Introduction
The glucocorticoid receptor (GR) is a
hormone-dependent transcription factor and a member of the nuclear
receptor superfamily. The functions of the receptor include the
transcription and regulation of a variety of genes. Glucocorticoids
(GCs) usually target GR-α, which is expressed in almost all tissues
and cells of the body (1). GR-α is
not only involved in signal transduction, but has also been
demonstrated to exert multiple functions, including
anti-inflammatory, antiproliferative and immunosuppressive effects
(1).
Psoriasis is a form of chronic inflammatory
dermatosis, which is characterized by the excessive proliferation
and abnormal differentiation of epidermal keratinocytes (2). The epidermal tissue has been reported
to be one of the target tissues for GCs. Thus, GR-α was
hypothesized to be involved in the overproliferation and abnormal
differentiation of epidermal keratinocytes (2) underlying the pathogenesis of psoriasis.
In the present study, immunohistochemistry was used to detect the
expression of GR-α in normal control subjects, and lesional and
non-lesional samples from patients with psoriasis. The aim of the
present study was to investigate the function of GR-α in the
molecular pathogenesis underlying psoriasis.
Materials and methods
Study population
The study population was composed of 26 patients
with psoriasis vulgaris and 10 healthy control subjects, who were
recruited from the Air Force General Hospital of the People's
Liberation Army (Beijing, China) in 2013. The patients had been
undergoing consultations at least once a week with a physician with
regard to their skin condition. However, prior to sampling, none of
the patients had received treatment with GCs, immunosuppressants or
vitamin A acid drugs, and had only used Vaseline or other
moisturizing creams. With regard to the effect of treatment with
GCs, studies were performed prior to and after using it in the 6
months follow-up survey independently.
Institutional Ethics Board approval was obtained
from the Medical Ethics Committee of the Air Force General Hospital
of People's Liberation Army (Beijing, China). All the patients had
previously provided written, informed consent to having their
clinical and pathological information used for this research.
Sample collection
The psoriasis area and severity index (PASI) was
used to detect and score the skin lesions of the 26 psoriasis
cases. The present study included three groups, the lesional group
contained samples from typical lesions of psoriasis cases, while
the non-lesional group consisted of samples from non-lesional
tissue within the range of 3 cm. In addition, normal control
samples were collected from the normal control group.
In the two psoriasis groups (lesional and
non-lesional), five specimens were obtained from the torso and 21
specimens were collected from the arms or legs. With regard to the
control group with normal skin tissue, five specimens were
collected from the head tissue, two specimens were obtained from
the torso and four specimens were collected from the arms or legs.
Every epidermis sample had a diameter of 3 mm and was obtained
using a trephine. All the samples were fixed in 10% formalin and
embedded in paraffin, immediately.
Section staining
Paraffin sections (4 µm) from the three groups were
analyzed using a streptavidin peroxidase-enzymatic (SP) method. Two
processes were utilized for sample preparation, which all samples
underwent. Firstly, after 2 h incubation at 60°C, the paraffin
sections were dewaxed in xylene and hydrated in graded ethanol.
Subsequently, the sections were blocked with 3% hydrogen peroxide
for 5 min at 25°C. In the second process of sample preparation, the
sections were subjected to a high temperature of 140°C for 2 min in
citrate buffer. At the end of each process, the samples were washed
three times with phosphate-buffered saline (PBS) for 2 min at
25°C.
GR-α expression was detected using a rabbit
anti-human GR-α primary antibody (1:300, EH-1657; Beijing
Biosynthesis Biotechnology Co., Ltd., Beijing, China).
Subsequently, samples were analyzed using an anti-rabbit
Ultra-Sensitive Immunohistochemistry Kit (#7770; Fuzhou Maixin
Biotechnology Development, Co., Ltd., Fuzhou, China), following the
manufacturer's instruction. The chromogenic reaction was observed
using a 3,3′-diaminobenzidine (DAB) kit (#0017; Maixin
Biotechnology Development, Co., Ltd.), following the manufacturer's
protocol. The primary antibody was incubated overnight under 4°C.
The secondary and horse radish peroxidase-conjugated tertiary
antibodies were incubated for 20 min at 37°C. The secondary and
tertiary antibodies were provided with the kit. DAB was used as a
substrate to visualize the reaction.
