Introduction
Parkinson's disease (PD) is the second most common
type of neurodegenerative disorder of aging, and it is
characterized by selective and progressive loss of dopaminergic
neurons in the substantia nigra pars compacta. While the etiology
of dopaminergic neuronal loss remains unclear, several mechanisms
have been suggested to be involved in the pathogenesis of PD, such
as abnormal protein aggregation, mitochondrial dysfunction and
oxidative stress (1). Together,
these mechanisms are thought to be responsible for dopaminergic
neurodegeneration via the induction of apoptosis.
1-Methyl-4-phenylpyridinium (MPP+) is the
active metabolite of 1-methyl-4-phenyl-2,3,6-tetrahydropyridine,
and is widely used in rodent and cellular models to elicit
PD-associated neurochemical alterations. MPP+ causes
apoptosis by inhibiting complex I of the mitochondrial electron
transport chain (1-3).
Notably, MPP+ can directly induce neuronal apoptosis
(3). Numerous genes and their
proteins are responsible for the progression of apoptosis; for
example, within the Bcl-2 family, Bcl-2 is anti-apoptotic, whereas
Bax is pro-apoptotic. MPP+-mediated cell death has been
reported to be attenuated by Bcl-2 but is not affected by Bax
(4). Glycogen synthase kinase-3β
(GSK-3β) is involved in MPP+-induced dopaminergic cell
death (5). A previous study showed
that MPP+ treatment causes cell death via the time- and
concentration-dependent activation of GSK-3β, as verified by
upregulation of the active form of this kinase, phosphorylated
(p)-GSK-3β at tyrosine 216(6).
Furthermore, GSK-3β inhibition significantly decreases
MPP+-induced neuron injury, ameliorates behavioral
impairments caused by MPP+ and is a therapeutic target
for PD (5).
Celastrus paniculatus (CP) Willd., belongs to
the Celastraceae family (7,8), and is primarily distributed in
tropical, subtropical and temperate zones of Asia. CP Willd. is
indigenous to India but also grows in Australia, China, Malaysia,
Cambodia, Taiwan, Indonesia, Laos, Myanmar and Thailand (9). Therefore, CP Willd. is not an
endangered species of plant. CP seeds have been reported to exert
antioxidant and neuroprotective activities (8,9). CP
seeds and organic extracts can attenuate hydrogen peroxide- and
glutamate-induced injury in embryonic rat neuronal cells (10). Moreover, the aqueous (A) extract of
CP seeds has been reported to significantly decrease brain levels
of malondialdehyde and significantly increase levels of glutathione
and catalase. The aforementioned findings demonstrated that the A
extract of CP seeds has an antioxidant effect on the brain
(11). The methanolic extract of CP
seeds has been shown to possess free radical-scavenging effects and
can reduce hydrogen peroxide-induced cytotoxicity and DNA damage in
human non-immortalized fibroblasts (12). Moreover, in vivo study has
revealed that solvent extracts of the seeds can markedly increase
and restore levels of antioxidant enzymes in acute and chronic
immobilized stress mice (13). The
antioxidant and anti-apoptotic activities of CP seed extract (CPSE)
have been assessed in tertiary butyl hydroperoxide-induced muscle
cell damage (14). Furthermore, CP
exhibits anti-apoptotic effects in murine C2C12 myoblasts by
regulating the expression levels of cytochrome c and heat
shock protein 70(14). However, the
underlying mechanisms by which CP mediates apoptotic inhibition in
neuronal cells in PD is unclear. Thus, in the present study,
MPP+ was used to induce cell death in SH-SY5Y neuronal
cells and the protective effect of CPSE on MPP+-induced
damage was investigated to provide novel insights into the
mechanism by which it prevents apoptosis.
Materials and methods
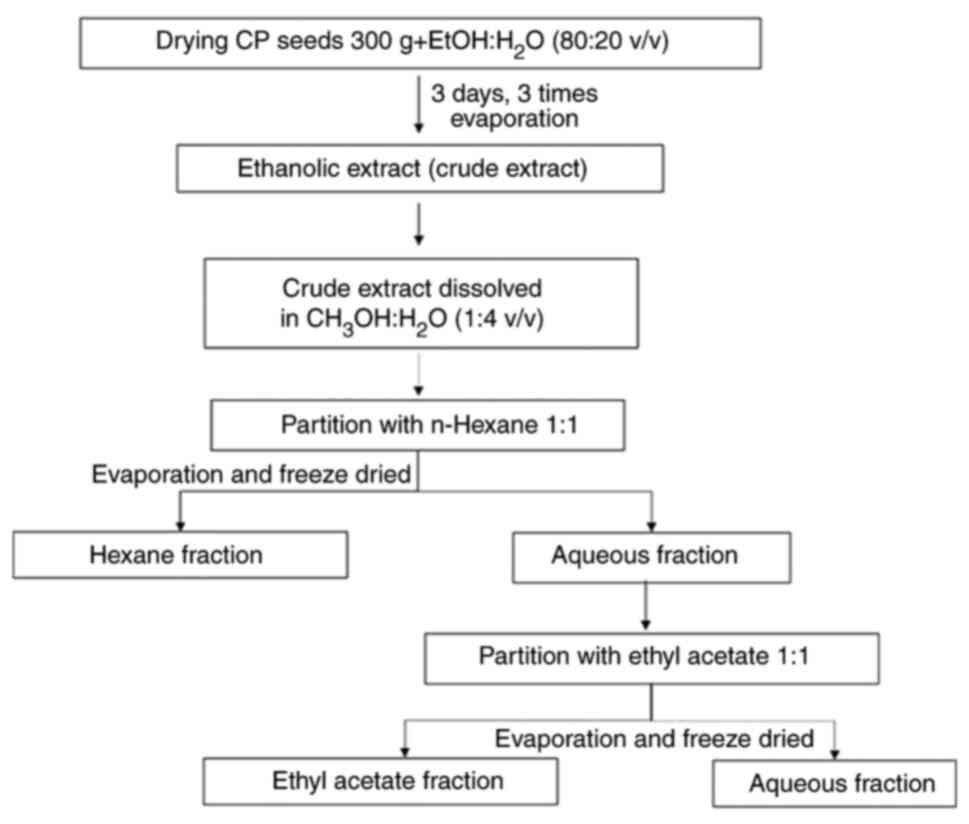
CPSE preparation
CP seeds were kindly provided by Queen Sirikit
Botanic Garden, Botanical Garden Organization of the Ministry of
Natural Resources and Environment (Chiang Mai, Thailand). Dried
plant material was ground into powder using a mill. The extraction
process was conducted according to the maceration method, where CP
seeds (300 g) were macerated with 95% ethanol at room temperature
for 72 h. Thereafter, the filtrate was separated from the residue.
This was repeated three times. The filtrate was combined and
evaporated for 16 h under reduced pressure at 50˚C to obtain the
crude ethanolic extract.
