Introduction
Worldwide, lung cancer is the leading cause of
cancer mortality (1). Major
progression in understanding molecular cancer biology and in
determining the mechanisms of oncogenesis during the last decade
has resulted in the development of molecular targets for non-small
cell lung cancer (NSCLC) treatments. Inhibition of the epidermal
growth factor receptor (EGFR) pathway with tyrosine kinase
inhibitors (TKIs), such as gefitinib and erlotinib, provides an
effective and promising treatment for NSCLC, as either first-line
or salvage therapy, with the added advantage of improved efficacy,
tolerability and quality of life compared with other chemotherapy
agents (2–9). It has been demonstrated that a subset
of patients (female, never smoked, East Asian with an
adenocarcinoma diagnosis) may respond better to EGFR-TKIs. A higher
prevalence of sensitive EGFR-activating mutations (deletion in exon
19 or point mutation of L858R in exon 21) has been found in this
subset of patients (10–12).
Gefitinib and erlotinib were each compared with a
placebo in phase III randomized trials (ISEL and BR.21,
respectively) in which the majority of enrolled patients were
Caucasian (2,3). The varying overall survival outcomes
with these two drugs compared with the placebo were widely debated,
although gefitinib demonstrated a significant survival benefit in a
subgroup of patients of Asian origin (4). There are many possible reasons for
this difference in survival, including the most frequently
mentioned - that the dose intensity or drug concentrations are
higher in patients receiving erlotinib treatment than in those
receiving gefitinib treatment (13–17).
Phase III, randomized trials comparing gefitinib or erlotinib with
docetaxel or pemetrexed have been performed, but the superiority of
one agent over the other has not been documented (5,18,19).
To our knowledge, there is no published prospective trial to date,
which compares gefitinib and erlotinib treatment, not to mention a
study performed based on the tumor EGFR mutation status. In the
present study, we retrospectively evaluated the difference in
efficacy between these two agents in Taiwanese patients with
advanced adenocarcinoma, whose tumor EGFR mutation status was
known. The aim of the study was to identify any difference in the
efficacy of these agents in patients with or without
EGFR-activating mutations.
Methods and materials
Patients
This study was approved by our Institutional Review
Board (VGHIRB 2011-04-0151A). Between January 2005 and December
2010, patients who received the standard dose of gefitinib (250 mg
daily) or erlotinib (150 mg daily) with assessable and inoperable
stage IIIB or IV adenocarcinoma were enrolled into this
retrospective study. Medical charts and images to evaluate
treatment response were retrospectively reviewed. Clinical
characteristics of the patients, including age, gender, Eastern
Cooperative Oncology Group (ECOG) performance status (PS), tumor
histological type, stage, smoking history and present EGFR-TKI
therapy, were recorded. Patients were defined as non-smokers if
they had smoked <100 cigarettes in their lifetime, or smokers if
they had smoked ≥100 cigarettes in their lifetime. The date that
EGFR-TKI treatment was commenced, time to disease progression and
date of death or last follow-up were also recorded.
Efficacy evaluation
Baseline assessments were performed within 3 weeks
prior to EGFR-TKI treatment. A chest computed tomography scan
(including liver and adrenal glands) was performed within 3 weeks
prior to commencing EGFR-TKI treatment, every 2 to 3 months
thereafter or when confirmation of treatment response or disease
progression was required. Treatment response evaluation was
performed according to the Response Evaluation Criteria in Solid
Tumors (RECIST) group criteria (20). Progression-free survival (PFS) was
calculated from the date of administration of the first dose of
EGFR-TKI to the earliest sign of disease progression, as determined
by RECIST (20), or death from any
cause. If disease progression had not occurred at the time of the
last follow-up visit, PFS was considered to have been censored at
that time. Survival was measured from administration of the first
dose of EGFR-TKI until the date of death or last follow-up.
EGFR mutation analysis
The EGFR mutation analysis was performed with
nucleotide sequence analysis. The VarientSEQr™
Resequencing Primer Set [Applied Biosystems, Inc. (ABI), Foster
City, CA, USA] was selected for mutational analysis of the tyrosine
kinase domain, exons 18–21 of the EGFR gene. Genomic DNA was
extracted from paraffin blocks, exons 18–21 were amplified and
uncloned polymerase chain reaction (PCR) fragments were sequenced
and analyzed in both sense and antisense directions for the
presence of heterozygous mutations. Normal, control DNA provided by
the ABI company was used for wild-type control. All sequence
variations were confirmed by multiple, independent PCR
amplifications and repeated sequencing reactions. EGFR-activating
mutations were defined as those with exon 19 deletions or point
mutation of L858R in exon 21.
Statistical analysis
All categorical variables were analyzed with
Chi-square tests. Median PFS and overall survival were estimated
using the Kaplan-Meier method and log-rank test. Hazard ratios
(HRs) in the overall population and in patient subsets were
calculated using the Cox proportional hazards model. All p-values
were 2-sided and a value of <0.05 was considered to be
statistically significant. All statistical analyses were performed
using SPSS software (SPSS Inc., Chicago, IL, USA).
