Introduction
Neuromyelitis optica (NMO) is an acute or subacute
lesion of demyelinating disease involving optic nerve and spinal
cord (1). Previously, NMO was
considered a clinical subtype of multiple sclerosis (MS), although
the effects of clinical treatment were unsatisfactory (1,2). The
development of imaging technology identified NMO and MS as
different with regard to the characteristics of lesions.
By applying two inversion pulses, three-dimensional
double inversion recovery (3D-DIR) sequence significantly improved
the signal contrast of different tissues and the imaging effects of
gray matter lesions (3). In recent
years, 3D-DIR has been gradually applied in the diagnosis of
epilepsy, MS and other diseases, and the effects have proven to be
satisfactory (4–6).
The aim of the present study was to examine the
application value of 3D-DIR in the early differential diagnostic
and prognostic evaluation of NMO.
Materials and methods
Patients
In total, 48 patients diagnosed with suspicious NMO
at the Rizhao People's Hospital between October 2013 and October
2015 were included into the present study. All 48 patients were
confirmed to be first onset and first treatment according to the
clinical symptoms and physical signs. Of the 48 patients, there
were 10 males and 38 females including 7 children. The patient age
range was 8–66 years, with a median age of 43.5 years, and a time
of onset ranging from 1 to 3 days with an average of 1.7±0.5 days.
The patients with central nervous demyelinating lesions such as MS,
definite infection history, cerebral tumor, epilepsy, pregnancy,
consciousness disturbance and those who refused to participate were
excluded.
Approval for the present study was obtained from the
ethics committee of the Rizhao People's Hospital. Patients and
their families/guardians provided informed consent.
Methods
The patients were given a combined NMO-IgG
quantitative detection and 3D-DIR examination as well as standard
medical drug therapy. For the 3D-DIR examination, a Siemens
MAGNETOM Skyra 3.0T superconducting scanner (Siemens Healthcare
GmbH, Erlangen, Germany) and 8-channel head coil were used to
perform the routine MR examination. Fast spin echo (TSE) T2WI,
fluid-attenuated inversion recovery (FLAIR) sequence and 3D-DIR
sequence were examined in turn. Parameters of the 3D-DIR sequence
included (TR 7,500 msec, TE 308 msec; T1 3,000 msec; matrix
190×192; visual field of FOV 256×256 mm; 128 layers; and thickness
of 1.3 mm), two reversal pulses, TI1 (time from the first 180°
reverse pulse to 90° drive pulse) 3,400 msec, and parameters of TI2
(time from the second 180° reverse pulse to 90° drive pulse) 325
msec. Subsequently, T2W TSE sequence (TR 5,000 msec, TE 87 msec;
matrix 256×256; FOV 256×256 mm; 35 layers; thickness of 4 mm)
scanning was performed. According to the anatomical location of the
lesions in MRI, the lesions were divided into: endodermal lesions,
gray and white matter mixed lesions, subcortical lesions, deep gray
matter lesions and white matter lesions.
Observation indexes
The signal characteristics of the 3D-DIR scan of the
cerebral and spinal cord lesions of NMO and MS patients were
compared, and the signal characteristics of 3D-DIR scan of NMO
patients prior and subsequent to surgery were compared.
Statistical methods
SPSS 20.0 statistical software (Chicago, IL, USA)
was used for statistical analysis. Measurement data were presented
as mean ± standard deviation. Enumeration data were expressed as a
percentage (%). The group comparison was made using the
χ2 test. P<0.05 was considered to be statistically
significant.
Results
Signal characteristics of 3D-DIR
scaning of NMO patients
The diagnostic criteria for NMOSD revised by
Wingerchuk et al in 2015 (7)
indicated: i) at least one core clinical characteristic, ii)
positive test for AQP4-IgG using the best available detection
method, and iii) exclusion of alternative diagnoses. At least one
core clinical characteristic (optic nerve) was neuritis and acute
myelitis, with the supporting standard (met at least two
requirements) being: spinal cord MRI lesion extended at least three
segments, head MRI failed MS diagnostic criteria, and positive
serum NMO-IgG. Forty cases (83.3%) were confirmed with NMO, and the
time from onset to diagnosis was 3.5±0.6 days on average.
