Introduction
Hepatocellular carcinoma (HCC) is the third most
common cause of cancer-related mortality worldwide (1) and the second most common cause of
cancer-related mortality in China (2). The majority of patients with advanced
HCC at the time of initial diagnosis exhibit poor outcomes
(3). In China, HCC is most
commonly caused by infection with the hepatitis B virus (HBV)
(4). The incidence of HCC has
increased in recent years, largely owing to chronic HBV
infection-related liver cirrhosis (5). Therapeutic options are
stage-dependent (6,7). Only approximately 30% of patients who
present with early stage tumors undergo resection, liver
transplantation and percutaneous ablation, due to various factors,
such as multi-focal tumor and poor liver function resulting from
underlying cirrhosis (8–10). Until recently, no effective
treatment was available for these conditions (11). Sorafenib is a newly developed,
molecular targeted agent. This multikinase inhibitor has
demonstrated significant survival benefits in phase III trials for
patients with advanced HCC (12,13).
However, its efficacy remains moderate and certain patients
continue to display a short period of survival following treatment
(14). The mechanisms causing
certain patients to become refractory to sorafenib are, at present,
unclear. High intratumoral microvessel density (MVD) has been
associated with a higher level of activity along the vascular
endothelial growth factor (VEGF)/VEGF receptor (VEGFR) signaling
pathway. As such, the presence of high intratumoral MVD in advanced
HCC patients may be associated with a positive response to
sorafenib treatment. However, it remains unknown as to whether the
presence of high intratumoral MVD is capable of effecting responses
to sorafenib treatment in advanced HCC patients. Previous findings
have provided a strong rationale for combining the two treatment
modalities. In mice with implanted renal tumors, the combination of
radiofrequency ablation (RFA) and sorafenib has been found to cause
an increase in the efficacy of tumor ablation that is dependent on
the dose of sorafenib (15).
Although the value of cryotherapy (cryoRx) in HCC is not yet as
well established as that of RFA, cryoRx has been found to be more
advantageous for improving immunity following treatment compared to
RFA. Osada et al found that not only the local tumor, but
also the adjacent tumor tissue was necrotic and shrunken in HCC
patients following cryoablation, which was regarded as ectopic
tumor suppression (16). This
response may be associated with the release of tumor antigens,
resulting in the host production of anti-tumor antibodies (17). The majority of the bias against
cryoRx for HCC is based on the theoretical risk associated with a
cryoablation modality that does not employ cauterization-like,
heat-based ablation therapy and as a result of the large probes,
which may cause serious bleeding when removed (18). The experimental evaluation has
identified no significant difference among the hemorrhages
encountered following an ablation with a single RF probe versus a
single cryoprobe (19). Therefore,
this technology has been used extensively in open surgical settings
and, more recently, applied percutaneously to treat renal tumors
and liver metastases (20,21). Nevertheless, the efficacy of the
use of cryoRx for the improvement of clinical outcomes of sorafenib
for the treatment of advanced HCC is, at present, unknown. The aim
of this study was to confirm the efficacy and safety of sorafenib
combined with cryoRx for the treatment of advanced HCC, as well as
the croyablation tumor burden impact for sorafenib therapy
responses.
Patients and methods
Patients
Based on the Barcelona Clinic Liver Cancer (BCLC)
staging classification (7), 296
consecutive patients with HBV-related advanced HCC were screened
between July 2008 and July 2010, at the Center of Therapeutic
Research for Hepatocellular Carcinoma (Beijing, China). A total of
57 patients were classified as Child-Pugh C, 38 patients were
classified as Child-Pugh B8 or B9, with serum bilirubin levels of
>51.3 μmol/l. The life expectancy of 23 patients was <12
weeks, 10 patients had an Eastern Cooperative Oncology Group
Performance Status (ECOG PS) of ≥3 and 64 patients had a history of
hepatectomy (8), preoperative
chemotherapy (6), prior
transarterial chemoembolization (TACE) or local ablation (44), and
radiotherapy (6). Consequently,
192 patients were excluded from this study. A total of 104 patients
with advanced HCC were eligible for evaluation (Table I). The diagnosis of HCC (6) was indicated by imaging findings and
confirmed by biopsy (single action biopsy device, 16 g; Promex
Technologies, Franklin, IN, USA). The histological grade of the
tumor differentiation was determined by the Edmondson
classification into well, moderately and poorly differentiated
(22). Portal vein thrombosis
(PVT), as a sign of macroscopic vascular invasion and extrahepatic
spread, was used to define advanced HCC; however, patients
exhibiting extrahepatic spread were excluded from the study.
Eligibility criteria also included ECOG PS of 0, 1 or 2; Child-Pugh
class A or B; life expectancy of at least 12 weeks; total bilirubin
concentration of ≤51.3 μmol/l and HBV DNA positivity. In addition,
patients considered for inclusion were required to exhibit at least
one tumor lesion that could be measured along one dimension
according to modified RECIST (mRECIST) assessment for HCC (23).
 | Table I.Demographic and baseline
characteristics of patients. |
Table I.
Demographic and baseline
characteristics of patients.
