Introduction
Partial nephrectomy is currently the standard
treatment for low-stage renal cell carcinoma, providing oncological
outcomes that are comparable to radical nephrectomy (1). Recently, advances in surgical
techniques have resulted in the development of minimally invasive
surgical approaches (2). Application
of a laparoscopic partial nephrectomy (LPN) facilitates a reduced
hospital duration, and decreased operative bleeding and operative
duration when compared with an open partial nephrectomy procedure
(3). However, a LPN remains a
challenging procedure for surgeons to perform.
In general, the main renal artery is clamped during
a LPN in order to reduce hemorrhage. Clamping of the main renal
artery allows for improved visualization, which enables the repair
of the renal collecting system and the resection of the tumor
(4). However, hilar occlusion may
result in renal ischemic injury and impair renal function. Loss of
renal function occurs if the duration of warm ischemia is >28
min (5). A number of techniques have
been developed with the aim of minimizing warm ischemic injury
(2,6,7). The
technique of renal segmental artery clamping involves the selective
clamping of renal arterial branches, and may eliminate renal
ischemia from the entire kidney and minimize renal functional loss
(4). It is crucial that surgeons
obtain preoperative knowledge of the renal arterial anatomy in
order to successfully perform selective renal arterial branch
clamping.
Computed tomography angiography (CTA) has previously
been used to accurately visualize renal vascular anatomy (8,9). In the
present study, patients underwent a LPN with CTA to enable the
superselective clamping of the renal arterial branches. The aim of
the present study was to evaluate the clinical value of CTA in the
preoperative assessment of renal arterial anatomy by comparing the
preoperative CTA results with the surgical observations obtained
during LPN.
Patients and methods
Ethical approval and patient
selection
The study protocol was approved by the Dongguan
Peoples Hospital ethical committee (Dongguan, China), and patients
provided written informed consent for the publication of the
present case details.
A total of 42 patients with renal masses of <4 cm
were retrospectively enrolled in the study between May 2008 and
December 2013. All the patients had undergone a LPN with
superselective clamping of the renal arterial branches. The
inclusion criteria for the LPN was a tumor size of <4 cm.
Patients were excluded from the study if the LPN surgical
observations were insufficiently documented to permit comparison
with the preoperative CTA images.
CTA protocol
Patients underwent a preoperative CTA examination. A
Brilliance iCT multidetector spiral CT scanner (Philips Medical
Systems, Inc., Cleveland, OH, USA) was used to examine the 42
patients. CTA was performed using the following parameters:
Standard modality; gantry rotation, 0.4 sec; pitch, 0.915; tube
potential, 120 kV; tube current, 300 mA; collimation, 128×0.625 mm;
matrix, 512×512; thickness, 0.9 mm; increment, −0.45 mm; and dose
length product, 802.3 mGy•cm.
An Ultravist contrast agent (iopromide, 370 mgI/ml;
Bayer HealthCare Pharmaceuticals, Berlin, Germany) was administered
via a CT high-pressure syringe, with a moderate injection rate (4–5
ml/sec) and dose (1.0–1.5 ml/kg). Following an unenhanced CT
examination of the abdomen, a high-resolution contrast-enhanced
scan was performed on the kidneys in the arterial, venous and
urographic phases.
Images were postprocessed using an Extended
Brilliance Workspace independent workstation (version 4.5; Philips
Medical Systems, Inc.) to obtain multiplanar reconstructions and
maximum intensity projections, in addition to surface and volume
renderings.
Image analysis
CTA images were independently reviewed by two
radiologists that were blinded to the study. The number, location
and branching patterns of the renal arteries were documented and
the extrarenal length of the tumor-feeding arteries (from the first
branch of the main renal artery to the distal part of segmental
artery reaching the renal capsule) was measured. For supernumerary
renal arteries, the artery with the maximum diameter was recorded
as the main renal artery and all others were classified as renal
accessory arteries.
Surgical protocol
All 42 cases underwent a LPN via the retroperitoneal
approach. Surgery was performed following the CTA examination by a
surgeon with 10 years experience of performing LPNs. Tumor-feeding
arteries were preoperatively selected by two radiologists on the
basis of the CTA images. Following the superselective renal
arterial clamping of the tumor-feeding arteries, the tumors were
resected. For each patient the surgeon recorded a number of
surgical observations, including the number, location, extrarenal
length and branching patterns of the renal arteries. In addition,
clinical, perioperative and follow-up outcomes (9–75 months) were
recorded.