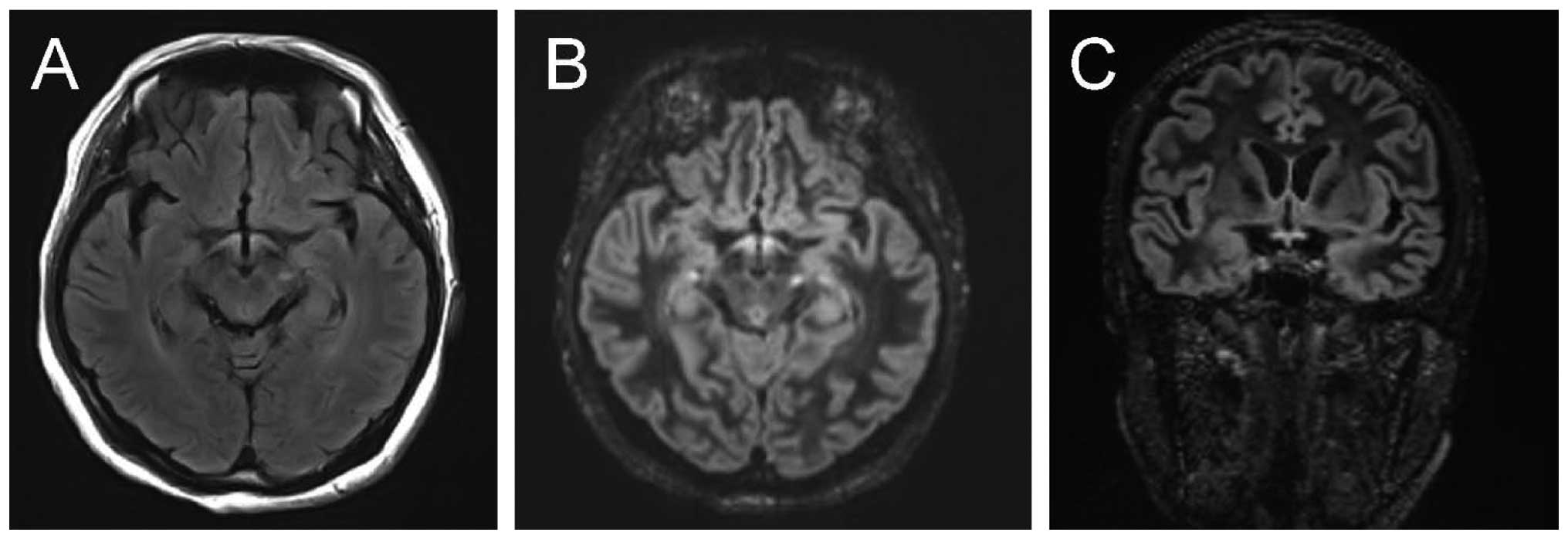
The brain of NMO patients showed low T1W signal,
high T2W and FLAIR signals, involving 5.8±1.2 sites on average, and
being distributed in the peripheral lateral ventricle, medulla,
cerebral white matter, the third ventricle, peripheral aqueduct of
sylvius, pons and diencephalon. The average T2W signal strength was
2.73±0.12, average FLAIR signal strength was 2.56±0.23 and the
average DIR signal strength was 2.89±0.32. The signal intensity of
T2W, FLAIR and DIR of NMO patients was significantly higher than
that of MS patients, with the average sites involved being more
than those of MS patients (P<0.05) (Table I and Fig.
1).
 | Table I.Brain signal characteristics of NMO
patients. |
Table I.
Brain signal characteristics of NMO
patients.
