Introduction
Chlamydophila pneumoniae (C.
pneumoniae) is a common respiratory pathogen that can cause a
number of respiratory diseases, including pneumonia, asthma,
chronic pharyngitis and chronic bronchitis (1–4).
C. pneumoniae activates macrophages to produce
proinflammatory cytokines, which may result in atherosclerosis.
Although C. pneumoniae is a serious threat to human health,
its underlying pathogenic mechanisms are not fully understood. It
has, however, been hypothesized that C. pneumoniae secretes
various toxic proteins.
It is widely accepted that gram-negative bacteria
secrete proteins through type I-V secretion systems. The type III
secretion system (T3SS) is an independent system, whose effector
proteins can change cytoskeletal structures, destroy signal
transduction pathways, suppress apoptotic activity and interfere
with host transcriptional regulation (5–7).
Techniques for the screening and identification of Cpn T3SS have
become increasingly studied. Previous studies have shown that the
coding sequences of T3SS effector proteins are always located next
to the chaperones (8–15). The Cpn 0810 gene is adjacent to Cpn
lcrH1, a chaperone homolog gene with Yersinia lcrH, and the
Cpn 0810 gene family is located within the coding clusters of the
T3SS. Therefore, Cpn 0810 has been hypothesized to be an effector
of the T3SS (16–19).
In the present study, Cpn 0810 was cloned, expressed
and purified from C. pneumoniae. The effects of Cpn 0810 on
inflammatory and apoptotic processes in human monocytic cells
(THP-1) were investigated, with the aim to provide a basis for the
further study of the pathogenic mechanisms underlying Cpn T3SS
effector proteins.
Materials and methods
Strains, plasmids and cell lines
An Escherichia coli (E. coli) BL21
strain and the THP-1 cell line were provided by the Department of
Pathogenic Biology, University of South China (Hengyang,
China).
Gene amplification and recombinant
plasmid construction
Amplification of Cpn 0810 was performed using
polymerase chain reaction (PCR), based on the following primer
pairs: P1, 5′-CGCGGATCCATGAATAAAAAGCCCAAGAAAAC-3′, and P2,
5′-TTTTCCTTTTGCGGCCGCTTACTCAGC GCCTTTAACCAT-3′.
Amplification was performed in a final reaction
volume of 50 μl, containing 39.6 μl ddH2O, 5 μl 10X Pfu
buffer, 1 μl dNTP mix (10mM), 1 μl P1 primer, 1 μl P2 primer, 0.4
μl DNA Polymerase (5 units) and 2 μl Cpn templates. The
amplification conditions were as follows: Initial polymerase
activation at 94°C (5 min); 30 cycles of 94°C (30 sec), 52°C (45
sec) and 72°C (3 min); and a final elongation step at 72°C for 10
min. Distilled water was used as a negative control. The
amplification products (363 bp) were subjected to 1.0% agarose gel
electrophoresis containing ethidium bromide.
The PCR products were digested with BamHI and
NotI (Promega Corporation, Madison, WI, USA), and ligated
into the pGEX6p-2 plasmid (GE Healthcare, Piscataway, NJ, USA). The
recombinant plasmid was transformed into E. coli BL21
competent cells, and the positive clones were screened by PCR and
sequencing.
Expression and purification of the
recombinant protein
Positive E. coli BL21 colonies, containing
pGEX6p-2/Cpn 0810, were cultured in Luria-Bertani (LB) solid medium
(with ampicillin) at 37°C overnight, after which the culture was
transferred to fresh LB liquid medium (with ampicillin). When the
optical density reached a wavelength of 600 nm, isopropyl
β-D-1-thiogalactopranoside (IPTG) was added with a final
concentration of 0.2 mM, and the culture was shaken at 30°C for 4
h. The bacteria were then collected, and phosphate-buffered saline
(8 ml/g cells) and lysozyme (4.0 g/l) were added to the cell
pellet. Following incubation at room temperature for 2 h, the cells
were subjected to sonication (10 sec on, 10 sec off) 30 times using
a MSE Soniprep 150 (SANYO, Osaka, Japan). Following centrifugation
at 10,000 × g for 20 min at 4°C, the supernatant was purified using
a glutathione S-transferase (GST) purification resin column
(Novagen; Merck KGaA, Darmstadt, Germany), according to the
manufacturer’s instructions. The GST-Cpn 0810 recombinant protein
was identified by western blot analysis using a mouse anti-Cpn AR39
primary antibody (1:2,000 dilution; ab190064, Abcam, Cambridge, MA,
USA), and the protein concentration was detected using
bicinchoninic acid kits (Pik-day Bio Co., Ltd., Beijing,
China).