Statistical analysis
LPN surgical observations were considered to be the
reference standard for comparison. Statistical analysis was
performed using SPSS software, version 17.0 (SPSS, Inc., Chicago,
IL, USA). Data are presented as the mean ± standard deviation, or
as the median and range. Continuous variables were compared using
the paired Wilcoxon test, while nominal variables were analyzed
using Pearson's χ2 test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
In total, 42 patients successfully underwent a LPN
for renal masses of <4 cm. Patient demographics and tumor
characteristics are presented in Table
I. The mean tumor diameter was 3.2±0.4 cm, the mean operative
time was 103±12 min, the estimated blood loss was 263±136 ml and
the mean warm ischemia time was 23±3.6 min. A total of three
patients (7.1%) required a blood transfusion (Clavien grade II);
however, no patients developed a renal arteriovenous fistula or
required embolotherapy following surgery. The mean absolute
reduction in the estimated glomerular filtration rate (eGFR) at 3–6
months following surgery was 24.9±4.4 ml/min/1.73 m2.
Pathological examination detected clear-cell cancer in 34 cases,
angiomyolipoma in seven cases and papillary cancer in one case,
without positive margins.
 | Table I.Patient demographics, tumor
characteristics, surgical details and outcome. |
Table I.
Patient demographics, tumor
characteristics, surgical details and outcome.
| Variables | Mean ± SD | Median (range) |
|---|
| Patients (n) | 42 | – |
| Age (years) | 58.4±14.8 | 56 (45–72) |
| Male/female (n) | 24/18 | – |
| BMI
(kg/m2) | 22.5±1.5 | 21.9 (19.0–25.3) |
| Right/left side
(n) | 17/25 | – |
| Tumor diameter
(cm) | 3.2±0.4 | 3.2 (1.8–3.9) |
| Tumor location
(n) |
|
|
|
Polar | 25 | – |
|
Anterior | 7 | – |
|
Posterior | 9 | – |
| R.E.N.A.L score | 5.2±1.6 | 5 (3–9) |
| Perioperative |
|
|
| Surgery
time (min) | 103±12 | 99 (86–145) |
| EBL
(ml) | 263±136 | 227 (105–725) |
| WIT
(min) | 23±3.6 | 22.5 (18–35) |
| Transfusion (n) | 3 | – |
| Postoperative
complication |
|
|
| Clavien grade
I/II | 4/3 | – |
| eGFR absolute change
of the |
|
|
| affected side
(ml/min/1.73 m2) | 24.9±4.4 | 24 (16–38) |
| Histology (n) |
|
|
|
Clear-cell RCC | 34 |
|
| Papillary
RCC | 1 | – |
|
Angiomyolipoma | 7 | – |
| Margins negative
(n) | 42 | – |
Comparison between CTA and LPN
surgical observations for renal arteries
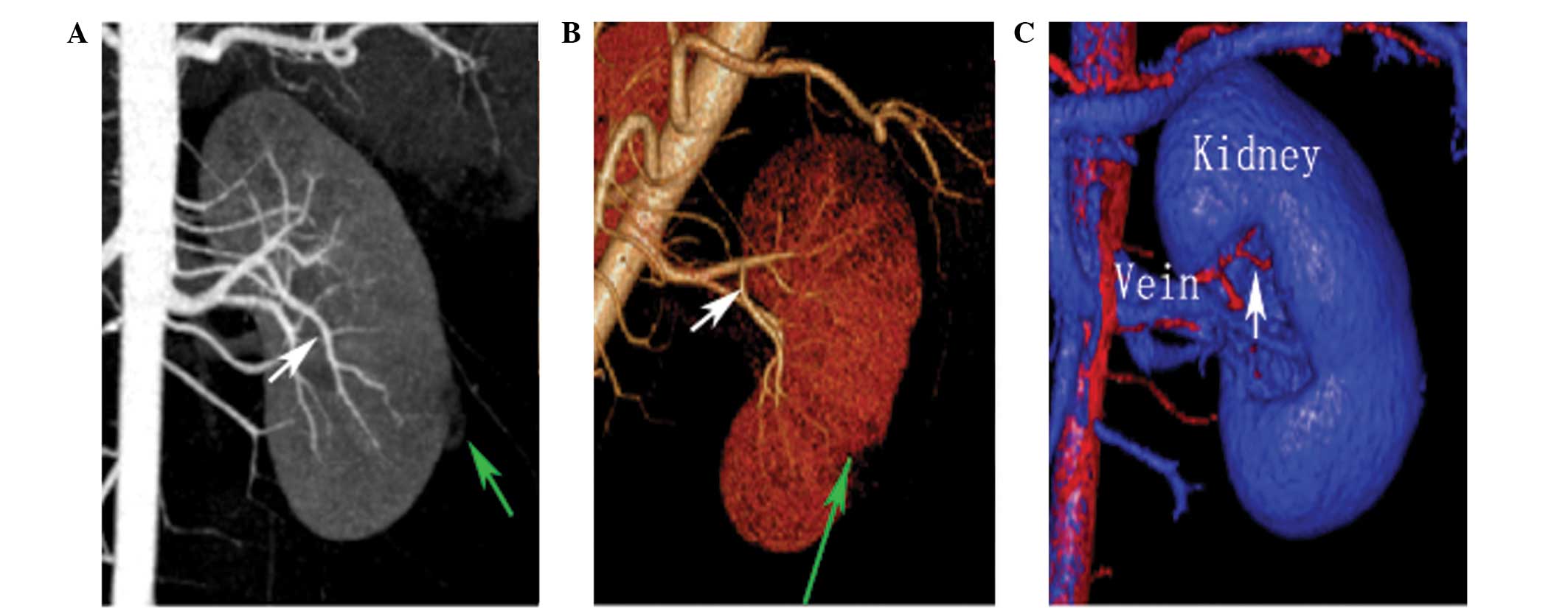
CTA was performed in 42 patients, providing a