| Group | T2W | FLAIR | DIR | Mean involved
parts |
|---|
| NMO patients | 2.73±0.12 | 2.56±0.23 | 2.89±0.32 | 5.8±1.2 |
| MS patients | 2.84±0.15 | 2.77±0.25 | 3.04±0.22 | 2.7±0.6 |
| t | 4.251 | 4.189 | 4.237 | 5.324 |
| P-value | 0.043 | 0.044 | 0.042 | 0.038 |
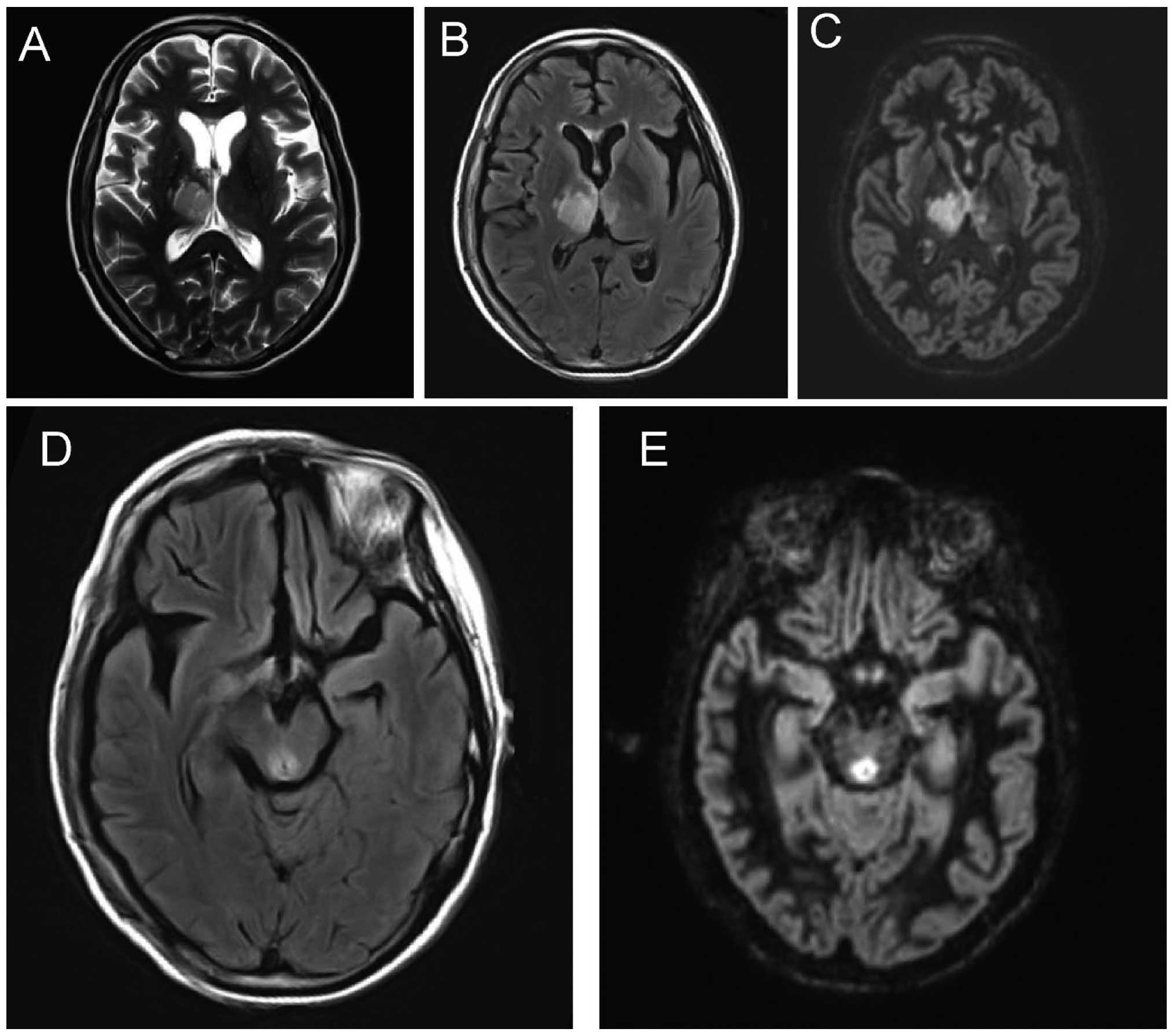
Signal characteristics of 3D-DIR scan
of NMO patients prior to and after treatment
Following treatment, the signal intensity of the
brain, optic nerve, optic chiasma and spinal cord decreased
significantly, and the differences were statistically significant
(P<0.05) (Table II, and Figs. 2 and 3).
 | Table II.Signal characteristics of 3D-DIR scan
of NMO patients before and after treatment. |
Table II.
Signal characteristics of 3D-DIR scan
of NMO patients before and after treatment.
|
| T2W | FLAIR | Brain 3D-DIR | Optic nerve
FLAIR | Optic nerve
3D-DIR | Spinal cord
3D-DIR | Spinal cord
FLAIR |
|---|
| Pre-treatment | 2.73±0.12 | 2.56±0.23 | 2.84±0.25 | 2.13±0.14 | 2.37±0.23 | 2.17±0.24 | 1.98±0.22 |
| Post-treatment | 1.35±0.13 | 1.42±0.21 | 1.63±0.24 | 1.77±0.15 | 1.98±0.17 | 1.83±0.21 | 1.63±0.18 |
| t | 4.647 | 4.795 | 5.321 | 5.102 | 5.624 | 5.698 | 4.968 |
| P-value | 0.037 | 0.036 | 0.032 | 0.034 | 0.021 | 0.017 | 0.035 |
Discussion
NMO is a type of demyelinating disease that occurs
in the central nerve system. Its pathological features include
failure of the nerve fiber myelin sheath and the presence of
multiple small disseminated disease or relatively large lesions
infused by one or more lesions, distributed in white matter and
infiltrating along the inflammatory cells surrounding veinlet;
neurons, axons, and supporting tissues that remain relatively
intact, free from wallerian degeneration or secondary bundle
degeneration (8). The etiology and
mechanism of NMO remain unknown, but may be associated with genetic
quality and ethnic differences (9).
