptors little information is available about the
alteration of expression of these P2X receptors in nociceptive
conditions. Based on the limited information, variable or
conflicting experimental results have also been reported regarding
the expression of these P2X receptors in different experimental
nociceptive conditions. For example, the gene expression of P2X6
receptors has been reported to decrease in the rat spinal nerve
ligation experiment (24). By
contrast, it has recently been demonstrated that the gene and
protein expression of P2X6 receptors markedly increased following
sciatic nerve CCI in rats (19).
In the present study, in order to evaluate the
regulation of expression of P2X receptors in the chronic
neuropathic pain condition, the expression of P2X1-6 receptor
subunits were analyzed in retrograde FG-labeled sensory neurons in
L4+L5 DRG following unilateral CCI of the rat sciatic nerve using
immunohistochemistry combined with retrograde fluorescence-tracing
method. It was demonstrated that the average proportions of the
FG-labeled neurons were 44 and 55% in the sham and CCI groups, and
there were no significant differences detected in different sizes
of neurons between these two groups. It was also demonstrated that
all P2X receptor proteins were expressed in DRG neurons of CCI and
sham groups, except the signal of P2X5 receptors was not detected
just like a previous study reported (7).
The authors' previous study demonstrated that the
expression of P2X1 receptors in rat DRG neurons increased following
sciatic nerve CCI (18). The present
study, however, revealed that in similar experimental conditions
the expression of P2X1 receptors did not change significantly. The
reason for this discrepancy is most likely due to the cells used
for analysis between these two studies being different: In the
previous study the cells were not labelled using retrograde
fluorescence-tracing method and the cells used for analysis may not
be directly associated with the nerve injury. Similarly, previous
studies demonstrated that the expression of P2X2 receptors in rat
DRG neurons increased following spinal nerve ligation (23) and sciatic nerve CCI (18). The experimental results of the present
study demonstrated that the expression of P2X2 receptors slightly
increased following CCI compared with the sham group, but the
difference between these two groups was not significant. Again, the
reason for this discrepancy is most likely due to different cells
being used for analysis in different studies. Consistent with
previous studies (17,18), the results of the present study
demonstrated that the expression of P2X3 receptors in rat DRG
neurons significantly increased following sciatic nerve CCI,
supporting the functional role of this receptor involved in
neuropathic pain sensation. It has been observed that the
expression of P2X4 receptors in rat DRG neurons did not
significantly alter following sciatic nerve CCI in the authors'
previous study (18). In the present
study, however, it was demonstrated that the expression of P2X4
receptors decreased compared with in the sham group. As mentioned
above, the reason for this discrepancy is most likely due to
different cells used for analysis between these two studies. The
expression of P2X6 receptor in rat DRG neurons following sciatic
nerve CCI has been demonstrated to increase in the authors'
previous study (18) and similar
results were demonstrated in the present study: In FG-labeled
neurons (including small-, medium- and large-diameter cells), the
percentage of P2X6-IR neurons in CCI group increased compared with
in the sham group.
Present study to the best of our knowledge, provides
the first evidence regarding the regulation of P2X1-6 receptors in
retrograde FG-labeled sensory neurons directly associated with
sciatic nerve injury in rats and it was demonstrated that among
P2X1-6 receptors only the expression of P2X3 and P2X6 receptors
increased. These results consistent with the previous studies
regarding the role of P2X3 receptors in peripheral neuropathic pain
sensation. Interestingly, the present study demonstrated that the
expression of P2X2 receptors did not significantly increase,
suggesting that compared with the P2X3 receptor, the P2X2/3
heteromeric receptor is not the major receptor involved in
peripheral neuropathic pain sensation. It is noteworthy that in
P2X2/3 double knockout mice the pain-associated behavior reduced in
response to intraplantar injection of formalin, suggesting that
heteromeric P2X2/3 receptors make an important contribution to
nociceptive responses (11). However,
the functional role of heteromeric P2X2/3 receptors in neuropathic
pain sensation has not been clearly established. In addition, the
present study revealed that the expression of P2X6 receptors
significantly increased, which is similar to the authors' previous
study (18). Based on the current
information, however, P2X6 receptors seem unable to form functional
homomultimers (1,2) and these receptors also do not appear to
form heteromultimers with P2X3 receptors which was observed to
significantly increase in the present study (24). Therefore, determining the functional
role of P2X6 receptors in peripheral neuropathic pain sensation
will be an interesting subject for future studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81371235 to CL).
Availability of data and materials
All data used and/or analyzed during this study are
available from the corresponding author on reasonable request.
Authors' contributions
LC and CLi made substantial contributions to the
conception and design of the study. CLeng and LC performed the
experiments and analyzed the data. CLeng drafted the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animals used in the experiments in the present
study were performed in accordance with the National Institutes of
Health Guide for the Care and Use of Laboratory Animals, with the
approval of Animal Care and Use Committee of Jianghan University
(Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Burnstock G: Introduction and perspective,
historical note. Front Cell Neurosci. 7(227)2013. View Article : Google Scholar
|
|
2
|
Burnstock G: Purinergic receptors and
pain. Curr Pharm Des. 15:1717–1735. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bennett GJ and Xie YK: A peripheral
mononeuropathy in rat that produces disorders of pain sensation
like those seen in man. Pain. 33:87–107. 1988.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hargreaves K, Dubner R, Brown F, Flores C
and Joris J: A new and sensitive method for measuring thermal
nociception in cutaneous hyperalgesia. Pain. 32:77–88.
