Introduction
The surgical outcome of patients with esophageal
squamous cell carcinoma (ESCC) has improved as a result of a
progress in diagnostic methods, surgical procedures, and
perioperative management. However, recent studies have emphasized
the utility of chemoradiotherapy (CRTx) for patients with ESCC
(1,2), since it enables a favorable
therapeutic outcome comparable to that of radical surgery while
avoiding surgical stress and maintaining a good quality of life
(QOL) (3,4). The regimen of standard CRTx usually
consists of the concurrent use of cisplatin/fluorouracil (CDDP/5FU)
with radiation (5).
However, other anticancer agents for concurrent use
with radiation therapy have not been examined thoroughly with
regard not only to their cytocidal effects, but also their
mechanisms of action.
Among the many agents assessed for therapeutic
efficacy against ESCC, docetaxel has been introduced during the
last decade. The anti-tumor mechanism of docetaxel is completely
different from that of 5FU and CDDP (6). Docetaxel promotes the polymerization
of tubulin and inhibits the disassembly of microtubules, thereby
blocking cell division at the M phase during the cell cycle. In
vitro colony formation assays have demonstrated the superior
anti-tumor activity of docetaxel against many cell lines including
ESCC (7,8). A phase II clinical trial of docetaxel
monotherapy for the treatment of advanced/recurrent ESCC reported a
response rate of 20.4%. These findings indicate that docetaxel may
be a useful chemotherapeutic agent for the treatment of ESCC
(9–12).
Concerning CRTx, previous studies have reported that
the combined use of docetaxel with radiation therapy yielded
excellent tumor-inhibition in patients with ESCC (13–15).
It was also also reported that CRTx including docetaxel produced a
response rate of 96% and a complete response rate of 50% in
patients with head and neck cancer (16). These findings demonstrated that the
combined use of docetaxel and radiation improved the tumor
response, compared with docetaxel or radiation alone (17,18).
Recent studies demonstrated the cell-killing effects of radiation
and docetaxel on squamous cell carcinoma (SCC) cell lines in
vitro, and also demonstrated the same effect on SCC cells in
vivo (19–24). Although these studies described a
significant anti-tumor effect under the concurrent use of docetaxel
with radiation, the docetaxel solution that was used in the
experiment was itself sufficient to kill the SCC cells, and the
synergistic aspects of docetaxel used in combination with radiation
were not reported (19–22). We performed CRTx using a very low
concentration of etoposide that is not capable of killing the
cancer cells by itself (i.e., a non-cytocidal concentration), and
demonstrated a marked anti-tumor effect in SCC cells (25). This observation indicates a
synergistic effect of anticancer agents used concurrently with
radiation.
In the present study, a colony formation assay and
flow cytometry were conducted to demonstrate the
radiation-sensitizing effect of docetaxel at a concentration that
did not exhibit cytotoxity in the SCC cell lines. Our hypothesis
proposed that a low concentration of docetaxel acts as a radiation
sensitizer, in the same manner as etoposide.
Materials and methods
SCC cells
Two ESCC cell lines (TE-2 and TE-3) were kindly
provided by Dr T. Nishihira (Tohoku University, Sendai, Japan);
vulvar carcinoma cells (A431) were also used in this study.
Although A431 is a vulvar carcinoma cell line, it is a well-known
SCC cell line and is easy to culture and handle. It was therefore
used as the control in the experiment. All cells were grown in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal calf serum and maintained in a humidified atmosphere of air
containing 5% carbon dioxide at 37°C.
Colony formation assay
The colony formation assay was used to calculate the
cell survival fraction. All cells were obtained at an exponential
growth phase 2 days after seeding and incubation in flasks with a
surface area of 25 cm2 and a cell concentration of
1×106 cells per flask. The cells used in the study had
more than 70% of the plating efficiency. Prior to the experiment,
the plating efficiency of A431, TE-1, TE-2, TE-3, TE-5, TE-8 and
TE-10 was assessed. A431, TE-2, and TE-3 were selected for the
study since these cell lines had a high plating efficiency. After
individual experiments, the cells were rinsed twice in
phosphate-buffered saline (PBS), trypsinized (0.05%), and seeded
into culture dishes (US-11900, Sumitomo, Japan). After having been
incubated for 1–3 weeks, the cells were stained with crystal violet
and the colonies (>50 cells) were counted.