preoperative map of the renal arteries. Postprocessing images of
all 42 cases accurately displayed the main renal trunks and vessel
branches (Figs. 1 and 2). Comparison between the CTA and LPN
observations for the renal arteries are summarized in Table II. Preoperatively, 57 renal arteries
were identified using the CTA images. Among the 42 cases, CTA
successfully detected 36 single renal arteries (97.3%).
Furthermore, a total of 5/6 accessory arteries were observed
preoperatively using the CTA images (Fig. 3), while one accessory artery of <2
mm in diameter was not detected. Thus, the accuracy of detection
for the renal arteries using preoperative CTA was 97.6% (41/42
arteries detected).
 | Table II.Comparison between preoperative CTA
predictions and LPN surgical observations for the renal
arteries. |
Table II.
Comparison between preoperative CTA
predictions and LPN surgical observations for the renal
arteries.
|
| LPN observations |
|
|
|---|
|
|
|
|
|
|---|
| CTA prediction | 1 artery | 2 artery | Accuracy (%) | Overall accuracy
(%) |
|---|
| CTA results | – | – |
| 97.6 |
| 1 artery | 36 | 1 | 97.3 |
|
| 2 artery | 0 | 5 | 100 |
|
Comparison between CTA and LPN
surgical observations for tumor-feeding arteries
Comparisons between preoperative CTA predictions and
LPN surgical observations for the number of tumor-feeding arteries
are summarized in Table III. The
accuracy of CTA for the detection of renal tumor-feeding branches
was 85.7%. Statistical analysis identified no statistically
significant differences between the CTA predictions and LPN
observations with regard to the number of tumor-feeding arteries
(P=0.839). The comparisons between the preoperative CTA and LPN
surgical results for the extrarenal length of the tumor-feeding
arteries are summarized in Table IV
(P=0.183).
 | Table III.Comparison between preoperative CTA
predictions and LPN surgical observations for tumor-feeding
arteries. |
Table III.
Comparison between preoperative CTA
predictions and LPN surgical observations for tumor-feeding
arteries.
|
| LPN observations |
|
|
|
|---|
|
|
|
|
|
|
|---|
| CTA prediction | 1 artery | 2 artery | 3 artery | Accuracy (%) | Overall accuracy
(%) | P-value |
|---|
| 1 artery | 28 | 4 | 0 | 87.5 |
|
|
| 2 artery | 1 | 7 | 1 | 77.8 | 85.7 | 0.839a |
| 3 artery | 0 | 0 | 1 | 100 |
|
|
 | Table IV.Comparison between CTA predictions and
LPN observations for the extrarenal length of the tumor-feeding
arteries. |
Table IV.