Samples were clarified using xylene and
counterstained with hematoxylin, in order to produce clearer and
identifiable images. PBS was used as a negative control. The
specific brown granules in the tissues and cells were observed
using an Olympus BX60 optical microscope (Olympus Corporation,
Tokyo, Japan).
Quantification of GR-α expression
Using five typical fields of view under the optical
microscope, images of the epidermis, excluding the stratum corneum,
were collected. A pathological image analysis system, known as
CMIAS (jointly developed by the Air Force General Hospital and
Beijing University of Aeronautics and Astronautics, Beijing, China)
(3), was used to convert the image
signal into a numeric value, according to the optical density per
unit area, which was measured using a Nikon DS-SMC-UI system (Nikon
Corporation, Tokyo, Japan) (4,5). The
mean value of the specimens was calculated.
Reagents
The polyclonal rabbit anti-human GR-α antibody was
purchased from Beijing Biosynthesis Biotechnology Co, Ltd.
(Beijing, China). The highly sensitive SP immunohistochemical kit
was obtained from Beijing Hantian Biotechnology Co., Ltd. (Beijing,
China), and the DAB chromogenic kit was obtained from Fuzhou Maxim
Biotechnology Co., Ltd. (Fuzhou, China).
Statistical analysis
All the data are represented as the mean ± standard
deviation of three or more independent experiments. If the data
exhibited homogeneity, analysis of variance, the
Student-Newman-Keuls test and Pearson's correlation analysis were
performed. If the data exhibited heterogeneity, the Kruskal-Wallis
test, Games-Howell test and Spearman's correlation analysis were
performed. All statistical analyses were conducted using SPSS 17.0
software (SPSS, Inc, Chicago, IL, USA), and P<0.05 was
considered to indicate a statistically significant difference.
Results
Population characteristics
Of the 26 psoriasis cases, 19 individuals were male