Results
Patients
A total of 224 patients who received EGFR-TKI
treatment and whose tumor EGFR mutation status was known were
enrolled; of these, 124 received gefitinib treatment and 100
received erlotinib treatment. Since 2 to 4 weeks are required for
the results of tumor EGFR mutation analysis to be available, a
large proportion (>90%) of the patients commenced gefitinib or
erlotinib treatment before these results were known. Of the 224
patients, 146 had tumors with EGFR-activating mutations (96 exon 19
deletions alone, 41 L858R mutations, and 9 with both exon 19
deletions and L858R mutations) and 78 did not (15 atypical
mutations in either exon 18, 20 and/or 21, and 63 wild-type).
Although more male patients received erlotinib
treatment and more patients who never smoked received gefitinib
treatment, there was no statistically significant difference in
gender, smoking, performance and treatment response between the
patients who received either treatment. However, more patients with
EGFR-activating mutations received gefitinib than erlotinib
(p<0.001; Table I). With regard
to the activating mutation incidence in relation to the clinical
predictors commonly used, males had a 67.6% mutation rate and
females had a 62.9% mutation rate (p=0.486); non-smokers had a
66.7% mutation rate, while smokers had a 61.3% mutation rate (p=
0.531). EGFR-TKI was the first-line treatment in 75 patients,
second-line treatment in 126 patients, third-line treatment in 3
patients and fourth-line treatment in 1 patient.
 | Table I.Clinical characteristics of the 224
patients who received gefitinib or erlotinib treatment. |
Table I.
Clinical characteristics of the 224
patients who received gefitinib or erlotinib treatment.
| Variables | Patient number (%)
| p-valuea |
|---|
| Gefitinib
(n=124) | Erlotinib
(n=100) |
|---|
| Gender | | | 0.081 |
| Male | 53 (49.1) | 55 (50.9) | |
| Female | 71 (61.2) | 45 (38.8) | |
| Smoking
history | | | 0.071 |
| No | 96 (59.3) | 66 (40.7) | |
| Yes | 28 (45.2) | 34 (54.5) | |
| Performance
status | | | 0.43 |
| 0 | 21 (61.8) | 13 (38.2) | |
| 1 | 72 (50.7) | 70 (49.3) | |
| 2 | 20 (66.7) | 10 (33.3) | |
| 3 | 6 (66.7) | 3 (33.3) | |
| 4 | 5 (55.6) | 4 (44.4) | |
| Type of response to
TKI | | | 0.141 |
| Partial
response | 52 (55.3) | 42 (44.7) | |
| Stable
disease | 46 (63) | 27 (37) | |
| Progressive
disease | 26 (45.6) | 31 (54.4) | |
| EGFR-activating
mutations | | | <0.001 |
| With | 94 (64.4) | 52 (35.6) | |
| Without | 30 (38.5) | 48 (61.5) | |
Treatment response
There was no difference in the type of response
between the 2 agents (p=0.141). Patients with the best partial
response, stable disease, or progressive disease during treatment
were the following: 52 (41.9%), 46 (37.1%) and 26 (21%),
respectively (among 124 patients who received gefitinib), and 42
(42%), 27 (27%) and 31 (31%), respectively (among 100 patients who
received erlotinib). The objective response rate to EGFR-TKI was
similar between patients who received gefitinib or erlotinib (41.9
vs. 42%, p=1.000; Table II). When
treatment response was classified according to tumor EGFR mutation
status, 78 out of 146 patients (53.4%) with EGFR-activating
mutations had a partial response to EGFR-TKI treatment, while only
16 out of 78 patients (20.5%) without EGFR-activating mutations
responded to the treatment (p<0.001).
 | Table II.Type of treatment response and the
relationship to EGFR mutation status |
Table II.
Type of treatment response and the
relationship to EGFR mutation status
| Gefitinib, no.
(%) | Erlotinib, no.
(%) | p-valuea |
|---|
| With activating
mutations | | | 0.277 |
| Partial
response | 48 (51.1) | 30 (57.7) | |
| Stable
disease | 35 (37.2) | 13 (25) | |
| Progressive
disease | 11 (11.7) | 9 (17.3) | |
| Without activating
mutations | | | 0.446 |
| Partial
response | 4 (13.3) | 12 (25) | 0.064b |
| Stable
disease | 11 (36.7) | 14 (29.2) | |
| Progressive
disease | 15 (50) | 22 (45.8) | |
When the type of treatment response was classified
according to the EGFR mutation status and EGFR-TKI treatment was
used, there was no significant difference in patients with
EGFR-activating mutations who received gefitinib or erlotinib. In
addition, there was no significant difference in patients without
EGFR-activating mutations, other than erlotinib, which had a
numerically higher response rate than gefitinib among these
patients, with borderline significance (p=0.064; Table II).
