Introduction
Painful bladder syndrome/interstitial cystitis
(PBS/IC) is a clinical condition that mainly occurs in females. The
prevalence of PBS/IC is approximately 1/1,000 (1,2). The
disease presents a variety of symptoms, including urinary
frequency, nocturia, pain on bladder filling and supra-pubic pain,
which may ultimately limit bladder capacity. Despite investigations
in the past few decades, IC remains an unresolved problem with
regard to its etiology, mechanisms and clinical management.
A number of studies have demonstrated a link between
PBS/IC and inflammation (3–7).
Members of the tumor necrosis factor (TNF) superfamily (TNFSF) have
been revealed to be associated with PBS/IC (6,8,9). TNF
ligand-related molecule 1A [TL1A; also known as vascular
endothelial growth inhibitor (VEGI) and TNFSF member 15 (TNFSF15)]
is an anti-angiogenic cytokine belonging to the TNFSF. TL1A
regulates tumor cell behavior and is involved in chronic
inflammatory disease (10–12). These studies collectively indicate
that TL1A has a role in PBS/IC.
The present study investigated the expression levels
of TL1A and its receptor, death receptor 3 (DR3), in bladder biopsy
tissues and revealed a potential link between TL1A, DR3 and
PBS/IC.
Materials and methods
Patients and tissue samples
A total of 8 patients who fulfilled the National
Institute of Diabetes, Digestive and Kidney Diseases (NIDDK)
diagnostic criteria (13) for
PBS/IC and 8 age-matched hematuric controls undergoing cystoscopy
for bladder carcinoma were consecutively enrolled in the present
study. All participants were at least 18 years old and were
enrolled in accordance with the guidelines of the Institutional
Review Board of the Beijing Chaoyang Hospital (Beijing, China) and
all subjects provided written informed consent.
Following evaluations of the patients through a
detailed medical history analysis, physical examination and voiding
diary, each patient underwent urinalysis, urine culture, urine
cytology and urinary tract ultrasonography analyses. None of the
patients had an intravesical treatment history due to urinary
infection and PBS/IC. All medications, including antidepressants,
antihistamines and steroidal drugs, were discontinued for at least
48 h prior to hydro-distention therapy. No intravesical malignant
lesions were detected during cystoscopic evaluations in which
hydro-distension up to a pressure of 80 cm H2O was
administered for 3 min and all biopsies were obtained using the
cold cut technique from an area of the posterior bladder which
appeared normal after the bladder was emptied. The bladder mucosa
biopsies were obtained from the same sites in the control patients
with the exception of carcinomas ≥2 cm and were prepared using the
same methods for comparison. A total of 4 sections of bladder
tissues which were ∼2×2 mm in size were obtained from each patient.
Samples were stored in liquid nitrogen immediately and maintained
at −80°C until used.
Western blot analysis
The frozen samples were rapidly thawed and
homogenized at 4°C in Buffer C (50 mM Tris-HCl, pH 7.5, containing
2 mM DTT, 2 mM EDTA, 2 mM EGTA, 50 mM
4-(2-aminoethyl)-benzenesulfonylfluoride hydrochloride, 5 mg/ml
each of leupeptin, aprotinin, pepstatin A and chymostatin, 50 mM
KF, 50 mM okadaic acid, 5 mM sodium pyrophosphate and 2% SDS) and
then sonicated to disrupt the tissues completely. Protein
concentrations were measured using a BCA kit (Thermo Scientific,
Pittsburgh, PA, USA).
SDS-polyacrylamide gel electrophoresis (PAGE) and
western blot analysis were performed according to the laboratory’s
standard procedure. Firstly, 40 μg total protein from each sample
was loaded onto the corresponding lane of a 10% SDS-PAGE gel.