Docetaxel exposure
Docetaxel (Taxotere®, RP56976;
Sanofi-Aventis, Tokyo, Japan) (6)
was dissolved in serum-free DMEM medium, and a 10 mg/ml stock
solution was prepared; this solution was diluted to the final
concentration with DMEM medium at the time of use. The cells were
then incubated with the docetaxel solution for 3 h to determine the
effect of docetaxel. The diluted solutions were prepared
immediately prior to the start of each experiment. Solutions that
had been prepared 1 hour or more prior to the experiment were
discarded. To plot the cell survival curves, the colonies were
divided by the initial number of cells. Each experiment consisted
of quintuplicate disks to minimize deviations in cell plating, and
were repeated at least three times to confirm the results. The
concentrations of docetaxel that did not kill individual cells
(i.e., the non-cytocidal concentrations) were determined from the
cell survival curves.
X-ray irradiation
X-ray irradiation was performed with an MBR-1520R
X-ray machine (Hitachi, Tokyo, Japan) set at 150 kVp, and 20 mA,
using 1.5-mm aluminum filtration with a dose rate of 1 Gy/min.
Individual cells were irradiated with 2, 4, 6, or 8 Gy X-rays at
room temperature. Cell survival curves were plotted as described in
the docetaxel experiment.
Combination of docetaxel and
radiation
Next, a combined assessment of docetaxel and X-ray
radiation was performed. First, individual cell lines were exposed
to non-cytocidal concentrations of docetaxel, as determined in the
docetaxel exposure experiment, at time points prior to, during, or
after irradiation with 2, 4, 6, or 8 Gy X-rays, to determine
whether the timing of docetaxel administration would affect cell
survival. A colony formation assay was performed using the method
described above. The survival fraction (SF) was corrected by the
plating efficiency obtained from the cell survival rate in the
absence of radiation. Individual experiments were repeated at least
two times, and were comprised of quintuplicate cultures.
Any changes in the shoulder and slope
(D0) of the survival curves for docetaxel plus radiation
and radiation alone were analyzed to evaluate the effect of
docetaxel itself in individual cells. The cytocidal effects were
determined to compare the survival rate between cells treated with
docetaxel plus radiation and those treated with radiation alone for
each radiation dose.
Determination of cell cycle
To analyze the action mechanism of docetaxel as a
radiation sensitizer, flow cytometry was performed to assess the
cell cycle prior to and after docetaxel exposure. A431, TE2, and
TE3 cells were exposed to 10-10 M docetaxel for 3 h. To prepare
isolated cell suspensions, the cells were trypsinized and then
rinsed twice in PBS. The isolated cells were suspended in 1 ml of
PI solution containing 50 μg/ml propidium iodide (Sigma-Aldrich,
St. Louis, MO, USA), 0.25 mg/ml RNAse, 0.1% sodium citrate, and
0.2% Nonidet P-40, then stained at 4°C for 30 min. The cells were
then passed through a 35-μm nylon mesh filter, and the nuclear DNA
content was determined using an F1000 flow cytometer. The
fluorescence of >10,000 nuclei was measured using an EPICS
profile flow cytometer (Beckman Coulter, Fullerton, CA), and DNA
histograms were obtained. The DNA histograms were analyzed using
Cell FIT™ 2.0 (Becton Dickinson, Franklin Lakes, NJ, USA). The
nuclear DNA content of each cell was measured prior to and after
treatment with low concentrations of docetaxel, and the
distribution of cells was calculated in different phases of the
cell cycle (26–28).
Statistical analysis
A statistical analysis of the data was carried out
using a one-factor ANOVA (Stat View 5.0). A two-sided p<0.05 was
considered statistically significant.