Cell culture and simulation
THP-1 cell lines were cultured in RPMI 1640 medium
(GE Healthcare Life Sciences, Logan, UT, USA), supplemented with
10% fetal bovine serum (FBS; GE Healthcare Life Sciences) and 2
mmol/l glutamine, in a humidified incubator at 37°C with 5%
CO2. For simulation, cells were seeded on plastic
culture plates (Corning Inc., Corning, NY, USA) and cultured in 1%
FBS overnight. Cells were then stimulated using specific
concentrations of GST-Cpn 0810 for predetermined time periods.
ELISA analysis
THP-1 cells were cultured in suspension, at a
density of 106 cells/ml, and seeded on 24-well plates.
The groups were treated with 0.5, 1, 2, 3, 4, 5 and 6 μg/ml GST-Cpn
0810 in serum-free culture medium for 24 h. Treatments of 5 μg/ml
GST and distilled water were used as negative controls, while 0.1
μg/ml lipopolysaccharide (LPS) treatment was used as a positive
control. After 24 h, the supernatant was collected for analysis of
tumor necrosis factor (TNF)-α and interleukin (IL)-6 by ELISA
(Jingmei Biological Engineering Co., Ltd., Shenzhen, China). When
the optimal concentration of GST-Cpn 0810 treatment was determined,
the cells were cultured with the specific concentration of GST-Cpn
0810 for 0, 6, 12, 24, 36 and 48 h before the culture supernatant
was used for TNF-α and IL-6 analysis.
Hoechst 33258 staining
THP-1 cells were seeded on six-well plates, at a
density of 5×105 cells/ml, and stimulated with 0, 5 and
10 μg/ml GST-Cpn 0810 for 24 h. Apoptosis was subsequently analyzed
using Hoechst staining kits (Beyotime Institute of Biotechnology,
Haimen, China), according to the manufacturer’s instructions.
Annexin V-fluorescein
isothiocyanate-propidium iodide (FITC-PI) assay
THP-1 cells were seeded on six-well plates, at a
density of 5×105 cells/ml, and stimulated with 0, 5, 10,
15 and 20 μg/ml GST-Cpn 0810 for 18 h. The rates of apoptosis were
analyzed using an annexin V-FITC apoptosis detection kit (KeyGen
Biotech, Nanjing, China). Treatment with 5 μg/ml GST was used as
negative control, 0.1 μg/ml LPS was used as a positive control and
untreated cells were used as a blank control.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analysis was performed using SPSS 13.0 software (SPSS,
Inc., Chicago, IL, USA). Paired t-test was used to analyze
comparisons between groups, and for analysis of paired data.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cloning, expression and purification of
recombinant GST-Cpn 0810
In order to obtain purified recombinant Cpn 0810, a
pGEX6p-2/Cpn 0810 plasmid was constructed and transformed into
E. coli BL21 cells. Following induction with 0.2 mM IPTG for
4 h, these cells were lysed and the supernatant was purified using
a GST purification column. Western blot analysis was performed
using anti-Cpn AR39 antibodies. A specific protein band was
observed at approximately 42 kD, indicating the expression of
recombinant GST-Cpn 0810 protein (Fig.
1A). This purified recombinant protein was used in the
following experiments.