Following electrophoresis and the transfer of proteins to a
polyvinylidene difluoride membrane (PVDF; GE Healthcare, Waukesha,
WI, USA) at 4°C, the PVDF membrane was blocked with 10% non-fat
milk in TTBS (20 mM Tris-HCl, pH 7.5, containing 0.15 M NaCl and
0.05% Tween-20) for 1 h. The blocked membrane was incubated with
primary rabbit polyclonal antibody against TL1A or DR3 (1:1,000,
Abcam Inc., Cambridge, UK) for 3 h. To confirm the uniform loading
of the protein, the same PVDF membrane was reprobed with primary
mouse monoclonal antibody against β-actin (Sigma-Aldrich Company,
St. Louis, MO, USA) at a 1:2,000 dilution for 1 h. Horseradish
peroxidase-conjugated goat anti-rabbit or anti-mouse IgG (ZSGB-BIO
Inc., Beijing, China) were used as secondary antibodies at a
1:4,000 dilution for a 1-h incubation. An enhanced
chemiluminescence (ECL) kit (Applygen Technologies Inc., Beijing,
China) was used to identify the signals in the X-ray film. The
order of detection of the target proteins was TL1A, DR3 and then
β-actin. With each new round of detection on the same PVDF
membrane, stripping buffer, containing 100 mM 2-mercaptoethanol, 2%
SDS and 62.5 mM Tris-HCl (pH 6.7), was applied and the PVDF
membrane was incubated at 55°C until no signals were identified in
the X-ray film, indicating that the previously bound antibodies had
been stripped from the membrane.
Total RNA extraction and real-time
quantitative RT-PCR
Bladder tissues were homogenized and total RNA was
isolated according to the standard operating procedure of the
mirVana™ miRNA Isolation kit (Ambion, Carlsbad, CA, USA). RNA was
eluted and stored at −70°C. The total RNA concentration was
measured using ultraviolet spectrophotometry at 260 nm (NANO 2000,
Thermo Scientific), the purity was determined using the 260/280 A
ratio and the quality of the isolated RNA was confirmed using 1%
agarose gel electrophoresis.
The reverse transcription reactions were performed
using the High Capacity cDNA Reverse Transcription kit
(GoTaq® 2-Step RT-qPCR System, Promega, Madison, WI,
USA) with poly A primers. Real-time RT-PCR for each sample was
performed in triplicate using the Fast Real-time PCR System STRATA
Mx3000 (Agilent, Boeblingen, Germany). The Ct values and the qPCR
were normalized to the GAPDH housekeeping gene using the
2−ΔΔCt method (14).
The primers were synthesized by Taihe Gene, Inc. (Beijing, China;
Table I).
 | Table IPCR primer sequences. |
Table I
PCR primer sequences.
| Primers | Sequences
(5′-3′) | Product length
(bp) |
|---|
| GAPDH | | 325 |
| Forward |
GGCGATGCTGGCGCTGAGTA | |
| Reverse |
ACAGTTTCCCGGAGGGGCCA | |
| TL1A | | 145 |
| Forward |
CAAACAAGCCAGACTCCATCACT | |
| Reverse |
GAGAACATGGCTCCGAGGTAGAT | |
| DR3 | | 66 |
| Forward |
TGCCGCCGAGACAGCCCCACGAC | |
| Reverse |
GACGGCACGCTCACACTCCTCAG | |
Statistical analysis
The experimental data are expressed as the mean ±
standard deviation (±s). Statistical analyses were performed using
the t-test. P≤0.05 was considered to indicate a statistically
significant difference. Statistical analyses were performed using
SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA).
Results
Description of patients
The clinical characteristics of the 8 IC and 8
control patients are shown in Table
II. All patients were female and all subjects who had suffered
from an acute bacterial infection within 3 months were excluded.
None of the control patients exhibited any symptoms related to IC
and biopsy samples were obtained ≥2 cm from the bladder tumor
margins. All but 1 of the subjects were postmenopausal. No common
medical history was identified among the patients.
 | Table IIClinical characteristics of the
patients and controls. |
Table II
Clinical characteristics of the
patients and controls.
| Characteristic | PBS/IC | Control |
|---|
| Median age (range,
years) | 57 (39–78) | 59 (42–75) |
| Gender
(female/male) | 8/0 | 8/0 |
| Type of bladder
procedure | Hydro-distention | Cystoscopy |
| Anesthesia | Intravenous | Topical |
| No. ulcer | 0 | 0 |
| No. glomerular
bleeding | 8 | 0 |
| Urinalysis
(WBC+-++++) | 0 | 0 |
| Urine culture
(+) | 0 | 0 |
Protein levels of TL1A and DR3
The TL1A and DR3 protein expression levels were
observed to be increased in the bladder biopsies of the patients
with IC compared with those of the controls using western blotting.