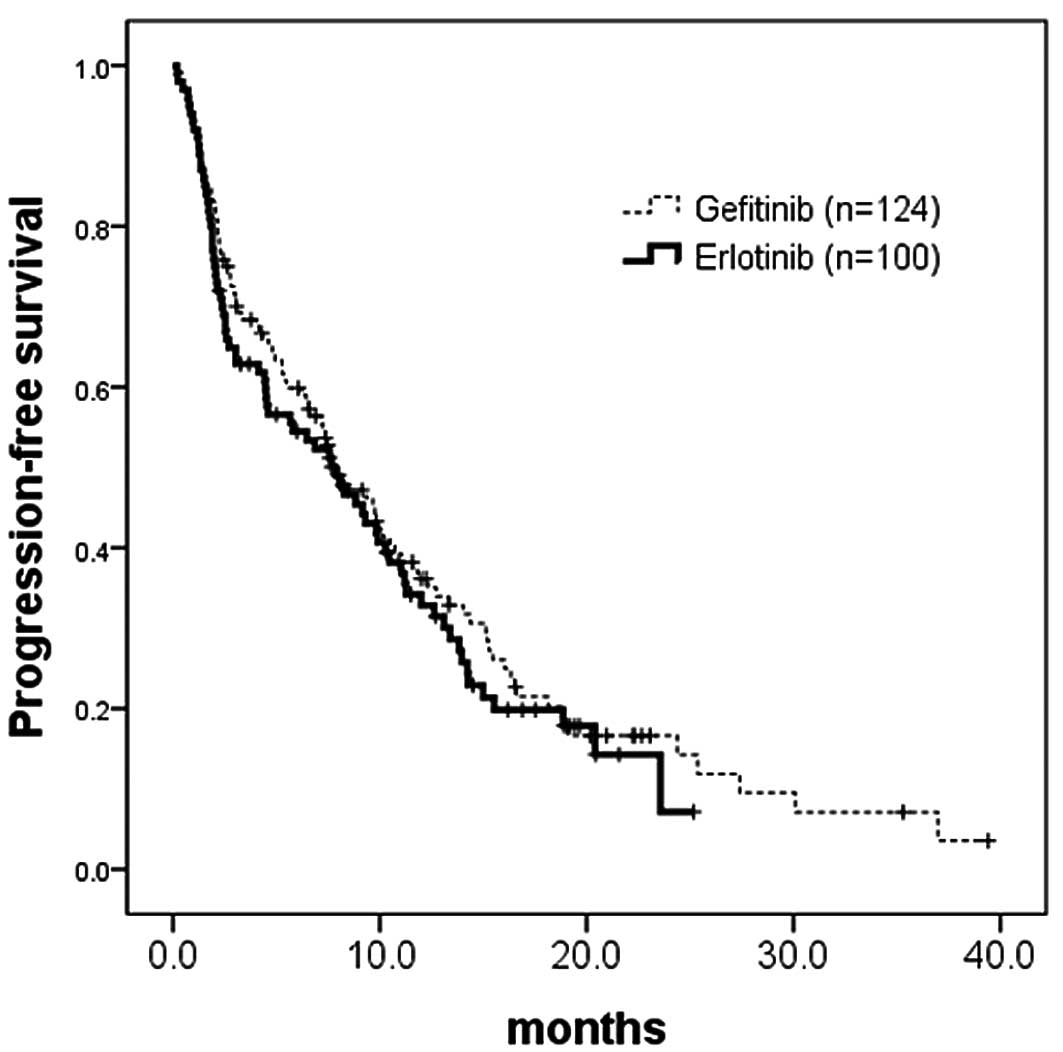
PFS
There was no difference in PFS between the 124
patients treated with gefitinib (censor, 28) and the 100 treated
with erlotinib (censor, 25) (median, 7.6 vs. 7.9 months; HR, 0.89;
95% CI, 0.66–1.21; p=0.4731) (Fig.
1). PFS was significantly longer in the 146 patients (censor,
40) whose tumors had EGFR-activating mutations than in the 78
patients (censor, 13) whose tumors did not have EGFR-activating
mutations (median, 10.5 vs. 2.5 months; HR, 0.48; 95% CI,
0.35–0.66; p<0.0001). There was no statistically significant
difference in PFS between males and females, and between EGFR-TKI
used as first-line or later lines of treatment. However, PFS was
significantly different in patients with different staging statuses
and varying PS (Table III).
 | Table III.PFS based on clinical characteristics
and EGFR mutation status. |
Table III.