NMO mainly involves the optic nerve, optic chiasma and spinal cord,
and lesions appearing in the thoracic and cervical segments, which
is different from typical MS. Additionally, its destructive lesions
are more obvious and axonal destruction may also be present
(10). Although NMO-IgG has 91%
specificity and 73% sensitivity in the diagnosis of NMO, the
NMO-IgG of most MS patients are negative. Nevertheless, in clinic,
imaging diagnosis remains the first choice for the early diagnosis
of NMO (11,12).
MRI is the first choice for the imaging diagnosis of
NOM, but the spatial and contrast resolution of conventional MR
pulse sequences is not efficient and requires improvement in
displaying gray matter lesions (13,14). The
3D-DIR pulse sequence uses pulses that may inhibit the
cerebrospinal fluid and white matter signals, better display the
gray matter of the brain, improve the sensitivity of gray matter
lesions, and identify cortical lesions that may not be detected by
conventional MR pulse sequence (13–15). In
the DIR sequence, selecting two TI values (TI1 and TI2) according
to the longitudinal relaxation time (T1) of the tissues to be
imaged, and in brain scanning, selecting corresponding TI1 and TI2
values may inhibit cerebrospinal fluid and white matter signals at
the same time and better display the gray matter of the brain
(16,17). At present, it is rarely used in the
study of NMO.
In the present study, by comparing the imaging
characteristics of NMO and MS, we found that: i) the brain of NMO
patients showed a low T1W signal, high T2W and FLAIR signals,
involving on average 5.8±1.2 sites, distributed in the peripheral
lateral ventricle, medulla, cerebral white matter, the third
ventricle, peripheral aqueduct of sylvius, pons and diencephalon.
AQP4 was similarly highly expressed around the aqueductus Sylvii,
ventriculus quartus cerebri and central canal (18). The enhancement of Gd-DTPA was varied,
periventricular, pia mater enhancement, corpus callosum linear, and
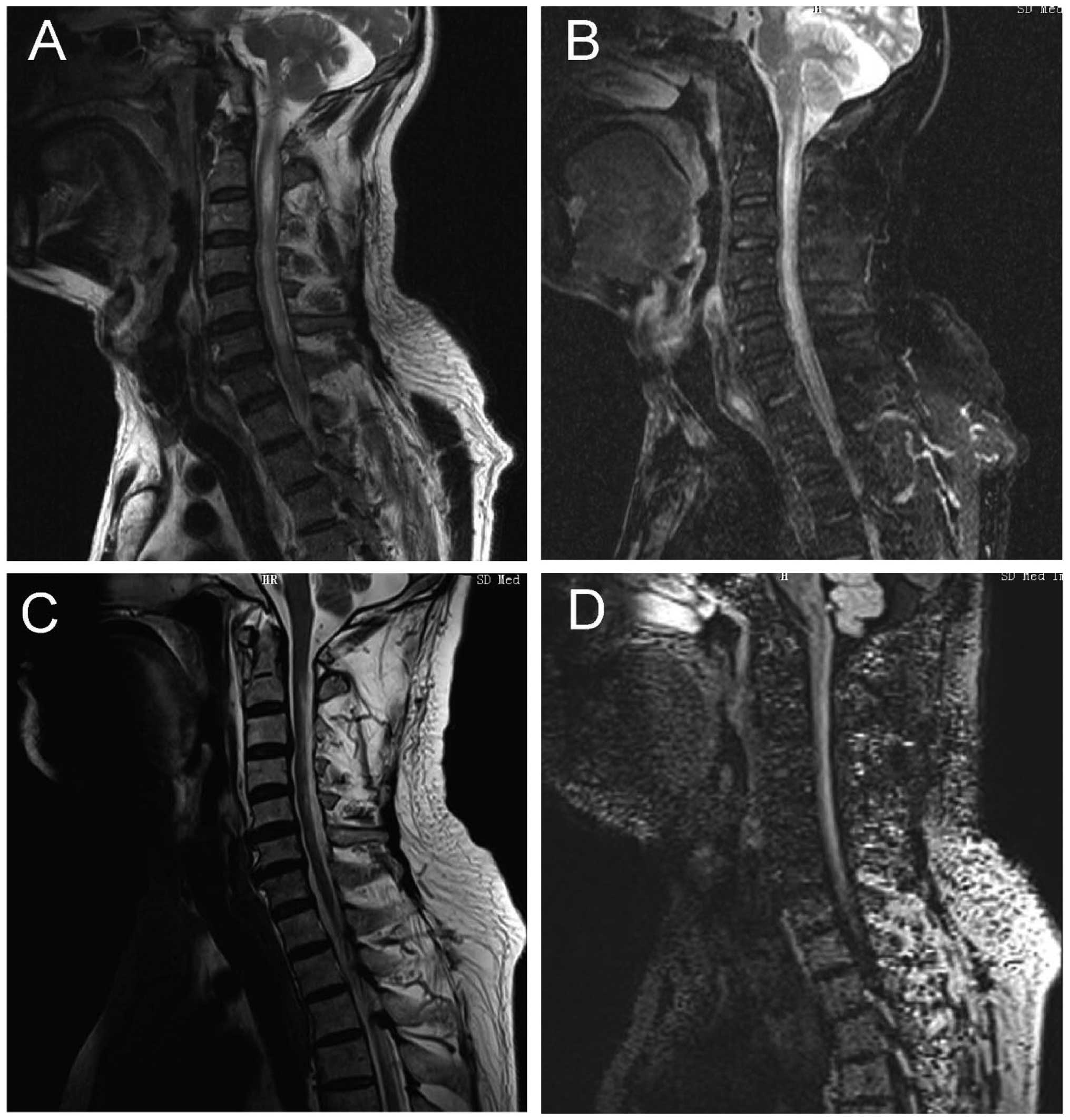
zonal enhancement exhibited specificity (19). The ii) optic nerve and chiasma showed
a high FLAIR signal. In the acute period, the spinal cord showed
swelling, necrosis and cavity lesions, lesions were enhanced after
enhanced scanning, involving primarily gray matters and partial
white matters of the central part, transversely, with the average
length of the lesion being 4.7±0.6 centrum (Fig. 4), mostly located in neck and thoracic