Results
Docetaxel sensitivity
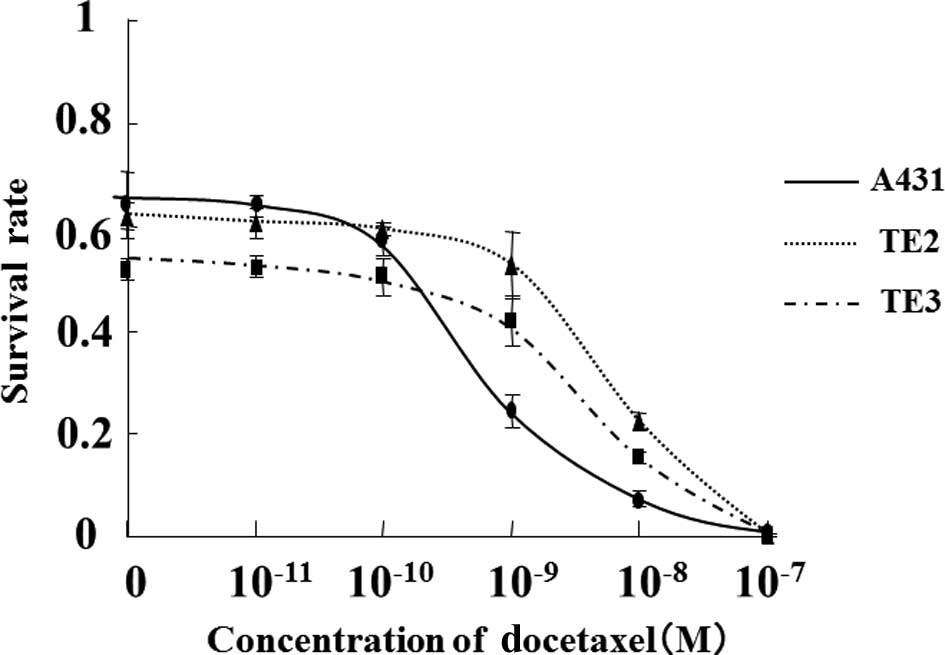
The cytocidal effect of docetaxel alone on cultured
cells was evaluated. The cell survival curves following 3 h of
treatment with docetaxel at concentrations ranging from
1.0×10−11 to 1.0×10−7 M are represented in
Fig. 1. These cell survival curves
clearly revealed a concentration-dependent cytocidal effect of
docetaxel, with a different IC50 for each cell type. The
IC50 for docetaxel treatment was 3.2×10−10 M for A431,
9.1×10−10 M for TE-3, and 1.3×10−9 M for
TE-2; thus, the sensitivity to docetaxel among these cell lines
increased according to this order. Moreover, almost no cytocidal
effect was observed at specific concentrations. A431 cell viability
decreased at docetaxel concentrations ≥1.0×10−9 M,
whereas exposure to docetaxel concentrations ≤1.0×10−10
M produced very slight cytocidal effects. The viability of TE-2 and
TE-3 cells decreased at docetaxel concentrations
≥1.0×10−8 M, whereas docetaxel concentrations
≤1.0×10−9 M had minimal effects. Based on these results,
the maximum docetaxel concentrations yielding no cytocidal effects
were arbitrarily designated as 1.0×10−11 M for A431, and
1.0×10−10 M for TE-2 and TE-3.
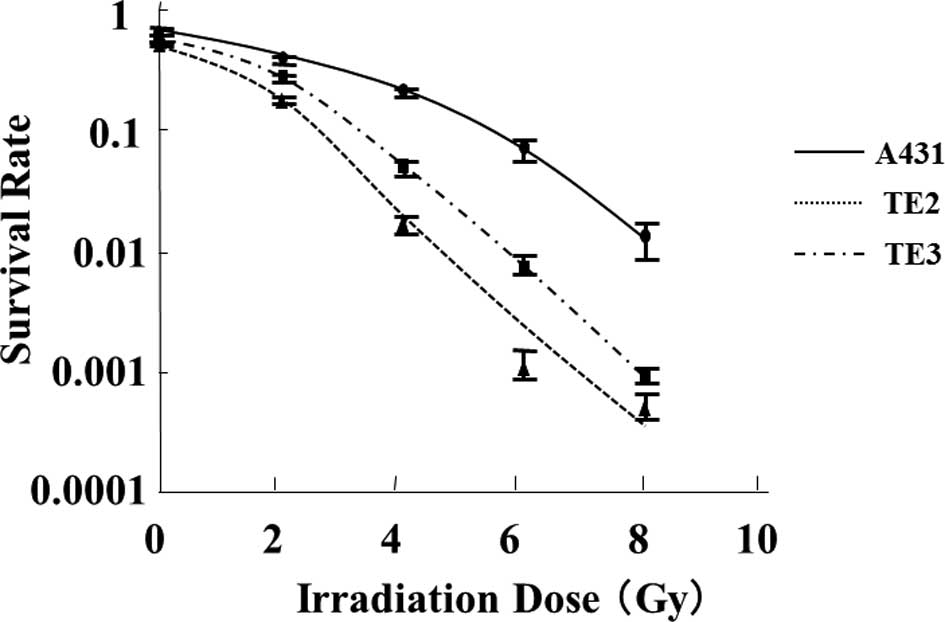
Radiosensitivity
The survival curves obtained after the X-ray
irradiation of individual cell lines are shown in Fig. 2. The cell survival curves
demonstrated that cell viability decreased in a radiation
dose-dependent manner in each cell line and that the sensitivity to
X-ray radiation differed among the cell lines. The TE-2 cell line
had the highest radiosensitivity to irradiation (D0
value = 0.97 Gy), followed by TE-3 (D0 value = 1.1 Gy) and A431
(D0 value = 2.2 Gy). Notably, A431 exhibited a markedly
high sensitivity to docetaxel, whereas TE-2 exhibited the lowest
sensitivity. These findings indicate that the radiosensitivity of
these cells was inversely correlated with the sensitivity to
docetaxel.