| Variable | Combination therapy
(n=52) | Sorafenib
(n=52) |
|---|
| Age (years) | 51.2±11.9 | 52.6±8.3 |
| Gender (%) | | |
| Male | 48 (92.3) | 47 (90.4) |
| Female | 4 (7.7) | 5 (9.6) |
| ECOG performance
status (%) | | |
| 0 | 16 (30.8) | 17 (32.7) |
| 1 | 29 (55.7) | 30 (58) |
| 2 | 7 (13.5) | 5 (9) |
| BCLC stage C
(%) | 52 (100) | 52 (100) |
| Tumor diameter - cm
(range) | 8.39±4.38
(3.5–12.8) | 8.32±2.72
(3.2–13.2) |
| Number of tumor
sites (%) | | |
| 1 | 7 (13.5) | 6 (11.5) |
| 2 | 9 (17.3) | 10 (19.2) |
| 3 | 10 (19.2) | 11 (21.2) |
| ≥4 | 26 (50) | 25 (48.1) |
| Macroscopic
vascular invasion (%) | | |
| Branch | 36 (69.3) | 37 (71.2) |
| Trunk | 16 (30.7) | 15 (28.8) |
| Differentiated
tumor (%) | | |
| Well | 9 (17.3) | 10 (19.2) |
| Moderately | 30 (57.7) | 30 (57.7) |
| Poorly | 13 (25) | 12 (23.1) |
| HBV DNA positive
(%) | | |
| 100-9,999 | 22 (42.3) | 24 (46.2) |
|
10,000–99,999 | 19 (36.5) | 18 (34.6) |
| ≥100,000 | 11 (21.2) | 10 (19.2) |
| Child-Pugh class
(%) | | |
| A | 41 (78.8) | 43 (83) |
| B | 11 (21.2) | 9 (17) |
Study design
According to the SHARP trial (13), the overall survival (OS) rate of
the advanced HCC patients for sorafenib at the 15-month time-point
was 37%. The sample size calculation is based on the detection of
significant differences in OS (the second end-point parameter of
this trial), assuming that the OS rate is 50% for the combination
therapy group at the 15-month interval. A total of 90 patients were
required for a log-rank test with an overall two-sided significance
level of 0.05 and power of 0.805. From our experience, it was
expected that 15–20% of the patients would drop-out following
randomization. In order to accommodate for the drop-out rate, the
total sample size was thereby increased to 104. Study randomization
was centralized, investigator-initiated and was approved by the
Ethics Committee of the Beijing 302nd Hospital (China). Written
informed consent was obtained from the patients prior to
enrollment. All eligible patients were randomly assigned (with a
1:1 ratio) to the sorafenib and cryoRx group (n=52) or the
sorafenib-alone group (n=52) using simple computer randomization to
achieve a balance between the two groups. All eligible patients
received continuous oral treatment with 0.1 g of lamivudine once
daily. None of the patients had received prior treatment, such as
chemotherapy or radiation therapy.
Treatment and disease assessment
Sorafenib administration
All patients received sorafenib at a dose of 400 mg
twice daily for at least 8 weeks. Treatment interruptions and dose
reductions (first 400 mg twice daily, then 200 mg twice daily) were
permitted for adverse drug reactions (ADRs) according to the
National Cancer Institute Common Toxicity Criteria (24). For ADRs of grade 3–4, the sorafenib
dose was decreased to 200 mg twice daily until the ADRs improved to
a grade of ≤2, then increased to 400 mg twice daily if well
tolerated. Therapy was discontinued if the following criteria were
met: ADRs that required termination of medication, deterioration of
ECOG PS score to 4 and withdrawal of consent. If disease
progression was observed, sorafenib was continued when the patient
was considered to have a good clinical status (e.g., PS, liver
function and tolerable side-effects) and wished to continue the
treatment. Following sorafenib treatment, cryoRx was conducted in
those without absolute contraindications, based on the potential
clinical benefits expected from the treatment and the patient’s
consent. Sorafenib therapy was continued without interruption
during local therapies.
CryoRx procedure
Cryoablation was performed as described previously
(25). Briefly, the size and
number of the probes depended on the location and average size of
the lesions to be ablated. An argon-helium gas-based CRYOcare
system (EndoCare, Inc., Irvine, CA, USA) and cryoprobes (2 and 3
mm) were used to freeze the tumor with a dual freeze-thaw cycle
under ultrasound-guidance. For optimum reduction of tumor burden,
cryoablation was carried out in single or repeated doses. For
tumors <5 cm in diameter, the aim was for complete ablation, for
larger tumors, the aim was to reduce the tumor burden by at least
60% of the original tumor burden. Cryoablation was limited to three
procedures at most.
Immunohistochemical staining for
CD34
All samples from HCC patients were reviewed
histologically using hematoxylin and eosin staining, the
paraffin-embedded samples were cut into 5-μm sections and processed
for immunohistochemistry according to the manufacturer’s
instructions, as previously described (26). Tumor sections were immunostained
with human CD34 monoclonal antibody (BioGenex, San Ramon, CA, USA).
Tissue sections were incubated with primary CD34 monoclonal
antibodies. Subsequently, secondary biotinylated antimouse
immunoglobulin (Dako North America, Inc., USA) was applied and
reacted with streptavidin biotinylated horseradish peroxidase
complex (Dako). The negative control was obtained by substituting
the primary antibodies with mouse immunoglobulin G.
Determination of MVD
The intratumoral MVD was evaluated by two
independent observers who were blinded to the patients’ clinical
data. The tissue sections were screened at a low power field (x40)
and the five areas with the most intense neovascularization (hot
spots) were selected. Microvessel counts of these areas were
performed with a high power field (x200). To reduce
observer-related variation, counting of microvessels was performed
using the computer image analyzer (MetaMorph Imaging System Version
3.0; Universal Imaging Corp, West Chester, PA, USA). Microvessels,
tumor cells and connective elements were counted as one
microvessel, irrespective of the presence of a vessel lumen. The
mean microvessel count of the five most vascular areas was taken to
constitute the MVD, which was expressed as the absolute number of
microvessels per 0.74 mm2 (x200 field).
Disease assessment
Based on the computed tomography/magnetic resonance
tomography (MRT) scans of the liver performed at baseline and every
8 weeks, tumor response (sorafenib combined with cryoRx or
sorafenib alone) was evaluated according to mRECIST (22) assessment for HCC by independent
radiologists. Patients who succumbed to the disease prior to their
first radiographic control were judged as having progressive
disease (PD). Patients who exhibited complete response (CR) or
partial response (PR) were defined as achieving a clinical efficacy
response (CER).
End-points
The primary end-point of the study was OS. The
secondary end-points included time to progression (TTP), the
disease-control rate (DCR) and tolerability. The OS was defined as
the time from sorafenib initiation to the date of expiration or the
patient’s last follow-up. TTP was defined as the time from
sorafenib initiation to the date of disease progression or
expiration. The disease progression was defined as the tumor
progression according to mRECIST criteria or progression of
cirrhosis.
Statistical analysis
Continuous data were expressed as the median and
range. All continuous data were classified into subgroups according
to the median for analysis. Associations between OS, TTP and
potential prognostic factors were assessed by the Kaplan-Meier
method (log-rank test) in a univariable analysis. The Cox
proportional hazards model was used for multivariate analyses with
a step-wise procedure and a significance level of 0.10 was used to
enter and remove variables. All statistical analyses were performed
using SPSS software version 16.0 for Windows (SPSS Inc., Chicago,
IL, USA). P-values <0.05 were considered to indicate
statistically significant differences.