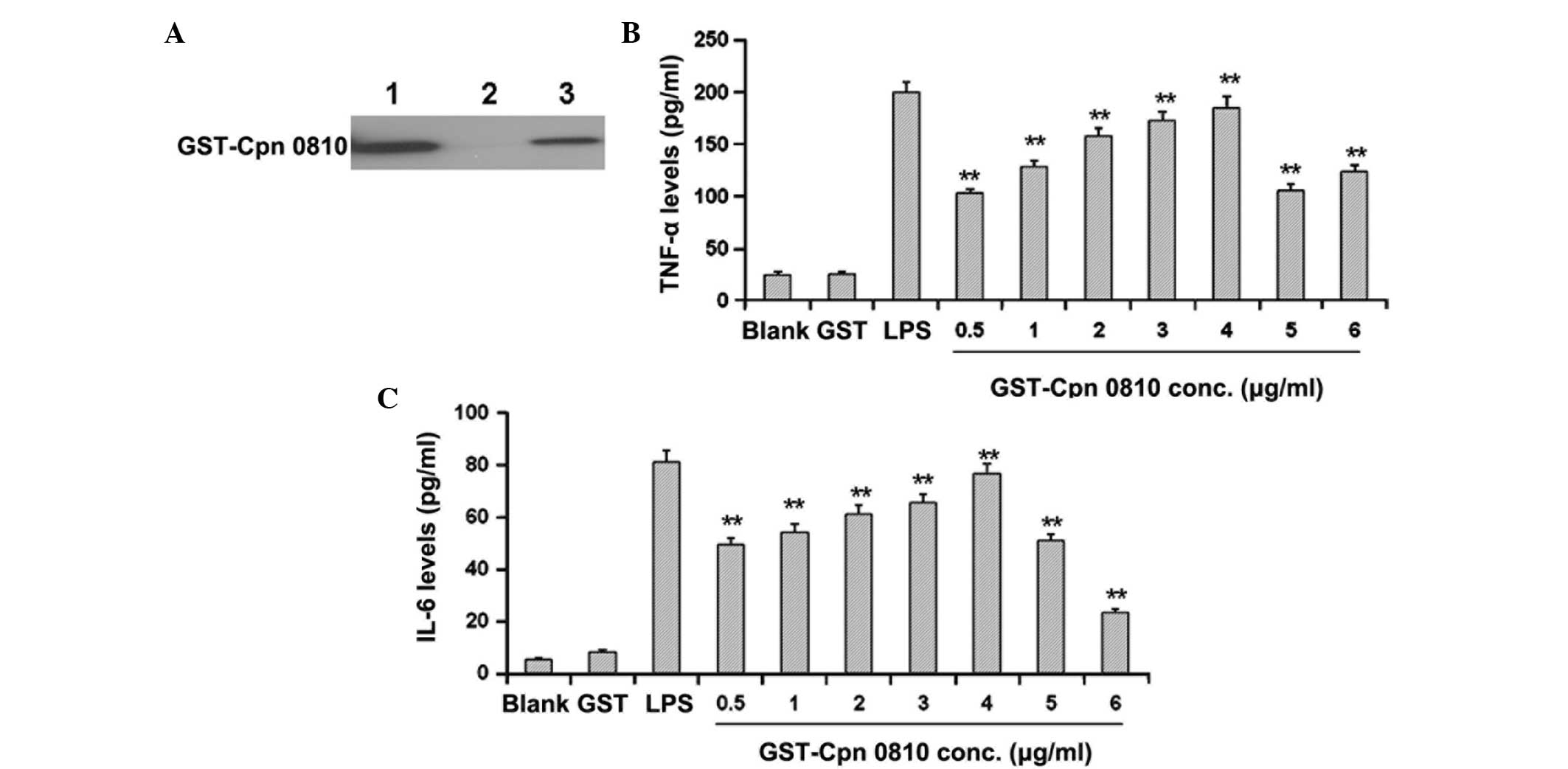 | Figure 1GST-Cpn 0810 increases the levels of
proinflammatory cytokines in THP-1 cells in a dose-dependent
manner. (A) Western blot analysis of the GST-Cpn 0810 recombinant
proteins was performed with an anti-Cpn AR39 primary antibody.
Lanes: 1, BL21 cells with pGEX6p-2/Cpn 0810 induced with 0.2 mmol/l
IPTG; 2, non-induced BL21 cells; 3, purified GST-Cpn 0810
recombinant proteins. THP-1 cells were treated with GST-Cpn 0810 at
gradient concentrations of 0.5, 1, 2, 3, 4, 5 and 6 μg/ml for 24 h,
and the expression levels of (B) TNF-α and (C) IL-6 were analyzed
with ELISA kits. **P<0.01, vs. GST-treated group.
GST, glutathione S-transferase; IPTG, isopropyl
β-D-1-thiogalactopyranoside; TNF, tumor necrosis factor; IL,
interleukin. |
Recombinant GST-Cpn 0810 elevates the
expression levels of proinflammatory cytokines in THP-1 cells
To investigate the effects of GST-Cpn 0810 on the
inflammation of THP-1 cells, purified recombinant GST-Cpn 0810 was
incubated with the cells and the levels of TNF-α and IL-6 were
detected using ELISA. THP-1 cells were treated with GST-Cpn 0810 at
gradient concentrations of 0.5, 1, 2, 3, 4, 5 and 6 μg/ml for 24 h.
The expression levels of TNF-α and IL-6 were found to increase over
the concentration range, 0.5–4 μg/ml GST-Cpn 0810, when compared
with the blank control group. Expression peaked at 4 μg/ml, where
the expression levels of TNF-α and IL-6 were 184.75±17.40 and
75.36±29.49 pg/ml, respectively (Fig.
