Introduction
Propofol, also known as 2,6-diisopropylphenol, is an
intravenous, short-acting anesthetic that is widely used because it
can induce and maintain general anesthesia, providing systematic
sedation (1). Propofol has a fast
onset of action of 10-50 sec and a short duration of action of 3-10
min, making anesthesia induction rapid and sensitive (2). Therefore, it is commonly used as a
sedative in patients who are critically ill (2). Due to its pharmacokinetic properties,
it can be infused continuously using an intravenous pump. In
particular, it can be combined with opioids as part of a ‘balanced
anesthesia’ program (3). It can
also be used as a hypnotic in patients receiving mechanical
ventilation and conscious sedation, especially during day surgery
or non-invasive surgery, such as radiotherapy, endoscopy and MRI.
This is because propofol carries reduced risks of nausea and
vomiting compared with inhalation anesthetics, such as sevoflurane,
in addition to the lack of abirritation (3,4).
However, propofol is not without side effects, such as injection
pain, apnea and low blood pressure. A serious adverse reaction is
propofol-related infusion syndrome or propofol infusion syndrome,
which is an umbrella term for a set of symptoms, including
bradycardia, metabolic acidosis, hepatomegaly, rhabdomyolysis
and/or myoglobinuria (5). Mortality
can occur in extreme cases (5).
The possible relationship between adverse reactions
after propofol infusion and microscopic changes in the body remain
elusive. A number of studies have previously shown that some
biologically active intestinal microbiota and microbial metabolites
are closely associated with the homeostasis, etiology and prognosis
of disease (6-8).
Gut microbes rely on high-fiber food residues to maintain their
normal structure and metabolism, with the production of the
metabolite butyrate having a notable role in maintaining gut
homeostasis and epithelial health (7). Butyrate can mediate the protective
effect of dietary fiber against colorectal cancer through various
mechanisms (7). Over the past
decade, extensive attention has been paid in the study of the gut
microbiome. In addition to 16S ribosomal RNA sequencing,
metabolomic analysis is an effective method of understanding how
the host and the microbiome communicate in the gut environment
(6). Microbial metabolites and
products are becoming increasingly recognized as important signals
regulating host physiology (8). In
addition, they can serve as diagnostic markers of disease. Several
studies have previously reported that short-chain fatty acid
levels, including acetate, butyrate and propionate, are markedly
reduced in patients with acute graft-vs.-host disease (aGVHD),
which is in turn associated with increased GVHD severity and
mortality (9). A recent study
showed that continuous infusion with propofol exerts little effect
on the intestinal flora (10). The
present study was an extension of a previous study (10) to investigate the effect of propofol
infusion on intestinal metabolites in rats.
In the current study, the feces of rats that
received continuous intravenous infusions with propofol were
collected before the intestinal signature of differential
metabolites was analyzed at different time periods. Furthermore, a
correlation analysis with existing intestinal flora results was
carried out to assess the relationship between the flora and
metabolite profiles.
Materials and methods
Ethics
The ethical approval reference number for the
present study was KY2018-02 and was approved by the Regional Ethics
Committee of the Cancer Hospital Affiliated to Harbin Medical
University (Harbin, China) in December 2018.
Animals
A total of 16 2-month-old specific pathogen-free
status, male Wistar International-Genetics-Standard rats (batch
qualification no. 1100111911030133) provided by Beijing Weitong
Lihua Experimental Animal Technology Co. Ltd. [license number: SCXK
(Beijing, China) 2016-0006] were kept at room temperature 23±1˚C
and 20-30% humidity, with 12-h light/night cycle lighting with free
movement. Food and water were available ad libitum. The
feeding of animals was performed in accordance with the ‘Guide for
the care and use of laboratory animals’. The tail of every rat was
labeled as T1-T16. Rats were adaptively housed for 1 month, after
which the experiments were performed.
Criterion of loss of righting reflex
(LORR) and recovery of righting reflex (RORR)
In the present study, LOOR was applied to reflect
the suppression of consciousness in rats, whereas RORR was used to
reflect the recovery of consciousness in rats. A rat was considered
to have regained consciousness if it could voluntarily return to
the prone position within 30 sec (11).
Deceased rats
Two unexplained rat deaths were confirmed upon
feeding. Another six rats were excluded for experimental purposes
because indwelling needles could not be inserted due to
unsuccessful venipunctures. A total of eight rats with successful
venipuncture were included in the present study, and these rats
were thereafter re-numbered as R1-R8. The weight of every rat was
measured before anesthesia (Table
I). The average weight of the 8 rats was 384.0±44.7 g.
 | Table IWeight and RORR time of every R
(n=8). |
Table I
Weight and RORR time of every R
(n=8).
| Rat parameter | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | x̄±s |
|---|
| Weight, in g | 350.0 | 340.0 | 367.0 | 381.0 | 335.0 | 420.5 | 420.0 | 458.5 | 384.0±44.7 |
| RORR time, in
min | 25.0 | 11.0 | 60.0 | 25.0 | 30.0 | 30.0 | 10.0 | 50.0 | 30.1±17.4 |
Experimental design
Tail vein puncture was performed on every rat and an
indwelling needle (no. 26) was inserted. After successful
operation, a single dose (11 mg/kg) of propofol (Diprivan; X22027B;
CordenPharma GmbH) was administered for 1 min to every rat via
tail-vein injection (i.v.) to induce LORR (12). After LORR, propofol was continuously
injected into the rats tail vein for 3 h through an injection pump
(SYS-52; Shenzhen Maiketian Biomedical Technology Co., Ltd.). The
anesthetic maintenance dose was initially set at 40 mg/kg/h
(12). The dose was adjusted based
on changes in the body weight and respiratory rate of the rats. The
respiratory rate was recorded every 5 min. The respiratory rate was
kept in the range of 60-85 times/min. The RORR time of every rat is
listed in Table I, where the
average RORR time of the 8 rats was 30.1±17.4 min. The rats were
put back into the cage for further feeding after their righting
reflex was completely recovered. Feces were collected from every
rat before and on days 1, 3 and 7 after propofol infusion. The
collection of feces was performed at 10 a.m. Feces were collected
in sterile frozen tubes before being labeled and stored in a -80˚C
refrigerator. The feces of the rats were divided into different
groups based on time periods, with before and after intervention on
days 1, 3 and 7 labeled as groups P, A1, A3 and A7, respectively.
Signs of severe distress in rats, such as apnea, behavioral
limitations due to excessive pain and avoidance behaviors were
considered the humane endpoints of the present study requiring
immediate intervention. However, there were no cases requiring
euthanasia due to observation of a humane endpoint and, therefore,
animals were euthanized only at the end of the experimental period.
After the completion of the current study, all experimental rats
were euthanized. Every rat was given a caudal vein infusion with 6
mg of propofol, and then cervical dislocation was performed.
Euthanasia was confirmed by loss of heartbeat and no response to
foot pinch test.
