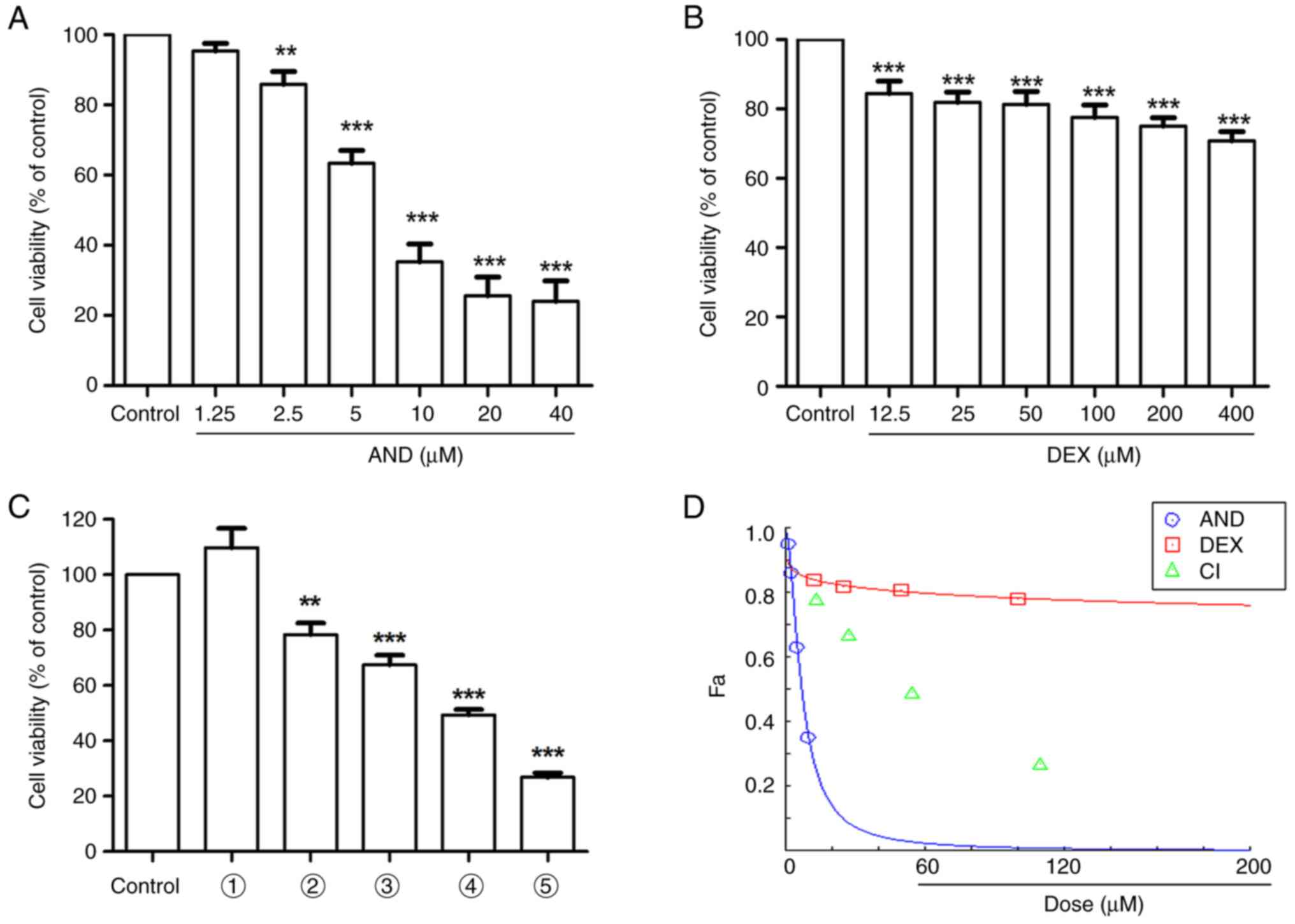
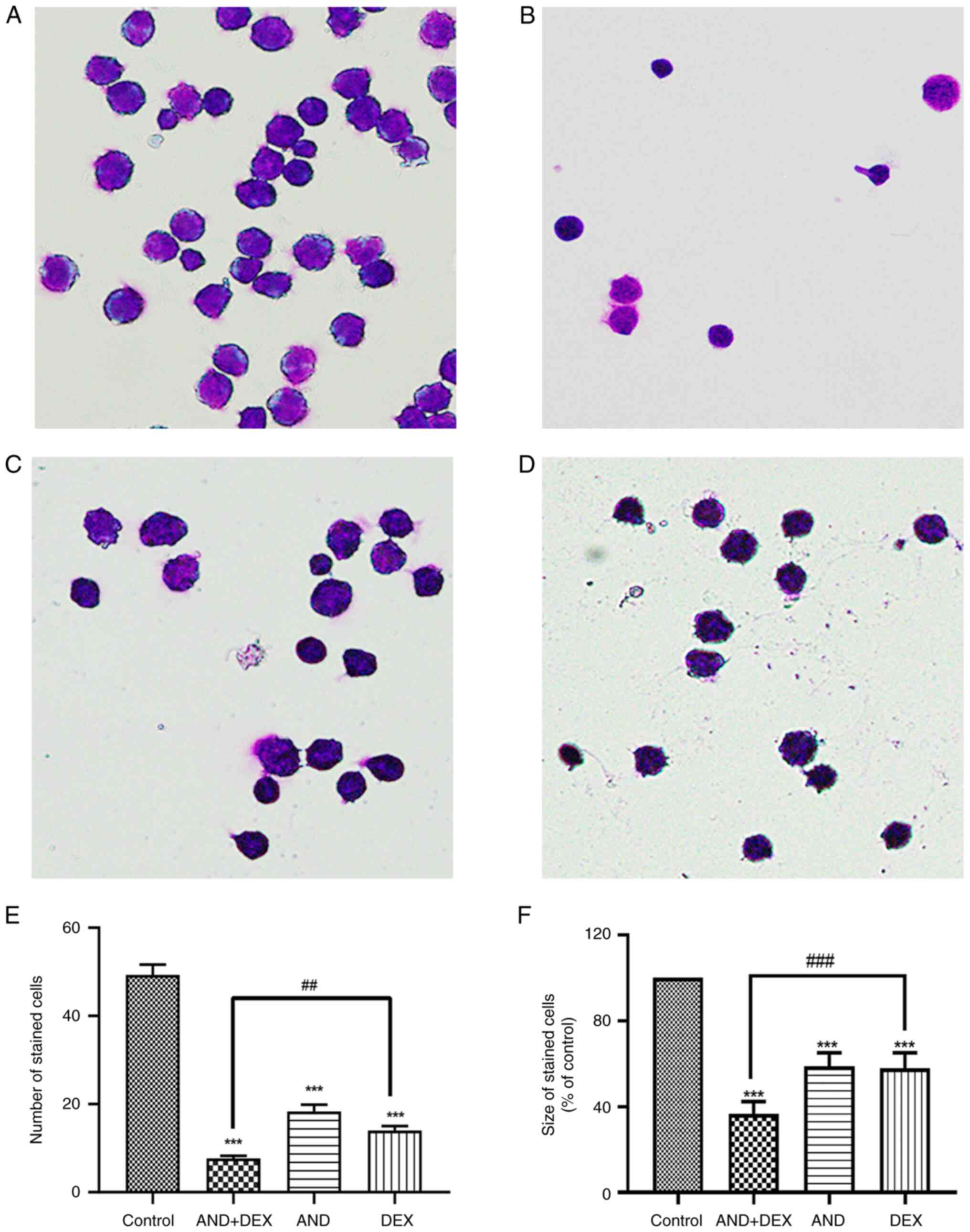
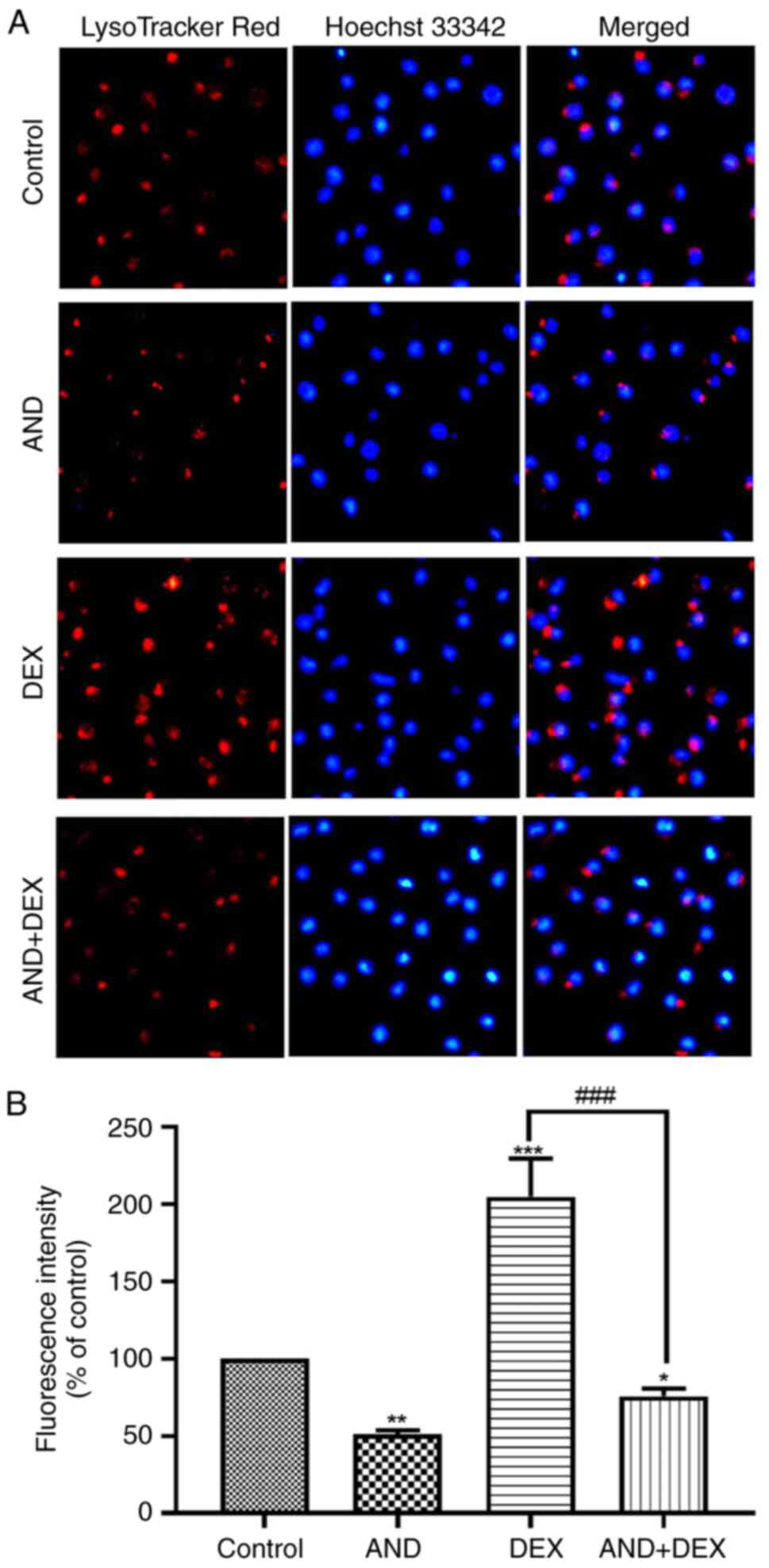
|
1
|
Olivas-Aguirre M, Torres-López L, Pottosin
I and Dobrovinskaya O: Overcoming glucocorticoid resistance in
acute lymphoblastic leukemia: repurposed drugs can improve the
protocol. Front Oncol. 11(617937)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gregory S: Adult acute lymphoblastic
leukemia: treatment and management updates. Semin Oncol Nurs.
35(150951)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Koh WJ, Greer BE, Abu-Rustum NR, Campos
SM, Cho KR, Chon HS, Chu C, Cohn D, Crispens MA, Dizon DS, et al:
Vulvar cancer, version 1.2017, NCCN clinical practice guidelines in
oncology. J Natl Compr Canc Netw. 15:92–120. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Huang FL, Yu SJ and Li CL: Role of
autophagy and apoptosis in acute lymphoblastic leukemia. Cancer
Control. 28(10732748211019138)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chennamadhavuni A, Lyengar V, Mukkamalla
SKR and Shimanovsky A: Leukemia. In: StatPearls [Internet].