The TL1A to β-actin ratio in the IC patients was 0.65±0.03 and that
in the control group was 0.25±0.02 which was statistically
significantly different (P<0.001). The TL1A protein levels of
the IC patients were 2.6 fold higher than those of the controls.
The DR3 to β-actin ratio in the IC patients was 0.66±0.06 and that
in the controls was 0.27±0.02 which was statistically significantly
different (P<0.001). The DR3 protein levels of the IC patients
were 2.44 fold higher than those of the controls (Fig. 1).
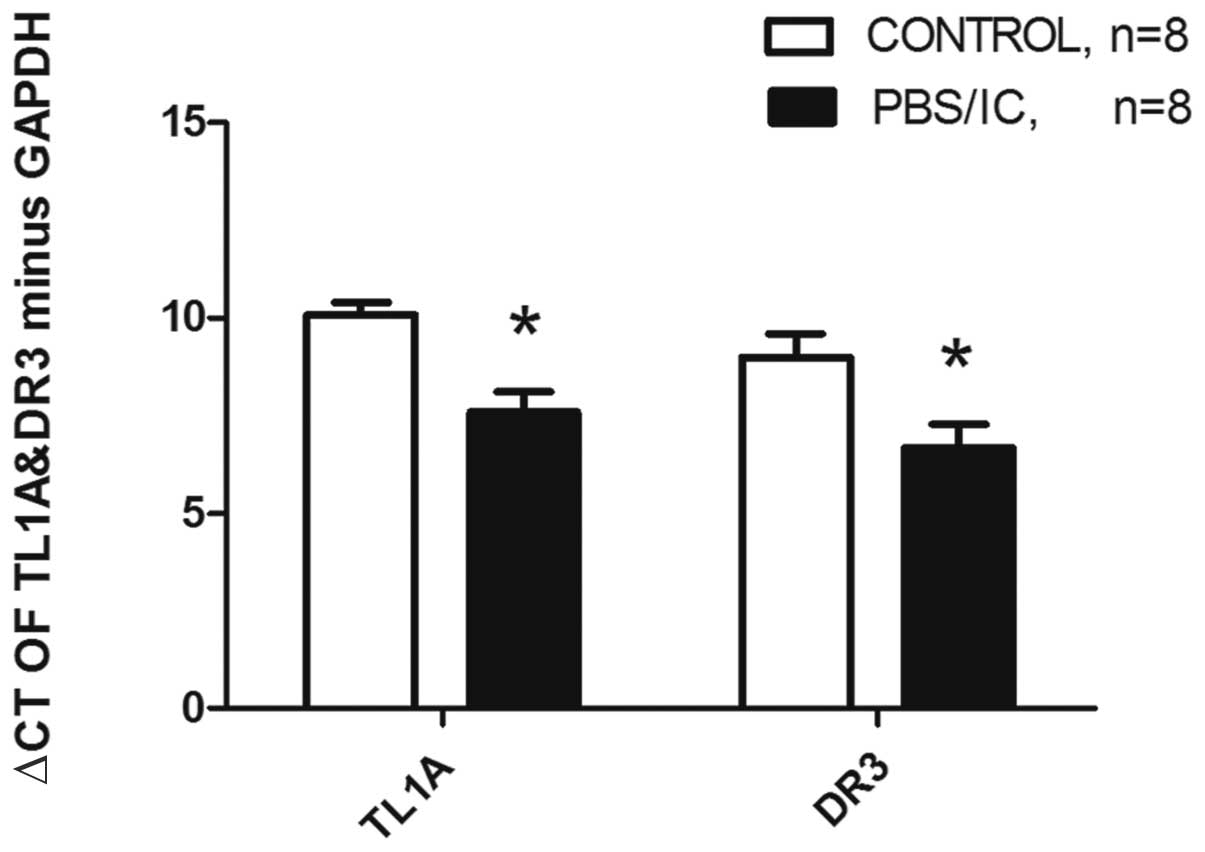
mRNA expression levels of TL1A and
DR3
The TL1A and DR3 mRNA expression levels were also
evaluated in the bladder biopsies using real-time RT-PCR. The ΔCts
of TL1A minus GAPDH in the patient and control groups were
7.60±0.52 and 10.08±0.32, respectively, and were statistically
significantly different (P<0.001). The TL1A mRNA levels of the
patients were upregulated 5.28-fold. The ΔCts of DR3 minus GAPDH in
the patient and control groups were 6.68±0.60 and 8.99±0.61,
respectively, which was statistically significantly different
(P=0.017). The DR3 mRNA levels of the patients were upregulated
4.92-fold (Fig. 2).