Combined effect of docetaxel and
radiation
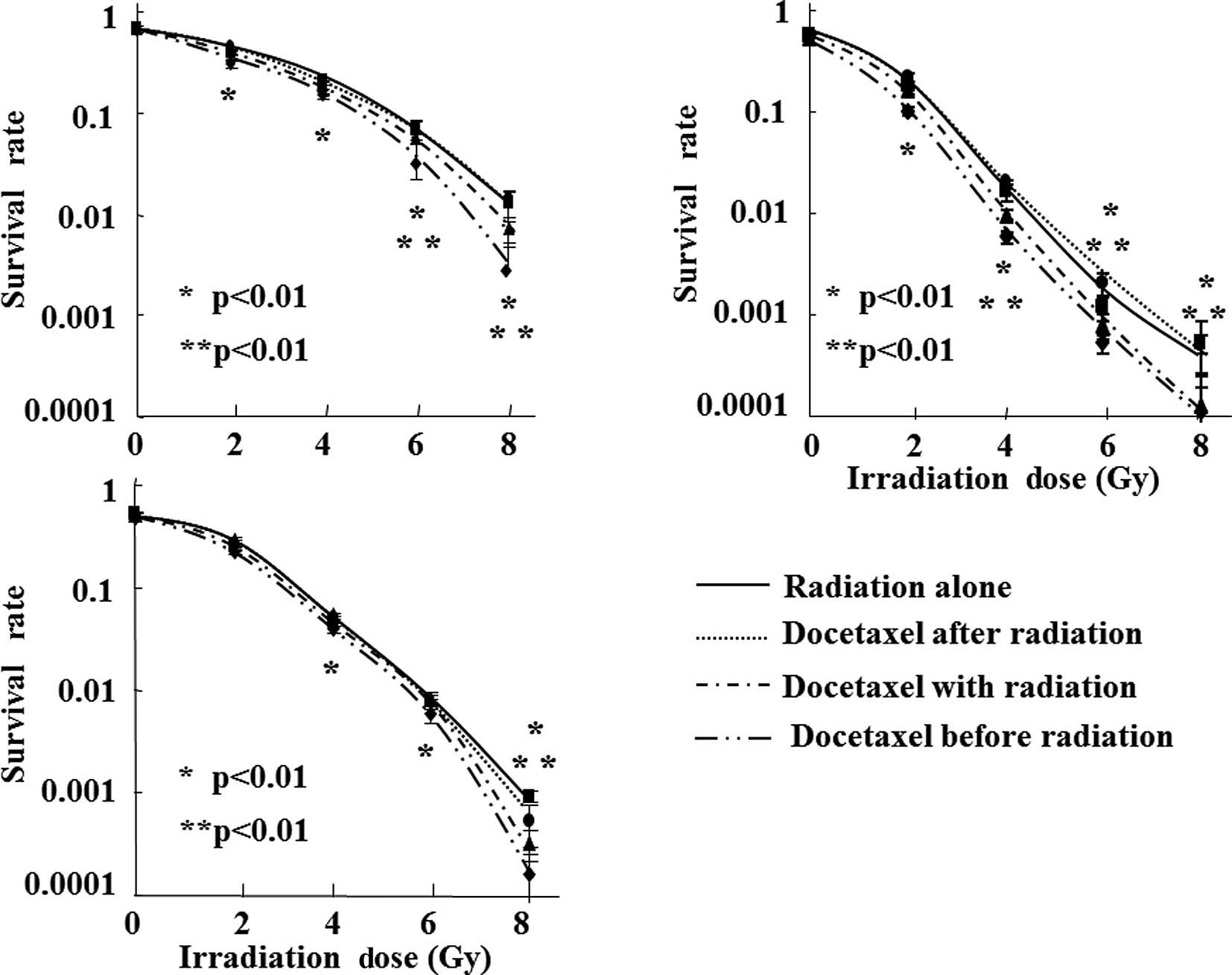
We then investigated whether docetaxel had a
radiation-sensitizing effect on SCC cells. Almost no decrease in
cell viability was observed after 3 h of treatment with docetaxel
alone at a concentration of 1.0×10−11 M or lower in the
A431 cells, and at a concentration of 1.0×10−10 M or
lower in the TE-2 or TE-3 cells. Based on the above results, the
changes in the cell survival curves following X-ray irradiation
with docetaxel exposure at these non-cytocidal concentrations were
analyzed (Fig. 3). We also
analyzed whether the treatment timing of docetaxel (prior to,
during, or after radiation) was correlated with cell survival.