and 7 patients were female, with an average male-to-female gender
ratio of 2.714. The average age was 36.0±11.2 months (range, 19–57
months), and the average PASI value was 21.9±6.1 (range,
11.6–31.8). In the normal control group, 7 subjects were male and 3
were female, with an average male-to-female gender ratio of 2.333.
The average age of the subjects was 35.5±14.0 months (range, 10–50
months). There were no statistically significant differences with
regard to patient gender and age between the groups.
Expression of GR-α
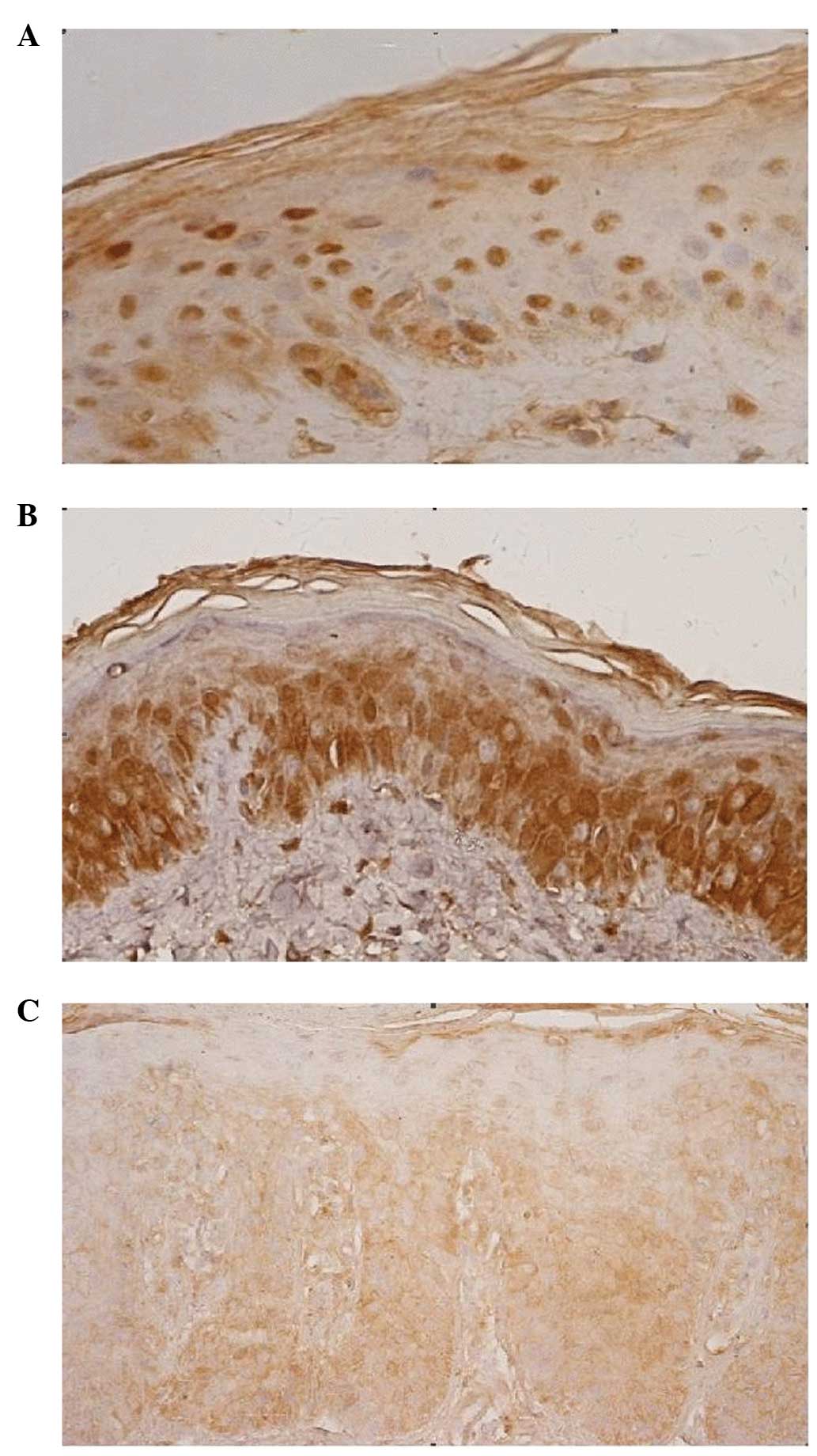
GR-α expression within the nucleus was observed in
the normal control group, while cytoplasmic expression was observed
in the lesions of the psoriatic group (Fig. 1A and C), excluding the stratum
corneum. However, in the non-lesional psoriasis samples, GR-α
expression was observed in the nucleus and cytoplasm (Fig. 1B). Thus, the microscopic observations
revealed that GR-α was differentially expressed among the three
groups.
The results from the quantitative image analysis
(Table I) revealed that there was no
statistically significant difference in the expression levels of
GR-α between the normal control group and the non-lesional
psoriasis samples (P=0.677). However, the expression levels of GR-α
in the psoriasis lesional group were significantly lower when
compared with the other two groups (P<0.001).
 | Table I.Analysis of GR-α expression. |
Table I.
Analysis of GR-α expression.
| Group | Cases (n) | Optical density |
|---|
| Normal epidermis
control | 10 |
0.166±0.032 |
| Non-lesional
psoriatic epidermis | 26 |
0.161±0.033 |
| Lesional psoriatic
epidermis | 26 |
0.093±0.025a |
According to the 6-month follow-up survey,
administration of GCs was not shown to affect GR-α expression.
There was no statistically significant difference prior to and
following the administration of GCs (P=0.300).
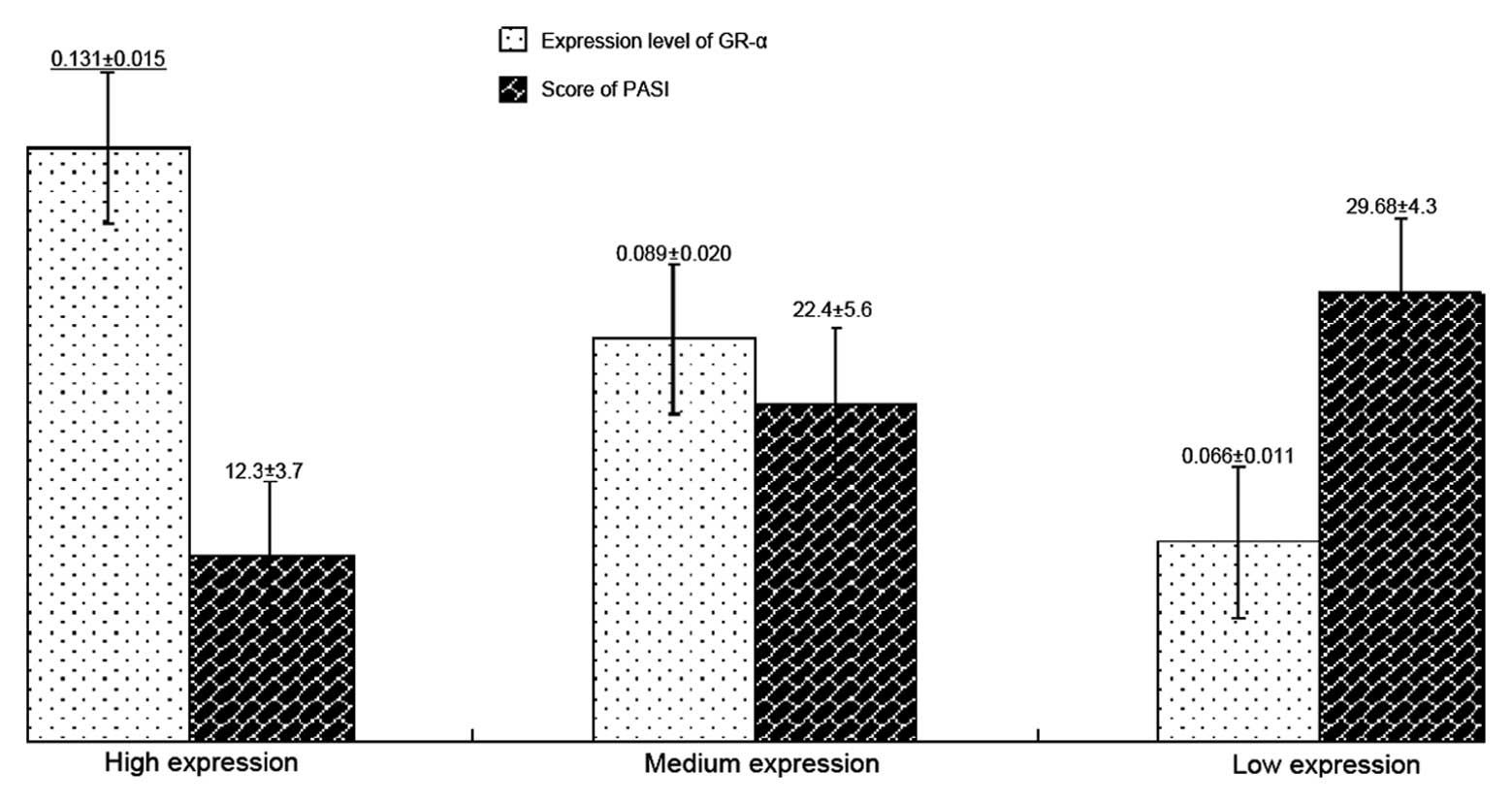
The association between GR-α expression and