Discussion
PBS/IC is a clinical condition that manifests mainly
as storage period symptoms, including frequency and supra-pubic
pain, suggesting a mainly sensation-based problem. In the present
study, patients with PBS/IC were revealed to have elevated levels
of the proteins and mRNA of TL1A and DR3. TL1A is a cytokine
belonging to the TNFSF. Three isoforms of TNFSF15 have been
reported; TL1A is the most predominant of these isoforms and may be
active in inflammation and apoptosis. TL1A has been demonstrated to
be significant in tumor cell behavior and chronic inflammatory
disease (10,11,15).
DR3 is the receptor for TL1A and tumor necrosis factor-like weak
inducer of apoptosis (TWEAK). After binding to its ligand, DR3
binds to the adaptor molecule TNF-related apoptosis death domain
(TRADD) through its cytoplasmic death domain. TRADD recruitment
causes downsteam molecules to activate NF-κB and mitogen-activated
protein kinase (MAPK) signaling or triggers caspase activation and
programmed cell death under certain conditions (16).
Although the results of the present study suggest an
upregulation of TL1A and DR3 in PBS/IC patients, their precise role
in this disease was not addressed. However, the known functions of
TL1A indicate that it is likely to be involved in the pathogenesis
of PBS/IC. Firstly, TL1A is active in inflammation and apoptosis
(17–19). The activation of NF-κB in the
bladder biopsies of PBS/IC patients (predominantly in the cells of
the urothelium and submucosal layer) and apoptosis of endothelial
cells in this condition have been reported (20–23).
There is also evidence that apoptosis in PBS/IC is mediated by
inflammation (23). Upregulation
of TL1A and its receptor may trigger inflammation and apoptosis in
the bladder, in particular in the elderly population (24). With regard to the evidence that
TNF-related apoptosis-inducing ligand and TNFSF14 are also
important in PBS/IC (6,8), it may be suggested that TL1A and DR3
elevation is one of the factors contributing to the inflammation
and apoptosis in PBS/IC. Secondly, TL1A has been shown to have an
anti-angiogenic function (25,26).
Upregulation of TL1A and DR3 may inhibit angiogenesis which then
causes bladder ischemia and destroys the blood-urine barrier.
Previous studies have demonstrated that there is ischemia in PBS/IC
patient bladders (27) and
impairment of the bladder surface in the PBS/IC condition (22,28).
The clinical identification of glomerular bleeding is the only
non-exclusive diagnostic evidence of PBS/IC according to the NIDDK
criteria. The underlying cause of glomerular bleeding may be the
abnormality of the blood vessels arising as a result of this
mechanism. Steroid hormone treatments are capable of alleviating
the symptoms, which provides supporting evidence for this
hypothesis since these drugs inhibit inflammation (29).
The present study has limitations, primarily due to
the limited number of patients. The mechanism should be further
tested in in vivo models. However, there are no widely
recognized animal models in which to test this hypothesis. At
present, we aim to conduct a larger scale clinical investigation
and possibly to specifically block this signaling pathway in a
suitable IC disease model, in order to elucidate the precise roles
of TL1A and DR3 in PBS/IC. The promising results of the present
study justify a larger population study and further mechanistic
studies to identify the precise pathogenic role of TL1A and DR3 in
PBS/IC and to explore their potential as a possible therapeutic
approach.
In conclusion, the present study revealed that TL1A
and its receptor DR3 were upregulated in patients with PBS/IC.