PFS based on clinical characteristics
and EGFR mutation status.
| Median PFS,
months | Hazard ratio (95%
CI) | p-valuea |
|---|
| Treatment | | 0.89
(0.66–1.21) | 0.4731 |
| Gefitinib,
n=124 | 7.6 | | |
| Erlotinib,
n=100 | 7.9 | | |
| EGFR-activating
mutations | | 0.48
(0.35–0.66) | <0.0001 |
| No, n=78 | 2.5 | | |
| Yes, n=146 | 10.5 | | |
| Gender | | 0.75
(0.56–1.03) | 0.0707 |
| Male, n=108 | 9.8 | | |
| Female,
n=116 | 7.6 | | |
| Staging | | 0.40
(0.18–0.90) | 0.0222 |
| IIIB, n=13 | 15.1 | | |
| IV, n=211 | 7.6 | | |
| Performance
status | | <0.0001 | |
| 0, n=34 | 7.4 | | |
| 1, n=142 | 9.8 | 0.42
(0.19–0.94) | |
| 2, n=30 | 4.6 | 0.51
(0.25–1.04) | |
| 3, n=9 | 1.3 | 0.81
(0.36–1.78) | |
| 4, n=9 | 2.8 | 3.57
(1.35–9.41) | |
| TKI treatment
as | | | 0.2917 |
| First-line,
n=75 | 7.4 | | |
| Second-line,
n=126 | 8 | 0.85
(0.50–1.45) | |
| Third-line,
n=22 | 4.6 | 1.08
(0.65–1.79) | |
| Fourth-line,
n=1 | >19.4 | | |
The Cox-regression test for multivariate analysis of
PFS, including EGFR mutation status (with or without activating
mutations), treatment regimen (gefitinib or erlotinib), gender
(male or female), PS (0, 1, 2, 3 or 4), staging (IIIB or IV) and
present line of treatment (first-, second-, third- or fourth-line),
revealed that only tumors with EGFR-activating mutations (HR, 0.37;
95% CI, 0.25–0.53; p<0.0001) and PS (p<0.0001) had a