A part of the crude extract was dissolved in
methanol and water (1:4 v/v). The extract solution was inserted
into a separating funnel and n-hexane (H) solvent was then added
with a volume ratio of 1:1 and shaken until the two solutions were
mixed into one phase. When A and H phase were separated, and the H
fraction was placed in a glass beaker. This process was repeated
three times until the H fraction was almost clear. Fractionation
was continued via the addition of ethyl acetate (EA) into the A
fraction using a separating funnel (100 ml or in a 1:1 ratio). The
same procedure was performed as in the H fraction. Then, each
fraction was evaporated until dry using a rotary evaporator to
obtain the H, EA and A fractions (Fig.
1). All extracts were refrigerated at 4˚C until further
use.
Assessment of total phenolic
content
The total phenolic content of extract was determined
according to the method reported by Khongkaew and Chaemsawang
(15). The phenolic component was
obtained from CPSE: 25 µl Folin-Ciocalteu reagent was added to
cause a reaction with the phenolic compound and then incubated at
room temperature in a dark room for 6 min. Then, 100 µl 7.5% sodium
carbonate was added to each well and incubated at room temperature
for 90 min in the dark. Light absorption was analyzed at 765 nm
using a microplate reader to calculate the total phenolic compound
content in the extract and to compare it with the standard gallic
acid. All samples were subtracted from the absorbance of the blank
sample. The result was reported as µg gallic acid equivalent/mg
extract.
Determination of the free
radical-scavenging activity of 2,2-diphenyl-1-picrylhydrazyl
(DPPH)
The antioxidant activity in terms of half-maximal
inhibitory concentration (IC50), CPSE antioxidant capacity was
measured on the basis of DPPH• scavenging. A 0.1 mM DPPH solution
was prepared using 1.97 mg DPPH in 50 ml methanol The CPSE (100 µl)
was added into 96-well plates with DPPH and incubated at room
temperature in the dark for 30 min, after which, the wavelength was
determined at 520 nm using a microplate reader. If the extract
contains antioxidants, the purple color will change to yellow.
Inhibition was calculated using the following equation: Inhibition
(%)=[(absorbance of control-absorbance of sample)/absorbance of
control) x100.
Determination of the free
radical-scavenging activity of 2,2'-Azino-bis
(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS)
ABTS solution was mixed with potassium persulfate
together in a 1:1 ratio and then incubated in the dark for 16 h at
room temperature. Then, the crude extract, hexane fraction, ethyl
acetate fraction and water fraction were taken and dissolved in
methanol to a concentration of 1,000, 500, 300, 100 µg/ml.
Subsequently, a volume of 100 µl/well was pipetted into a 96-well
plate and 100 µl ABTS solution was added, and then incubated in the
dark for 6 min at room temperature. The absorbance was then
calculated at a wavelength of 734 nm. Inhibition was calculated
using the following equation: Inhibition (%)=[(absorbance of
control-absorbance of sample)/absorbance of control) x100.
SH-SY5Y cell culture
The SH-SY5Y neuroblastoma cell line was purchased
from American Type Culture Collection. The cells were maintained in
a 1:1 mixture of modified Eagle medium and Ham's F-12 nutrient
mixture with 10% fetal bovine serum (Thermo Fisher Scientific,
Inc.) at 37˚C in a humidified environment containing 5%
CO2. The culture medium was changed every 3-4 days.
SH-SY5Y cells were grown in a 6-well plate. CPSE was dissolved in
dimethyl sulfoxide (DMSO) to a concentration of 100 µg/ml as a
stock solution. The stock solution was diluted with complete medium
before use. For pre-treatment, CPSE (10 µg/ml) was added to SH-SY5Y
cells. Then, cells were maintained in a humidified 5%
CO2 atmosphere at 37˚C for 24 h. After washing with 1X
PBS, 2 mM MPP+ was added into the culture plate and
incubated at room temperature for 3 h. In the CPSE post-treatment
group, MPP+ was added 3 h before CPSE treatment.
Cell viability assay
Cell viability was assessed by MTT assay, which is
based on the cleavage of tetrazolium salts by mitochondrial
succinate reductase in viable cells to form formazan dye. SH-SY5Y
cells were cultured in 96-well plates at a density of
1x104 cell/ml for 24 h. Following the aforementioned
treatments, MTT (0.5 mg/ml) was added to each well and incubated
for 3 h at 37˚C. The formazan crystals formed were then dissolved
in DMSO. The absorbance was measured at 540 nm using a microplate
reader and cell viability was expressed as the percentage of
control.
Lactate dehydrogenase (LDH) leakage
assay
LDH activities were measured spectrophotometrically
by a CyQUANT™ LDH Cytotoxicity Assay (cat. no. C20300,
Invitrogen™), using pyruvate as substrate, at 340 nm. Following the
aforementioned treatments, an aliquot of the culture medium was
taken to determine the activity of LDH that leaked through the cell
membranes. Data are presented as the percentage of LDH leakage
relative to the control. Four independent assays were conducted in
triplicate.
WST-1 assay
Following 24 h treatment with all CP extract, cell
vitality was measured quantitatively using the reagent WST-1. Cells
were incubated with the reagent for 4 h at 37˚C. The absorption of
the color product was measured at a 450/620 nm wavelength with a
microplate reader. Each condition was tested in duplicate.
Western blotting
SH-SY5Y cells were harvested following the
aforementioned treatments and lysed using Pierce® RIPA
Buffer (Thermo Fisher Scientific, Inc.). For measuring the
concentrations of proteins, Bradford protein assay is used.
Subsequently, equal amounts of protein (30 µg) were separated by
sodium dodecyl-sulfate polyacrylamide gel electrophoresis on 10%
gels and transferred to nitrocellulose membranes. The membrane were
blocked with 5% skim milk in TBST for 1 h at room temperature. The
membranes were incubated with primary antibodies at a dilution of
1:1,000 as follows: Mouse anti-p-GSK-3β (Ser9; cat. No. 05-643),
anti-GSK-3β (cat. No. G7914), anti-Bcl-2 (Cat. No. SAB4500003) and
anti-β-actin (Cat. No. A2228) antibodies (all Sigma-Aldrich) for 24
h at 4˚C. Membranes were then probed with horseradish
peroxidase-conjugated secondary antibodies (Cat. No. sc-2357; Santa
Cruz Biotechnology, Inc.) at a dilution of 1:5,000 for 3 h at 4˚C
and detected with Thermo Scientific SuperSignal® West
Pico Substrate (Thermo Fisher Scientific, Inc.). Densitometric
analysis was performed using the ImageJ analysis software version
1.48p (ImageJ, NIH, Bethesda, Maryland, USA) and results were
normalized to β-actin.