Metabolite extraction and gas
chromatography coupled with a time-of-flight mass spectrometer
(GC-TOF-MS) analysis
In total, 50±1 mg of fecal sample was transferred
into a 2-ml tube, before 500 µl of pre-cold extraction mixture
[methanol/chloroform (volume:volume)=3/1] with 10 µl internal
standard (adonitol, 0.5 mg/ml stock) were added, mixed by vortex
for 30 sec. The steel ball was then added, before the samples were
processed with a 35-Hz grinding instrument for 4 min followed by
ultrasonic treatment in an ice water bath for 5 min. This process
was repeated three times. After centrifugation at 4˚C for 15 min at
13,800 x g, 200 µl supernatant were transferred into a fresh 2-ml
tube. To prepare the quality control (QC) sample, 50 µl of every
sample were removed and mixed together. After evaporation in a
vacuum concentrator, 30 µl of methoxyamination hydrochloride (20
mg/ml in pyridine) were added and incubated at 80˚C for 30 min,
before being derivatized by 40 µl
N,O-Bis(trimethylsilyl)trifluoroacetamide reagent (1% Tri Methyl
Chloro Silane, volume/volume) at 70˚C for 1.5 h. Fecal samples from
four different time points were used as pairwise control groups and
GC-TOF-MS analysis was performed on them using an Agilent 7890 gas
chromatograph (Agilent Technologies, Inc.), detailed by a previous
study (13).
Data preprocessing and annotation
Raw data analysis, including peak extraction,
baseline adjustment, deconvolution, alignment and integration, was
performed using ChromaTOF® (version 4.3x; LECO
Corporation) software. The LECO-Fiehn Rtx5 database (https://fiehnlab.ucdavis.edu/projects/fiehnlib) was
used for metabolite identification by matching the mass spectrum
and retention index. Finally, the peaks detected in <50% of the
QC samples or relative standard deviation >30% in the QC
samples, were removed.
Metabolome and microbe association
analysis
Pearson's correlation and Spearman's correlation
analyses were used to determine whether there was a significant
linear (Pearson's correlation) or monotonic (Spearman's
correlation) relationship between the microbiome components or
genes and a single metabolite of the metabolome. The redundancy
analysis (RDA) or canonical correlation analysis (CCA) is a sorting
method developed based on the corresponding analysis, combining the
corresponding analysis with multiple regression analysis to perform
multiple linear regressions of two sets of data for every
calculation during the iteration of the corresponding analysis. The
RDA is based on a linear model, whereas the CCA is based on a
unimodal model. Decision Curve Analysis was performed with the
species-sample data to deduce the size of the first axis of lengths
of gradient in the analysis results. If >4.0, then CCA would be
preferred. If 3.0-4.0, both RDA and CCA were used. However, if
<3.0, then RDA was preferred. Differential genes and
differential metabolites were simultaneously mapped to the Kyoto
Encyclopedia of Genes and Genomes (KEGG; http://www.kegg.jp/kegg/pathway.html) pathway database
through the ‘Pathview’ package of R (v1.26.0; https://pathview.uncc.edu/) to obtain their common
pathway information. Nodes enriched for the differential compounds
and differential genes in the significant metabolic pathways were
then shown in different colors, representing differential compounds
and genes in metabolic pathways.
Statistical analysis
In the present study SPSS (v21.0; IBM Corp.) was
used for statistical analysis. SIMCA® software (v16.0.2;
Sartorius Stedim Data Analytics AB) was used to model and analyze
data, including principal component analysis (PCA) and orthogonal
projections to latent structures-discriminant analysis (OPLS-DA).
Differential metabolites were screened using the paired Student's
t-test and the variable importance in the projection (VIP) of the
first principal component of OPLS-DA model (P<0.05 and VIP >1
were considered to indicate a statistically significant
difference). The quantitative values of differential metabolites
were calculated using the Euclidean distance matrix, and the
differential metabolites were clustered using complete linkage
method. Finally, the heatmap of differential metabolites following
hierarchical clustering analysis was shown. Key pathways with the
highest correlation with the metabolite differences were found by
metabolic pathway enrichment analysis. Spearman correlation
analysis was used to assess the correlation between the
microorganisms and metabolites.
Results
Changes in the types and contents of
metabolites
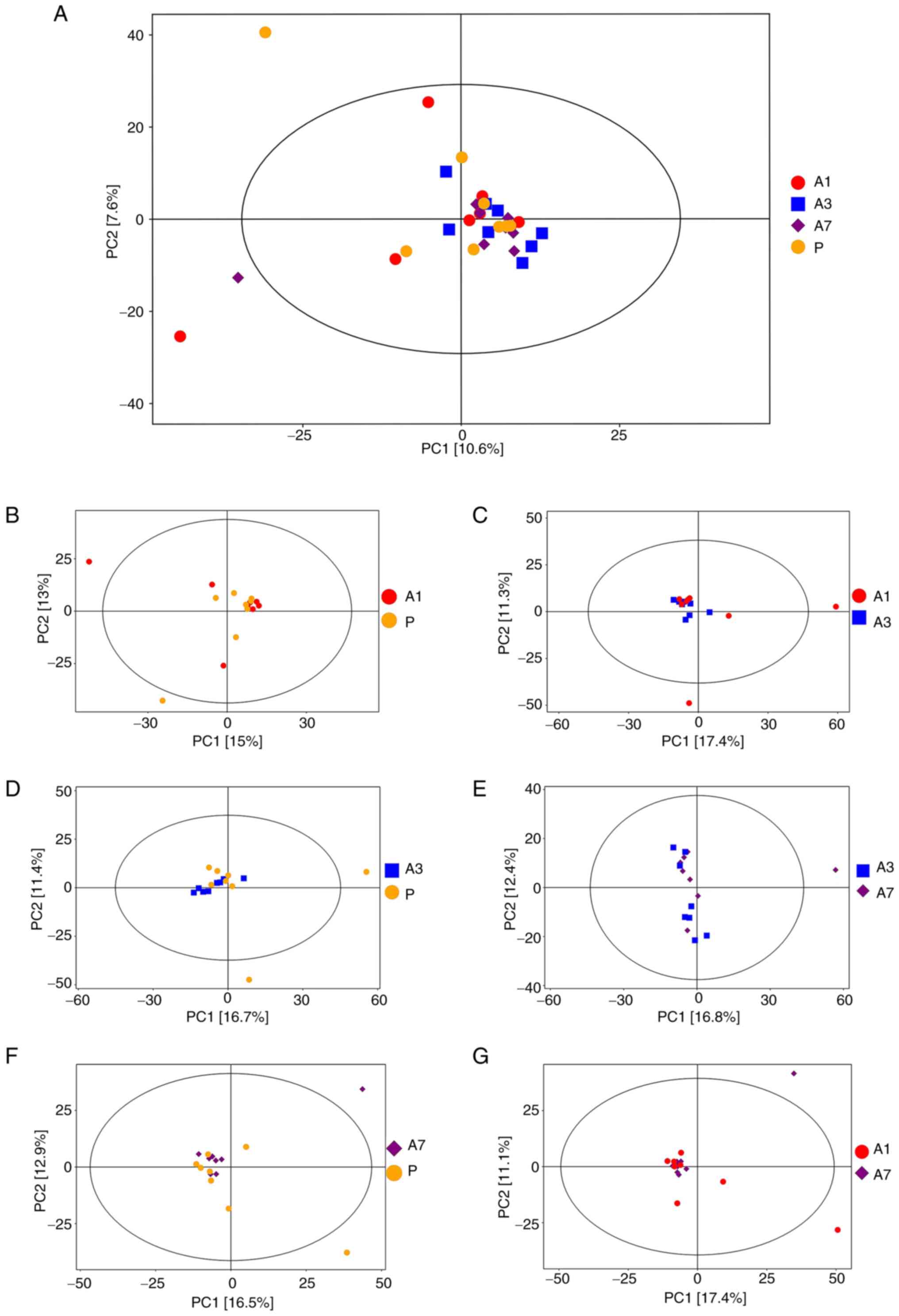
In the score scatterplot from the PCA model, the
distribution of the four sample groups was found to be relatively
concentrated on a whole (Fig. 1A).