Results
Patient characteristics
The combination therapy and sorafenib-alone
treatment groups were well balanced with regard to baseline
demographic and disease characteristics (Table I). A total of 84 (80.8%) patients
were classified as Child-Pugh class A and 20 (19.2%) patients were
classified as Child-Pugh class B. A total of 33 (31.7%) patients
were ECOG PS 0, 59 patients (56.7%) were ECOG PS 1, and 12 patients
(11.6%) were ECOG PS 2. The tumor differentiation was high in 19
(18.3%), intermediate in 60 (57.7%) and low in 25 patients (24.0%).
The HBV DNA loads were low in 46 (44.2%), intermediate in 37
(35.6%) and high in 21 patients (20.2%).
Adverse events
With regard to non-hematological toxicity, rash was
observed most commonly (62%), followed by hypertension (56%),
weight loss (52.9%), alopecia (50%), diarrhea (46%), fatigue
(43.3%), hand-foot skin reaction (HFSR) (42%), liver dysfunction
(34.6%), voice change (18%), abdominal pain (12.5%) and upper
gastrointestinal tract bleeding (16%). Furthermore, the grade 3 or
4 non-hematological toxicities were HFSR, diarrhea, liver
dysfunction and upper gastrointestinal tract bleeding, which
occurred in 12.2, 12, 6.4 and 6% of patients, respectively. With
regard to hematological toxicity, leukopenia (24%) was the most
common sign of toxicity, followed by thrombocytopenia (12%) and
anemia (8%).
Overall response and efficacy
The median time of follow-up was 10.5 months (range
4.0–26.0) and the median duration of sorafenib treatment was 7.5
months (2.5–26.0). A total of 10 (9.6%) patients discontinued
sorafenib treatment at 6–24 weeks due to liver function
deterioration (6 cases) and bleeding from gastroesophageal varices
(4 cases), 21 (20.2%) patients reduced sorafenib dosage to 200 mg
twice daily due to grade 3–4 ADRs. However, all these patients
restored the dose to 400 mg twice daily after 2 to 3 weeks.
Overall, patients receiving the combination therapy had a median OS
of 12.5 months (95% CI 10.6–16.4), compared to 8.6 months (95% CI
7.3–10.4) for those receiving sorafenib (log-rank, P=0.009;
Fig. 1B). In addition, patients
receiving combination therapy had a significantly longer median TTP
[9.5 months (95% CI 8.4–13.5)] than patients receiving sorafeinb
[5.3 months (95% CI 3.8–6.9), log-rank P=0.024; Fig. 1C). In the analysis for best
response, 4 out of 52 patients receiving combination therapy (7.6%,
Fig. 1A) exhibited a CR, 9
patients (17.3%) exhibited a PR, 22 patients (42.3%) exhibited
stable disease (SD), whereas out of the patients receiving
sorafenib treatment alone, 4 (7.6%) and 19 (36.5%) patients
exhibited PR and SD, respectively. The rates of CER and DCR were
significantly higher in the combination therapy group (CER, 22% and
DCR, 66%) than in the sorafenib group [CER, 7.6% (P=0.04) and DCR,
44.2% (P=0.03); Table II]. A total
of 67 advanced HCC patients receiving lamivudine treatment with HBV
DNA-negative status had a median OS of 11.5 months (95% CI
6.3–14.8), compared to 8.0 months (95% CI 5.8–11.5) for the 37
patients receiving lamivudine treatment with HBV DNA-positive
status (log-rank, P=0.035). There were no significant differences
between the median TTP for the two groups (log-rank, P=0.544).
Disease progression occurred in 86 (82.6%) patients, the OS was
significantly longer in 50 patients with a clinically stable
presentation who continued sorafenib treatment than in those with
clinical deterioration who discontinued therapy (11 vs. 7.5 months,
P<0.001; Fig. 1D). Furthermore,
53 (50.9%) patients succumbed to the disease due to
recurrence/metastasis in 25 (24%), liver failure in 13 (12.5%),
bleeding from gastroesophageal varices in 8 (7.7%), refractory
ascites-induced renal failure in 4 (3.8%) and tumor
rupture/hemorrhage in 3 patients (2.9%).
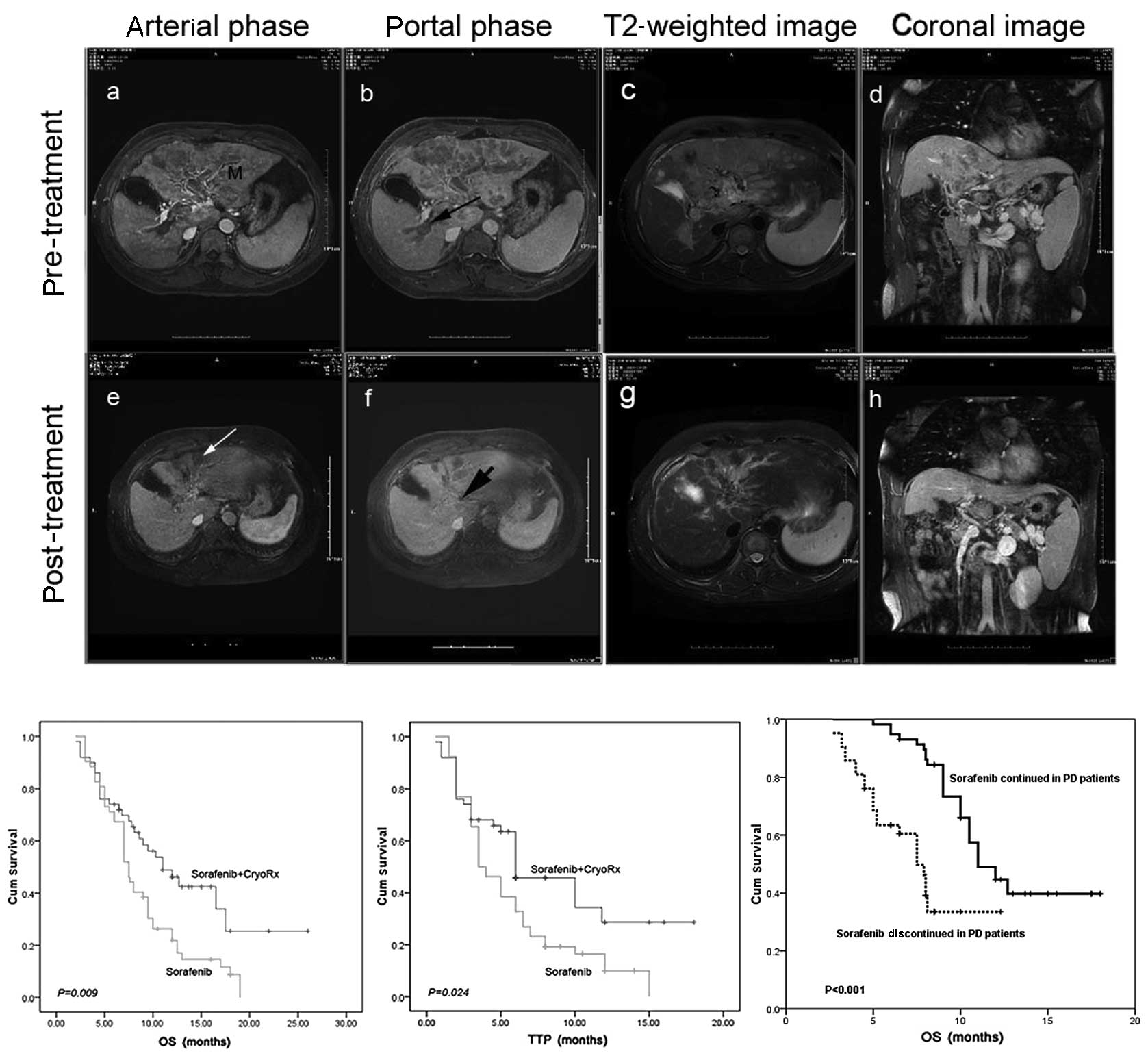 | Figure 1.Overall response and efficacy of the
combined sorafenib and cryotherapy in advanced HCC. (A) Overall
response was a complete response in a live 55-year-old male over a
24-month period at end-point. (A-a) MRI scan reveals a huge mass
(M) at the dome of the left hepatic lobe (upper section). (A-b)
Long black arrow indicates portal vein thrombosis,
histopathological diagnosis was HCC, Edmondson classification to
moderately differentiated. (A-c) The large mass at the dome of the
left hepatic lobe is shown on T2-weighted image of MRI. (A-d)
Portal vein thrombosis is shown on coronal image of MRI. Following