Statistical analysis
All experiments were performed in triplicate. Data
are presented as the mean ± SD. Statistical significance was
analyzed with one-way analysis of variance followed by Tukey's post
hoc test. All data were analyzed by GraphPad Prism version 7.0
(GraphPad Software Inc.; Dotmatics). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of CPSE and its fractions on
SH-SY5Y cell viability
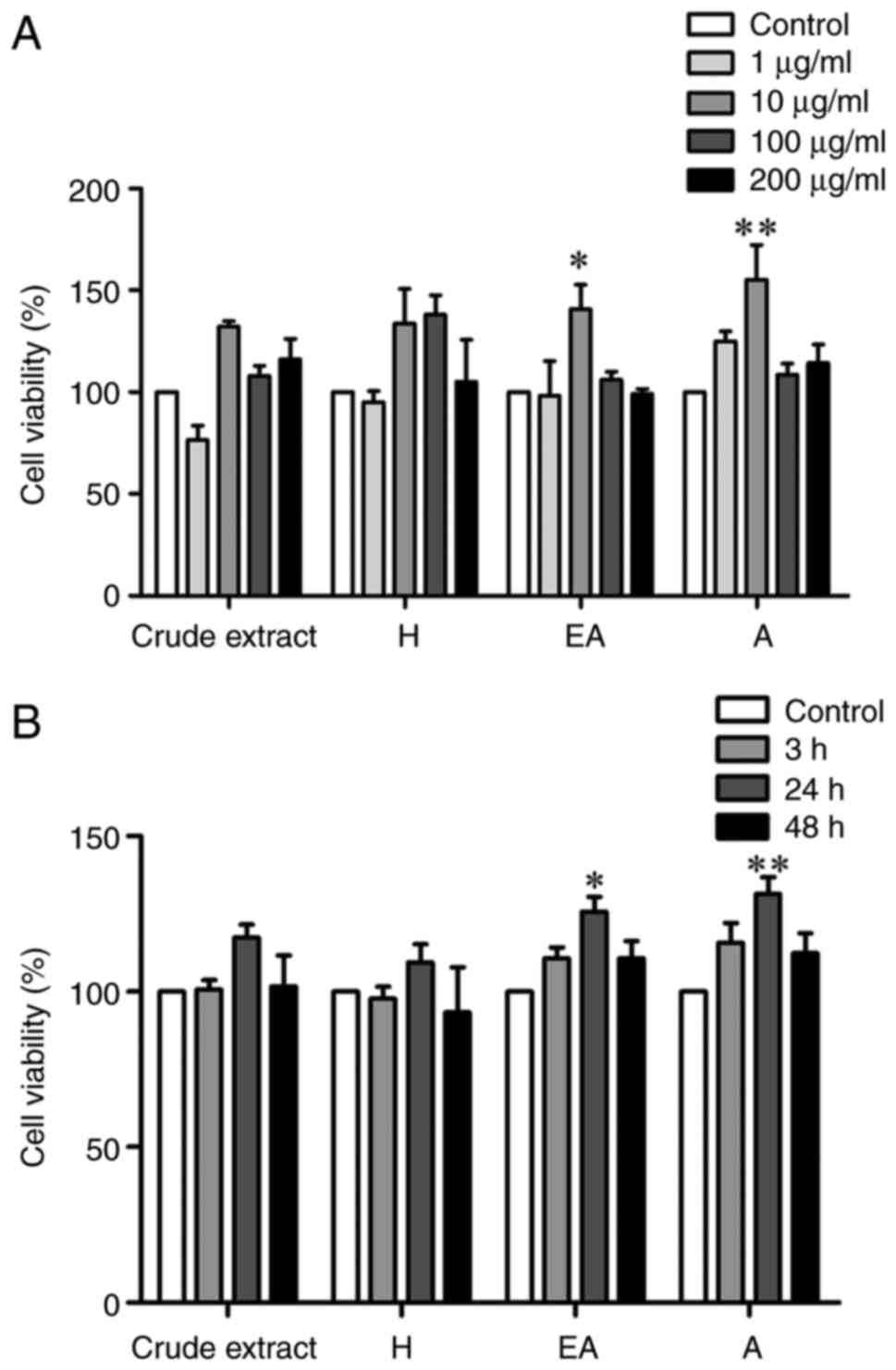
The present study determined the effect of different
concentrations of CPSE and its fractions including H, EA, and A on
SH-SY5Y cell viability. Cell viability was significantly increased
following treatment with 10 µg/ml EA and A fractions but not in
response to the crude extract or H fraction (Fig. 2A). In addition, treatment with EA
and A fractions for 24 h significantly increased the viability of
SH-SY5Y cells (Fig. 2B).
High concentrations of all CP fractions (200 µg/ml)
and long-term CP treatment (48 h) did not affect cell viability.
Viability was highest following treatment with all fractions 10
µg/ml for 24 h; therefore, this treatment condition was selected
for further experiments.
Total phenolic content of CPSE and its
fractions
The total phenolic content of CPSE and its fractions
was quantified (Table I). The A
fraction presented the highest levels of individual phenolic
compounds (30.20 µg/100 g), followed by EA (21.40 µg/100 g) and H
fractions (8.83 µg/100 g). The crude extract had a low phenolic
content (3.01 µg/100 g).
 | Table ITotal phenolic content of
Celastrus paniculatus seed fractions extracted with hexane,
ethyl acetate and water. |
Table I
Total phenolic content of
Celastrus paniculatus seed fractions extracted with hexane,
ethyl acetate and water.
| Sample | GAE/mg extract,
µg |
|---|
| Crude extract | 3.01±1.09 |
| N-hexane
fraction | 8.83±0.54 |
| Ethyl acetate
fraction |
21.40±2.00a |
| Aqueous
fraction |
30.20±4.30b |
Antioxidant activity of CPSE and its
fractions
The antioxidative mechanisms of CPSE and its
fractions were assessed using ABTS and DPPH assays (Table II). In the EA fraction,
half-maximal inhibitory concentration (IC50) values tested by ABTS
and DPPH were 492.11±43.92 and 1462.84±233.20 µg/ml, respectively;
in the A fraction, the IC50 values were 393.35±32.53 and
719.17±107.50 µg/ml, respectively. In the crude extract and H
fraction, higher IC50 values indicated low antioxidant
capacity.
 | Table IIAntioxidant activity of Celastrus
paniculatus seed extracts assessed by ABTS and DPPH assays. |
Table II
Antioxidant activity of Celastrus
paniculatus seed extracts assessed by ABTS and DPPH assays.