Comparison between CTA predictions and
LPN observations for the extrarenal length of the tumor-feeding
arteries.
| Variable | CTA prediction | LPN
observation | P-value |
|---|
| Extrarenal length
of the tumor feeding arteries (mm) | 30.2±2.8 | 30.4±2.7 | 0.183 |
Discussion
A partial nephrectomy results in comparable
oncological outcomes to a radical nephrectomy for small renal
tumors, and is associated with a reduced risk of chronic kidney
disease (1). As surgical techniques
have improved, LPN has replicated the techniques of open partial
nephrectomy for small renal tumors (10). Temporary hilar occlusion is commonly
employed during a LPN; however, hilar occlusion may result in warm
ischemic injury, which frequently impairs renal function (11). The safe upper limit for a period of
warm ischemia remains controversial, ranging between 20 and 40 min
(5,12). Thompson et al suggested that
the warm ischemic period should be restricted as much as possible
during a partial nephrectomy (13).
In the present study, the warm ischemic period was 23±3.6 min.
Hilar occlusion involves clamping the main renal
vasculature to allow for improved visualization; however, the
process may result in global renal ischemia. A number of techniques
have been developed with the aim to minimize warm ischemic injury
(2,6,7). One
approach, namely segmental renal artery clamping, eliminates global
renal ischemia and minimizes the loss of renal function (4). Segmental renal artery clamping involves
the selective clamping of renal arterial branches in order to
eliminate global renal ischemia. In the present study, between May
2008 and December 2013, patients underwent a LPN with
superselective clamping of the renal arterial branches. The mean
absolute reduction in the eGFR at 3–6 months following surgery was
24.9±4.4 ml/min/1.73 m2.
As the field of view during a LPN is limited, it is
crucial that surgeons obtain preoperative knowledge of the renal
arterial anatomy prior to performing selective clamping of the
renal arterial branches. Although digital subtraction angiography
is currently regarded as the gold standard for inspecting renal
vascular anatomy, CTA is becoming increasingly used to evaluate
renal arterial anatomy since the technique is less invasive, more
accurate and more widely available (14). A previous study indicated that the
sensitivity of CTA for preoperatively identifying renal arteries
was 98.5% (15). Furthermore,
Pozniak et al reported that the sensitivity of CTA was 100
and 93% for the detection of accessory arteries and prehilar
arterial branches, respectively (16). In the present study, CTA correctly
identified 36/37 single renal arteries, resulting in a 97.3% rate
of accuracy.
In the majority of individuals, the kidney is
supplied with blood by a single renal artery, and the prevalence of
multiple renal arteries reportedly varies between 9 and 76%
(17,18). The location of accessory arteries is
crucial knowledge for surgeons to obtain prior to surgery, as the
ligation of an accessory renal artery may lead to ischemic injury
to the portion of the kidney supplied by that artery. However,
hemorrhage may occur if a tumor-feeding accessory renal artery is
not clamped, which may further affect the visualization of the
laparoscopic field. Among the 42 cases investigated in the present
study, 5/6 accessory arteries were preoperatively detected using
CTA images, with only one accessory artery (diameter, <2 mm)
undetected (Fig. 3). Therefore,
preoperative CTA may aid surgeons in anticipating potential