1988.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rose RD and Rohrlich D: Counting sectioned
cells via mathematical reconstruction. J Comp Neurol. 263:365–386.
1987.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zeng JW, Cheng SY, Liu XH, Zhao YD, Xiao
Z, Burnstock G and Ruan HZ: Expression of P2X5 receptors in the
rat, cat, mouse and guinea pig dorsal root ganglion. Histochem Cell
Biol. 139:549–557. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jarvis MF, Burgard EC, McGaraughty S,
Honore P, Lynch K, Brennan TJ, Subieta A, Van Biesen T, Cartmell J,
Bianchi B, et al: A-317491, a novel potent and selective
non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces
chronic inflammatory and neuropathic pain in the rat. Proc Natl
Acad Sci USA. 99:17179–17184. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
McGaraughty S, Wismer CT, Zhu CZ, Mikusa
J, Honore P, Chu KL, Lee CH, Faltynek CR and Jarvis MF: Effects of
A-317491, a novel and selective P2X3/P2X2/3 receptor antagonist, on
neuropathic, inflammatory and chemogenic nociception following
intrathecal and intraplantar administration. Br J Pharmacol.
140:1381–1388. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wu G, Whiteside GT, Lee G, Nolan S, Niosi
M, Pearson MS and Ilyin VI: A-317491, a selective P2X3/P2X2/3
receptor antagonist, reverses inflammatory mechanical hyperalgesia
through action at peripheral receptors in rats. Eur J Pharmacol.
504:45–53. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Barclay J, Patel S, Dorn G, Wotherspoon G,
Moffatt S, Eunson L, Abdel'al S, Natt F, Hall J, Winter J, et al:
Functional downregulation of P2X3 receptor subunit in rat sensory
neurons reveals a significant role in chronic neuropathic and
inflammatory pain. J Neurosci. 22:8139–8147. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Honore P, Mikusa J, Bianchi B, McDonald H,
Cartmell J, Faltynek C and Jarvis MF: TNP-ATP, a potent P2X3
receptor antagonist, blocks acetic acid-induced abdominal
constriction in mice: Comparison with reference analgesics. Pain.
96:99–105. 2002.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Inoue K, Tsuda M and Koizumi S: ATP
induced three types of pain behaviors, including allodynia. Drug
Dev Res. 59:56–63. 2003. View Article : Google Scholar
|
|
14
|
Dorn G, Patel S, Wotherspoon G,
Hemmings-Mieszczak M, Barclay J, Natt FJ, Martin P, Bevan S, Fox A,
Ganju P, et al: siRNA relieves chronic neuropathic pain. Nucleic
Acids Res. 32(e49)2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cockayne DA, Hamilton SG, Zhu QM, Dunn PM,
Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L,
et al: Urinary bladder hyporeflexia and reduced pain-related
behaviour in P2X3-deficient mice. Nature. 407:1011–1015.
2000.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Souslova V, Cesare P, Ding Y, Akopian AN,
Stanfa L, Suzuki R, Carpenter K, Dickenson A, Boyce S, Hill R, et
al: Warm-coding deficits and aberrant inflammatory pain in mice
lacking P2X3 receptors. Nature. 407:1015–1017. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Cockayne DA, Dunn PM, Zhong Y, Rong W,
Hamilton SG, Knight GE, Ruan HZ, Ma B, Yip P, Nunn P, et al: P2X2
knockout mice and P2X2/P2X3 double knockout mice reveal a role for
the P2X2 receptor subunit in mediating multiple sensory effects of
ATP. J Physiol. 567:621–639. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen L, Liu YW, Yue K, Ru Q, Xiong Q, Ma
BM, Tian X and Li CY: Differential expression of ATP-gated P2X
receptors in DRG between chronic neuropathic pain and visceralgia
rat models. Purinergic Signal. 12:79–87. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Novakovic SD, Kassotakis LC, Oglesby IB,
Smith JA, Eglen RM, Ford AP and Hunter JC: Immunocytochemical
localization of P2X3 purinoceptors in sensory neurons in naive rats
and following neuropathic injury. Pain. 80:273–282. 1999.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Eriksson J, Bongenhielm U, Kidd E,
Matthews B and Fried K: Distribution of P2X3 receptors in the rat
trigeminal ganglion after inferior alveolar nerve injury. Neurosci
Lett. 254:37–40. 1998.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kage K, Niforatos W, Zhu CZ, Lynch KJ,
Honore P and Jarvis MF: Alteration of dorsal root ganglion P2X3
receptor expression and function following spinal nerve ligation in
the rat. Exp Brain Res. 147:511–519. 2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Biggs JE, Yates JM, Loescher AR, Clayton
NM, Robinson PP and Boissonade FM: P2X(3) expression is not altered
by lingual nerve injury. Neurosci Lett. 441:110–114.
2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kim C, Chung JM and Chung K: Changes in
the gene expression of six subtypes of P2X receptors in rat dorsal
root ganglion after spinal nerve ligation. Neurosci Lett.
337:81–84. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Torres GE, Egan TM and Voigt MM:
Hetero-oligomeric assembly of P2X receptor subunits. Specificities
exist with regard to possible partners. J Biol Chem. 274:6653–6659.
1999.PubMed/NCBI View Article : Google Scholar
|