Treasure Island (FL): StatPearls Publishing, 2023.
|
|
6
|
Park H, Youk J, Shin DY, Hong J, Kim I,
Kim NJ, Lee JO, Bang SM, Yoon SS, Park WB and Koh Y: Micafungin
prophylaxis for acute leukemia patients undergoing induction
chemotherapy. BMC Cancer. 19(358)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rafei H, Kantarjian HM and Jabbour EJ:
Recent advances in the treatment of acute lymphoblastic leukemia.
Leuk Lymphoma. 60:2606–2621. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kato M and Manabe A: Treatment and biology
of pediatric acute lymphoblastic leukemia. Pediatr Int. 60:4–12.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Martinelli G, Papayannidis C, Piciocchi A,
Robustelli V, Soverini S, Terragna C, Marconi G, Lemoli RM, Guolo
F, Fornaro A, et al: INCB84344-201: Ponatinib and steroids in
frontline therapy for unfit patients with Ph+ acute lymphoblastic
leukemia. Blood Adv. 6:1742–1753. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Imai K: Acute lymphoblastic leukemia:
Pathophysiology and current therapy. Rinsho Ketsueki. 58:460–470.
2017.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
11
|
Aureli A, Marziani B, Venditti A,
Sconocchia T and Sconocchia G: Acute lymphoblastic leukemia
immunotherapy treatment: Now, next, and beyond. Cancers (Basel).
15(3346)2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lato MW, Przysucha A, Grosman S,
Zawitkowska J and Lejman M: The new therapeutic strategies in
pediatric T-cell acute lymphoblastic leukemia. Int J Mol Sci.
22(4502)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Guo XM, Fang YJ, Lv CL, Wang YR and Sun
XY: Changes of peripheral blood marrow-derived suppressor cell
level after chemotherapy induction remission by VDLP regimen and
their relationship with immune system in B-ALL children. Zhongguo
Shi Yan Xue Ye Xue Za Zhi. 25:1611–1614. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
14
|
Follini E, Marchesini M and Roti G:
Strategies to overcome resistance mechanisms in T-cell acute
lymphoblastic leukemia. Int J Mol Sci. 20(3021)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Samii B, Jafarian A, Rabbani M, Zolfaghari
B, Rahgozar S and Pouraboutaleb E: The effects of Astragalus
polysaccharides, tragacanthin, and bassorin on
methotrexate-resistant acute lymphoblastic leukemia. Res Pharm Sci.
18:381–391. 2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Inaba H and Pui CH: Glucocorticoid use in
acute lymphoblastic leukaemia. Lancet Oncol. 11:1096–1106.
2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kruth KA, Fang M, Shelton DN, Abu-Halawa
O, Mahling R, Yang H, Weissman JS, Loh ML, Müschen M, Tasian SK, et
al: Suppression of B-cell development genes is key to
glucocorticoid efficacy in treatment of acute lymphoblastic
leukemia. Blood. 129:3000–3008. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bedewy AM, El-Maghraby SM, Kandil NS and
El-Bendary WR: The prognostic value of glucocorticoid receptors for
adult acute lymphoblastic leukemia. Blood Res. 50:235–241.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xu JY and Luo JM: Association between BIM
gene and glucocorticoid resistance in children with acute
lymphoblastic leukemia. Zhongguo Dang Dai Er Ke Za Zhi. 19:945–949.
2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
20
|
Gong H, Liu L, Cui L, Ma H and Shen L:
ALKBH5-mediated m6A-demethylation of USP1 regulated T-cell acute
lymphoblastic leukemia cell glucocorticoid resistance by Aurora B.