| Sample | IC50 for ABTS
(µg/ml) | IC50 for DPPH
(µg/ml) |
|---|
| Crude extract | 2084.68±167.20 | 7221.86±627.00 |
| N-hexane
fraction | 1278.65±102.90 | 9012.25±599.10 |
| Ethyl acetate
fraction |
492.11±43.92a |
1462.84±233.20a |
| Aqueous
fraction |
393.35±32.53b |
719.17±107.50b |
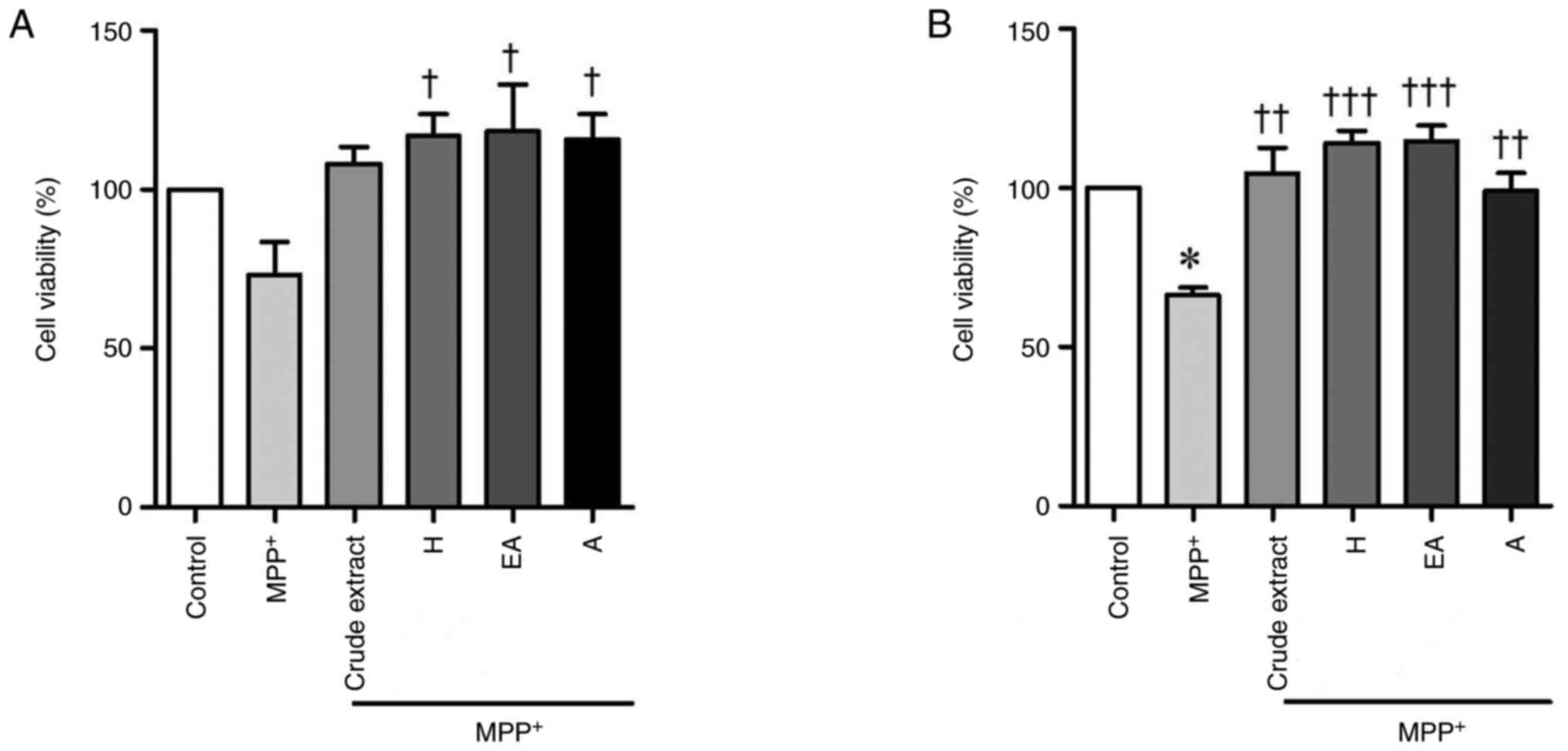
CPSE and its fractions decrease
MPP+-treated SH-SY5Y cell death
Oxidative stress and activation of the apoptotic
cascade serve central roles in the pathogenesis of PD (1). Thus, to investigate the potential
effect of CPSE and its fractions on MPP+-induced SH-SY5Y
cell injury, SH-SY5Y cells treated with crude extract, H, EA or A
fractions were stimulated with 2 mM MPP+ for 3 h before
or after CPSE treatment, followed by the MTT assay (Fig. 3A and B). Treatment with 2 mM MPP+
resulted in decreased viability of SH-SY5Y cells, whereas
treatments with all types of CPSE did not significantly affect the
viability of SH-SY5Y cells compared with control. Both CPSE pre-
and post-treatment reversed the MPP+-induced reduction
of SH-SY5Y cell viability, especially H, EA or A fractions.
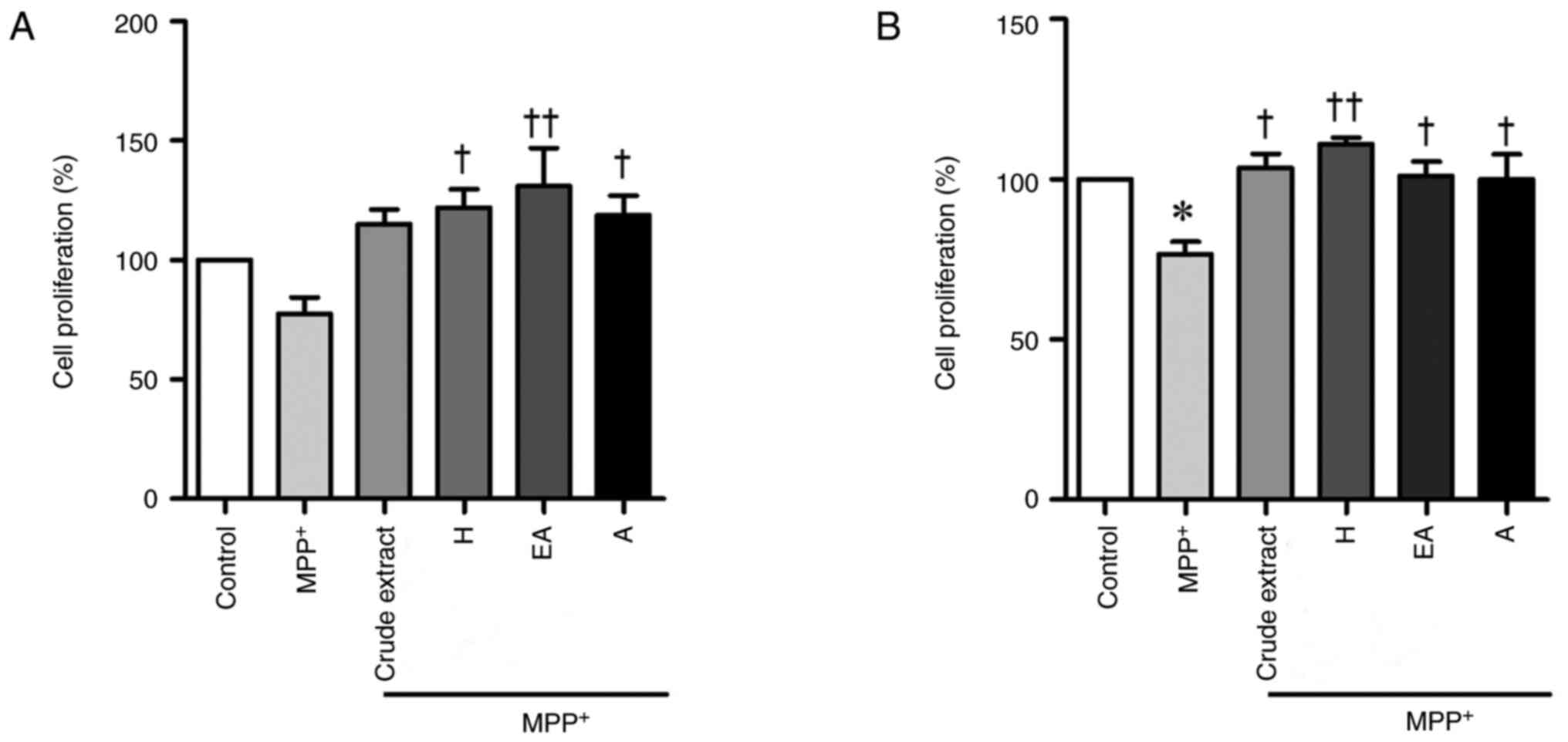
CPSE and its fractions increase the
proliferation of MPP+-induced SH-SY5Y cells
The balance between apoptosis and proliferation is
key to maintaining normal cell activity. Cell proliferation was
evaluated by WST-1 assay. Similar to the MTT assay, pre-treatment
with H, EA and A fractions significantly increased the
proliferation of MPP+-treated cells. (Fig. 4A). Post-treatment with all CPSE
fractions increased the proliferation of MPP+-treated
cells (Fig. 4B). This confirmed the
significant neuroprotective effect of CPSE when administered either
before or after MPP+ treatment.