1B and 1C). At higher concentrations of 5–6 μg/ml GST-Cpn, the
expression levels of TNF-α and IL-6 were reduced when compared with
the peak levels. Accordingly, a concentration of 4 μg/ml GST-Cpn
was used in the following time-course experiments.
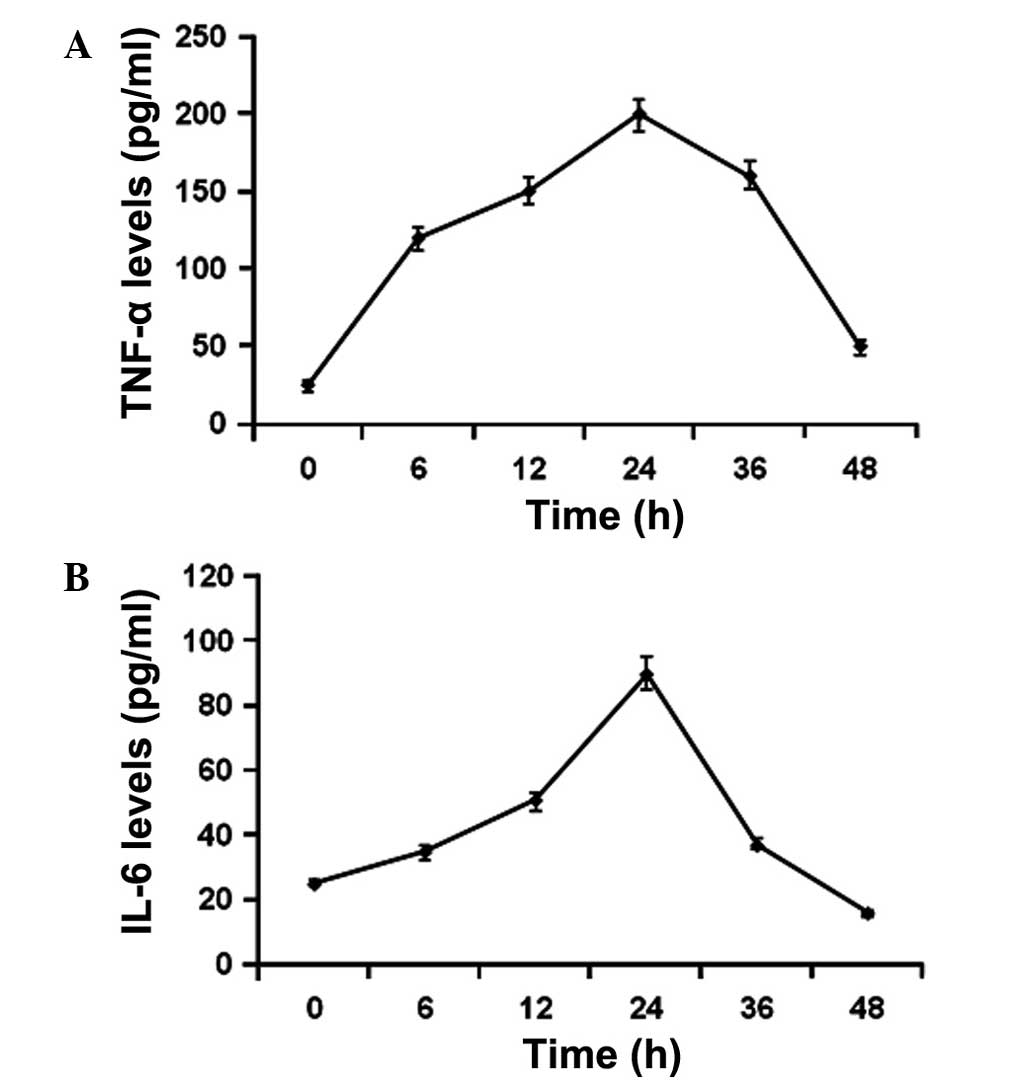
THP-1 cells were subsequently treated with 4 μg/ml
GST-Cpn 0810 for 6, 12, 24, 36 and 48 h, and the supernatant was
collected for the TNF-α and IL-6 assays. The results showed that
the stimulated expression levels of TNF-α and IL-6 were detectable
6 h after GST-Cpn 0810 treatment, peaking at 24 h (Fig. 2). Therefore, the results indicated
that GST-Cpn 0810 may elevate the levels of TNF-α and IL-6
expression in a dose- and time-dependent manner.