Mol Carcinog. 60:644–657. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Yuan N, Song L, Zhang S, Lin W, Cao Y, Xu
F, Fang Y, Wang Z, Zhang H, Li X, et al: Bafilomycin A1 targets
both autophagy and apoptosis pathways in pediatric B-cell acute
lymphoblastic leukemia. Haematologica. 100:345–356. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wandler AM, Huang BJ, Craig JW, Hayes K,
Yan H, Meyer LK, Scacchetti A, Monsalve G, Dail M, Li Q, et al:
Loss of glucocorticoid receptor expression mediates in vivo
dexamethasone resistance in T-cell acute lymphoblastic leukemia.
Leukemia. 34:2025–2037. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kaveh K, Takahashi Y, Farrar MA, Storme G,
Guido M, Piepenburg J, Penning J, Foo J, Leder KZ and Hui SK:
Combination therapeutics of Nilotinib and radiation in acute
lymphoblastic leukemia as an effective method against
drug-resistance. PLoS Comput Biol. 13(e1005482)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Burgos RA, Alarcón P, Quiroga J, Manosalva
C and Hancke J: Andrographolide, an anti-inflammatory multitarget
drug: All roads lead to cellular metabolism. Molecules.
26(5)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Guo BJ, Liu Z, Ding MY, Li F, Jing M, Xu
LP, Wang YQ, Zhang ZJ, Wang Y, Wang D, et al: Andrographolide
derivative ameliorates dextran sulfate sodium-induced experimental
colitis in mice. Biochem Pharmacol. 163:416–424. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li X, Yuan K, Zhu Q, Lu Q, Jiang H, Zhu M,
Huang G and Xu A: Andrographolide ameliorates rheumatoid arthritis
by regulating the apoptosis-NETosis balance of neutrophils. Int J
Mol Sci. 20(5035)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ahmed S, Kwatra M, Ranjan Panda S, Murty
USN and Naidu VGM: Andrographolide suppresses NLRP3 inflammasome
activation in microglia through induction of parkin-mediated
mitophagy in in-vitro and in-vivo models of Parkinson disease.
Brain Behav Immun. 91:142–158. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Doi H, Matsui T, Dijkstra JM, Ogasawara A,
Higashimoto Y, Imamura S, Ohye T, Takematsu H, Katsuda I and
Akiyama H: Andrographolide, isolated from Andrographis paniculata,
induces apoptosis in monocytic leukemia and multiple myeloma cells
via augmentation of reactive oxygen species production. F1000Res.
10(542)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Latif R and Wang CY: Andrographolide as a
potent and promising antiviral agent. Chin J Nat Med. 18:760–769.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Adiguna SP, Panggabean JA, Atikana A,
Untari F, Izzati F, Bayu A, Rosyidah A, Rahmawati SI and Putra MY:
Antiviral activities of andrographolide and its derivatives:
Mechanism of action and delivery system. Pharmaceuticals (Basel).
14(1102)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang L, Bao M, Liu B, Zhao H, Zhang Y, Ji
X, Zhao N, Zhang C, He X, Yi J, et al: Effect of andrographolide
and its analogs on bacterial infection: A review. Pharmacology.
105:123–134. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kumar G, Singh D, Tali JA, Dheer D and
Shankar R: Andrographolide: Chemical modification and its effect on
biological activities. Bioorg Chem. 95(103511)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mussard E, Cesaro A, Lespessailles E,
Legrain B, Berteina-Raboin S and Toumi H: Andrographolide, a
natural antioxidant: An update. Antioxidants (Basel).
8(571)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jadhav AK and Karuppayil SM: Andrographis
paniculata (Burm. F) wall ex nees: Antiviral properties. Phytother
Res. 35:5365–5373. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tandoh A, Danquah CA, Benneh CK, Adongo
DW, Boakye-Gyasi E and Woode E: Effect of diclofenac and
andrographolide combination on carrageenan-induced paw edema and
hyperalgesia in rats. Dose Response.
20(15593258221103846)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yuwen D, Mi S, Ma Y, Guo W, Xu Q, Shen Y
and Shu Y: Andrographolide enhances cisplatin-mediated anticancer
effects in lung cancer cells through blockade of autophagy.
Anticancer Drugs. 28:967–976. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mi S, Xiang G, Yuwen D, Gao J, Guo W, Wu
X, Wu X, Sun Y, Su Y, Shen Y and Xu Q: Inhibition of autophagy by
andrographolide resensitizes cisplatin-resistant non-small cell
lung carcinoma cells via activation of the Akt/mTOR pathway.