statistically significant effect on PFS.
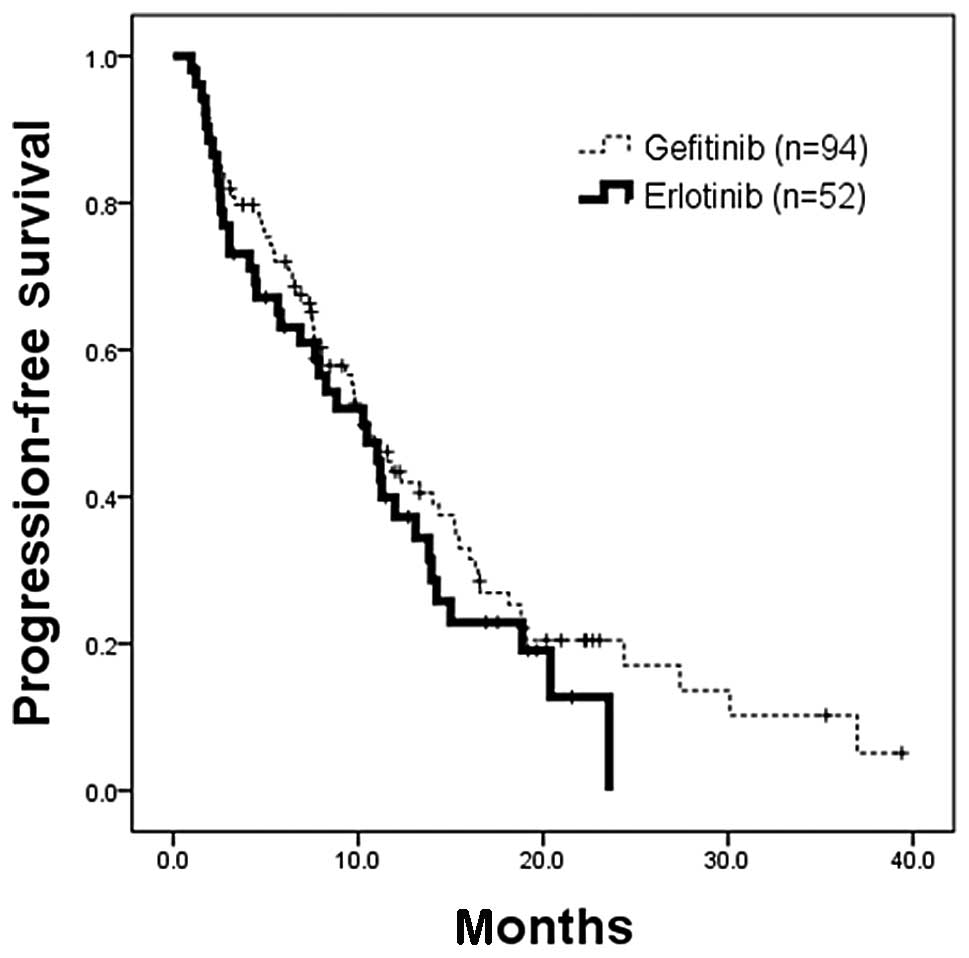
When an EGFR-TKI regimen was administered to the
patients whose tumors had EGFR-activating mutations, there was no
statistical difference in PFS between the 94 patients (censor, 26)
who received gefitinib and 52 (censor, 14) who received erlotinib
(median, 10.5 vs. 10.3 months; HR, 1.22; 95% CI, 0.82–1.83;
p=0.3224; Fig. 2). When EGFR-TKI
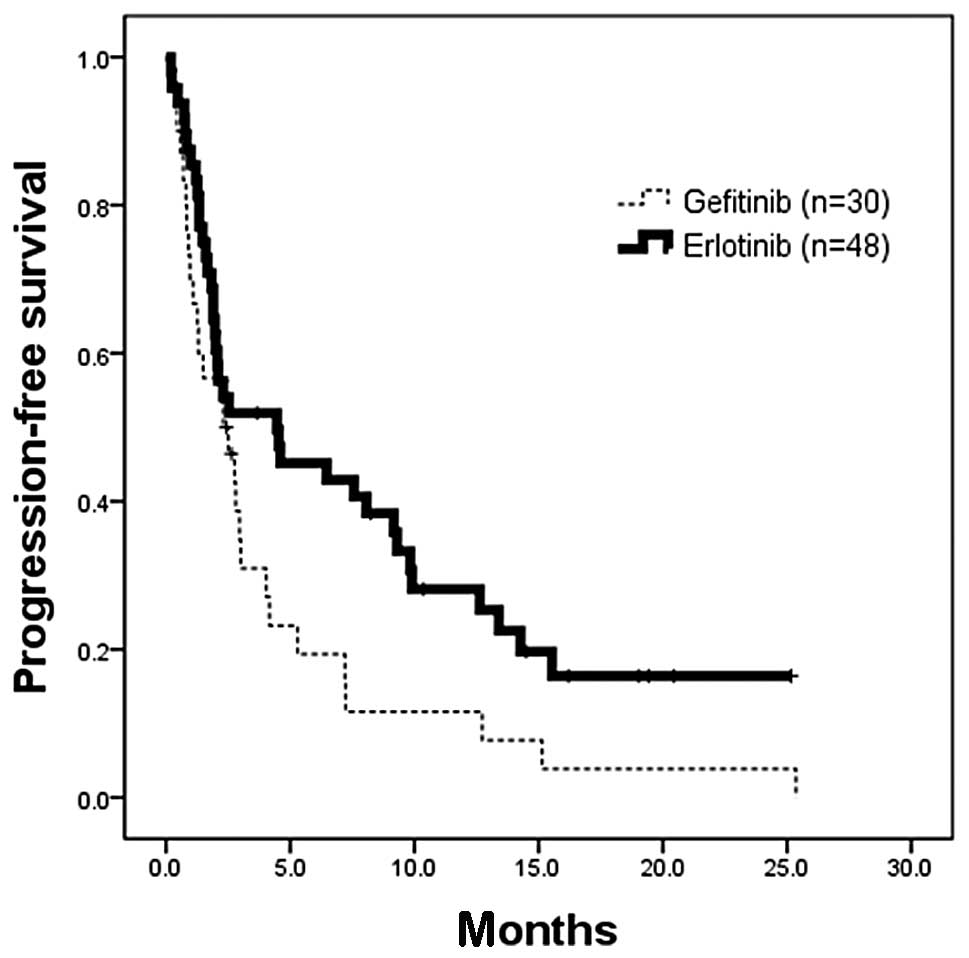
was administered to the patients whose tumors did not have
EGFR-activating mutations, the 48 (censor, 11) who received
erlotinib had a significantly longer PFS than the 30 (censor, 2)
who received gefitinib (median, 4.5 vs. 2.3 months; HR, 0.58; 95%
CI, 0.35–0.96; p=0.0315; Fig.
3).
Overall survival analysis revealed no survival
difference in patients with EGFR-activating mutations who received
either gefitinib (n=94; censor, 54; median, 26 months) or erlotinib
treatment (n=52; censor, 40; median, not reached) (HR, 0.49; 95%
CI, 0.36–1.22; p=0.2728; Fig. 4A).
There was also no survival difference in patients without
EGFR-activating mutations who received either gefitinib treatment
(n=30; censor, 14; median, 8.6 months) or erlotinib treatment
(n=48; censor, 31; median, not reached) (HR, 0.58; 95% CI,
0.26–1.14; p=0.1104; Fig. 4B).
Overall survival could not be interpreted due to the inadequate
number of deaths.