Recombinant GST-Cpn 0810 promotes
apoptosis in THP-1 cells
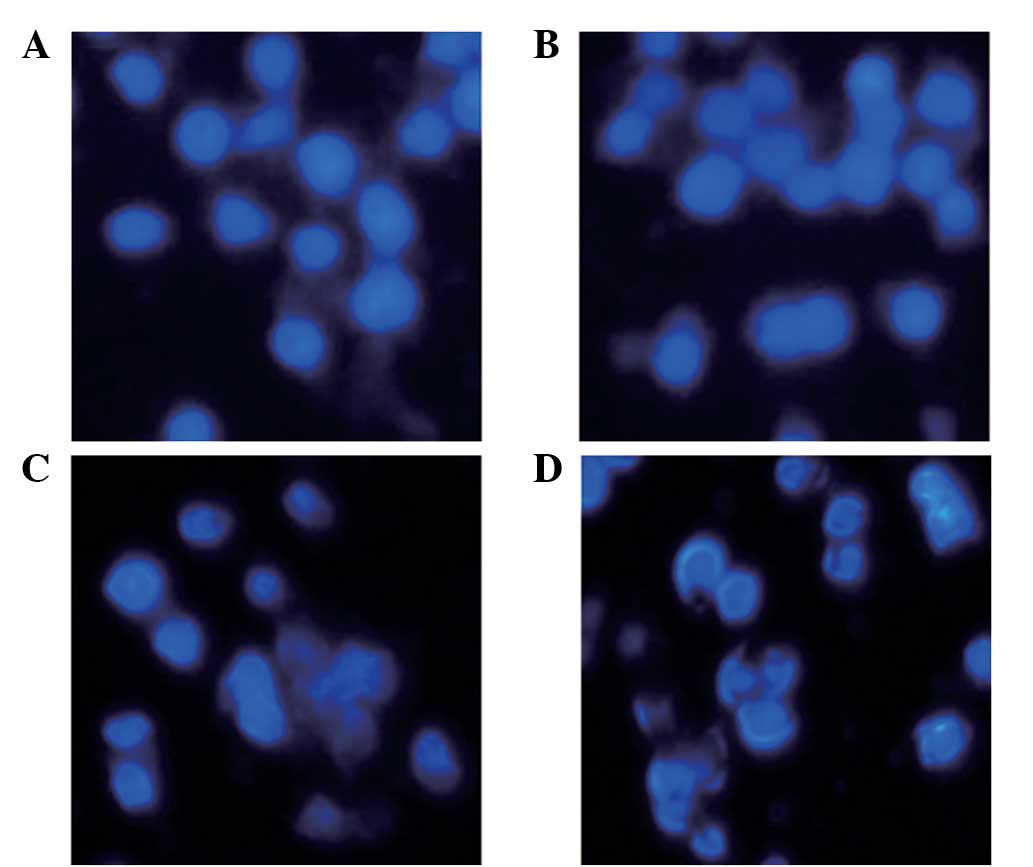
Hoechst 33258 staining and annexin V binding
analyses were performed to investigate the impact of GST-Cpn 0810
on the apoptotic process of THP-1 cells. For Hoechst 33258
staining, the THP-1 cells were stimulated with 0, 5 and 10 μg/ml
GST-Cpn 0810 for 24 h. Reduced nuclear size and blocky/granular
particles were observed in the cytoplasm of the THP-1 cells
following treatment with 5 μg/ml GST-Cpn 0810. When the
concentration was increased to 10 μg/ml GST-Cpn 0810, the
morphological changes were more evident (Fig. 3).