CPSE and its fractions alleviate
MPP+-induced SH-SY5Y cell damage
To explore the neuroprotective effect of CPSE on
MPP+-induced cell injury, SH-SY5Y cells were treated
with CPSE 24 h before MPP+ treatment or after 3 h of
MPP+ treatment. LDH is rapidly released into the cell
culture supernatant when the plasma membrane is damaged, a key
feature of cells undergoing apoptosis (14). MPP+ treatment
significantly increased cytoplasmic LDH release; this was partially
restored by CPSE and its fractions (Fig. 5). Therefore, both pre- and
post-treatment of CPSE alleviated MPP+-induced SH-SY5Y
cell damage. However, post-treatment with CPSE and H fraction did
not significantly affect LDH levels (Fig. 5B).
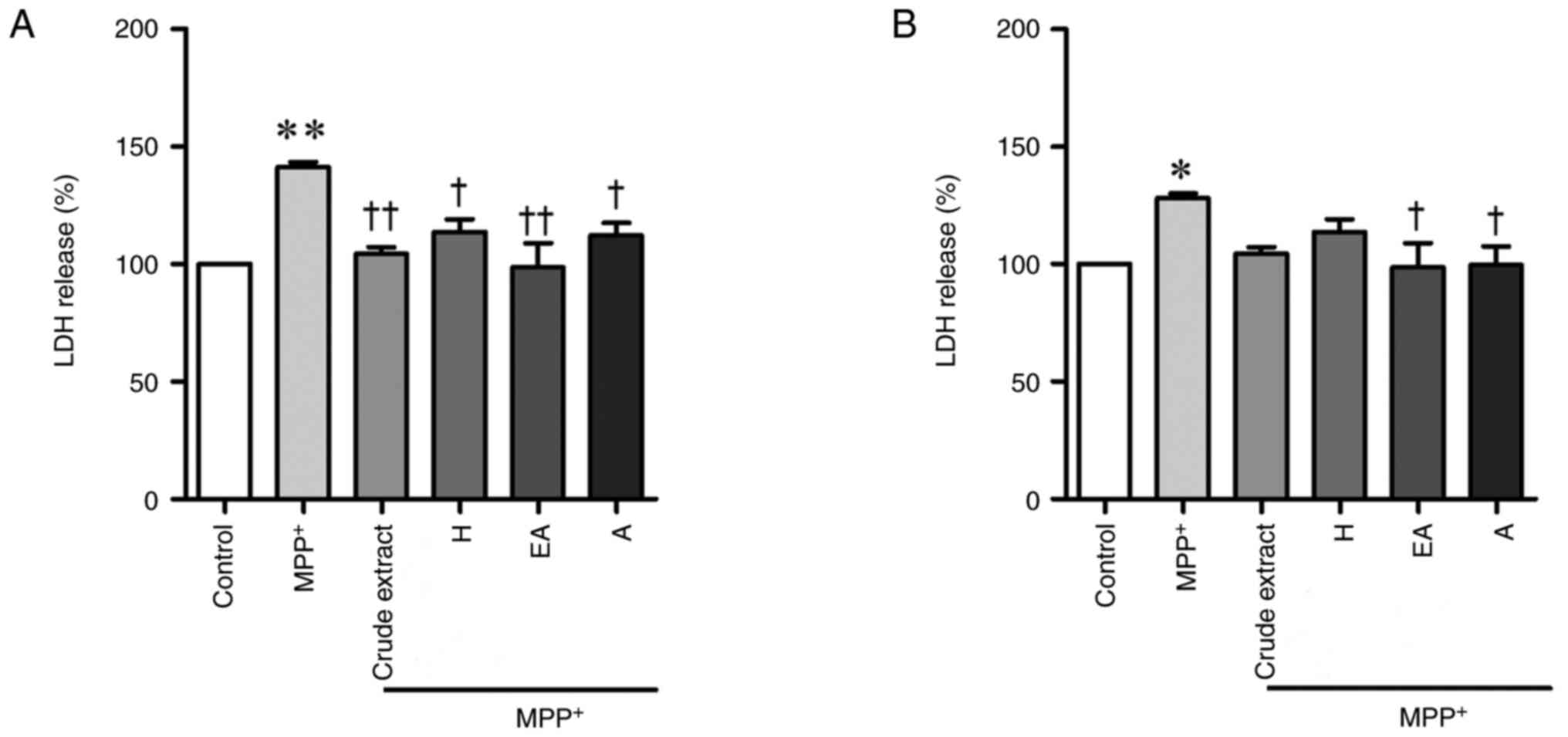 | Figure 5Neuroprotective effects of CPSE and
its fractions on MPP+-induced SH-SY5Y cell death, as
determined by the LDH assay. Cells were exposed to 2 mM
MPP+ alone, or (A) after or (B) before treatment with
CPSE. Data are presented as the mean ± standard error of the mean.
*P<0.05, **P<0.01 vs. control;
†P<0.05, ††P<0.01 vs. MPP+.