Therefore, TL1A, a cytokine which triggers NF-κB activation,
induces apoptosis and inhibits vascular formation via its receptor
DR3, may be important in the pathogenesis of PBS/IC.
Acknowledgements
This study was financially supported
by the National Natural Science Foundation of China (no. 81070603).
The authors appreciate the valuable comments from the other members
of their laboratories.
References
|
1
|
Clemens JQ, Meenan RT, Rosetti MC, Gao SY
and Calhoun EA: Prevalence and incidence of interstitial cystitis
in a managed care population. J Urol. 173:98–102. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choe JH, Son H, Song YS, Kim JC, Lee JZ
and Lee KS: Prevalence of painful bladder syndrome/interstitial
cystitis-like symptoms in women: A population-based study in Korea.
World J Urol. 29:103–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang ZY and Bjorling DE: Tumour necrosis
factor-α induces expression and release of interleukin-6 by human
urothelial cells. Inflammation Res. 60:525–532. 2011.
|
|
4
|
Imamura T, Igawa Y, Ogawa T, Homma T, Seki
S, Ishizuka O, Satoshi A, Homma Y and Nishizawa O: Expression level
of CXCL10 peptide in bladder urothelium and urine as possible
biomarkers for diagnosis of ulcerative interstitial cystitis. Eur
Urol Suppl. 9:2122010. View Article : Google Scholar
|
|
5
|
Richter B, Roslind A, Hesse U, Nordling J,
Johansen JS, Horn T and Hansen AB: YKL-40 and mast cells are
associated with detrusor fibrosis in patients diagnosed with
bladder pain syndrome/interstitial cystitis according to the 2008
criteria of the European Society for the Study of Interstitial
Cystitis. Histopathology. 57:371–383. 2010. View Article : Google Scholar
|
|
6
|
Ogawa T, Homma T, Igawa Y, Seki S,
Ishizuka O, Imamura T, Akahane S, Homma Y and Nishizawa O: CXCR3
binding chemokine and TNFSF14 over expression in bladder urothelium
of patients with ulcerative interstitial cystitis. J Urol.
183:1206–1212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lv J, Luo Y, Leng J, Xue W, Liu D and
Huang Y: Aberrant expression of monocyte chemoattractant protein-1
(mcp-1) in interstitial cystitis patients. Sci Res Essays.
5:663–667. 2010.
|
|
8
|
Kutlu O, Akkaya E, Koksal IT, Bassorgun
IC, Ciftcioglu MA, Sanlioglu S and Kukul E: Importance of
TNF-related apoptosis-inducing ligand in pathogenesis of
interstitial cystitis. Int Urol Nephrol. 42:393–399. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen MC, Keshavan P, Gregory GD and Klumpp
DJ: RANTES mediates TNF-dependent lamina propria mast cell
accumulation and barrier dysfunction in neurogenic cystitis. Am J
Physiol Renal Physiol. 292:F1372–F1379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang N, Sanders AJ, Ye L, Kynaston HG and
Jiang WG: Expression of vascular endothelial growth inhibitor
(VEGI) in human urothelial cancer of the bladder and its effects on
the adhesion and migration of bladder cancer cells in vitro.
Anticancer Res. 30:87–95. 2010.PubMed/NCBI
|
|
11
|
Song YJ, Meylan F, Botson J,
Goldbach-Mansky R, Lee D and Siegel R: TL1A-DR3 interactions are
important in both adaptive and innate immunity in inflammatory
arthritis. Clin Immunol. 135:S882010. View Article : Google Scholar
|
|
12
|
Tan KB, Harrop J, Reddy M, Young P,
Terrett J, Emery J, Moore G and Truneh A: Characterization of a
novel TNF-like ligand and recently described TNF ligand and TNF
receptor superfamily genes and their constitutive and inducible
expression in hematopoietic and non-hematopoietic cells. Gene.
204:35–46. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gillenwater JY and Wein AJ: Summary of the
National Institute of Arthritis, Diabetes, Digestive and Kidney
Diseases Workshop on Interstitial Cystitis, National Institutes of
Health, Bethesda, Maryland, August 28–29, 1987. J Urol.