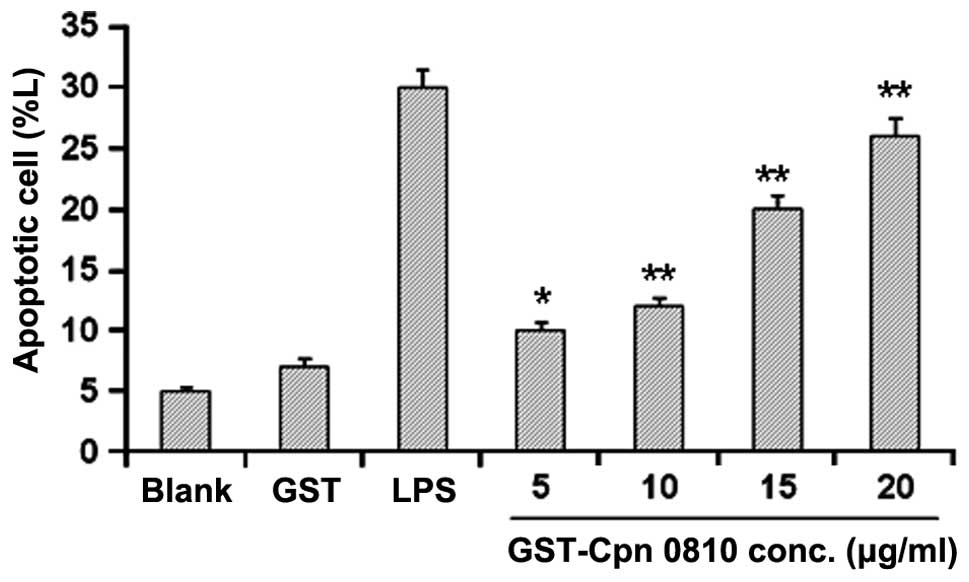
Analysis of annexin V binding in the THP-1 cells was
performed using annexin V-FITC detection kits, following treatment
with GST-Cpn 0810 for 24 h. In the flow cytometric analysis,
control cells exhibited weak FITC and PI signals, early apoptotic
cells showed high PI but low FITC fluorescence, and late apoptotic
or necrotic cells showed strong FITC and PI staining. GST-Cpn 0810
treatment for 24 h was shown to induce apoptosis in the THP-1 cells
in a dose-dependent manner, with a significantly increased
apoptotic rate of 25.84±5.50% at 20 μg/ml (P<0.01). The
apoptotic rate in these cells was 30.05±3.18% when treated with LPS
(positive control), indicating that GST-Cpn 0810 significantly
promoted apoptosis in the THP-1 cells (Fig. 4).
Discussion
C. pneumoniae causes a variety of diseases in
humans, including chronic infection, lung disease and
cardiovascular disease; however, its pathogenic mechanism is not
fully understood. Previous studies have shown that Cpn T3SS may
play an important role in these pathogenic processes, and Cpn 0810
has been predicted to be one of its effector proteins. In the
present study, the Cpn 0810 gene was subcloned into the prokaryotic
expression vector, pGEX6p-2, and successfully transformed into
E. coli BL21 cells. The recombinant proteins were purified
using a GST purification resin column, and applied to THP-1 cells
in order to study the effects.
The Cpn 0810 recombinant protein was shown to
stimulate THP-1 cells to produce proinflammatory cytokines,
including TNF-α and IL-6, in a dose- and time-dependent manner. The
TNF-α and IL-6 levels increased as the GST-Cpn 0810 concentrations
were increased from 0.5 to 4 μg/ml. However, as the Cpn 0810
concentration continued to increase, the inflammatory cytokine
levels decreased. These results indicated that high concentrations
of GST-Cpn 0810 can have toxic effects in THP-1 cells, as evidenced
by the decreased levels of inflammatory cytokines. Since the
proinflammatory cytokine levels in the GST control group were
similar to the negative control group, the possibility of direct
induction of these proinflammatory cytokines by GST was excluded.
Cpn 0810 may interact with the host cell and participate in
pathogenic processes. The expression levels of proinflammatory
cytokines were stimulated 6 h after the administration of GST-Cpn
0810, and peaked at 24 h, demonstrating the time-course of the
inflammation-stimulating effects of Cpn 0810.
TNF-α and IL-6 are important inflammatory mediators.
TNF-α exerts a wide range of biological effects, and is one of the
main cytokines involved in inflammatory cascades. TNF-α plays a key
role in the regulation of inflammatory processes in
atherosclerosis, mainly through the TNF-receptor 1 (p55) signaling
pathway (20–24). Early in inflammation, TNF-α can
promote immune cells in response to the invasion of pathogenic
microorganisms. In addition, high levels of TNF-α can induce the
instability of atherosclerotic plaques. Therefore, TNF-α has been
regarded as an inflammatory biological marker for atherosclerotic
plaque inflammation. TNF-α has been shown to be produced by local
atherosclerotic plaque macrophages, blood neutrophils and
monocytes, particularly in cases of arterial injury, plaque rupture
and ulceration (25). TNF-α also
activates endothelial and white blood cells, promotes the
aggregation of inflammatory cells and promotes the release of
inflammatory mediators (26).
IL-6 is an additional inflammatory cytokine with a
variety of biological functions, known to be involved in immune
regulation and the inflammatory response. IL-6 can increase the
activation of platelets and fibrinogen, leading to increased blood
viscosity and endothelial damage (27). In addition, IL-6 is closely
associated with TNF-α in the acute phase inflammatory response,
where TNF-α induces IL-6 and other factors to stimulate the
production of C-reactive protein (CRP) in the liver (28). Furthermore, IL-6 and CRP are
independent risk factors for stroke and myocardial infarction.