CPSE, Celastrus paniculatus seed extract; H, n-hexane; A,
aqueous; EA, ethyl acetate; MPP+,
1-methyl-4-phenylpyridinium ion; LDH, lactate dehydrogenase. |
CPSE and its fractions attenuate
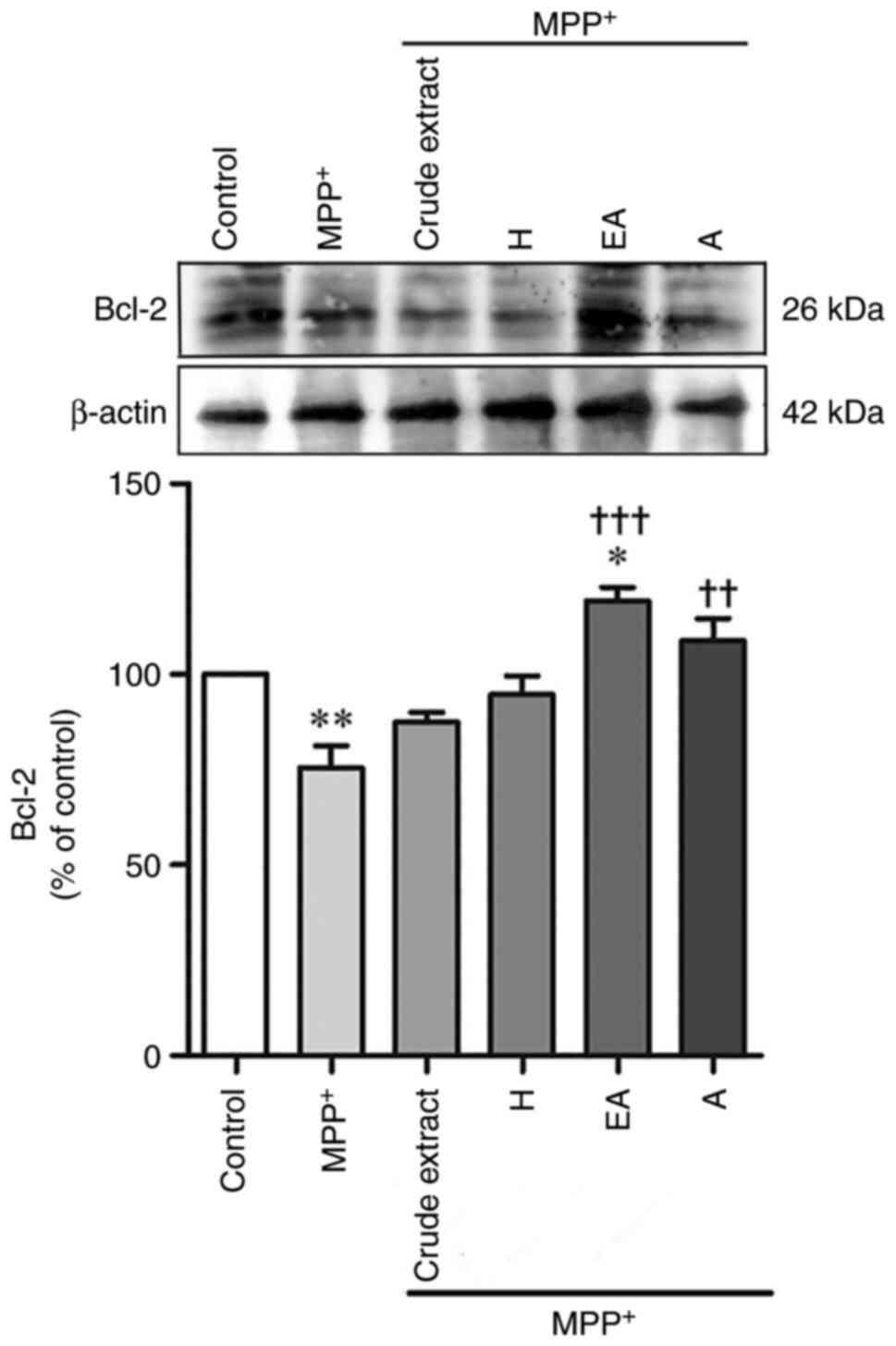
MPP+-decreased Bcl-2 protein expression
The expression levels of anti-apoptotic proteins
following MPP+ treatment with CPSE post-treatment were
examined by western blot analysis. SH-SY5Y cells treated with
MPP+ had decreased levels of the anti-apoptotic protein
Bcl-2 (63.86±4.2%) compared with control; however, the protein
expression levels of Bcl-2 were significantly increased in the EA-
and A-treated groups (Fig. 6). The
expression levels of Bcl-2 indicate the susceptibility of cells to
apoptosis (4,16). These data suggested that
MPP+ can induce the apoptosis of SH-SY5Y cells and CPSE
treatment can protect SH-SY5Y cells by upregulating Bcl-2
expression.
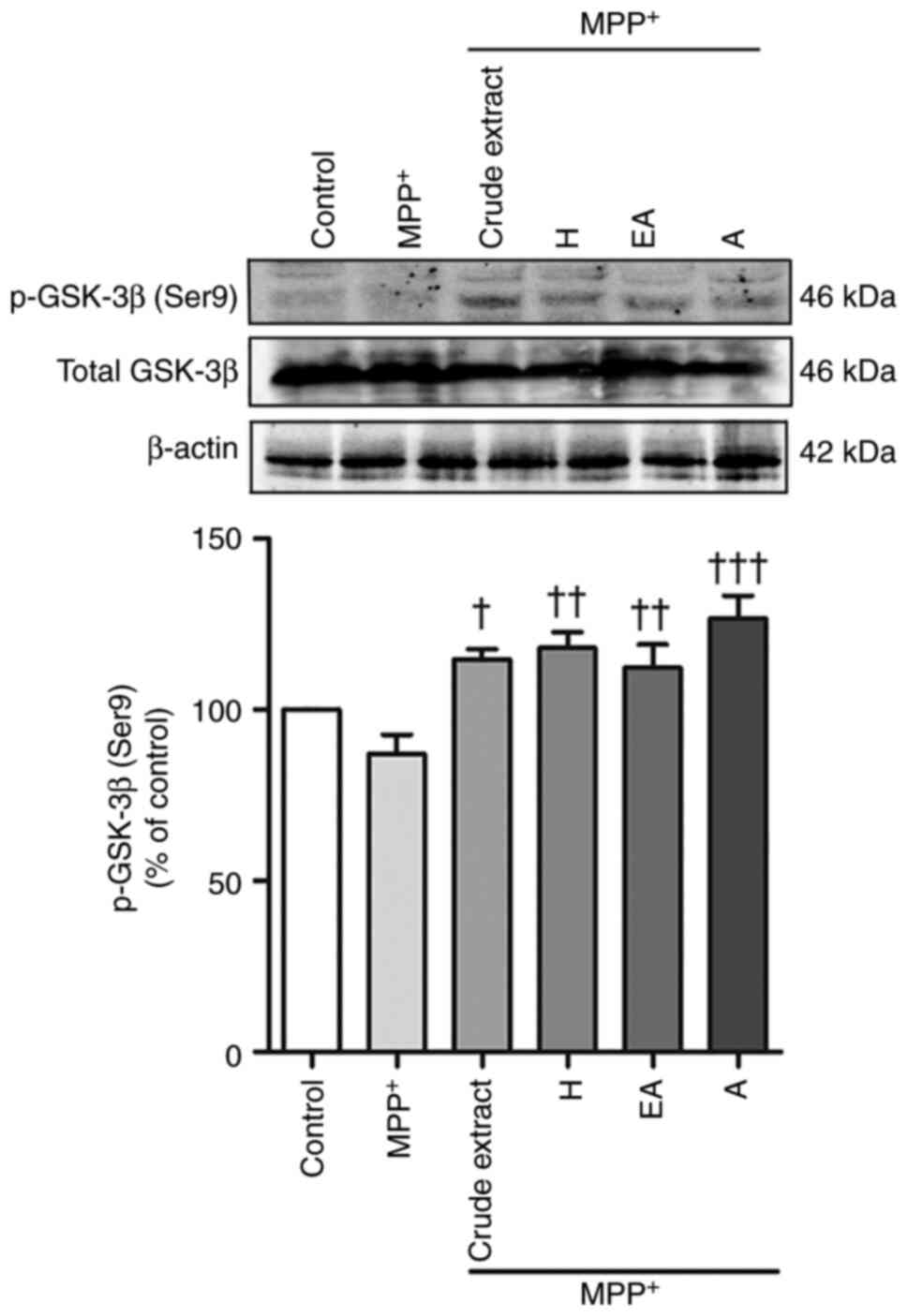
Neuroprotective effects of CPSE and
its fractions are involved in p-GSK-3β (Ser9) expression
Since a previous study reported that GSK-3β is
involved in MPP+-induced SH-SY5Y cells, the present
study investigated the expression levels of this protein (5). The results showed that MPP+
treatment reduced expression (but not but not statistically
significant) of p-GSK-3β (Ser9), whereas post-treatment with crude
extract, H, EA and A fractions significantly increased the
expression levels of p-GSK-3β (Ser9) compared with those in the
MPP+-treated group (Fig.