Among the three different timings of docetaxel
administration, cell survival was lowest when the cells were
treated using X-ray radiation immediately after 3 h of exposure to
docetaxel at a non-cytocidal concentration. Significant decreases
in cell viability were observed compared with radiation alone (≥2
Gy in A431 and TE-2, and ≥4 Gy in TE-3). When the cells were
treated with radiation (≥6 Gy in A431, ≥4 Gy in TE-2, and ≥8 Gy in
TE-3) during docetaxel exposure, significant decreases in cell
survival were also observed in all the cell lines. The cytocidal
effects were lower than those obtained by irradiation immediately
after docetaxel exposure, and the extent of the
radiation-sensitizing effect was highest in TE-2 cells. On the
other hand, no decreases in cell viability were observed in any of
the cell lines treated with docetaxel immediately after X-ray
irradiation at any radiation dose.
The correlation between the IC50 of
docetaxel and the radiation-sensitizing effect was then assessed.
The cells were treated with 8 Gy of radiation immediately after
exposure to a non-cytocidal concentration of docetaxel, and the
cell survival rates were compared. The cell viability of the
concurrent use of radiation and docetaxel was 20.3% in A431, 69.2%
in TE-2, and 21.7% in TE-3, compared with radiation alone,
indicating that cell lines with a lower IC50 had a lower
cell survival rate after concurrent treatment.
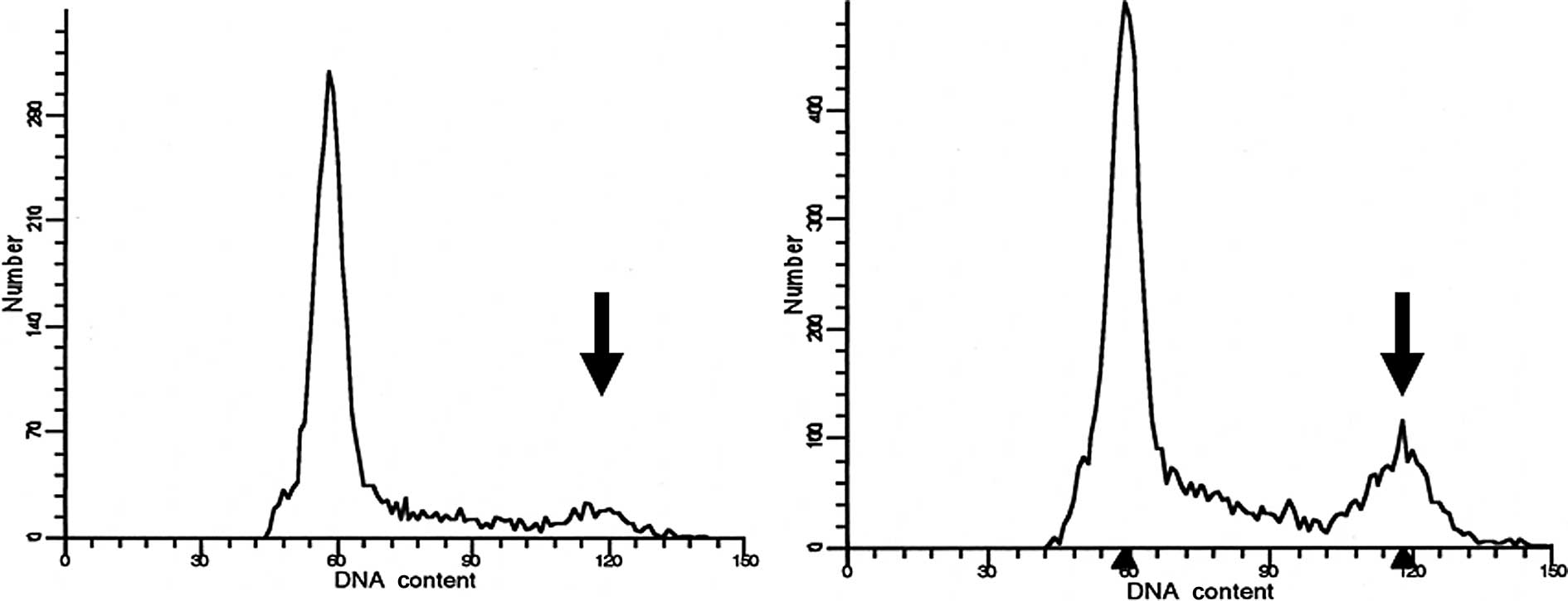
Determination of the cell cycle
distribution in each cell line
The cell cycle profiles prior to and after 3 hours
of treatment with docetaxel at a non-cytocidal concentration of
1.0×10−10 M were determined using flow cytometry
(Fig. 4). The cell fraction in the
G2/M stage had clearly increased by approximately 2 times in the
A431 cells and 5 times in the TE-2 cells (Table I). Although the cell fraction in
the G2/M stage had also increased in the TE-3 cells, the increase
was not as obvious.