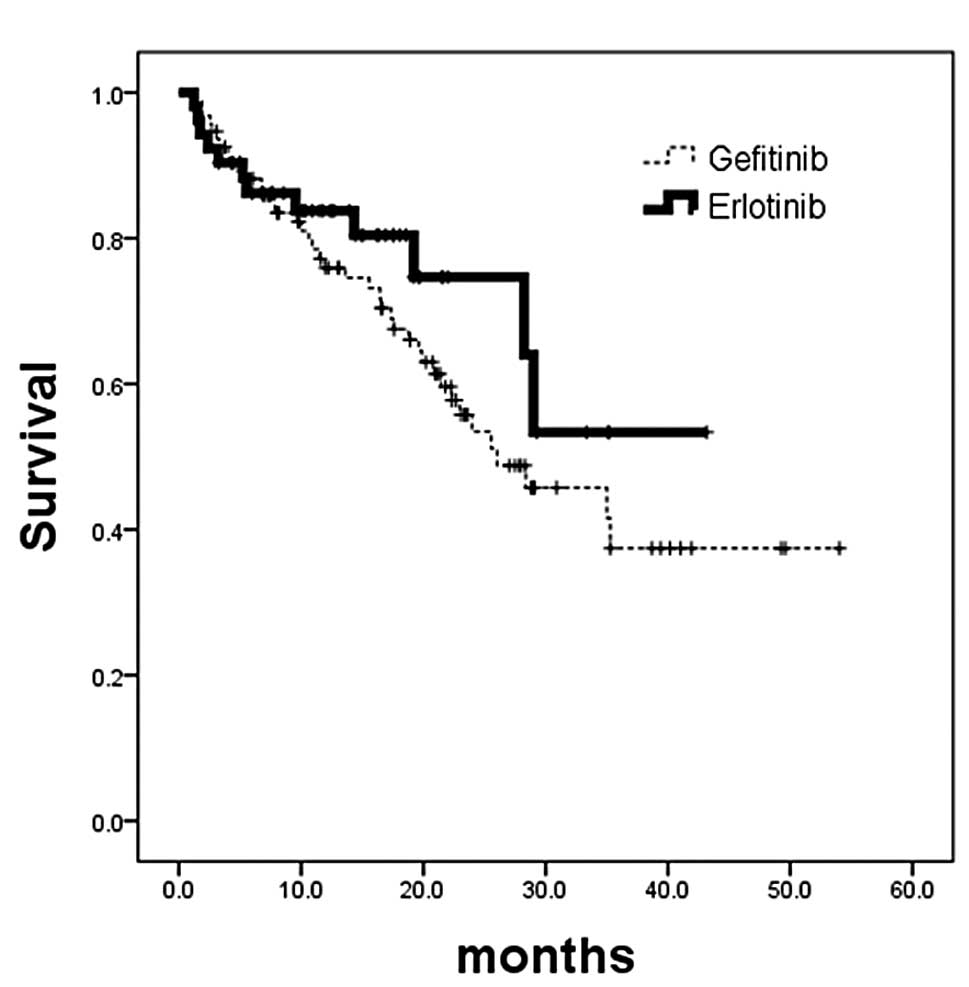 | Figure 4.Kaplan-Meier analysis of overall
survival calculated from patients commencing EGFR-TKI treatment.
(A) Patients whose tumors had EGFR-activating mutations. No
survival difference was noted in patients who received gefitinib
treatment (n=94; censor, 54; median, 26 months) or erlotinib
treatment (n=52; censor, 40; median, not reached) (p=0.2728; HR,
0.49; 95% CI, 0.36–1.22). (B) Patients whose tumors did not have
EGFR-activating mutations. No survival difference was noted in
patients who received gefitinib treatment (n=30; censor, 14;
median, 8.6 months) or erlotinib treatment (n=48; censor, 31;
median, not reached) (p=0.1104; HR, 0.58; 95% CI, 0.29–1.14). TKI,
tyrosine kinase inhibitor; EGFR, epidermal growth factor receptor;
HR, hazard ratio. |
Discussion
Treatment with one of the EGFR-TKIs, gefitinib or
erlotinib, has become an important option for patients with
advanced NSCLC. The tumor EGFR mutation rate is approximately 3
times more prevalent in Asian patients than in Caucasians. An
effect on overall survival in genotypically uncharacterized NSCLC
patients was observed with erlotinib (BR.21 trial) (3), but not gefitinib (ISEL trial)
(2), although the response rates
were similar. Several possible explanations have been offered, and
the focus has mainly been on the suboptimal dosage of gefitinib
(17). Erlotinib was administered
at its maximum tolerated dose (MTD), while gefitinib was
administered at approximately one third of its MTD (13–16).
Erlotinib treatment may be more efficacious than gefitinib in
EGFR-wild type or atypical mutated lung tumors, since erlotinib
inhibits the activity of wild-type EGFR-TKI in tumor cells at 50%
inhibitory concentrations of 2–20 nmol/l. By contrast, several-fold
higher drug concentrations of gefitinib are required to block
wild-type or atypical mutated EGFR signaling (21–24).
This result was supported by evidence from patients lacking
EGFR-activating mutations who still achieved a benefit from
erlotinib following failure of gefitinib treatment (25). By contrast, for patients or
cell-lines with EGFR-activating mutations, such as exon 19
deletions and exon 21 L858R point mutations, both gefitinib and
erlotinib are highly effective (26,27).