continuation of sorafenib treatment, concurrent receipt of 2-times
percutaneous cryoablation reduced the tumor burden up to 60% of the
original tumor burden 2 weeks, 10 months later. Not only did the
treated tumor shrink, but the non-treated tumor also decreased
(lower). (A-e) Long white arrow reveals that the treated tumor was
necrotic. (A-f) Short black arrow indicates that the portal vein
tumor thrombus was almost invisible. (A-g) T2-weighted image of MRI
shows the treated tumor. (A-h) Coronal image of MRI indicates that
the portal vein tumor thrombus was almost invisible. (B)
Kaplan-Meier survival curves for 52 patients with the combination
therapy and 52 patients with sorafenib-alone treatment; the median
OS was significantly different between groups. (C) Kaplan-Meier
survival curves displaying significant differences in TTP in
patients between the combination therapy and sorafenib-alone
treatment groups. (D) Kaplan-Meier analysis for the effect of
continuing sorafenib therapy in patients with radiologic
progressive disease on OS. TTP, time to progression; OS, overall
survival; HCC, hepatocellular carcinoma; MRI, magnetic resonance
imaging; PD, progressive disease; Cum, cumulative. |
 | Table II.Summary of efficacy measures. |
Table II.
Summary of efficacy measures.
| Outcome | Combination therapy
(n=52) | Sorafenib
(n=52) | P-value |
|---|
| Overall survival
(months) | 0.009 | | |
| Median | 12.5 | 8.6 | |
| (95% CI) | 10.6–16.4 | 7.3–10.4 | |
| Time to
progression | 0.024 | | |
| Median | 9.5 | 5.3 | |
| (95% CI) | 8.4–13.5 | 3.8–6.9 | |
| Level of response
(%) | | | |
| Complete
response | 3 (5.7) | 0 | NA |
| Partial
response | 9 (17.3) | 4 (7.6) | 0.1186 |
| Stable
disease | 22 (42.3) | 19 (36.5) | 0.4423 |
| CER (%) | 12 (23.0) | 4 (7.6) | 0.0314 |
| DCR (%) | 34 (65.4) | 23 (44.2) | 0.0212 |
Response and efficacy according to
intratumoral MVD
Specific staining of capillary-like vessels by
anti-CD34 was observed in intratumoral specimens in all outcome
groups (CR+PR, mean MVD-CD34, 111±49/0.74 mm2; SD,
206±74/0.74 mm2; PD, 339±92/0.74 mm2;
Fig. 2A and B). The mean MVD-CD34
in the responsive (CR+PR) patients was significantly lower than
that of PD patients (P<0.001). At the time of analysis, all
patients were divided into two groups by the median MVD value
(median, 219.5/0.74 mm2; range, from 34 to 512/0.74
mm2). When the entire cohort of 104 patients was
analyzed, 53 patients with low intratumoral MVD (≤219.5/0.74
mm2), receiving combination therapy, had longer TTP and
OS than that of patients receiving sorafenib-alone treatment
[log-rank, P=0.018 (TTP); P=0.023 (OS); Fig. 2C-a and b]. TTP and OS were not
significantly different between groups [log-rank, P=0.312 (TTP);
P=0.062 (OS); Fig. 2C-c and d] in
51 patients exhibiting a high intratumoral MVD (>219.5/0.74
mm2).
 | Figure 2.Comparison of intratumoral MVD-CD34
in advanced HCC patients displaying various overall responses. (A)
Intratumoral MVD by anti-CD34 immunostaining (pink staining) (left,
low MVD-CD34; right, high MVD-CD34; x200). (B) The mean
intratumoral MVD-CD34 significantly increased with poor overall
response. (C) Analysis of a cohort of 104 patients. Kaplan-Meier
survival curves for 51 patients with low MVD-CD34 (≤219.5/0.74
mm2); (C-a) compared to the TTP, revealed significant
differences in patients between the combination therapy and
sorafenib-alone treatment groups; (C-b) the median OS was
significantly different between groups. Kaplan-Meier survival
curves for 53 patients with high MVD-CD34 (>219.5/0.74
mm2); (C-c) the TTP and (C-d) the OS displayed no
significant differences between the two groups. OS, overall
survival; PD, progressive disease; SD, stable disease; CR, complete
response; PR, partial response; MVD, microvessel density; TTP, time
to progression; Cum, cumulative. |
Univariate and multivariate analysis
of predictive factors for TTP and OS
The combination therapy or the sorafenib-alone
treatment groups exhibited a clinical benefit in all preplanned
subgroup analyses, despite certain patients having characteristics
associated with poor prognosis, including poorer ECOG PS ≥1, tumor
diameter >7 cm, PVT within the trunk, high HBV DNA load,
Child-Pugh class B, fatigue, weight loss, α-fetoprotein (AFP)
levels of >895 ng/ml and high intratumoral MVD (Table III).
 | Table III.Univariate analysis of patient
demographics and clinical characteristics for predictive factors of
TTP and OS. |
Table III.