Comparisons between the A1 and P groups (Fig. 1B), A1 and A3 groups (Fig. 1C), P and A3 groups (Fig. 1D), P and A7 groups (Fig. 1F) and between the A7 and A1 groups
(Fig. 1G), showed a relatively
centralized trend. Only the A7 and A3 group comparison revealed
some degree of separation (Fig.
1E). These results suggested that continuous intravenous
infusion with propofol exerted little effect on the type and
content of metabolites in the fecal samples.
Differential metabolites
To deduce the actual difference among the groups and
screen for effective differential metabolites, OPLS-DA was used to
analyze the results. The results of OPLS-DA analysis showed that
the samples between A1 and P (Fig.
2A), A3 and P (Fig. 2C), A7 and
P (Fig. 2E), A3 and A1 (Fig. 2G), A7 and A1 (Fig. 2I) and between A7 and A3 (Fig. 2K) were all separated. This suggested
that there were differential metabolites among the groups. Bringing
the samples further into the permutation tests within OPLS-DA,
comparisons between the models of A1 and P (Fig. 2B), A3 and P (Fig. 2D), A7 and P (Fig. 2F), A3 and A1 (Fig. 2H) and between A7 and A3 (Fig. 2L), yielded robustness and no
over-fitting phenomenon. Only comparison between A7 and A1 did not
yield robustness (Fig. 2J). This
finding suggested that there was no significant difference in
intestinal metabolites between days 1 and 7 after intravenous
propofol anesthesia. Metabolites with VIP >1 and P<0.05 were
considered to be differential metabolites. Table II listed the differential
metabolites' P-value and VIP. As it can be seen in Table II, from day 3 to 7 the content of
3-hydroxyphenylacetic and palmitic acid increased significantly.
The heatmap of differential metabolites following hierarchical
clustering analysis is shown in Fig.
3. It was revealed that a number of differential metabolites
showed continuous changes. In particular, the levels of cytosin and
aconitic acid were consistently and significantly decreased
throughout the 7 days after anesthesia (Fig. 3A-C). In addition, the levels of
linoleic acid methyl ester and zymosterol 1 decreased throughout
the 3 days after anesthesia, but they returned to pre-anesthetic
levels on day 7 (Fig. 3A, B and F).
The levels of linoleic acid dropped to a minimum on day 3, but they
recovered on day 7 (Fig. 3B and
F). By contrast, pipecolinic acid
and guanosine levels were both significantly elevated on day 1, and
then gradually recovered on day 3 (Fig.
3A and D).
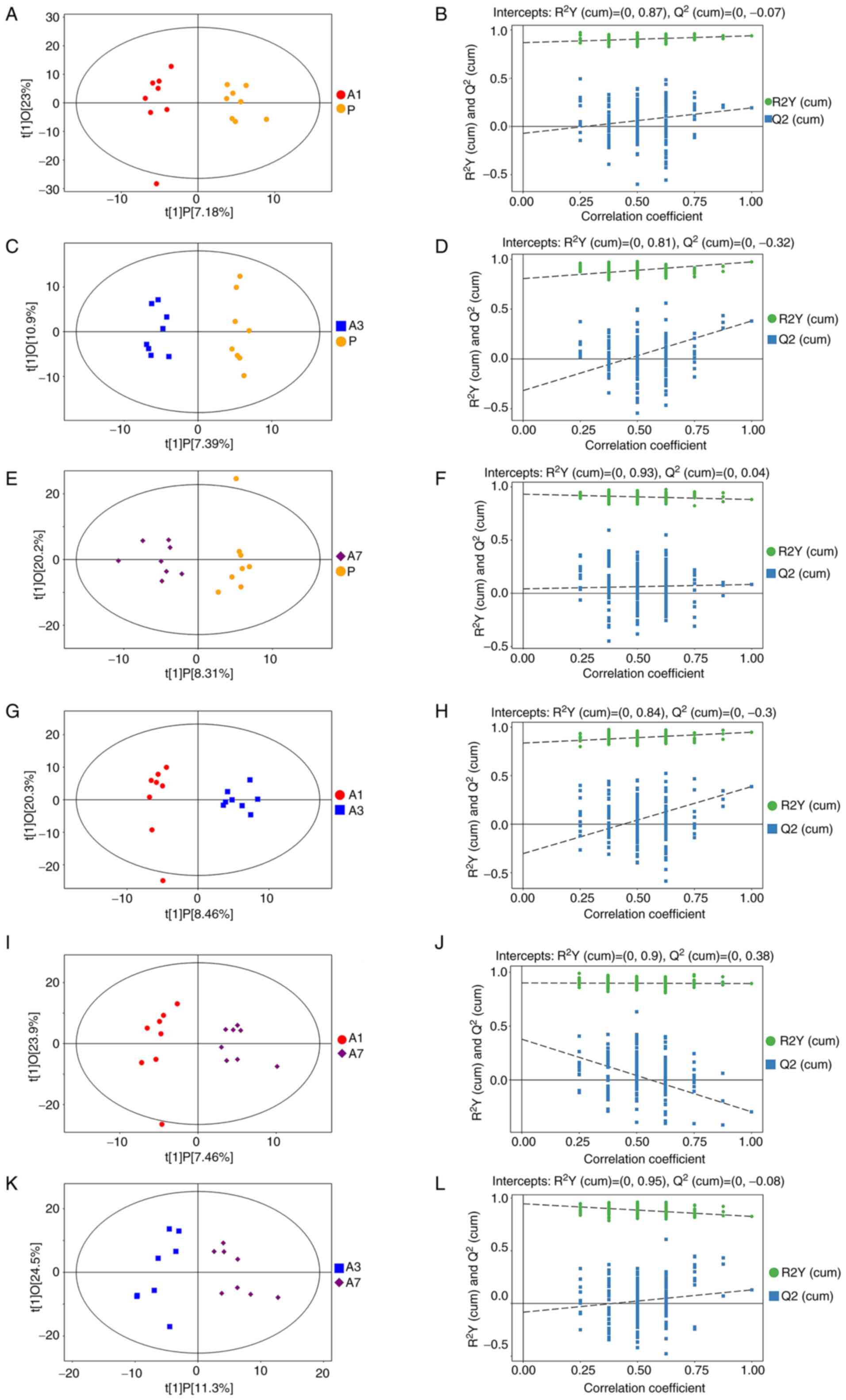 | Figure 2Score scatter plot and permutation
test of OPLS-DA model. (A and B) Group P vs. A1, (C and D) group P
vs. A3, (E and F) group P vs. A7, (G and H) group A1 vs. A3, (I and
J) group A1 vs. A7 and (K and L) group A3 vs. A7. In the score
scatter plot of OPLS-DA model, horizontal coordinate t[1]P
represents the predicted principal component score of the first
principal component, showing the differences between sample groups;
vertical coordinate t[1]O represents the orthogonal principal
component score, showing the differences within the sample group.
Every scatter represents a sample, and the shape and color of the
scatter represent different experimental groups. The farther the
transverse distance between samples, the greater the differences
between groups. The closer the longitudinal distance, the more
improved the repeatability was within the group. n=8 per group, the
samples were all within 95% confidence intervals. Permutation test
of OPLS-DA model verified the robustness of the OPLS-DA model.