Clinical data from a recent molecular analysis of the BR.21 trial
also demonstrated that the survival impact of erlotinib was not
confounded significantly by tumor cell EGFR mutation status
(28). Furthermore, the SATURN
trial, which tested maintenance erlotinib following chemotherapy
revealed that PFS and overall survival were prolonged in patients
with or without EGFR-activating mutations (29). These results suggest that erlotinib
treatment is beneficial, irrespective of EGFR mutation status. The
present study revealed a better response rate and PFS in patients
whose tumors had EGFR-activating mutations. In addition, response
rate and PFS did not differ in patients with EGFR-activating
mutations, regardless of whether they received gefitinib or
erlotinib. These findings are compatible with cell line findings of
hypersensitive cell lines with EGFR-activating mutations responding
well to both agents (26,27). Multivariate analysis also revealed
that having, or not having, an EGFR-activating mutation is a
significant prognostic factor for PFS, while the type of EGFR-TKI
used is not a significant prognostic factor.
Our previous findings of a similar response rate,
better disease control rate, longer PFS and overall survival, in a
multi-center, retrospective analysis of NSCLC patients with unknown
tumor EGFR mutation status, were again partially documented in the
present study. There was an improved response rate and longer PFS
in patients without tumor EGFR-activating mutations (17). This effectiveness in patients
without EGFR-activating mutations is supported by cell line
findings and is also possibly due to the higher dose intensity of
erlotinib than gefitinib.
Since the EGFR-activating mutation rate was high, up
to 67.6% (73 out of 108), in the male patients in our study and in
61.3% (38 out of 62) of patients who were smokers, and both
clinical characteristics were considered to be poor clinical
predictors for EGFR-activating mutations, all of our Taiwanese or
Chinese adenocarcinoma patients should receive tumor EGFR mutation
analysis.
This study had several limitations, such as the
retrospective study design with its inherent potential for bias,
and the fact that no toxicity profiles were reported. It was
considered that there may be some differences in the frequency of
adverse effects between the two agents due to varying dose
intensities.
In conclusion, there was no difference in treatment
response rate when one or the other agent was administered to
patients with tumors either with or without EGFR-activating
mutations. PFS also did not differ with either agent in patients
whose tumors had EGFR-activating mutations, while PFS was
significantly longer for patients receiving erlotinib treatment
whose tumors did not have EGFR-activating mutations. Thus, both
agents may be used in patients whose tumors have EGFR-activating
mutations with similar efficacy, but not in patients whose tumors
do not have EGFR-activating mutations; for these, the treatment
should be erlotinib.
Acknowledgements
This research was supported in part by
grants from the National Science Council of the Republic of China,
grant number NSC99-2314-B-075-035-MY3, Department of Health of the
Republic of China, grant number DOH100-TD-C-111-007, and Taipei
Veterans General Hospital, grant number V99C1-050.
References
|
1.
|
DM ParkinF BrayJ FerlayGlobal cancer
statisticsCA Cancer J Clin5574108200510.3322/canjclin.55.2.74
|
|
2.
|
N ThatcherA ChangP ParikhGefitinib plus
best supportive care in previously treated patients with refractory
advanced non-small-cell lung cancer: results from a randomised,
placebo-controlled, multicentre study (Iressa Survival Evaluation
in Lung
Cancer)Lancet36615271537200510.1016/S0140-6736(05)67625-8
|
|
3.
|
FA ShepherdPJ RodriguesT CiuleanuErlotinib
in previously treated non-small-cell lung cancerN Engl J
Med353123132200510.1056/NEJMoa05075316014882
|
|
4.
|
A ChangP ParikhS ThongprasertGefitinib
(IRESSA) in patients of Asian origin with refractory advanced
non-small cell lung cancer: Subset analysis from the ISEL studyJ
Thorac Oncol1847855200610.1097/01243894-200610000-0001417409969
|
|
5.
|
ES KimV HirschT MokGefitinib versus
docetaxel in previously treated non-small-cell lung cancer
(INTEREST): a randomised phase III
trialLancet37218091818200810.1016/S0140-6736(08)61758-419027483
|
|
6.
|
TS MokY-L WuS ThongprasertGefitinib or
carboplatin-paclitaxel in pulmonary adenocarcinomaN Engl J
Med361947957200910.1056/NEJMoa081069919692680
|
|
7.
|
R RosellT MoranC QueraltScreening for
epidermal growth factor receptor mutations in lung cancerN Engl J
Med361958967200910.1056/NEJMoa090455419692684
|
|
8.
|
C ZhouYL WuG ChenEfficacy results from the
randomized phase III OPTIMAL (CTONG 0802) study comparing
first-line erlotinib versus carboplatin (CBDCA) plus gemcitabine
(GEM), in Chinese advanced non-small-cell lung cancer (NSCLC)
patients (PTS) with EGFR activating mutationsAnn Oncol21Suppl
8LBA132010
|
|
9.
|
YM ChenJ Whang-PengCM ChenReview of
first-line systemic therapy for metastatic non-small cell lung
cancerJ Exp Clin Med3116120201110.1016/j.jecm.2011.04.008
|
|
10.
|
PA JanneBE JohnsonEffect of epidermal
growth factor receptor tyrosine kinase domain mutations on the
outcome of patients with non-small cell lung cancer treated with
epidermal growth factor receptor tyrosine kinase inhibitorsClin
Cancer Res12Suppl 1444164420200610.1158/1078-0432.CCR-06-0555
|
|
11.