Univariate analysis of patient
demographics and clinical characteristics for predictive factors of
TTP and OS.
| Parameter | No. of patients who
expired | TTP (months)
| OS (months)
|
|---|
| Median | P-value | Median | P-value |
|---|
| Gender | | | 0.514 | | 0.781 |
| Male | 48 | 6.0 | | 10.5 | |
| Female | 5 | 4.5 | | 9.0 | |
| Age (years) | | | 0.668 | | 0.228 |
| ≤51 | 28 | 5.0 | | 9.0 | |
| >51 | 25 | 6.0 | | 10.5 | |
| ECOG PS | | | <0.001 | | <0.001 |
| 0 | 7 | 8.5 | | 17.0 | |
| 1 | 35 | 6.0 | | 11.0 | |
| 2 | 11 | 3.0 | | 6.5 | |
| Tumor
differentiation | | | 0.155 | | 0.401 |
| Well | 10 | 4.5 | | 9.0 | |
| Moderate | 29 | 4.0 | | 8.0 | |
| Poor | 14 | 3.0 | | 7.0 | |
| Tumor diameter
(cm) | | | 0.025 | | 0.007 |
| ≤7 | 19 | 6.0 | | 12.0 | |
| >7 | 34 | 5.0 | | 8.1 | |
| Tumor number | | | 0.165 | | 0.995 |
| 1 | 15 | 6.0 | | 12.7 | |
| 2 | 11 | 6.0 | | 11.0 | |
| 3 | 11 | 5.0 | | 10.0 | |
| 4 | 21 | 4.0 | | 10.0 | |
| Invasion of portal
vein | | | 0.019 | | 0.040 |
| Branch | 30 | 6.0 | | 11.0 | |
| Trunk | 23 | 4.0 | | 9.0 | |
| HBV DNA
(IU/ml) | | | <0.001 | | <0.001 |
| 0–9,999 | 26 | 6.0 | | 12.7 | |
|
10,000–99,999 | 10 | 4.0 | | 10.0 | |
| ≥100,000 | 22 | 3.0 | | 8.0 | |
| Therapy | | | <0.001 | | <0.001 |
| Combined
treatment | 21 | 9.5 | | 12.5 | |
| Sorafenib
alone | 32 | 5.3 | | 8.6 | |
| Child-Pugh
class | | | <0.001 | | <0.001 |
| A | 39 | 6.0 | | 11.5 | |
| B | 14 | 3.5 | | 7.0 | |
| Weight loss | | | 0.001 | | <0.001 |
| Grade 0 | 13 | 6.5 | | 11.2 | |
| Grade 1–4 | 40 | 4.0 | | 7.0 | |
| Fatigue | | | <0.001 | | <0.001 |
| Grade 0 | 21 | 7.0 | | 13.0 | |
| Grade 1–4 | 32 | 4.0 | | 8.1 | |
| AFP levels
(ng/ml) | | | 0.005 | | 0.001 |
| ≤895 | 20 | 6.0 | | 12.7 | |
| >895 | 33 | 4.0 | | 9.0 | |
| Intratumoral
MVD | | | <0.001 | | <0.001 |
| High | 34 | 3.0 | | 7.0 | |
| Low | 19 | 8.8 | | 13.5 | |
Cox proportional hazards model analyses (Table IV) revealed that cryoRx was
independently associated with improved TTP and OS of sorafenib
treatment for advanced HCC. High intratumoral MVD was independently
associated with poor clinical outcome of sorafenib for treatment of
advanced HCC. Whereas poor ECOG PS (OR 5.213, 95% CI 3.052–9.309;
P<0.001) and high Child-Pugh class (OR 2.628, 95% CI
1.416–4.878; P= 0.002) were independently associated with worse
TTP. Moreover, good ECOG PS (OR 8.698, 95% CI 4.315–18.396;
P<0.001) and low AFP levels (OR 2.260, 95% CI 1.174–4.352;
P=0.015) were independently associated with better OS.
 | Table IV.Multivariable analysis of predictive
factors for TTP and OS by Cox proportional hazards model. |
Table IV.
Multivariable analysis of predictive
factors for TTP and OS by Cox proportional hazards model.
| Variable | Risk ratio (95%
CI) | P-value |
|---|
| TTP | | |
| ECOG PS | 5.213
(3.052–9.309) | <0.001 |
| Combined
treatment | 0.576
(0.399–0.831) | 0.003 |
| Child-Pugh
class | 2.628
(1.416–4.878) | 0.002 |
| Low MVD | 1.626
(0.604–3.516) | 0.006 |
| OS | | |
| ECOG PS | 8.689
(4.315–18.396) | <0.001 |
| Combined
treatment | 0.245
(0.071–0.846) | 0.026 |
| AFP | 2.260
(1.174–4.352) | 0.015 |
| Low MVD | 4.618
(3.213–11.236) | <0.001 |
Discussion
To the best of our knowledge, this report is the
first to describe cryoRx as being associated with improved clinical
outcomes of sorafenib treatment for advanced HCC. Sorafenib
demonstrated a significant survival benefit and high tolerance in
patients with advanced HCC in a phase III clinical trial (13), and was simultaneously found to
impede tumor burden limit in advanced HCC (27). It is crucial to reduce tumor burden
in order to increase the clinical responses of drugs (28). CryoRx has been proposed as a valid
alternative to surgery for the treatment of HCC in patients with
cirrhosis (29). Recent studies
have examined the outcomes of percutaneous cryoRx for HCC using CT
monitoring and MR guidance, reporting that it is safe and
efficacious (30). Moreover, it
was found that not only was the local tumor necrotic, but also that
the adjacent tumor tissue was necrotic and shrunken in HCC patients
following cryoRx, which was regarded as reflecting ectopic tumor
suppression (16). We report that
following cryoablation for small HCC the 1-, 2- and 3-year
recurrence-free survival rates were 72, 56 and 43%, respectively
(25). As such, local recurrence
following cryoRx represents one of the major problems of this
therapy, and limits its associated survival benefits. We hope that
the combinaion of sorafenib and cryoRx may be used to overcome
tumor burdens and local recurrence for advanced HCC patients, and
provide significant survival benefits.