7). This phosphoserine exerts an inhibitory effect on the
kinase by blocking the catalytic site; therefore, low p-GSK-3β
(Ser9) levels can indicate increased kinase activity (6).
Discussion
The present study showed the effect of CPSE on cell
viability according to the MTT assay. Treatment with 10 µg/ml EA or
A fraction for 24 h effectively increased the viability of SH-SY5Y
cells. Furthermore, the A fraction had the highest total phenolic
content and free radical-scavenging activities. In addition, H, EA
and A fractions consistently attenuated MPP+-induced
toxicity both pre- and post-treatment according to the MTT, WST-1
and LDH assays. Post-treatment with CPSE could inhibit
MPP+-induced cell death via the induction of the
anti-apoptotic protein Bcl-2, and Bcl-2 increased with the
expression of p-GSK-3β (Ser9) measured in SH-SY5Y cells. However,
the present study lacked verification in another neuronal cell line
or animal models. Therefore, MPP+-induced PD rat models
should be further used.
Despite several reports regarding the
neuroprotective effect of CP seeds on damage induced by several
toxins, including N-methyl-D-aspartate (17), 3-nitropropionic acid and
MPP+ (18), the
mechanism by which CP affects neurons exposed to MPP+ is
unknown. MPP+ inhibits complex I activity of the
mitochondrial transport chain, and it enters dopaminergic neurons
via dopamine transporters and is transported into the mitochondria
according to the mitochondrial membrane potential (2). Since MPP+ treatment induces
selective dopaminergic neuronal degeneration, this agent is
commonly used to study the pathogenesis of PD (4,6). In a
recent study, overnight treatment with 9 µg/ml CPSE yielded a
significant neuroprotective effect against MPP+-induced
dopaminergic degeneration in a Caenorhabditis elegans model
of PD (18). Moreover, a previous
study showed that CPSE decreases the intraneuronal Ca2+
concentration in CP-treated mice. Furthermore, CPSE treatment
ameliorates the expression of Bax, Bcl-2 and caspase-3 in the brain
tissue of glutamate-induced brain-injured mice (16). Here, 24 h treatment with 10 µg/ml
CPSE exerted a significant anti-apoptotic effect by increasing the
expression levels of Bcl-2. Therefore, CPSE treatment may attenuate
MPP+-induced neuronal apoptosis by increasing the
expression levels of the anti-apoptotic protein Bcl-2. These
findings indicated that CPSE may attenuate MPP+-induced
neuronal toxicity in a cell model of PD, which may be associated
with inhibition of apoptosis. Notably, mitochondrial dysfunction
serves a central role in the pathogenesis of PD; however, the
present study did not directly explore mitochondrial function.
The PI3K/Akt signaling pathway is significantly
associated with neuronal survival/degeneration. Inactivation of Akt
triggers GSK-3β activity by decreasing its phosphorylation at
serine 9, which serves a key role in the neuronal loss that occurs
in neurodegenerative diseases, including PD (5). MPP+ has been reported to
induce GSK-3β-dependent neurodegeneration in cells, including
SH-ST5Y cells, thus suggesting that GSK-3β may be considered a key
mediator of neuronal death (6). In
the present study, MPP+ treatment reduced the expression
levels of p-GSK-3β (Ser9). Moreover, this result was consistent
with previous evidence that CPSE treatment inhibits the occurrence
of lead acetate-induced degenerative changes in the renal tubules
with significantly increased PI3K and AKT mRNA expression levels in
CP-treated rats (19) This previous
finding indicated that CPSE may inhibit lead acetate-induced
detrimental changes in the kidneys by regulating the PI3K/AKT
signaling pathway (19). Since the
phosphorylation status of GSK-3β is primarily regulated by AKT, and
various stimulating factors activate the PI3K/AKT pathway (5), the present study suggested that CPSE
inhibited the MPP+-induced model of PD in SH-SY5Y cells
by regulating PI3K/AKT/GSK-3β signaling.
To the best of our knowledge, the present study is
the first to provide the therapeutic potential of three fractions
extracted from CP seed, as H, EA and A. The A fraction of CPSE had
the highest antioxidant capacity, whereas the crude extract had low
phenolic content, since the crude extract contains compounds other
than phenolic compounds, such as fat, which is commonly found in
plant seeds (20). This finding
information supports the idea that phenolic compounds are soluble
in polar or semipolar solvents such as water, ethanol, or EA
(21). Accordingly, compounds
mostly found in the A layer were low molecular weight phenolics,
such as gallic acid and flavonoids (21). Similarly, a previous study using
okra seeds found significant levels of flavonoid glycosides, such
as high-polar isoquercitrin (22).
According to a study by Popoola (23) on the extraction of Nigerian
Amaranthus viridis seeds, in addition to oils, phenolics and
flavonoids, such as vanillic and caffeic acid, quercetin
diglycoside and kaempferol diglycoside were found. This is
consistent with the present phenolic content analysis because
phenolics and flavonoids are reported to have good antioxidant
properties (21). As the phenolic
content in the A fraction in the present study was high, its
antioxidation potential is high. Antioxidant capacity detected by
ABTS assay has been reported to be significantly higher for a
variety of foods compared with that of the DPPH assay, partly
because the highly pigmented and hydrophilic antioxidants are
better reflected by the ABTS assay than the DPPH assay (24). Recently, a study showed that the
active metabolites of CP comprise dihydro-β-agarofuran
sesquiterpenoids, characterized by a tricyclic architecture
comprising trans-decalin and tetrahydrofuran rings and oxygen
functionalities (25). The
biological activity of dihydro-β-agarofuran sesquiterpenoids
includes multidrug resistance reversal activity, cytotoxicity,
antitumor activity and insecticidal activity (26). Dihydro-β-agarofuran sesquiterpenoids
have been evaluated for their in vitro cytotoxic activity
against human cancer cell lines, including HepG2 and MCF-7
(26,27). However, further studies on normal
cells are needed to confirm the neuroprotective effects of
dihydro-β-agarofuran sesquiterpenoids.
In conclusion, the present results suggested that
CPSE exhibited neuroprotective effects on an
MPP+-induced cell model of PD. The neuroprotective
effect of CPSE may be due to its stimulatory actions on the
upregulation of Bcl-2 and p-GSK-3β (Ser9) expression levels, and
improvement of mitochondrial function. Therefore, CPSE may serve as
a preventative and therapeutic agent for PD, and its application in
animal models of PD needs further exploration.
Acknowledgements
The authors would like to thank Queen Sirikit
Botanic Garden, Botanical Garden Organization of the Ministry of
Natural Resources and Environment, (Chiang Mai, Thailand) for
kindly providing the Celastrus paniculatus Willd. seeds.