 | Table I.Changes in the cell cycle following
exposure to 1×10−10 M of docetaxel, as determined using
flow cytometry. |
Table I.
Changes in the cell cycle following
exposure to 1×10−10 M of docetaxel, as determined using
flow cytometry.
| | Cell cycle (%)
|
|---|
| Cell line | G0/G1 | S | G2/M |
|---|
| A431 | Before treatment | 67.29 | 32.53 | 0.18 |
| After treatment | 66.85 | 32.22 | 0.93 |
| TE2 | Before treatment | 74.79 | 21.59 | 3.63 |
| After treatment | 63.43 | 28.57 | 8.00 |
| TE3 | Before treatment | 83.23 | 16.59 | 0.18 |
| After treatment | 77.95 | 21.75 | 0.30 |
Discussion
CRTx has been widely applied to patients with ESCC
due to its marked therapeutic effect, although its basic mechanism
has not been thoroughly examined. In this study, the in
vitro radiation-sensitizing effect of docetaxel was elucidated.
It was observed that a non-cytocidal concentration of docetaxel may
have affected the cell cycle at the G2/M stage and improved the
anti-tumor effect of radiotherapy.
Although previous studies have revealed the utility
of combined anticancer drug and radiation treatment, few studies
have demonstrated the synergistic effect of anticancer agents with
radiation (19–22). The majority of previous studies
have used a high concentration of an anticancer agent capable of
reducing the cell survival rate by itself; consequently, the slopes
of the cell survival curves for combined CRTx and radiation alone
were similar, indicating that the anticancer agents only had an
additive effect. By contrast, the noncytocidal concentration of
docetaxel was determined in the present study prior to any
treatments; chemoradiotherapy using these very low concentrations
of docetaxel was capable of reducing the cell survival rate,
increasing the slope of the survival curve. These findings clearly
indicated that docetaxel had a synergistic effect on radiation,
i.e., a radiation-sensitizing effect. When the various cell lines
were compared, the order of sensitivity to docetaxel was the
inverse of the order of radiosensitivity among the cells used in
the present study, and the cell-killing effect of combined
chemoradiotherapy reflected the sensitivity of the cells to
docetaxel. These findings also supported the hypothesis that
docetaxel has a radiation-sensitizing effect on SCC cell lines.
We have previously used etoposide at a concentration
that did not have a cell-killing effect and performed CRTx in
vitro on Chinese hamster-related V79 cells, human bladder
cancer-derived T24 cells, human breast cancer-derived MDA-MB231
cells, and human ovarian cancer-derived RGMT cells, and
demonstrated that etoposide had a radiation-sensitizing effect
among certain types of cell lines (25). These findings indicated that a
low-concentration method was useful for assessing the synergistic
effect of a chemotherapy agent on radiation. The present study was
performed on the basis of these previous findings.
Significantly, the timing of docetaxel may affect
the therapeutic efficacy of chemoradiotherapy. Previous studies
have revealed a radiation-sensitizing effect after the contact of
ESCC cells with CDDP. As we expected, radiation produced the
strongest cell-killing effect immediately after contact with
docetaxel. With regard to the action mechanism of this effect,
intracellular and extracellular factors require further
investigation. Since the cell cycle may be related to the radiation
efficacy, flow cytometry was performed to assess the cell cycle
distribution, and it was demonstrated that the administration of
docetaxel increased the cell fraction in the G2/M stage. The G2/M
phase is considered to be the most radiosensitive period, resulting
in a high radiation-sensitizing effect after contact with docetaxel
(26–28).
The pharmacological action of docetaxel is unique.
Docetaxel mainly affects tubulin (especially β-tubulin), which is a
protein that is suspended in cells; docetaxel promotes
polymerization and arrests cell division by inhibiting microtubular
depolymerization (6). This
mechanism results in the synchronization of the cell cycle during
G2/M phase, when radiosensitivity is at its highest, due to the
large quantity of microtubes (26,27).