OPLS-DA, orthogonal projections to latent structures-discriminant
analysis; P, before intervention; A3, after intervention on day 3;
after intervention on 7. |
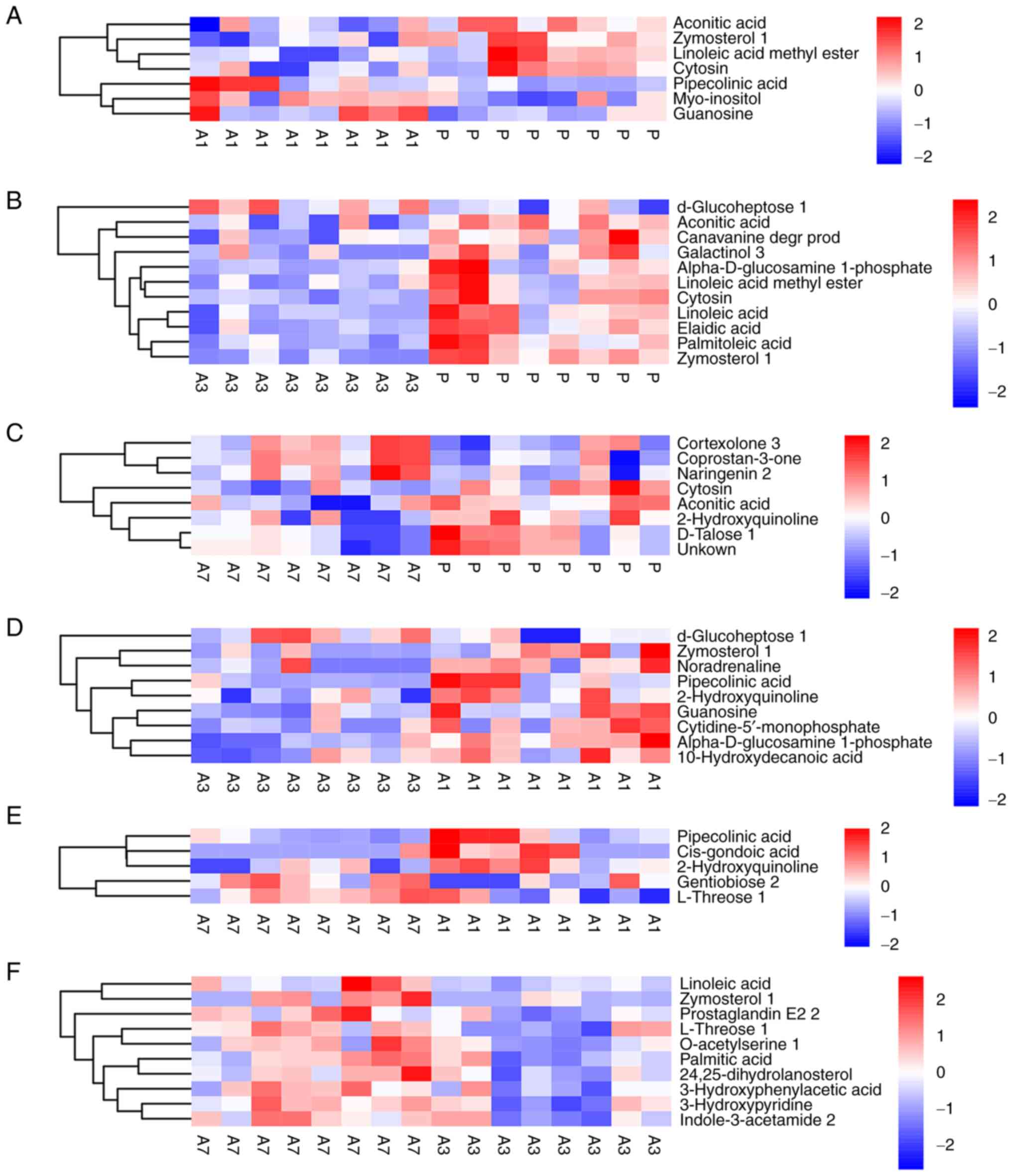 | Figure 3Heatmap of hierarchical clustering
analysis. (A) Group P vs. A1, (B) group P vs. A3, (C) group P vs.
A7, (D) group A1 vs. A3, (E) group A1 vs. A7, (F) group A3 vs. A7.
The abscissa represents different experimental groups, the ordinate
represents the different metabolites compared in the group, and the
color blocks at different positions represent the relative
expression levels of metabolites at corresponding positions. Red
indicates that the substance is highly expressed in the group, and
blue indicates that the substance is low expressed in the group.
n=8 per group. The figures show the changes of quantitative values
of differential metabolites in different groups. P, before
intervention; A3, after intervention on day 3; after intervention
on 7. |
 | Table IIStatistics of differential metabolite
outcomes between groups. |
Table II
Statistics of differential metabolite
outcomes between groups.
| Group | Peak | VIP | P-VALUE | Q-VALUE | FOLD CHANGE | LOG_
FOLDCHANGE | Variation
tendency |
|---|
| P-A1 | Linoleic acid
methyl ester | 1. 3551 | 0.0103 | 1 | 0.4355 | -1.1994 | Down |
| P-A1 | Zymosterol 1 | 1. 6643 | 0.0162 | 1 | 0.4971 | -1.0085 | Down |
| P-A1 | Cytosin | 1. 4682 | 0.0383 | 1 | 0.5220 | -0.9379 | Down |
| P-A1 | Pipecolinic
acid | 1.2355 | 0.0424 | 1 | 3.3086 | 1.7262 | Up |
| P-A1 | Aconitic Acid | 1.1256 | 0.0313 | 1 | 0.6182 | -0.6938 | Down |
| P-Al | Guanosine | 2.3050 | 0.0438 | 1 | 1.9521 | 0.9650 | Up |
| P-A3 | Linoleic acid | 2.8319 | 0.0014 | 0.2425 | 0.6045 | -0.7262 | Down |
| P-A3 | Elaidic acid | 2.7986 | 0.0017 | 0.2635 | 0.6515 | -0.6181 | Down |
| P-A3 | Linoleic acid
methyl ester | 2.3945 | 0.0217 | 0.7570 | 0.5256 | -0.9279 | Down |
| P-A3 | Zymosterol 1 | 2.4409 | 0.0000 | 0.0080 | 0.1252 | -2.9976 | Down |
| P-A3 | Cytosin | 2.4346 | 0.0055 | 0.4427 | 0.4020 | -1.3149 | Down |
| P-A3 | Galactinol 3 | 1.6365 | 0.0273 | 0.7969 | 0.3273 | -1.6113 | Down |
| P-A3 | Aconitic Acid | 1.9367 | 0.0026 | 0.2942 | 0.3882 | -1.3650 | Down |
| P-A7 | Cytosin | 1.0576 | 0.0115 | 1 | 0.4319 | -1.2113 | Down |
| P-A7 |
Coprostan-3-one | 1.2726 | 0.0112 | 1 | 1.8257 | 0.8685 | Up |
| P-A7 | Cortexolone 3 | 1.7122 | 0.0351 | 1 | 1.5252 | 0.6090 | Up |
| P-A7 | Naringenin 2 | 1.2024 | 0.0426 | 1 | 1.7064 | 0.7709 | Up |