psoriasis severity, according to the PASI score, is illustrated in
Fig. 2. According to the GR-α
expression level, the samples were divided into high, medium and
low expression groups. A higher PASI score was observed in those
with lower expression levels of GR-α. In addition, Pearson's
correlation analysis revealed that there was a negative correlation
between the average optical density of GR-α and the PASI score in
26 patients with psoriasis (r=-0.627, P=0.007).
Discussion
Chrousos and Kino (6)
suggested that the expression of 20% of genes in the human genome
was influenced by GCs. Thus, GCs were hypothesized to participate
in the regulation of a number physiological and pathological
processes, such as cell growth and differentiation, metabolism,
immunity and inflammation, through activating the corresponding
GR-α in cells (6). The inactive form
of GR-α, which is not combined with GC, has been demonstrated to
primarily exist in the cytoplasm. By contrast, the active form of
GR-α, which is combined with GC, can be rapidly transported into
the nucleus, where the receptor is able to recognize and bind to
the GC reactive element, which is located upstream of the target
gene (6,7). As a transcription factor, activated
GR-α is able to activate or inhibit the expression of the target
gene by directly or indirectly regulating the transcription of the
target gene, to subsequently produce various biological effects,
including antiproliferation, anti-inflammation and
immunosuppression (7). In the study
by Fan et al, the level of serum GC in patients with
psoriasis was demonstrated to be higher than normal, while the mRNA
expression level of GR in peripheral blood leukocytes was
significantly lower than normal. These results indicated that the
decreased expression of GR in psoriasis patients may prevent GC
from effectively exerting an anti-inflammatory effect, subsequently
inducing or aggravating the psoriasis immune inflammatory reaction
(8).