Using flow cytometry, an increase in the G2/M cell fraction was
observed even after contact with a non-cytocidal concentration of
docetaxel, consistent with the basic pharmacological mechanism of
docetaxel and indicating that this mechanism may be partly
responsible for docetaxel's radiation-sensitizing effects.
In this study, three cells lines (TE-2, TE-3, and
A431) with a high plating efficiency were selected. Plating
efficiency is significant when conducting a colony formation assay
where the assessment of cells with a lower cellular proliferation
capability is insufficient. This may be clinically related to tumor
aggressiveness, and further investigation is required.
With regard to preserving the esophagus and
maintaining the patient's QOL, CRTx has several advantages to
surgery; however, lethal adverse events have also been reported.
Therapies with a marked local effect and fewer general effects are
usually considered as being ideal, and CRTx is promising for this
reason. Nevertheless, the frequency of adverse events affecting
other organs is still being debated. Conventional FP therapy, which
is a chemotherapy regimen combined with radiation for esophageal
cancer, often requires hospitalization for renal preservation and
involves a high treatment cost. Although the usual dose of
docetaxel administration evokes adverse events at a rate of
approximately 50% (9–12), it may be possible to decrease this
rate by reducing the administered dose, since the occurrence of
adverse events depends on the concentration of docetaxel. Our
findings demonstrated radiation-sensitizing effects after the
administration of a low concentration of docetaxel, which may
establish a rationale for CRTx using an anticancer agent with a
radiation-sensitizing effect that could increase the clinical
response and decrease the risk of adverse events. Moreover, in
addition to its clinical benefit, the administration of low
concentrations of docetaxel might also facilitate administration of
CRTx on an outpatient basis, enabling a cost benefit.
In conclusion, this study demonstrated that the
administration of a low, non-cytocidal concentration of docetaxel
prior to radiation exposure increases the cell-killing effect on
SCC cell lines. The increase of the G2/M fraction as a result of
the administration of docetaxel may affect this
radiation-sensitizing effect.
References
|
1.
|
Herskovic A, Martz K, Al-Sarraf M, et al:
Combined chemotherapy and radiotherapy compared with radiotherapy
alone in patients with cancer of the esophagus. N Engl J Med.
326:1593–1598. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Al-Sarraf M, Martz K, Herskovic A, et al:
Progress report of combined chemoradiotherapy versus radiotherapy
alone in patients with esophageal cancer: an intergroup study. J
Clin Oncol. 15:277–284. 1997.PubMed/NCBI
|
|
3.
|
Cooper JS, Guo MD, Herskovic A, et al:
Chemoradiotherapy of locally advanced esophageal cancer: long-term
follow-up of a prospective randomized trial (RTOG 85-01). Radiation
Therapy Oncology Group. JAMA. 281:1623–1627. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Coia LR, Minsky BD, Berkey BA, et al:
Outcomes of patients receiving radiation for cancer of the
esophagus: results of the 1992–1994 patterns of care study. J Clin
Oncol. 18:455–462. 2000.PubMed/NCBI
|
|
5.
|
Ando N, Iizuka T, Ide H, et al: Surgery
plus chemotherapy compared with surgery alone for localized
squamous cell carcinoma of the thoracic esophagus: A Japan Clinical
Oncology Group Study-JCOG9204. J Clin Oncol. 21:4592–4596. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ringel I and Horwitz SB: Studies with RP
56976 (Taxotere): a semisynthetic analogue of taxol. J Natl Cancer
Inst. 83:288–291. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Garcia P, Braguer D, Carles G, et al:
Comparative effects of taxol and taxotere on two different human
carcinoma cell lines. Cancer Chemother Pharmacol. 34:335–343. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Hanauske AR, Degen D, Hilsenbeck SG,
Bissery MC and von Hoff DD: Effects of taxotere and taxol on in
vitro colony formation of freshly explanted human tumor cells.