| P-A7 | Aconitic Acid | 1.6468 | 0.0221 | 1 | 0.5612 | -0.8333 | Down |
| P-A7 |
2-Hydroxyquinoline | 2.3604 | 0.0343 | 1 | 0.5065 | -0.9813 | Down |
| A1-A3 | Zymosterol 1 | 1.7887 | 0.0373 | 1 | 0.2519 | -1.9891 | Down |
| AI-A3 | Pipecolinic
acid | 1.9042 | 0.0332 | 1 | 0.2546 | -1.9736 | Down |
| A1-A3 | Guanosine | 1.3697 | 0.0183 | 1 | 0.3933 | -1.3462 | Down |
| A1-A3 |
2-Hydroxyquinoline | 1.4066 | 0.0285 | 1 | 0.5283 | -0.9206 | Down |
| A3-A7 | Linoleic acid | 2.1390 | 0.0418 | 0.6866 | 1.6807 | 0.7491 | Up |
| A3-A7 | Palmitic acid | 1.7825 | 0.0275 | 0.6866 | 1.3943 | 0.4796 | Up |
| A3-A7 |
3-hydroxyphenylacetic acid | 1.7011 | 0.0253 | 0.6866 | 1.3354 | 0.4172 | Up |
| A3-A7 |
3-Hydroxypyridine | 1.6606 | 0.0285 | 0.6866 | 1.2469 | 0.3183 | Up |
| A3-A7 | Zymosterol 1 | 1.2452 | 0.0398 | 0.6866 | 4.7028 | 2.2335 | Up |
| A3-A7 | L-Threose 1 | 1.6325 | 0.0286 | 0.6866 | 1.2289 | 0.2974 | Up |
| A1-A7 | Pipecolinic
acid | 1.2104 | 0.0423 | 1 | 0.2981 | -1.7462 | Down |
| A1-A7 | L-Threose 1 | 2.0590 | 0.0366 | 1 | 1.2338 | 0.3031 | Up |
| A1-A7 |
2-Hydroxyquinoline | 2.1475 | 0.0138 | 1 | 0.4385 | -1.1894 | Down |
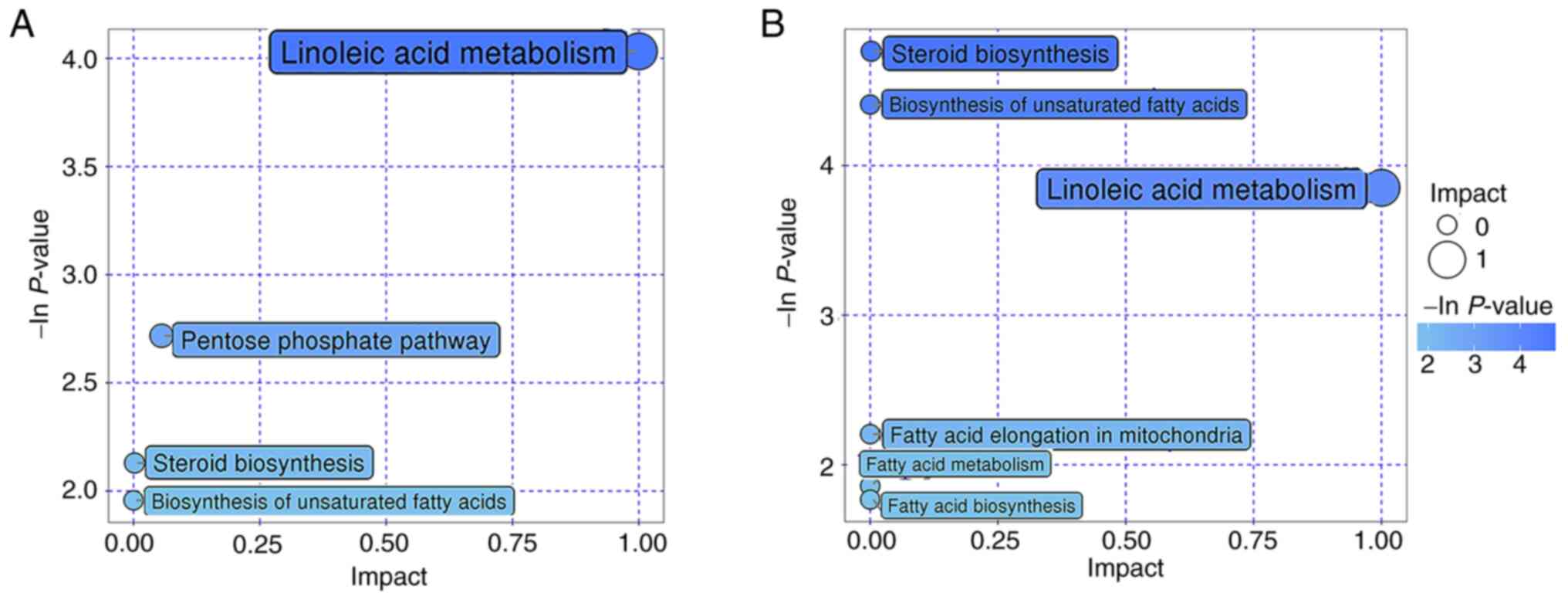
Metabolic pathways
The differential metabolites in the present study
were then incorporated into KEGG and PubChem database (https://pubchem.ncbi.nlm.nih.gov/) for metabolic
pathway analysis, the results of which are shown in the bubble map
in Fig. 4. ‘Linoleic acid
metabolism’ was found to be a commonly enriched metabolic pathway
in the A3 vs. P and A7 vs. A3 groups (Fig. 4A and B).
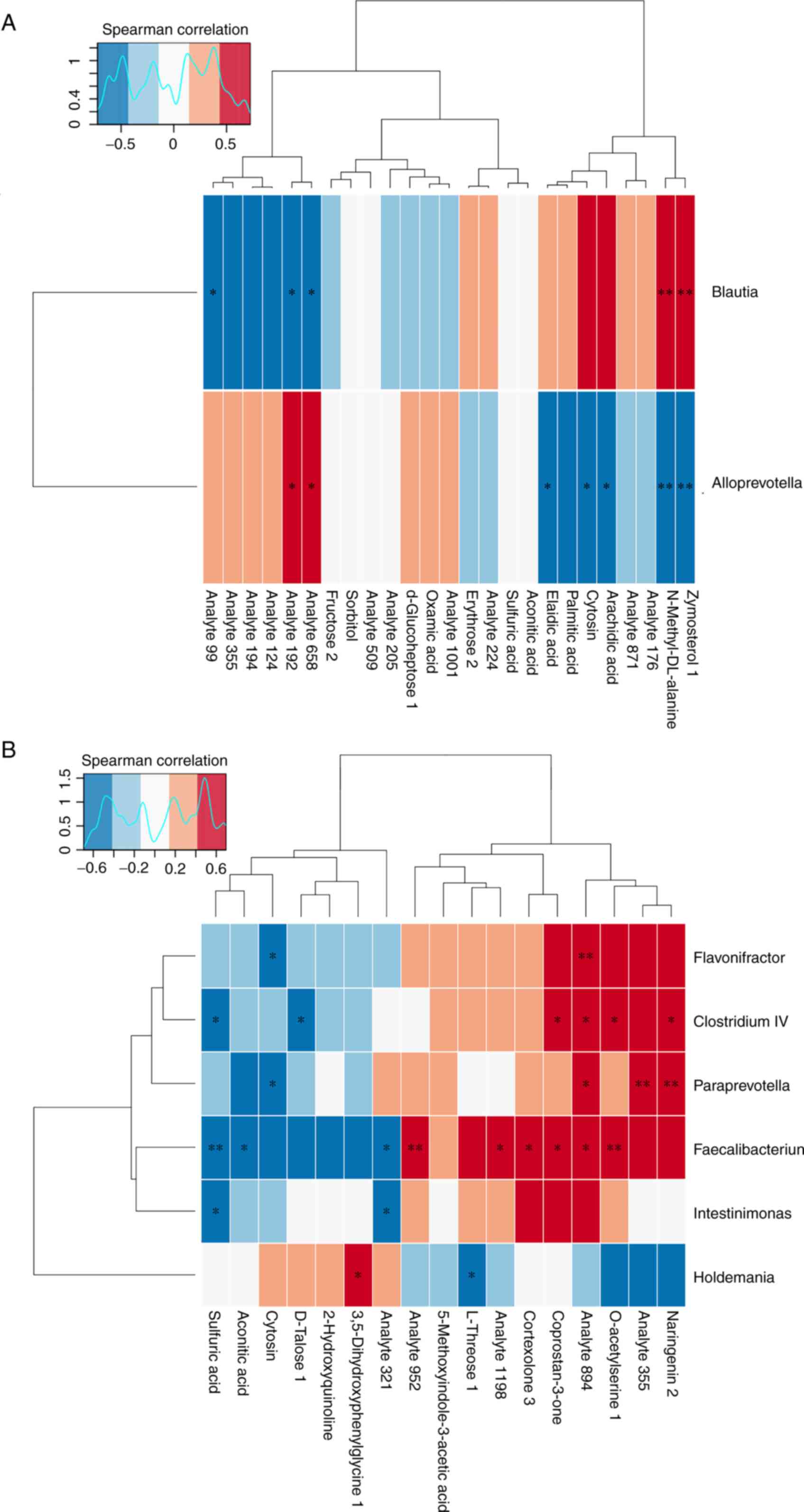
Metabolome and microbe association
analysis
A previous study showed that continuous intravenous
infusion with propofol ~3 h resulted in changes in the gut microbe
profile (10). Spearman correlation
analysis was therefore performed to explore whether there was a
correlation between different species of microorganisms and various
metabolites among the aforementioned groups (Fig. 5). It was observed that zymosterol 1,
cytosin and elaidic acid were negatively correlated with
Alloprevotella in the A3 vs. P group (Fig. 5A). In the A7 vs. P group,
cortexolone 3 and coprostan-3-one were positively correlated with
Faecalibacterium, whilst aconitic acid was negatively
correlated with it (Fig. 5B). In
addition, Alloprevotella and Faecalibacterium were
the different species of microbes found in the previous studies
(10).