Toxicol Appl Pharmacol. 310:78–86. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yang T, Yao S, Zhang X and Guo Y:
Andrographolide inhibits growth of human T-cell acute lymphoblastic
leukemia Jurkat cells by downregulation of PI3K/AKT and
upregulation of p38 MAPK pathways. Drug Des Devel Ther.
10:1389–1397. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kocak M, Ezazi Erdi S, Jorba G, Maestro I,
Farrés J, Kirkin V, Martinez A and Pless O: Targeting autophagy in
disease: Established and new strategies. Autophagy. 18:473–495.
2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chen Y and Gibson SB: Three dimensions of
autophagy in regulating tumor growth: Cell survival/death, cell
proliferation, and tumor dormancy. Biochim Biophys Acta Mol Basis
Dis. 1867(166265)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wei H, Wang C, Croce CM and Guan JL:
p62/SQSTM1 synergizes with autophagy for tumor growth in vivo.
Genes Dev. 28:1204–1216. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li X, He S and Ma B: Autophagy and
autophagy-related proteins in cancer. Mol Cancer.
19(12)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Amaravadi RK, Kimmelman AC and Debnath J:
Targeting autophagy in cancer: Recent advances and future
directions. Cancer Discov. 9:1167–1181. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Pugsley HR: Quantifying autophagy:
Measuring LC3 puncta and autolysosome formation in cells using
multispectral imaging flow cytometry. Methods. 112:147–156.
2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Shi B, Ma M, Zheng Y, Pan Y and Lin X:
mTOR and Beclin1: Two key autophagy-related molecules and their
roles in myocardial ischemia/reperfusion injury. J Cell Physiol.
234:12562–12568. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Nishimura T and Tooze SA: Emerging roles
of ATG proteins and membrane lipids in autophagosome formation.
Cell Discov. 6(32)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Tran S, Fairlie WD and Lee EF: BECLIN1:
Protein structure, function and regulation. Cells.
10(1522)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Dunn WA Jr: Autophagy and related
mechanisms of lysosome-mediated protein degradation. Trends Cell
Biol. 4:139–143. 1994.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Gu X, Guo W, Zhao Y, Liu G, Wu J and Chang
C: Deoxynivalenol-induced cytotoxicity and apoptosis in IPEC-J2
cells through the activation of autophagy by inhibiting
PI3K-AKT-mTOR signaling pathway. ACS Omega. 4:18478–18486.
2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Song G, Lu H, Chen F, Wang Y, Fan W, Shao
W, Lu H and Lin B: Tetrahydrocurcumin-induced autophagy via
suppression of PI3K/Akt/mTOR in non-small cell lung carcinoma
cells. Mol Med Rep. 17:5964–5969. 2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Fattahi S, Amjadi-Moheb F, Tabaripour R,
Ashrafi GH and Akhavan-Niaki H: PI3K/AKT/mTOR signaling in gastric
cancer: Epigenetics and beyond. Life Sci.
262(118513)2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Xu Z, Han X, Ou D, Liu T, Li Z, Jiang G,
Liu J and Zhang J: Targeting PI3K/AKT/mTOR-mediated autophagy for
tumor therapy. Appl Microbiol Biotechnol. 104:575–587.
2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Li X, Zhang W, Liang L, Duan X, Deng J and
Zhou Y: Natural product-derived icaritin exerts anti-glioblastoma
effects by positively modulating estrogen receptor β. Exp Ther Med.
19:2841–2850. 2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Duarte D, Falcão SI, El Mehdi I,
Vilas-Boas M and Vale N: Honeybee venom synergistically enhances
the cytotoxic effect of CNS drugs in HT-29 colon and MCF-7 breast
cancer cell lines. Pharmaceutics. 14(511)2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Feng L, Lu CK, Wu J, Chan LL and Yue J:
Identification of anhydrodebromoaplysiatoxin as a dichotomic
autophagy inhibitor. Mar Drugs. 21(46)2023.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Pierro J, Hogan LE, Bhatla T and Carroll
WL: New targeted therapies for relapsed pediatric acute
lymphoblastic leukemia. Expert Rev Anticancer Ther. 17:725–736.