In the present study, the median OS time of the
combination therapy was 12.5 months, significantly longer than that
of patients receiving the sorafenib-alone therapy (8.6 months). In
addition, the combination therapy significantly prolonged TTP and
CER or DCR compared with the sorafenib-alone therapy. The
significant improvements in OS and TTP in the combination therapy
group provide encouraging evidence that combination therapy may
overcome the tumor burden and local tumor recurrence. The OS we
found is longer than that reported in all previous studies of
sorafenib treatment for advanced HCC patients (12,13,31–33).
All patients in our study suffered from advanced HCC (100% BCLC
stage C and HBV DNA-positive) with macroscopic vascular invasion.
In 50% of our patients, the largest tumor diameter was >7 cm;
these characteristics suggest that the patients enrolled in our
study may have had greater tumor burden than those studied in
previously reported trials (12,13).
In the Asian-Pacific study, the median OS and TTP were 6.5 and 2.8
months, respectively, and the population displayed poorer
performance (74% ECOG PS ≥1) and a more advanced stage of cancer
(96% BCLC stage C) (12). In the
current study, 35 out of 52 patients (67%) in the sorafenib-alone
treatment group exhibited ECOG PS ≥1, all received continuous oral
treatment with 0.1 g of lamivudine once daily, advanced HCC
patients with antiviral therapy had the opportunity for sorafenib
maintenance therapy by improving liver function. Thus, the
sorafenib-alone treatment group had a much better OS and TTP than
those reported in the Asian-Pacific trial of sorafenib (12). In the SHARP study (13), the median OS and TTP were 10.7 and
4.1 months, respectively, and the population exhibited a more
advanced stage of cancer (82% BCLC stage C; 38% macroscopic
vascular invasion and 51% extrahepatic spread). The 12.5-month OS
and 8.5-month TTP observed in patients with PVT in the combination
therapy treatment groups are particularly impressive, in accordance
with the rationale for the combination treatments by previous
findings. Although we found that sorafenib was capable of
prolonging survival in advanced HCC patients, monotherapy of
sorafenib has not yet been found to produce tumor regression in
HCC. High tumor burden may render patients refractory to sorafenib
(27).
In accordance with the evidence from previous
studies, our results suggest that combination therapy has numerous
advantages. First, cryoRx may reduce the tumor burden, thus
increasing the efficacy of sorafenib. Second, sorafenib-mediated
blockage of the Raf/mitogen-activated protein kinase and VEGFR
pathways might enhance the efficacy of local cryoRx. These
possibilities are supported by the current data. The clinical
benefits of the combination therapy may be largely due to the
reduction of tumor burden by cryoRx, in accordance with previous
findings (15). More importantly,
the addition of cryoRx to sorafenib treatment could further improve
OS in these HCC patients. The profile, frequency and degree of
sorafenib-related ADRs were comparable to those from previous
reports, and cryoRx did not increase the frequency and degree of
sorafenib-related ADRs. These encouraging results indicate that
sorafenib combined with cryoRx may provide the best therapeutic
benefit in patients suffering from advanced HCC.
A crucial difference between the present study and
previous studies was the continuous administration of sorafenib,
which may have also contributed to the survival benefit we
observed. In the SHARP trial a survival time of 5.2 months was
reported following disease progression (12). In a Japanese phase I study of
sorafenib, despite the median TTP being only 4.9 months, the median
OS was relatively long, at 15.6 months (34). The results of Yau et al
indicate that even in patients who did not demonstrate any clinical
benefits with sorafenib, OS was substantially improved compared
with their historical cohort (27). Wörns et al reported that
radiological disease stabilization (PR+SD) was achieved in 50% of
patients following a median of 3.2 months, or at least a clinically
stable presentation in a subset of patients with radiological PD
leading to continuation of therapy (35). These findings suggest that even
patients who exhibited radiological tumor progression with
sorafenib treatment might obtain a survival benefit from the drug.
Therefore, the application of radiological progression criteria
would be likely to lead to a discontinuation of sorafenib therapy
after 3–4 months in these cases, potentially denying these patients
the opportunity to continue to receive clinical benefit and
improved OS. We propose that the decision to continue therapy with
sorafenib following radiological progression is justified in
patients with sustained clinically stable presentation.
Sub-analyses were conducted on the basis of various
factors associated with the prognosis of patients with HCC,
including age, largest tumor diameter, tumor difference, ECOG PS,
Child-Pugh class and HBV DNA load. Our data showed that the
combination therapy or the sorafenib-alone treatment groups
exhibited a clinical benefit in all preplanned subgroup analyses.
However, patients with an ECOG PS of 2, tumor diameter >7 cm,
PVT within the trunk, high HBV DNA load, Child-Pugh class B, AFP
levels >895 ng/ml and high intratumoral MVD were associated with
poor prognosis. We also analyzed the correlation of
treatment-related toxicities with prognosis. Similar to the results
of Vincenzi et al, the fatigue, weight loss and liver toxicity
correlated with poor DCR, TTP and OS to a certain extent (36). In a study by Yau et al, fatigue was
observed in 50% of patients (27).
In the present study, 44% of patients exhibited fatigue. As such,
we believe that severe fatigue may be predictive of poor prognosis
to a certain extent. Liver toxicity is a key issue during sorafenib
and local treatment. Compared to previous reports, adding cryoRx
did not further increase frequency and degree of sorafenib-related
ADRs. This finding suggests that liver toxicity can be induced by
sorafenib. Sorafenib may induce liver failure not only in
Child-Pugh B patients but also in Child-Pugh A patients (37). However, in the present study, the
majority of sorafenib-induced liver failure occurred in Child-Pugh
B patients.