Funding
Funding: The present study was supported by the Burapha
University and Thailand Science Research and Innovation (grant no.
47/2565), the Burapha University through the National Research
Council of Thailand (grant no. 16/2562) and the Faculty of Allied
Health Sciences, Burapha University (grant nos. AHS3/2562 and
AHS04/2565).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TP and SC conceived the study. TP performed the
experiments. WC, NT and AA verified the statistical analysis. All
authors discussed the results and commented on the manuscript. SC
wrote the manuscript. All authors have read and approved the final
manuscript. TP and SC confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lin MT and Beal MF: Mitochondrial
dysfunction and oxidative stress in neurodegenerative diseases.
Nature. 443:787–795. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Singer TP and Ramsay RR: Mechanism of the
neurotoxicity of MPTP. An update. FEBS Lett. 274:1–8.
1990.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Enogieru AB, Haylett W, Hiss DC and Ekpo
OE: Regulation of AKT/AMPK signaling, autophagy and mitigation of
apoptosis in rutin-pretreated SH-SY5Y cells exposed to MPP. Metab
Brain Dis. 36:315–326. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
O'Malley KL, Liu J, Lotharius J and Holtz
W: Targeted expression of BCL-2 attenuates MPP+ but not 6-OHDA
induced cell death in dopaminergic neurons. Neurobiol Dis.
14:43–51. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li J, Ma S, Chen J, Hu K, Li Y, Zhang Z,
Su Z, Woodgett JR, Li M and Huang Q: GSK-3β contributes to
parkinsonian dopaminergic neuron death: Evidence from conditional
knockout mice and tideglusib. Front Mol Neurosci.
13(81)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Petit-Paitel A, Brau F, Cazareth J and
Chabry J: Involvement of cytosolic and mitochondrial GSK-3beta in
mitochondrial dysfunction and neuronal cell death of
MPTP/MPP-treated neurons. PLoS One. 4(e5491)2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kulkarni YA, Agarwal S and Garud MS:
Effect of Jyotishmati (Celastrus paniculatus) seeds in animal
models of pain and inflammation. J Ayurveda Integr Med. 6:82–88.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bhagya V, Christofer T and
Shankaranarayana Rao BS: Neuroprotective effect of Celastrus
paniculatus on chronic stress-induced cognitive impairment. Indian
J Pharmacol. 48:687–693. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bhanumathy M, Harish MS, Shivaprasad HN
and Sushma G: Nootropic activity of Celastrus paniculatus seed.
Pharm Biol. 48:324–327. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Godkar PB, Gordon RK, Ravindran A and
Doctor BP: Celastrus paniculatus seed oil and organic extracts
attenuate hydrogen peroxide- and glutamate-induced injury in
embryonic rat forebrain neuronal cells. Phytomedicine. 13:29–36.
2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kumar MH and Gupta YK: Antioxidant
property of Celastrus paniculatus willd.: A possible mechanism in
enhancing cognition. Phytomedicine. 9:302–311. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Russo A, Izzo AA, Cardile V, Borrelli F
and Vanella A: Indian medicinal plants as antiradicals and DNA
cleavage protectors. Phytomedicine. 8:125–132. 2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lekha G, Mohan K and Samy IA: Effect of
Celastrus paniculatus seed oil (Jyothismati oil) on acute and
chronic immobilization stress induced in swiss albino mice.
Pharmacogn Res. 2:169–174. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kumar KH, Venuprasad MP, Jayashree GV,
Rachitha P, Krupashree K, Pal A and Khanum F: Celastrus paniculatus
Willd. mitigates t-BHP induced oxidative and apoptotic damage in
C2C12 murine muscle cells. Cytotechnology. 67:955–967.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Khongkaew P and Chaemsawang W: Development
and characterization of stingless bee propolis properties for the
development of solid lipid nanoparticles for loading lipophilic
substances. Int J Biomater. 2021(6662867)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
John E, Mishra B, Tripathi AS and Aleesha
R: Protective effect of Celastrus paniculatus on cognitive function
in glutamate-induced brain-injured mice by reducing the
intracellular influx of Ca. Int J Dev Neurosci. 82:331–338.
2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Godkar PB, Gordon RK, Ravindran A and
Doctor BP: Celastrus paniculatus seed water soluble extracts
protect against glutamate toxicity in neuronal cultures from rat
forebrain. J Ethnopharmacol. 93:213–219. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Anjaneyulu J, R V and Godbole A:
Differential effect of ayurvedic nootropics on C. elegans models of
Parkinson's disease. J Ayurveda Integr Med. 11:440–447.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Balaji K, Vijayakumar J, Sankaran PK,
Senthilkumar S, Vijayaraghavan R, Selvaraj J and Yuvaraj MF:
Molecular studies on the nephroprotective potential of Celastrus
paniculatus against lead-acetate-induced nephrotoxicity in
experimental rats: Role of the PI3K/AKT signaling pathway.
Molecules. 26(6647)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Martins-Noguerol R, Pérez-Ramos IM, Matías
L, Moreira X, Francisco M, García-González A, Troncoso-Ponce AM,
Thomasset B, Martínez-Force E, Moreno-Pérez AJ and Cambrollé J:
Crithmum maritimum seeds, a potential source for high-quality oil
and phenolic compounds in soils with no agronomical relevance. J
Food Compos Anal. 108(104413)2022.
|
|
21
|
Kagan IA: Soluble phenolic compounds of
perennial ryegrass (Lolium perenne L.): Potential effects on animal
performance, and challenges in determining profiles and
concentrations. Anim Feed Sci Technol. 277(114960)2021.
|
|
22
|
Chaemsawang W, Prasongchean W,
Papadopoulos KI, Ritthidej G, Sukrong S and Wattanaarsakit P: The
effect of Okra (Abelmoschus esculentus (L.) Moench) eeed extract on
human cancer cell lines delivered in its native form and loaded in
polymeric micelles. Int J Biomater. 2019(9404383)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Popoola OO: Phenolic compounds composition
and in vitro antioxidant activity of Nigerian Amaranthus viridis
seed as affected by autoclaving and germination. Meas Food.
6(100028)2022.
|
|
24
|
Thaipong K, Boonprakob U, Crosby K,
Cisneros-Zevallos L and Hawkins Byrne D: Comparison of ABTS, DPPH,
FRAP, and ORAC assays for estimating antioxidant activity from
guava fruit extracts. J Food Compos Anal. 19:669–675. 2006.
|
|
25
|
Aleem M: Phytochemistry and pharmacology
of Celastrus paniculatus Wild: A nootropic drug. J Complement
Integr Med. 20:24–46. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yang T, Wang S, Zheng H, Wang L, Liu D,
Chen X and Li R: Understanding dihydro-β-agarofuran sesquiterpenes
from Tripterygium hypoglaucum as the modulators of multi-drug
resistance in HepG2/Adr cells. Biochem Biophys Res Commun.
508:742–748. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Weng JR, Yen MH and Lin WY: Cytotoxic
constituents from Celastrus paniculatus induce apoptosis and
autophagy in breast cancer cells. Phytochemistry. 94:211–219.
2013.PubMed/NCBI View Article : Google Scholar
|