Anticancer Drugs. 3:121–124. 1992.
|
|
9.
|
Catimel G, Verweij J, Mattijssen V, et al:
Docetaxel (Taxotere): an active drug for the treatment of patients
with advanced squamous cell carcinoma of the head and neck. EORTC
Early Clinical Trials Group. Ann Oncol. 5:533–537. 1994.
|
|
10.
|
Couteau C, Chouaki N, Leyvraz S, et al: A
phase II study of docetaxel in patients with metastatic squamous
cell carcinoma of the head and neck. Br J Cancer. 81:457–462. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Bang YJ, Kang WK, Kang YK, et al:
Docetaxel 75 mg/m2 is active and well tolerated in
patients with metastatic or recurrent gastric cancer: a Phase II
Trial. Jpn J Clin Oncol. 32:248–254. 2002.
|
|
12.
|
Dreyfuss AI, Clark JR, Norris CM, et al:
Docetaxel: An active drug for squamous cell carcinoma of the head
and neck. J Clin Oncol. 14:1672–1678. 1996.PubMed/NCBI
|
|
13.
|
Mauer AM, Masters GA, Haraf DJ, et al:
Phase I study of docetaxel with concomitant thoracic radiation
therapy. J Clin Oncol. 16:159–164. 1998.PubMed/NCBI
|
|
14.
|
Matsuura M, Hasegawa M, Hayakawa K, et al:
Experimental study of the effects on apoptosis of docetaxel alone
and in combination with irradiation. Oncol Rep. 7:289–293.
2000.PubMed/NCBI
|
|
15.
|
Kim ES and Khuri FR: Docetaxel and
radiation as combined-modality therapy. Oncol (Williston Park).
16(Suppl 6): 97–105. 2002.PubMed/NCBI
|
|
16.
|
Fujii M, Tsukuda M, Sakata B, et al: Japan
Cooperative Head and Neck Oncology Group (JCHNOG). Phase I/II trial
of weekly docetaxel and concomitant radiotherapy for squamous cell
carcinoma of the head and neck. Int J Clin Oncol. 9:107–112. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Koukourakis M, Kourousis C, Kamilaki M, et
al: Weekly docetaxel and concomitant boost radiotherapy for
non-small-cell lung cancer. A phase I/II dose escalation trial. Eur
J Cancer. 34:838–844. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Segawa Y, Kiura K, Takigawa N, et al:
Phase III trial comparing docetaxel and cisplatin combination
chemotherapy with mitomycin, vindesine, and cisplatin combination
chemotherapy with concurrent thoracic radiotherapy in locally
advanced non-small-cell lung cancer: OLCSG 0007. J Clin Oncol.
28:3299–3306. 2010. View Article : Google Scholar
|
|
19.
|
Choy H, Rodriguez F, Koester S, et al:
Synergistic effect of taxol/taxotere on radiation sensitivity on
human tumor cell lines. Int J Radiat Oncol Biol Phys. 24(Suppl):
274–275. 1992. View Article : Google Scholar
|
|
20.
|
Pradier O, Rave-Fränk M, Lehmann J, et al:
Effects of docetaxel in combination with radiation on human head
and neck cancer cells (ZMK-1) and cervical squamous cell carcinoma
cells (CASKI). Int J Cancer. 91:840–845. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Hennequin C, Giocanti N and Favaudon V:
Interaction of ionizing radiation with paclitaxel (Taxol) and
docetaxel (Taxotere) in HeLa and SQ20B cells. Cancer Res.
56:1842–1850. 1996.PubMed/NCBI
|
|
22.
|
Creane M, Seymour CB, Colucci S and
Mothersill C: Radiobiological effect of docetaxel (Taxotere): a
potential radiation sensitizer. Int J Radiat Biol. 75:731–737.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Mason KA, Hunter NR, Milas M, Abbruzzese
JL and Milas L: Docetaxel enhances tumor radioresponse in
vivo. Clin Cancer Res. 3:2431–2438. 1997.PubMed/NCBI
|
|
24.
|
Mason KA, Kishi K, Hunter N, et al: Effect
of docetaxel on the therapeutic ratio of fractionated radiotherapy
in vivo. Clin Cancer Res. 5:4191–4198. 1999.PubMed/NCBI
|
|
25.
|
Shigematsu N, Kawata T, Ihara N, et al:
Effect of combined treatment with radiation and low dose etoposide
on cell survival. Anticancer Res. 21:325–328. 2001.PubMed/NCBI
|
|
26.
|
Withers HR, Mason K, Reid BO, et al:
Response of mouse intestine to neutrons and gamma rays in relation
to dose fractionation and division cycle. Cancer. 34:39–47. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Hartwell LH and Weinert TA: Checkpoints:
controls that ensure the order of cell cycle events. Science.
246:629–634. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Dolbeare F, Beisker W, Pallavicini MG,
Vanderlaan M and Gray JW: Cytochemistry for bromodeoxyuridine/DNA
analysis: stoichiometry and sensitivity. Cytometry. 6:521–530.
1985. View Article : Google Scholar : PubMed/NCBI
|