140:203–206. 1988.PubMed/NCBI
|
|
14
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taraban VY, Slebioda TJ, Willoughby JE,
Buchan SL, James S, Sheth B, Smyth NR, Thomas GJ, Wang EC and
Al-Shamkhani A: Sustained TL1A expression modulates effector and
regulatory T-cell responses and drives intestinal goblet cell
hyperplasia. Mucosal Immunol. 4:186–196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pobezinskaya YL, Choksi S, Morgan MJ, Cao
X and Liu ZG: The adaptor protein TRADD is essential for TNF-like
ligand 1A/death receptor 3 signaling. J Immunol. 186:5212–5216.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang YJ, Kim WJ, Bae HU, Kim DI, Park YB,
Park JE, Kwon BS and Lee WH: Involvement of TL1A and DR3 in
induction of pro-inflammatory cytokines and matrix
metalloproteinase-9 in atherogenesis. Cytokine. 29:229–235. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Young HA and Tovey MG: TL1A: a mediator of
gut inflammation. Proc Natl Acad Sci USA. 103:8303–8304. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Altamirano CV, Pang S, Li LY and Berenson
JR: Vascular endothelial growth inhibitor (vegi) inhibits the
growth of multiple myeloma plasma cells and induces their
apoptosis. Blood. 98:638A2001.
|
|
20
|
Abdel-Mageed AB and Ghoniem GM: Potential
role of rel/nuclear factor-kappaB in the pathogenesis of
interstitial cystitis. J Urol. 160:2000–2003. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamada T, Nishimura M and Mita H:
Increased number of apoptotic endothelial cells in bladder of
interstitial cystitis patients. World J Urol. 25:407–413. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsieh JH, Chen CY and Kuo HC: Increased
apoptosis and decreased junction protein expression of urothelium
due to suburothelial inflammation in patients with interstitial
cystitis/painful bladder syndrome. Neurourol Urodynamics.
29:1138–1140. 2010.
|
|
23
|
Shie JH, Liu HT and Kuo HC: Increased cell
apoptosis of urothelium mediated by inflammation in interstitial
cystitis/painful bladder syndrome. Urology. 79:484 e7–e13.
2012.
|
|
24
|
Wang W, Zhang N, Zhu XH, He ZS, Wahafu W,
Xu ZQ and Yang Y: Involvement of TL1A and DR3 in induction of
ischaemia and inflammation in urinary bladder dysfunction in the
elderly. Mol Med Rep. 6:434–438. 2012.PubMed/NCBI
|
|
25
|
Wang L, Pan W, Zhu FL, Jiao BH, Lou YH,
Xiao Y and Qi ZT: Cloning, expression and biological activity of
VEGI(151), a novel vascular endothelial cell growth inhibitor. Acta
Biochimica Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai).
32:485–489. 2000.PubMed/NCBI
|
|
26
|
Chen X, Wu JH, Liu HN, He Z, Gu M, Wang N,
Ma J, Hu J, Xia L, He H, Yuan J, Li J, Li L, Li M and Zhu X:
Approaches to efficient production of recombinant angiogenesis
inhibitor rhVEGI-192 and characterization of its structure and
antiangiogenic function. Protein Sci. 19:449–457. 2010.PubMed/NCBI
|
|
27
|
Pontari MA, Hanno PM and Ruggieri MR:
Comparison of bladder blood flow in patients with and without
interstitial cystitis. J Urol. 162:330–334. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Altuntas CZ, Daneshgari F, Sakalar C,
Goksoy E, Gulen MF, Kavran M, Qin J, Li X and Tuohy VK:
Autoimmunity to uroplakin II causes cystitis in mice: a novel model
of interstitial cystitis. Eur Urol. 61:193–200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hanno PM, Burks DA, Clemens JQ, Dmochowski
RR, Erickson D, Fitzgerald MP, Forrest JB, Gordon B, Gray M, Mayer
RD, Newman D, Nyberg L Jr, Payne CK, Wesselmann U and Faraday MM;
Interstitial Cystitis Guidelines Panel of the American Urological
Association Education and Research Inc: AUA guideline for the
diagnosis and treatment of interstitial cystitis/bladder pain
syndrome. J Urol. 185:2162–2170. 2011. View Article : Google Scholar
|