2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Habiel DM, Krepostman N, Lilly M,
Cavassani K, Coelho AL, Shibata T, Elenitoba-Johnson K and Hogaboam
CM: Senescent stromal cell-induced divergence and therapeutic
resistance in T cell acute lymphoblastic leukemia/lymphoma.
Oncotarget. 7:83514–83529. 2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Rose-James A, Shiji R, Kusumakumary P,
Nair M, George SK and Sreelekha TT: Profiling gene mutations,
translocations, and multidrug resistance in pediatric acute
lymphoblastic leukemia: A step forward to personalizing medicine.
Med Oncol. 33(98)2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Palmer AC, Chidley C and Sorger PK: A
curative combination cancer therapy achieves high fractional cell
killing through low cross-resistance and drug additivity. Elife.
8(e50036)2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Jaaks P, Coker EA, Vis DJ, Edwards O,
Carpenter EF, Leto SM, Dwane L, Sassi F, Lightfoot H, Barthorpe S,
et al: Effective drug combinations in breast, colon and pancreatic
cancer cells. Nature. 603:166–173. 2022.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Pemovska T, Bigenzahn JW and Superti-Furga
G: Recent advances in combinatorial drug screening and synergy
scoring. Curr Opin Pharmacol. 42:102–110. 2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Li YJ, Lei YH, Yao N, Wang CR, Hu N, Ye
WC, Zhang DM and Chen ZS: Autophagy and multidrug resistance in
cancer. Chin J Cancer. 36(52)2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Sarang Z, Gyurina K, Scholtz B, Kiss C and
Szegedi I: Altered expression of autophagy-related genes might
contribute to glucocorticoid resistance in precursor B-cell-type
acute lymphoblastic leukemia. Eur J Haematol. 97:453–460.
2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Li Y, Qu M, Xing F, Li H, Cheng D, Xing N
and Zhang W: The protective mechanism of dexmedetomidine in
regulating Atg14L-Beclin1-Vps34 complex against myocardial
ischemia-reperfusion injury. J Cardiovasc Transl Res. 14:1063–1074.
2021.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Wu S, He Y, Qiu X, Yang W, Liu W, Li X, Li
Y, Shen HM, Wang R, Yue Z and Zhao Y: Targeting the potent Beclin
1-UVRAG coiled-coil interaction with designed peptides enhances
autophagy and endolysosomal trafficking. Proc Natl Acad Sci USA.
115:E5669–E5678. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Wu W, Wang X, Sun Y, Berleth N, Deitersen
J, Schlutermann D, Stuhldreier F, Wallot-Hieke N, José Mendiburo M,
Cox J, et al: TNF-induced necroptosis initiates early autophagy
events via RIPK3-dependent AMPK activation, but inhibits late
autophagy. Autophagy. 17:3992–4009. 2021.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Nakahira K, Pabon Porras MA and Choi AM:
Autophagy in pulmonary diseases. Am J Respir Crit Care Med.
194:1196–1207. 2016.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Wesch N, Kirkin V and Rogov VV:
Atg8-family proteins-structural features and molecular interactions
in autophagy and beyond. Cells. 9(2008)2020.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Prieto-Domínguez N, Ordóñez R, Fernández
A, García-Palomo A, Muntané J, González-Gallego J and Mauriz JL:
Modulation of autophagy by sorafenib: Effects on treatment
response. Front Pharmacol. 7(151)2016.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Yang G, Li Z, Dong L and Zhou F: lncRNA
ADAMTS9-AS1 promotes bladder cancer cell invasion, migration, and
inhibits apoptosis and autophagy through PI3K/AKT/mTOR signaling
pathway. Int J Biochem Cell Biol. 140(106069)2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Petrulea MS, Plantinga TS, Smit JW,
Georgescu CE and Netea-Maier RT: PI3K/Akt/mTOR: A promising
therapeutic target for non-medullary thyroid carcinoma. Cancer
Treat Rev. 41:707–713. 2015.PubMed/NCBI View Article : Google Scholar
|