vascular variants and facilitating LNP.
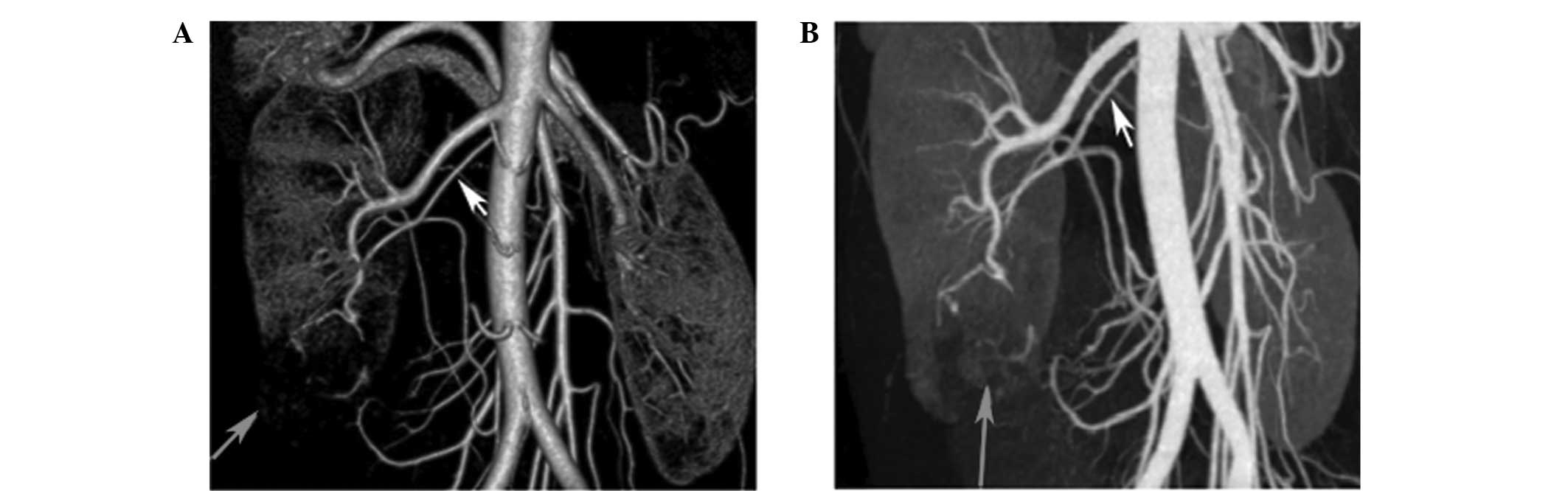
The preoperative identification of tumor-feeding
arteries may crucially affect the outcome of a LPN. Without
effective guidance, renal arteries may go undetected or may be
excessively clamped during the LPN, affecting visualization during
surgery or resulting in a loss of renal function. The renal artery
is typically divided into segmental arteries at the renal hilum.
With reformatting techniques, CTA can be used to accurately
elucidate the number, branching pattern and location of the renal
arteries. Thus, surgeons may preoperatively identify tumor-feeding
arteries using CTA images. In the present study, the accuracy of
the identification of tumor-feeding arteries via 256-channel CTA
was evaluated by comparing the CTA prediction with the actual
segmental arteries clamped during surgery. The accuracy of CTA in
detecting the renal tumor-feeding branches was 85.7%, and
statistical analysis indicated no statistically significant
difference between the CTA predictions and LPN observations with
regard to the number of tumor-feeding arteries (P=0.839; Fig. 2).
The extrarenal length of the tumor-feeding arteries
is a significant factor for the selective clamping of renal
arterial branches. Previously, Nohara et al reported that
tumor-feeding arteries of >10 mm in extrarenal length were
necessary for LPN to be performed (19). Furthermore, a previous study by Weld
et al (20) indicated that
the required extrarenal length of tumor-feeding arteries for
conducting a LPN was 31 mm. In addition, Kang et al reported
that tumors in the lower pole may be more amenable to segmental
artery clamping due to the presence of longer tumor-feeding
arteries (8). In the present study,
the extrarenal length of the tumor-feeding arteries was
preoperatively evaluated using CTA, and statistical analysis
indicated no statistically significant difference between the CTA
prediction and LPN observation of the extrarenal length of the
tumor-feeding arteries (P=0.183). In total, 42 patients
successfully underwent a LPN with superselective clamping of the
renal arterial branches, and no patients were converted to a LPN
with hilar occlusion. Therefore, the use of CTA may allow surgeons
to preoperatively evaluate the feasibility of segmental artery
clamping, and thus avoid unnecessary dissection of the segmental
artery.
A number of limitations were identified in the
present study. Firstly, the number of enrolled patients was
relatively limited; thus, further studies with larger population
samples are required to verify the present results. Secondly, there
may be a selection bias due to the retrospective nature of the
study.
In conclusion, CTA may be used to preoperatively
produce a map of the renal arteries, subsequently providing a
surgical advantage by optimizing the detection of tumor-feeding
arteries. Therefore, the use of CTA may facilitate successful
segmental renal artery clamping during a LPN.
Acknowledgements
The authors thank the medical staff from the
Department of Urology, Dongguan People's Hospital (Dongguan, China)
for supporting the study, and Professors Xiaolin Zheng and Tao Hu
of the Department of Radiology, Dongguan People's Hospital for
providing CT materials. In addition, the authors thank the patients
for volunteering to participate in the study. The study was
supported by the Science and Technological Program (no.
2012105102027) for Dongguan's Higher Education, Science and
Research and Health Care Institutions.