C. pneumoniae may stimulate smooth muscle cells to produce
IL-6, and C. pneumoniae-infected mononuclear cells may also
secrete IL-6 in response to pathological factors.
A previous study found that the mortality rate of
individuals with cardiovascular diseases in a five-year follow-up
period was able to be predicted based on the elevated concentration
of IL-6 in the peripheral blood (29). Arterial wall injury and repair, and
atherosclerotic plaque formation are always accompanied by
inflammation. It is widely accepted that inflammation is not only
associated with the formation of atherosclerotic plaques, but is
also involved in their stability, increasing the deterioration of
acute coronary syndrome (30).
Based on these studies, C. pneumoniae infection was
hypothesized to increase the levels of TNF-α and IL-6, in which
GST-Cpn 0810 may play a key role.
Apoptosis is a gene-regulated process of programmed
cell death. Through detecting atherosclerotic plaques in the
coronary and carotid arteries using TUNEL, previous studies
(31,32) found that there were DNA fragments
in the damaged intima. These fragments, which indicated apoptotic
processes, were not detected in healthy blood vessels. In the
present study, GST-Cpn 0810 was shown to inhibit THP-1 cell
proliferation by inducing apoptosis. When treated with GST-Cpn
0810, typical morphological changes of apoptosis, including cell
shrinkage, nuclear fragmentation, cell foam and apoptotic body
formation, were observed in the THP-1 cells. With annexin V-FITC
apoptosis detection kits, apoptotic processes were also detected at
24 h after GST-Cpn 0810 stimulation in these cells.
In conclusion, the results of the present study
demonstrated that recombinant GST-Cpn 0810 induces the expression
and secretion of the proinflammatory cytokines, TNF-α and IL-6, and
promotes apoptotic processes in THP-1 cells. Therefore, Cpn 0810
may function as an important pathogenic factor in interactions with
host cells. However, further studies are required to reveal the
underlying mechanisms of these processes.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 30870134).
References
|
1
|
Lui G, Ip M, Lee N, et al: Role of
‘atypical pathogens’ among adult hospitalized patients with
community-acquired pneumonia. Respirology. 14:1098–1105. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Damy SB, Higuchi ML, Timenetsky J, et al:
Mycoplasma pneumoniae and/or Chlamydophila pneumoniae inoculation
causing different aggravations in cholesterol-induced
atherosclerosis in apoE KO male mice. BMC Microbiol. 9:1942009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Papaetis GS, Anastasakou E and Orphanidou
D: Chlamydophila pneumoniae infection and COPD: more evidence for
lack of evidence? Eur J Intern Med. 20:579–585. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jupelli M, Shimada K, Chiba N, et al:
Chlamydia pneumoniae infection in mice induces chronic lung
inflammation, iBALT formation, and fibrosis. PLoS One.
8:e774472013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mota LJ and Cornelis GR: The bacterial
injection kit: type III secretion systems. Ann Med. 37:234–249.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mota LJ, Sorg I and Cornelis GR: Type III
secretion: the bacteria-eukaryotic cell express. FEMS Microbiol
Lett. 252:1–10. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Journet L, Hughes KT and Cornelis GR: Type
III secretion: a secretory pathway serving both motility and
virulence (review). Mol Membr Biol. 22:41–50. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Müller N, Sattelmacher F, Lugert R and
Gross U: Characterization and intracellular localization of
putative Chlamydia pneumoniae effector proteins. Med Microbiol
Immunol. 197:387–396. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stone CB, Johnson DL, Bulir DC, Gilchrist
JD and Mahony JB: Characterization of the putative type III
secretion ATPase CdsN (Cpn0707) of Chlamydophila pneumoniae. J
Bacteriol. 190:6580–6588. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Herrmann M, Schuhmacher A, Mühldorfer I,
Melchers K, Prothmann C and Dammeier S: Identification and
characterization of secreted effector proteins of Chlamydophila
pneumoniae TW183. Res Microbiol. 157:513–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cortes C, Rzomp KA, Tvinnereim A, Scidmore
MA and Wizel B: Chlamydia pneumoniae inclusion membrane protein
Cpn0585 interacts with multiple Rab GTPases. Infect Immun.
75:5586–5596. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo J, Jia T, Zhong Y, Chen D, Flores R
and Zhong G: Localization of the hypothetical protein Cpn0585 in
the inclusion membrane of Chlamydia pneumoniae-infected cells.