|
T KosakaY YatabeH EndohMutations of the
epidermal growth factor receptor gene in lung cancer: biological
and clinical implicationsCancer
Res6489198923200410.1158/0008-5472.CAN-04-2818
|
|
12.
|
H ShigematsuL LinT TakahashiClinical and
biological features associated with epidermal growth factor
receptor gene mutations in lung cancersJ Natl Cancer
Inst97339346200510.1093/jnci/dji05515741570
|
|
13.
|
J BaselgaD RischinM RansonPhase I safety,
pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective
oral epidermal growth factor receptor tyrosine kinase inhibitor, in
patients with five selected solid tumor typesJ Clin
Oncol2042924302200210.1200/JCO.2002.03.100
|
|
14.
|
RS HerbstAM MaddoxML RothenbergSelective
oral epidermal growth factor receptor tyrosine kinase inhibitor
ZD1839 is generally well-tolerated and has activity in
non-small-cell lung cancer and other solid tumors: results of a
phase I trialJ Clin Oncol2038153825200210.1200/JCO.2002.03.038
|
|
15.
|
M HidalgoLL SiuJ NemunaitisPhase I and
pharmacologic study of OSI-774, an epidermal growth factor receptor
tyrosine kinase inhibitor, in patients with advanced solid
malignanciesJ Clin Oncol1932673279200111432895
|
|
16.
|
SV SharmaDW BellJ SettlemanEGFR growth
factor mutations in lung cancerNat Rev
Cancer7169181200710.1038/nrc2088
|
|
17.
|
WC FanCJ YuCM TsaiDifferent efficacies of
erlotinib and gefitinib in Taiwanese patients with advanced
non-small cell lung cancer: a retrospective multi-center studyJ
Thor Oncol6148155201110.1097/JTO.0b013e3181f77b2721107294
|
|
18.
|
T CiuleanuL StelmakhS CicenasErlotinib
versus docetaxel or pemetrexed as second-line therapy in patients
with advanced non-small-cell lung cancer (NSCLC) and poor
prognosis: efficacy and safety results from the phase III TITAN
studyChicago Multidisciplinary Symposium in Thoracic Oncol:
LBOA52010
|
|
19.
|
L VamvakasS AgelakiNK
KentepozidisPemetrexed (MTA) compared with erlotinib (ERL) in
pretreated patients with advanced non-small cell lung cancer
(NSCLC): results of a randomized phase III Hellenic Oncology
Research Group trialProc ASCO2875192010
|
|
20.
|
P TherasseSG ArbuckEA EisenhauerNew
guidelines to evaluate the response to treatment in solid tumors.
European Organisation for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of CanadaJ Natl Cancer
Inst92205216200010.1093/jnci/92.3.205
|
|
21.
|
TJ LynchDW BellR SordellaActivating
mutations in the epidermal growth factor receptor underlying
responsiveness of non-small cell lung cancer to gefitinibN Engl J
Med35021292139200410.1056/NEJMoa04093815118073
|
|
22.
|
JG PaezPA JanneJC LeeEGFR mutations in
lung cancer: correlation with clinical response to gefitinib
therapyScience30414971500200410.1126/science.109931415118125
|
|
23.
|
JD MoyerEG BarbacciKK IwataInduction of
apoptosis and cell cycle arrest by OSI-774, an inhibitor of
epidermal growth factor receptor tyrosine kinaseCancer
Res574838484819979354447
|
|
24.
|
VA PolackDM SavageDA BakerInhibition of
epidermal growth factor receptor-associated tyrosine
phosphorylation in human carcinoma with OSI-774: dynamics of
receptor inhibition in situ and antitumor effects in athymic miceJ
Pharmacol Exp Ther2917397481999
|
|
25.
|
BC ChoCK ImMS ParkPhase II study of
erlotinib in advanced non-small cell lung cancer after failure of
gefitinibJ Clin
Oncol2525282533200710.1200/JCO.2006.10.416617577030
|
|
26.
|
SV SharmaDW BellJ SettlemanEpidermal
growth factor receptor mutations in lung cancerNat Rev
Cancer7169181200710.1038/nrc208817318210
|
|
27.
|
J GandhiJ ZhangY XieAlterations in genes
of the EGFR signaling pathway and their relationship to EGFR
tyrosine kinase inhibitor sensitivity in lung cancer cell linesPLoS
One4e4576200910.1371/journal.pone.000457619238210
|
|
28.
|
CQ ZhuGC SantosK DingRole of KRAS and EGFR
as biomarkers of response to erlotinib in National Cancer Institute
of Canada Clinical Trials Group Study BR.21J Clin
Oncol2642684275200810.1200/JCO.2007.14.892418626007
|
|
29.
|
F CappuzzoT CiuleanuL StelmakhErlotinib as
maintenance treatment in advanced non-small cell lung cancer: a
multicentre, randomised, placebo-controlled phase 3 studyLancet
Oncol11521529201010.1016/S1470-2045(10)70112-120493771
|