References
|
1
|
Van Poppel H, Da Pozzo L, Albrecht W, et
al: A prospective, randomised EORTC intergroup phase 3 study
comparing the oncologic outcome of elective nephron-sparing surgery
and radical nephrectomy for low-stage renal cell carcinoma. Eur
Urol. 59:543–552. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gill IS, Eisenberg MS, Aron M, Berger A,
Ukimura O, et al: Zero ischemia partial nephrectomy: Novel
laparoscopic and robotic technique. Eur Urol. 59:128–134. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gill IS, Kavoussi LR, Lane BR, et al:
Comparison of 1,800 laparoscopic and open partial nephrectomies for
single renal tumors. J Urol. 178:41–46. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shao P, Qin C, Yin C, Meng X, Ju X, Li J,
Lv Q, Zhang W and Xu Z: Laparoscopic partial nephrectomy with
segmental renal artery clamping: Technique and clinical outcomes.
Eur Urol. 59:849–855. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choi JD, Park JW, Choi JY, Kim HS, Jeong
BC, Jeon SS, Lee HM, Choi HY and Seo SI: Renal damage caused by
warm ischaemia during laparoscopic and robot-assisted partial
nephrectomy: An assessment using Tc 99m-DTPA glomerular filtration
rate. Eur Urol. 58:900–905. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peyronnet B, Baumert H, Mathieu R, et al:
Early unclamping technique during robot-assisted laparoscopic
partial nephrectomy can minimise warm ischaemia without increasing
morbidity. BJU Int. 114:741–747. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hsi RS, Macleod LC, Gore JL, Wright JL and
Harper JD: Comparison of selective parenchymal clamping to hilar
clamping during robotic-assisted laparoscopic partial nephrectomy.
Urology. 83:339–344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang WY, Sung DJ, Park BJ, et al:
Perihilar branching patterns of renal artery and extrarenal length
of arterial branches and tumour-feeding arteries on multidetector
CT angiography. Br J Radiol. 86:201203872013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rastogi N, Sahani DV, Blake MA, Ko DC and
Mueller PR: Evaluation of living renal donors: Accuracy of
three-dimensional 16-section CT. Radiology. 240:136–144. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gill IS, Desai MM, Kaouk JH, et al:
Laparoscopic partial nephrectomy for renal tumor: Duplicating open
surgical techniques. J Urol. 167:469–7; discussion 475–476. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Porpiglia F, Fiori C, Bertolo R, Morra I,
Russo R, Piccoli G, Angusti T and Podio V: Long-term functional
evaluation of the treated kidney in a prospective series of
patients who underwent laparoscopic partial nephrectomy for small
renal tumors. Eur Urol. 62:130–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Porpiglia F, Fiori C, Bertolo R, et al:
The effects of warm ischaemia time on renal function after
laparoscopic partial nephrectomy in patients with normal
contralateral kidney. World J Urol. 30:257–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thompson RH, Lane BR, Lohse CM, et al:
Every minute counts when the renal hilum is clamped during partial
nephrectomy. Eur Urol. 58:340–345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Türkvatan A, Ozdemir M, Cumhur T and Olçer
T: Multidetector CT angiography of renal vasculature: Normal
anatomy and variants. Eur Radiol. 19:236–244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raman SS, Pojchamarnwiputh S, Muangsomboon
K, Schulam PG, Gritsch HA and Lu DS: Utility of 16-MDCT angiography
for comprehensive preoperative vascular evaluation of laparoscopic
renal donors. AJR Am J Roentgenol. 186:1630–1638. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pozniak MA, Balison DJ, Lee FT Jr,
Tambeaux RH, Uehling DT and Moon TD: CT angiography of potential
renal transplant donors. Radiographics. 18:565–587. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uflacker R: Abdominal aorta and
branchesAtlas of Vascular Anatomy: An Angiographic Approach. 2nd.
Lippincott Williams & Wilkins; Philadelphia, PA: pp. 111–222.
2006
|
|
18
|
Satyapal KS, Haffejee AA, Singh B,
Ramsaroop L, Robbs JV and Kalideen JM: Additional renal arteries:
Incidence and morphometry. Surg Radiol Anat. 23:33–38. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nohara T, Fujita H, Yamamoto K, Kitagawa
Y, Gabata T and Namiki M: Modified anatrophic partial nephrectomy
with selective renal segmental artery clamping to preserve renal
function: A preliminary report. Int J Urol. 15:961–966. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weld KJ, Bhayani SB, Belani J, Ames CD,
Hruby G and Landman J: Extrarenal vascular anatomy of kidney:
Assessment of variations and their relevance to partial
nephrectomy. Urology. 66:985–989. 2005. View Article : Google Scholar : PubMed/NCBI
|