Discussion
The present study was the first to investigate the
effects of continuous intravenous administration of propofol on
fecal metabolites in rats using an untargeted metabolomics
technique. PCA and OPLS-DA analyses showed that although the types
and levels of excreta metabolites in rats changed on days 1, 3 and
7 after the continuous intravenous infusion with propofol, the
changes were not statistically significant. A similar previous
study found that continuous intravenous infusion with propofol has
little effect on the intestinal flora of male Wistar rats (10). The present study served as an
extension of this previous study (10), which further revealed the effects of
continuous intravenous infusion with propofol on the intestinal
microenvironment of rats from the perspective of metabolomics.
Propofol is a potent intravenous hypnotic drug and
is typically formulated as an Intralipid®-based emulsion
(Fresenuis Kabi Ltd.) containing the same ingredients, namely
soybean oil (100 mg/ml), glycerol (22.5 mg/ml) and egg lecithin (12
mg/ml) (14). Due to the infusion
with this lipid formulation, propofol can cause adverse effects,
such as metabolic acidosis, rhabdomyolysis, hyperlipidemia and
enlarged fatty liver (15). The
liver is the main site of propofol metabolism, but the small
intestine also participates in this process (14). A study has previously indicated that
compared with inhalation anesthesia, propofol anesthesia is
associated with lower incidence of early postoperative cognitive
dysfunction (POCD) in elderly patients (16). Another study on the effects of
anesthetics on the human serum metabolome showed that after the
continuous infusion with propofol for 1 h (1.7 µg/ml; n=40), 37
metabolites demonstrate notable changes in all intergroup
comparisons. These were found in the metabolite subgroups of
lipoprotein, fatty acid, glycerides and phospholipids (17). However, the effect of continuous
infusion with propofol on fecal metabolites remains elusive.
The present study showed that there were various
differential metabolites in the stool samples of rats collected at
different time periods after anesthesia, with comparisons carried
out between the pre- and post-treatment time periods. From day 3 to
7 after the continuous intravenous infusion with propofol, the
content of 3-hydroxyphenylacetic and palmitic acid increased
significantly. The metabolite 3-hydroxyphenylacetic acid belongs to
the aromatic family. A previous study has shown that
3-hydroxyphenylacetic acid is notably elevated in the urine of
children with autism, and it is positively correlated with the
Clostridium species of bacteria (18). In a previous study,
Clostridium was revealed to be a differential microbe
(10), before and after the
continuous intravenous infusion with propofol, which has also
coincidentally been found to be markedly enriched in the feces of
patients with autism (19).
Palmitic acid is the most abundant saturated fatty acid in the
human body; it can either be provided through diet, or synthesized
endogenously from other fatty acids, carbohydrates and amino acids
(20). In the intestines, palmitic
acid has been shown to be able to modulate the immune system by
inducing monocyte activation and stimulating proinflammatory
responses in human immune cells (21,22).
Disruption of the palmitic acid homeostatic balance has been
previously implicated in a range of pathophysiological conditions,
such as atherosclerosis, neurodegenerative diseases and cancer,
mainly due to aberrant endogenous palmitic acid biosynthesis
(20). Palmitatic acid in urine has
been previously reported to serve as a potential biomarker for
post-stroke depression (23).
A number of other differential metabolites also
showed dynamic changes after propofol anesthesia, such that
Spearman correlation analysis revealed significant correlation
between some differential metabolites and differential
microorganisms. Pipecolinic acid was significantly increased on day
1 after anesthesia, but returned to pre-anesthesia levels on day 3.
A serum metabolomics study in patients with new-onset type 2
diabetes (T2D) revealed that pipecolinic acid can serve as a
biomarker for T2D prediction, with a notable association between
pipecolinic acid and cholinergic receptor muscarinic 3 (rs535514)
(24). Pipecolinic acid has not
been well studied as an intestinal metabolite, with the
relationship to its host therefore being poorly understood. Ou
et al (25) previously
revealed that pipecolinic acid levels in the fecal metabolites have
a notable positive correlation with defecation frequency in
patients with constipation. In addition, the alleviation of
constipation has been found to be induced by Lactobacillus
casei, a Shirota strain (25).
The present study demonstrated that pipecolinic acid was
significantly negatively correlated with Alloprevotella but
positively correlated with Blautia in groups A1-A3. This
suggested that continuous infusion with propofol may cause the
fluctuation of pipecolinic acid content by altering the physiology
of Alloprevotella and Blautia. However, further
verification is required.
In the present study, the levels of cytosin and
aconitic acid continued to decrease significantly within 7 days
after propofol infusion. These are rare metabolites that showed a
one-way fluctuation, among the differential metabolites that were
found. Since information on cytosin remains insufficient, whether
cytosin serves an important mechanism of action in living organisms
remains unknown. By contrast, aconitic acid, also known as
propene-1,2,3-tricarboxylic acid, is an unsaturated tricarboxylic
acid used in the chemical industry as raw material for the organic
synthesis of other compounds, such as flavoring agents (26). Aconitic acid can also serve a
variety of biological roles in cells; serving as an intermediate in
the tricarboxylic acid cycle and providing certain plants unique
survival advantages as an antifeedant and antifungal agent, and a
means of storing fixed carbon pools (27). In a study on urine metabolomics,
Kwan et al (28) previously
revealed that 13 metabolites, including aconitic acid, are
associated with diabetic kidney disease. In addition, in a further
prospective cohort study (28),
3-hydroxyisobutyrate and aconitic acid levels were found to be
associated with higher and lower risk of kidney failure with
replacement therapy, that is initiation of dialysis or receipt of
transplant, respectively. Another untargeted metabolomics study of
serum (29) found that 11
metabolites, including aconitic acid, were the optimal markers for
the diagnosis of intracerebral hemorrhage. In addition, concerning
the metagenomics and metabolomic integration analysis of biofecal
matter, a controlled trial in patients with arteriosclerosis and
normal control individuals (30),
showed a high abundance and centrality of Flavonifractor
plautii in normal control individuals and the absence of this
species in the microbiome of patients with arteriosclerosis.