Microb Pathog. 42:111–116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kariagina AS, Alekseevskiĭ AV, Spirin SA,
Zigangirova NA and Gintsburg AL: Effector proteins of Clamidia. Mol
Biol (Mosk). 43:963–983. 2009.(In Russian).
|
|
14
|
Luo J, Jia T, Flores R, Chen D and Zhong
G: Hypothetical protein Cpn0308 is localized in the Chlamydia
pneumoniae inclusion membrane. Infect Immun. 75:497–503. 2007.
View Article : Google Scholar :
|
|
15
|
Flores R, Luo J, Chen D, Sturgeon G,
Shivshankar P, Zhong Y and Zhong G: Characterization of the
hypothetical protein Cpn1027, a newly identified inclusion membrane
protein unique to Chlamydia pneumoniae. Microbiology. 153:777–786.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fields KA and Hackstadt T: Evidence for
the secretion of Chlamydia trachomatis CopN by a type III secretion
mechanism. Mol Microbiol. 38:1048–1060. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lugert R, Kuhns M, Polch T and Gross U:
Expression and localization of type III secretion-related proteins
of Chlamydia pneumoniae. Med Microbiol Immunol. 193:163–171. 2004.
View Article : Google Scholar
|
|
18
|
Beeckman DS, Geens T, Timmermans JP, Van
Oostveldt P and Vanrompay DC: Identification and characterization
of a type III secretion system in Chlamydophila psittaci. Vet Res.
39:272008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jamison WP and Hackstadt T: Induction of
type III secretion by cell-free Chlamydia trachomatis elementary
bodies. Microb Pathog. 45:435–440. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Häcker H and Karin M: Regulation and
function of IKK and IKK-related kinases. Sci STKE. 2006:re132006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pfeffer K: Biological functions of tumor
necrosis factor cytokines and their receptors. Cytokine Growth
Factor Rev. 14:185–191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Perrins CJ and Bobryshev YV: Current
advances in understanding of immunopathology of atherosclerosis.
Virchows Arch. 458:117–123. 2011. View Article : Google Scholar
|
|
23
|
Hu S, Liang S, Guo H, et al: Comparison of
the inhibition mechanisms of adalimumab and infliximab in treating
tumor necrosis factor α-associated diseases from a molecular view.
J Biol Chem. 288:27059–27067. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paladino N, Mul Fedele ML, Duhart JM,
Marpegan L and Golombek DA: Modulation of mammalian circadian
rhythms by tumor necrosis factor-α. Chronobiol Int. 1–12. 2014.
|
|
25
|
Li L, Li DH, Qu N, Wen WM and Huang WQ:
The role of ERK1/2 signaling pathway in coronary
microembolization-induced rat myocardial inflammation and injury.
Cardiology. 117:207–215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tisato V, Zauli G, Rimondi E, et al:
Inhibitory effect of natural anti-inflammatory compounds on
cytokines released by chronic venous disease patient-derived
endothelial cells. Mediators Inflamm. 2013:4234072013.
|
|
27
|
Johnston SC, Zhang H, Messina LM, Lawton
MT and Dean D: Chlamydia pneumoniae burden in carotid arteries is
associated with upregulation of plaque interleukin-6 and elevated
C-reactive protein in serum. Arterioscler Thromb Vasc Biol.
25:2648–2653. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ridker PM, Rifai N, Stampfer MJ and
Hennekens CH: Plasma concentration of interleukin-6 and the risk of
future myocardial infarction among apparently healthy men.
Circulation. 101:1767–1772. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sharma J, Dong F, Pirbhai M and Zhong G:
Inhibition of proteolytic activity of a chlamydial
proteasome/protease-like activity factor by antibodies from humans
infected with Chlamydia trachomatis. Infect Immun. 73:4414–4419.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Biasucci LM, Santamaria M and Liuzzo G:
Inflammation, atherosclerosis and acute coronary syndromes. Minerva
Cardioangiol. 50:475–486. 2002.(In Italian). PubMed/NCBI
|
|
31
|
Geng YJ and Libby P: Evidence for
apoptosis in advanced human atheroma. Colocalization with
interleukin-1 beta-converting enzyme. Am J Pathol. 147:251–266.
1995.PubMed/NCBI
|
|
32
|
Hayakawa Y, Takemura G, Misao J, et al:
Apoptosis and overexpression of bax protein and bax mRNA in smooth
muscle cells within intimal hyperplasia of human radial arteries:
analysis with arteriovenous fistulas used for hemodialysis.
Arterioscler Thromb Vasc Biol. 19:2066–2077. 1999. View Article : Google Scholar : PubMed/NCBI
|