In previous research regarding intratumoral MVD as a
prognostic measure of tumor angiogenesis (26,38),
a prospective study found a significant positive correlation
between MVD and post-operative recurrence in patients undergoing
resection of HCCs ≤5 cm (39).
Therefore, we analyzed the correlation between intratumoral MVD and
the response to sorafenib therapy. Our results showed that the mean
MVD of patients with CER was significantly lower than that of
patients with PD (P<0.001). The results suggest that
intratumoral MVD may affect the clinical response to therapy in
advanced HCC patients. Among patients with low intratumoral MVD, we
found that those receiving the combination therapy exhibited a
significantly longer median TTP and OS than those receiving the
sorafenib-alone therapy; however, in patients with high
intratumoral MVD, TTP and OS were not significantly different
between treatments. The current data indicate that the
anti-angiogenic effects of sorafenib for advanced HCC patients with
a high intratumoral MVD are mild.
Multivariable analysis revealed that cryoRx was
independently associated with improved TTP and OS of sorafenib
treatment for advanced HCC, and that poor ECOG PS or high
intratumoral MVD predicted poor TTP and OS, which is consistent
with the results of previous studies (33). In the current study, none of the
patients had an ECOG PS >2 or a Child-Pugh class worse than B.
Ideally, the tumor control rate increases with the sorafenib dose
and the completion of local treatment. Patients with a better PS
had the opportunity for sorafenib maintenance therapy and
successful local treatment due to the acceptable adverse effects.
Certain well-established prognostic predictors, including tumor
differentiation and vascular invasion, were not associated with
survival. Although tumor size was significantly associated with OS
based on the log-rank test, which suggests that tumor burden might
be a mechanism involved in refraction to sorafenib, it was excluded
from multivariate analyses. Similar to the results of previous
studies, Child-Pugh class and AFP were independently associated
with TTP and OS, respectively. The precise mechanism by which AFP
affects prognosis remains unclear. However, several studies have
reported that AFP is a novel protein-binding partner for caspase-3,
which blocks the apoptotic signaling pathway, and promotes the
growth of human hepatoma cells as a co-repressor in retinoic acid
(RA)-RA receptor signaling (40,41).
Therefore, the low AFP level was independently associated with
better OS.
In conclusion, this clinical study demonstrates that
compared to the sorafenib-alone treatment group, the addition of
cryoRx to sorafenib treatment significantly improves the clinical
outcomes of sorafenib for the treatment of advanced HCC, with
acceptable tolerance and similar safety profiles as previously
reported. High intratumoral MVD was predictive of poor responses to
sorafenib therapy for advanced HCC patients. These results provide
further validation for sorafeinb treatment for advanced HCC.
Acknowledgements
The authors would like to thank all
the patients enrolled in this study for their kind understanding
and support. The present study was supported by a grant from the
Key Scientific and Technological Research Foundation of the
National Special-purpose Program (No. 2008ZX10002-018) and the
Capital Medical Development and Research Fund (2007-1021,
2009-2041).
References
|
1.
|
DM ParkinGlobal cancer statistics in the
year 2000Lancet Oncol253343200111905707
|
|
2.
|
ZY TangPerspective of clinical onology
from the viewpoint of liver cancer studiesTumor (China)29142009
|
|
3.
|
HY YooCH PattJF GeschwindThe outcome of
liver transplantation in patients with hepatocellular carcinoma in
the United States between 1988 and 2001: 5-year survival has
improved significantly with timeJ Clin
Oncol2143294335200314581446
|
|
4.
|
HI YangSN LuYF LiawSL YouCA SunLY WangCK
HsiaoPJ ChenDS ChenCJ ChenHepatitis B e antigen and the risk of
hepatocellular carcinomaN Engl J
Med347168174200210.1056/NEJMoa01321512124405
|
|
5.
|
M ShermanHepatocellular carcinoma:
epidemiology, risk factors, and screeningSemin Liver
Dis25143154200510.1055/s-2005-87119415918143
|
|
6.
|
JM LlovetA BurroughsJ BruixHepatocellular
carcinomaLancet36219071917200310.1016/S0140-6736(03)14964-1
|
|
7.
|
JM LlovetA BurroughsJ BruixPrognosis of
hepatocellular carcinoma: the BCLC staging classificationSemin
Liver Dis19329338199910.1055/s-2007-100712210518312
|
|
8.
|
J BruixJM LlovetPrognosis prediction and
treatment strategy in hepatocellular
carcinomaHepatology35519524200210.1053/jhep.2002.3208911870363
|
|
9.
|
J BruixM ShermanManagement of
hepatocellular
carcinomaHepatology4212081236200510.1002/hep.20933
|
|
10.
|
KW ParkJW ParkJI ChoiSurvival analysis of
904 patients with hepatocellular carcinoma in a hepatitis B
virus-endemic areaJ Gastroenterol
Hepatol23467473200810.1111/j.1440-1746.2007.05112.x17764529
|
|
11.
|
JM LlovetJ FusterJ Bruixthe
Barcelona-Clinic Liver Cancer GroupThe Barcelona approach:
diagnosis, staging, and treatment of hepatocellular carcinomaLiver
Transpl10Suppl 1115120200410.1002/lt.2003414762851
|
|
12.
|
AL ChengYK KangZ ChenRandomized phase III
trial of sorafenib versus placebo in Asian patients with advanced
hepatocellular carcinomaLancet
Oncol102534200910.1016/S1470-2045(08)70285-7
|
|
13.
|
JM LlovetS RicciV MazzaferroSHARP
Investigators Study GroupSorafenib in advanced hepatocellular
carcinomaN Engl J Med359378390200810.1056/NEJMoa070885718650514
|
|
14.
|
J FuruseSorafenib for the treatment of
unresectable hepatocellular
carcinomaBiologics2779788200819707458
|
|
15.
|
A HakiméA Hines-PeraltaH PeddiCombination
of radiofrequency ablation with antiangiogenic therapy for tumor
ablation efficacy: study in miceRadiology244464470200717641366
|
|
16.
|
S OsadaH ImaiH TomitaSerum cytokine levels
in response to hepatic cryoablationJ Surg
Oncol95491498200710.1002/jso.2071217219394
|
|
17.
|
G PostonCryosurgery for colorectal liver
metastasesHepatogastroenterol48323324200111379300
|
|
18.