Furthermore, increased cis-aconitic acid has been found to be the
main effector of the protective effect of Flavonifractor
plautii against arteriosclerosis. Cis-aconitic acid is the
isomer of aconitic acid (30).
These observations suggest that aconitic acid likely serves an
important role as a marker in different biological materials. The
levels of aconitic acid continued to decrease in the present study.
However, whether this specific metabolite can be used as a
potential biomarker for the occurrence of propofol-related
complications or for the mechanism of action of propofol anesthesia
on the human body remains a topic of further research.
Linoleic acid is not only the most abundant
polyunsaturated fatty acid (PUFA) in human nutrition, but also an
important component of the cellular membrane structure (31). Linoleic acid is the direct precursor
of a wide range of other bioactive ω6 PUFAs, such that the
metabolites of linoleic acid, represented by arachidonic acid, can
promote a potent proinflammatory response in rats (32,33).
In the gut, the dynamic balance of linoleic acid is maintained by
both dietary intake and bacterial metabolism (34). Linoleic acid can be metabolized to
10-hydroxy-cis-12-octadecenoic acid by the gut microbiota, which
can prevent epithelial barrier impairment in Caco-2 cells and
ameliorate intestinal inflammation by suppressing TNF receptor 2
upregulation (34). A previous
study found that novel propofol-AA esters exhibit unique anticancer
activity and can inhibit tumor growth (35). The present study found that linoleic
acid metabolism was the metabolic pathway that was particularly
enriched in both the P vs. A3 and A3 vs. A7 groups, suggesting that
propofol is likely to cause continuous changes in fecal linoleic
acid levels by affecting the linoleic acid metabolism pathway.
Another study revealed that the most notable changes
in the intestinal flora of mice are observed on day 7 after the
continuous inhalation of sevoflurane for 4 h, accompanied by
changes in metabolites associated with different microorganisms
(36). However, this previous study
(36) and the present study
revealed that after the continuous intravenous administration of
propofol, there are no significant changes in the intestinal flora
or intestinal metabolites of mice (10). The effects of pathological changes
during disease conditions after general anesthesia on changes in
the intestinal microecological environment caused by these two
anesthetics remain outstanding issues that require future
clarification. For example, the incidence of POCD after propofol
anesthesia is lower compared with that after sevoflurane anesthesia
(16), but the potential role of
the intestinal microecosystem in this remains unknown.
The present study is associated with a number of
limitations. Propofol is widely used in patients of all ages.
However, in the present study, 2-month-old adult male rats were
used, meaning that the study is lacking in experimental data from
juvenile, elderly and female rats. The effects of age and sex on
the effects of propofol anesthesia will need verification in future
studies. Another limitation refers to the potential effects of
hypoxia and respiratory depression on intestinal microbes and
metabolites. Intubation and oxygen delivery were not performed in
the present study. Although the respiratory rate of rats was
closely observed, this possibility cannot be completely ruled out.
In addition to hypoxia, anesthesia may cause changes in
physiological indicators, such as hypothermia, hypercapnia and
hypotension in rats. Tripathi et al (37) previously simulated sleep apnea
syndrome by exposing mice to intermittent hyperoxia and hypercapnia
for 6 weeks. The authors found that this intervention can lead to
changes in the intestinal microbiome and metabolites in mice
(37). Few studies have
investigated these contents, where the described interventions of
mice that exhibited positive results are long term, ranging from 30
days to 6 weeks. The short 3-h term effects of hypoxia, hypothermia
and hypercapnia on the intestinal microbiome and metabolites have
not been verified. In addition, as continuous database
improvements, the utility of measurement instruments is also
constantly improving. Only 6,810 metabolites were identified in the
feces of the human metabolome database. This may be an order of
magnitude lower compared with the true chemical diversity of the
metabolites in the human gastrointestinal flora (38). Resolution of these issues relies on
the development and improvement of metabolomics software tools
related to metabolite identification and diverse data types. In the
present study, only GC-TOF-MS was performed based on a metabolomic
analysis, and it was shown that continuous intravenous infusion
with propofol had little effect on rat intestinal metabolites. The
mechanism by which propofol can regulate gut microbiota metabolites
will need to be investigated. In addition, attempts can be made to
explain the underlying mechanism of the side effects or adverse
post-operative consequences following propofol application from the
perspective of intestinal microbiota metabolites for prevention
purposes.
In conclusion, the present study revealed
statistically insignificant effects of continuous intravenous
propofol on the intestinal metabolites in rats. However, additional
studies are required to confirm this, in particular in human
samples.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Outstanding Youth
Project Foundation of Harbin Medical Univercity Cancer Hospital
(grant no. JCQN2021-05), the Haiyan Research Foundation (grant no.
JJZD2022-04), and the Natural Science Foundation of Heilongjiang
Province (grant nos. YQ2020H038 and JJ2020YX0472).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and ZhoZ conceived the study, designed
experiments, analyzed and interpreted data, and wrote the
manuscript. HL, XQ, XY, NG and LC interpreted data. ZhaZ and CW
designed experiments, confirmed the authenticity of all the raw
data and gave final approval of the manuscript to be published. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The ethical approval reference number for the study
was KY2018-02 and was approved by the Regional Ethics Committee of
the Cancer Hospital Affiliated to Harbin Medical University in
December 2018.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Budic I, Jevtovic Stoimenov T, Pavlovic D,
Marjanovic V, Djordjevic I, Stevic M and Simic D: Clinical
importance of potential genetic determinants affecting propofol
pharmacokinetics and pharmacodynamics. Front Med (Lausanne).
9(809393)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Koriyama H, Duff JP, Guerra GG and Chan
AW: Sedation Withdrawal and Analgesia Team. Is propofol a friend or
a foe of the pediatric intensivist? Description of propofol use in
a PICU*. Pediatr Crit Care Med. 15:e66–e71. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dinis-Oliveira RJ: Metabolic profiles of
propofol and fospropofol: Clinical and forensic interpretative
aspects. Biomed Res Int. 2018(6852857)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Asghar MU, Cheema HA, Tanveer K and
Leinwand J: Propofol infusion and acute pancreatitis: A review. Am
J Ther. 27:e371–e374. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Walsh CT: Propofol: Milk of Amnesia. Cell.
175:10–13. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sanada S, Suzuki T, Nagata A, Hashidume T,
Yoshikawa Y and Miyoshi N: Intestinal microbial metabolite
stercobilin involvement in the chronic inflammation of ob/ob mice.
Sci Rep. 10(6479)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wu X, Wu Y, He L, Wu L, Wang X and Liu Z:
Effects of the intestinal microbial metabolite butyrate on the
development of colorectal cancer. J Cancer. 9:2510–2517.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xing PY, Pettersson S and Kundu P:
Microbial metabolites and intestinal stem cells tune intestinal
homeostasis. Proteomics. 20(e1800419)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lin D, Hu B, Li P, Zhao Y, Xu Y and Wu D:
Roles of the intestinal microbiota and microbial metabolites in
acute GVHD. Exp Hematol Oncol. 10(49)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Guo N, Zhang Z, Han C, Chen L, Zheng X, Yu
K, Zhang Z and Wang C: Effects of continuous intravenous infusion
with propofol on intestinal flora in rats. Biomed Pharmacother.