The results of the present study demonstrated that
GR-α was expressed in the nucleus, where the receptor played a role
in the cell proliferation and differentiation of normal human
epidermal keratinocytes. Abnormal expression of GR-α was observed
in the psoriatic epidermis, where the receptor was abundantly
expressed in the cytoplasm; however, the expression level of GR-α
in the non-lesional psoriasis epidermis did not decrease. These
results indicated that the translocation of GR-α into the nucleus
was blocked. In the psoriasis lesions, the expression level of GR-α
decreased significantly; thus, GR-α failed to enter the nucleus,
resulting in complete malfunction with regard to gene
regulation.
Results from Pearson's correlation analysis revealed
that there was a negative correlation between the psoriasis
severity and the expression of GR-α in the psoriasis lesions. This
observation indicates that the more serious the lesions of the
psoriasis, the lower the expression of GR-α in the epidermis.
A previous study found that the decreasing
expression of GR was the result of decreased levels of GC (9). However, the results of the present
study indicate that reduced expression of GR-α was not associated
with long-term exposure to GC. To eliminate this hypothesis, in the
present study, the psoriasis patients were divided into two groups.
One group did not receive GC treatment, while the other group
received partial GC treatment (no third GC administration) over 6
months. The results demonstrated that there was no statistically
significant difference in the expression levels of GR-α in the
psoriasis lesions between the two groups, indicating that partial
GC administration was not the fundamental reason for the abnormal
expression of GR-α in the psoriasis epidermis.
In conclusion, the present study demonstrated that
psoriasis epidermal keratinocytes exhibit decreased expression and
abnormal localization of GR-α. Thus, GR-α may play an important
role in the excessive proliferation and abnormal differentiation
observed in the epidermal cells of patients with psoriasis.
However, the factors contributing to this phenomenon remain
unclear. These factors may be crucially involved in the
pathogenesis of psoriasis vulgaris and require further
investigation. In summary, the results of the present study
provides insights relevant to the investigation of the mechanisms
underlying the pathogenesis of psoriasis vulgaris, and may aid the
development of novel therapies.
Abbreviations:
|
GR-α
|
glucocorticoid receptor-α
|
|
SP
|
streptavidin peroxidase
|
|
GC
|
glucocorticoid
|
|
PASI
|
psoriasis area and severity index
|
|
DAB
|
3,3′-diaminobenzidine
|
|
PBS
|
phosphate-buffered saline
|
References
|
1
|
Luft FC: Glucocorticoid receptor function
and clinical medicine. J Mol Med Berl. 80:267–269. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Serres M, Viac J and Schmitt D:
Glucocorticoid receptor localization in human epidermal cells. Arch
Dermatol Res. 288:140–146. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu P, Bu H, Wang H, Zhao G, Zhang J and
Zhou Q: Comparative study on image analysis and manual counting of
immunohistochemistry. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi.
20:288–290. 2003.(In Chinese). PubMed/NCBI
|
|
4
|
Ying C, Chunmin Y, Qingsen L, Mingzhou G,
Yunsheng Y, Gaoping M and Ping W: Effects of simulated
weightlessness on tight junction protein occludin and Zonula
Occluden-1 expression levels in the intestinal mucosa of rats. J
Huazhong Univ Sci Technolog Med Sci. 31:26–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li CH, Yang ZW, Yin ZR, Jin Z, Xing DG,
Piao LH, Kim YC and Xu WX: Relationship between atrial natriuretic
peptide-immunoreactive cells and microvessels in rat gastric
mucosa. Acta Pharmacol Sin. 27:205–211. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chrousos GP and Kino T: Intracellular
glucocorticoid signaling: A formerly simple system turns
stochastic. Sci STKE. 304:pe482005.
|
|
7
|
Pujols L, Mullol J, Roca-Ferrer J, et al:
Expression of glucocorticoid receptor alpha- and beta-isoforms in
human cells and tissues. Am J Physiol Cell Physiol.
283:C1324–C1331. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan JY, Yang XQ, Zhang LJ and Liu XQ:
Determination of level of glucocorticoid and its receptor gene mRNA
in the blood of psoriatic patients and its significance. Di 4 Jun
Yi Da Xue Xue Bao. 22:2091–2093. 2001.(In Chinese).
|
|
9
|
Yu B, He W, Zhang B, Yang L and Wu J:
Effects of dexamethasone on the expression of glucocorticoid
receptor α in human keratinocyte HaCaT cell line. Lin Chuang Pi Fu
Ke Za Zhi. 37:216–218. 2008.(In Chinese).
|