|
FT Lee JrDM MahviSG ChosyGM OnikWS WongPJ
LittrupKA ScanlanHepatic cryosurgery with intraoperative US
guidanceRadiology202624632199710.1148/radiology.202.3.90510059051005
|
|
19.
|
SA ShockPF LaesekeLA SampsonWD LewisTC
Winter IIIJP FineFT LEE JrHepatic hemorrhage caused by percutaneous
tumor ablation: radiofrequency ablation versus cryoablation in a
porcine
modelRadiology236125131200510.1148/radiol.236104053315987968
|
|
20.
|
TD AtwellMA FarrellMR CallstromJW
CharboneauBC LeibovichDE PattersonGK ChowML BlutePercutaneous
cryoablation of 40 solid renal tumors with US guidance and CT
monitoring: initial
experienceRadiology24327683200710.1148/radiol.243105213317329689
|
|
21.
|
W JungraithmayrD BurgerM OlschewskiS
EggsteinCryoablation of malignant liver tumor: results of a single
center studyHepatobiliary Pancreat Dis Int4554560200516286261
|
|
22.
|
J YamamotoT KosugeA SaiuraEffectiveness of
hepatic resection for early-stage hepatocellular carcinoma in
cirrhotic patients: subgroup analysis according to Milan
CriteriaJpn J Clin Ocncol34287295200710.1093/jjco/hym025
|
|
23.
|
R LencioniJM LlovetModified RECIST
(mRECIST) assessment for hepatocellular carcinomaSemin Liver
Dis305260201010.1055/s-0030-124713220175033
|
|
24.
|
A TrottiAD ColevasA SetserCTCAE v 3.0:
development of a comprehensive grading system for the adverse
effects of cancer treatmentSemin Radiat
Oncol13176181200310.1016/S1053-4296(03)00031-6
|
|
25.
|
C WangY LuY ChenY FengL AnX WangS SuW BaiL
ZhouY YangD XuPrognostic factors and recurrence of hepatitis
B-related hepatocellular carcinoma after argon-helium cryoablation:
a prospective studyClin Exp
Metastasis26839848200910.1007/s10585-009-9283-619784786
|
|
26.
|
RT PoonIO NgC LauWC YuZF YangST FanJ
WongTumor microvessel density as a predictor recurrence after
resection of hepatocellular carcinoma: a prospective studyJ Clin
Oncol2017751785200210.1200/JCO.2002.07.08911919234
|
|
27.
|
T YauP ChanKK NgPhase 2 open-label study
of single-agent sorafenib in treating advanced hepatocellular
carcinoma in a hepatitis B-endemic Asian population: presence of
lung metastasis predicts poor
responseCancer115428436200910.1002/cncr.24029
|
|
28.
|
TF GretenF KorangyMP MannsNP
MalekMolecular therapy for the treatment of hepatocellular
carcinomaBr J Cancer1001923200910.1038/sj.bjc.660478419018262
|
|
29.
|
LM ZuroED StarenCryosurgical ablation of
unresectable hepatic tumorsAORN J6423162394419968853781
|
|
30.
|
T ShimizuY SakuharaD AboY HasegawaY
KodamaH EndoH ShiratoK MiyasakaOutcome of MR-guided percutaneous
cryoablation for hepatocellular carcinomaJ Hepatobiliary Pancreat
Surg16816823200910.1007/s00534-009-0124-419466377
|
|
31.
|
JH ShimJW ParkJI ChoiBJ ParkCM
KimPractical efficacy of sorafenib monotherapy for advanced
hepatocellular carcnoma patients in a Hepatitis B virus-endemic
areaJ Cancer Res Clin
Oncol135617625200910.1007/s00432-008-0496-x18846384
|
|
32.
|
M PinterW SieghartI GraziadeiW VogelA
MaieronR KönigsbergA WeissmannG KornekC PlankM
Peck-RadosavljevicSorafenib in unresectable hepatocellular
carcinoma from mild to advanced stage liver
cirrhosisOncologist147076200910.1634/theoncologist.2008-019119144684
|
|
33.
|
RC KaneAT FarrellR MadabushiSorafenib for
the treatment of unresectable hepatocellular
carcinomaOncologist1495100200910.1634/theoncologist.2008-018519144678
|
|
34.
|
J FuruseH IshiiK NakachiPhase I study of
sorafenib in Japanese patients with hepatocellular carcinomaCancer
Sci99159165200817953709
|
|
35.
|
MA WörnsA WeinmannK PfingstSafety and
efficacy of sorafenib in patients with advanced hepatocellular
carcinoma in consideration of concomitant stage of liver cirrhosisJ
Clin Gastroenterol43489495200919247201
|
|
36.
|
B VincenziD SantiniA RussoEarly skin
toxicity as a predictive factor for tumor control in hepatocellular
carcinoma patients treated with
sorafenibOncologist158592201010.1634/theoncologist.2009-014320051477
|
|
37.
|
C SchrammG SchuchAW LohseSorafenib-induced
liver failureAm J
Gastroenterol10321622163200810.1111/j.1572-0241.2008.01982_19.x18796127
|
|
38.
|
D SemelaJF DufourAngiogenesis and
hepatocellular carcinomaJ
Hepatol41864880200410.1016/j.jhep.2004.09.00615519663
|
|
39.
|
L LiuY CaoC ChenSorafenib blocks the
RAF/MEK/ ERK pathway, inhibits tumor angiogenesis, and induces
tumor cell apoptosis in hepatocellular carcinoma model
PLC/PRF/5Cancer
Res661185111858200610.1158/0008-5472.CAN-06-137717178882
|
|
40.
|
M LiH LiC LiL GuoH LiuS ZhouX LiuZ ChenS
ShiJ WeiMA McNuttG LiCytoplasmic alpha-fetoprotein functions as a
co-repressor in RA-RAR signaling to promote the growth of human
hepatoma Bel 7402 cellsCancer
Lett285190199200910.1016/j.canlet.2009.05.01419501957
|
|
41.
|
M LiH LiC LiS ZhouL GuoH LiuW JiangX LiuP
LiMA McNuttG LiAlpha fetoprotein is a novel protein-binding partner
for caspase-3 and blocks the apoptotic signaling pathway in human
hepatoma cellsInt J
Cancer12428452854200910.1002/ijc.2427219267404
|
