134(111080)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shirasaka T, Yonaha T, Onizuka S and
Tsuneyoshi I: Effects of orexin-A on propofol anesthesia in rats. J
Anesth. 25:65–71. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang Y, Yu T, Yuan C, Yuan J, Luo Z, Pan
Y, Zhang Y, Zhang Y and Yu B: Effects of propofol on the dopamine,
metabolites and GABAA receptors in media prefrontal cortex in
freely moving rats. Am J Transl Res. 8:2301–2308. 2016.PubMed/NCBI
|
|
13
|
Liu H, Yin X, Li J, Cao Y, Wang Y, Mu W,
Zhuo Z, Chen L, Zhang Z, Qu X, et al: Preoperative intestinal
microbiome and metabolome in elderly patients with delayed
neurocognitive recovery. Anaesth Crit Care Pain Med.
41(101140)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sahinovic MM, Struys MMRF and Absalom AR:
Clinical pharmacokinetics and pharmacodynamics of propofol. Clin
Pharmacokinet. 57:1539–1558. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kam PC and Cardone D: Propofol infusion
syndrome. Anaesthesia. 62:690–701. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xu D, Yang W and Zhao G: Effect of
propofol and inhalation anesthesia on postoperative cognitive
dysfunction in the elderly: A meta-analysis. Nan Fang Yi Ke Da Xue
Xue Bao. 32:1623–1627. 2012.PubMed/NCBI(In Chinese).
|
|
17
|
Nummela AJ, Laaksonen LT, Laitio TT,
Kallionpaa RE, Langsjo JW, Scheinin JM, Vahlberg TJ, Koskela HT,
Aittomaki V, Valli KJ, et al: Effects of dexmedetomidine, propofol,
sevoflurane and S-ketamine on the human metabolome: A randomised
trial using nuclear magnetic resonance spectroscopy. Eur J
Anaesthesiol. 39:521–532. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xiong X, Liu D, Wang Y, Zeng T and Peng Y:
Urinary 3-(3-Hydroxyphenyl)-3-hydroxypropionic Acid,
3-Hydroxyphenylacetic Acid, and 3-Hydroxyhippuric acid are elevated
in children with autism spectrum disorders. Biomed Res Int.
2016(9485412)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Finegold SM, Molitoris D, Song Y, Liu C,
Vaisanen ML, Bolte E, McTeague M, Sandler R, Wexler H, Marlowe EM,
et al: Gastrointestinal microflora studies in late-onset autism.
Clin Infect Dis. 35 (Suppl 1):S6–S16. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Carta G, Murru E, Banni S and Manca C:
Palmitic Acid: Physiological role, metabolism and nutritional
implications. Front Physiol. 8(902)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nicholas DA, Zhang K, Hung C, Glasgow S,
Aruni AW, Unternaehrer J, Payne KJ, Langridge WHR and De Leon M:
Palmitic acid is a toll-like receptor 4 ligand that induces human
dendritic cell secretion of IL-1beta. PLoS One.
12(e0176793)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tran TT, Postal BG, Demignot S, Ribeiro A,
Osinski C, Pais de Barros JP, Blachnio-Zabielska A, Leturque A,
Rousset M, Ferre P, et al: Short term palmitate supply impairs
intestinal insulin signaling via ceramide production. J Biol Chem.
291:16328–16338. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen J, Lv YN, Li XB, Xiong JJ, Liang HT,
Xie L, Wan CY, Chen YQ, Wang HS, Liu P and Zheng HQ: Urinary
metabolite signatures for predicting elderly stroke survivors with
depression. Neuropsychiatr Dis Treat. 17:925–933. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ouyang Y, Qiu G, Zhao X, Su B, Feng D, Lv
W, Xuan Q, Wang L, Yu D, Wang Q, et al: Metabolome-Genome-Wide
association study (mGWAS) reveals novel metabolites associated with
future type 2 diabetes risk and susceptibility loci in a
case-control study in a Chinese prospective cohort. Glob Chall.
5(2000088)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ou Y, Chen S, Ren F, Zhang M, Ge S, Guo H,
Zhang H and Zhao L: Lactobacillus casei strain shirota alleviates
constipation in adults by increasing the pipecolinic acid level in
the Gut. Front Microbiol. 10(324)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Takiguchi A, Yoshioka I, Oda Y, Ishii Y
and Kirimura K: Constitutive production of aconitate isomerase by
Pseudomonas sp. WU-0701 in relation to trans-aconitic acid
assimilation. J Biosci Bioeng. 131:47–52. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bruni GO and Klasson KT: Aconitic acid
recovery from renewable feedstock and review of chemical and
biological applications. Foods. 11(573)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kwan B, Fuhrer T, Zhang J, Darshi M, Van
Espen B, Montemayor D, de Boer IH, Dobre M, Hsu CY, Kelly TN, et
al: Metabolomic markers of kidney function decline in patients with
diabetes: Evidence from the chronic renal insufficiency cohort
(CRIC) study. Am J Kidney Dis. 76:511–520. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen L, Wang S, Zhang Y, Li Y, Zhang X, Ma
J, Zou X, Yao T, Li S, Chen J, et al: Multi-omics reveals specific
host metabolism-microbiome associations in intracerebral
hemorrhage. Front Cell Infect Microbiol. 12(999627)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Luo S, Zhao Y, Zhu S, Liu L, Cheng K, Ye
B, Han Y, Fan J and Xia M: Flavonifractor plautii protects against
elevated arterial stiffness. Circ Res. 132:167–181. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Whelan J and Fritsche K: Linoleic acid.
Adv Nutr. 4:311–312. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Choque B, Catheline D, Rioux V and Legrand
P: Linoleic acid: Between doubts and certainties. Biochimie.
96:14–21. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fritsche KL: Too much linoleic acid
promotes inflammation-doesn't it? Prostaglandins Leukot Essent
Fatty Acids. 79:173–175. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Miyamoto J, Mizukure T, Park SB, Kishino
S, Kimura I, Hirano K, Bergamo P, Rossi M, Suzuki T, Arita M, et
al: A gut microbial metabolite of linoleic acid,
10-hydroxy-cis-12-octadecenoic acid, ameliorates intestinal
epithelial barrier impairment partially via GPR40-MEK-ERK pathway.
J Biol Chem. 290:2902–2918. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Khan AA, Alam M, Tufail S, Mustafa J and
Owais M: Synthesis and characterization of novel PUFA esters
exhibiting potential anticancer activities: An in vitro study. Eur
J Med Chem. 46:4878–4886. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Han C, Zhang Z, Guo N, Li X, Yang M, Peng
Y, Ma X, Yu K and Wang C: Effects of sevoflurane inhalation
anesthesia on the intestinal microbiome in mice. Front Cell Infect
Microbiol. 11(633527)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tripathi A, Melnik AV, Xue J, Poulsen O,
Meehan MJ, Humphrey G, Jiang L, Ackermann G, McDonald D, Zhou D, et
al: Intermittent hypoxia and hypercapnia, a hallmark of obstructive
sleep apnea, alters the gut microbiome and metabolome. mSystems.
3:e00020–18. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pettersen VK, Antunes LCM, Dufour A and
Arrieta MC: Inferring early-life host and microbiome functions by
mass spectrometry-based metaproteomics and metabolomics. Comput
Struct Biotechnol J. 20:274–286. 2022.PubMed/NCBI View Article : Google Scholar
|