cord, and the lesions of cervical segment can extend up to the
lower part of the medulla, and during recovery, the spinal cord in
lesion site was able to shrink (19,20). The
results of the present study show that the signal intensity of T2W,
FLAIR and DIR of NMO patients were significantly higher than those
of the MS patients, the average of sites involved were
significantly more than those of the MS patients. In addition,
after the follow-up of the present study, it was found that the
signal intensity of head, optic nerve, optic chiasma and spinal
cord following treatment were significantly lower than those prior
to treatment.
In summary, 3D-DIR was not only of great reference
value for the early differential diagnosis of NMO, but also
valuable for the evaluation of prognosis of patients.
References
|
1
|
Juryńczyk M, Craner M and Palace J:
Overlapping CNS inflammatory diseases: differentiating features of
NMO and MS. J Neurol Neurosurg Psychiatry. 86:20–25. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mealy MA, Wingerchuk DM, Greenberg BM and
Levy M: Epidemiology of neuromyelitis optica in the United States:
a multicenter analysis. Arch Neurol. 69:1176–1180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simon B, Schmidt S, Lukas C, Gieseke J,
Träber F, Knol DL, Willinek WA, Geurts JJ, Schild HH, Barkhof F, et
al: Improved in vivo detection of cortical lesions in multiple
sclerosis using double inversion recovery MR imaging at 3 Tesla.
Eur Radiol. 20:1675–1683. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Riederer I, Karampinos DC, Settles M,
Preibisch C, Bauer JS, Kleine JF, Mühlau M and Zimmer C: Double
inversion recovery sequence of the cervical spinal cord in multiple
sclerosis and related inflammatory diseases. AJNR Am J Neuroradiol.
36:219–225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morimoto E, Kanagaki M, Okada T, Yamamoto
A, Mori N, Matsumoto R, Ikeda A, Mikuni N, Kunieda T, Paul D, et
al: Anterior temporal lobe white matter abnormal signal (ATLAS) as
an indicator of seizure focus laterality in temporal lobe epilepsy:
comparison of double inversion recovery, FLAIR and T2W MR imaging.
Eur Radiol. 23:3–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vural G, Keklikoğlu HD, Temel Ş, Deniz O
and Ercan K: Comparison of double inversion recovery and
conventional magnetic resonance brain imaging in patients with
multiple sclerosis and relations with disease disability.
Neuroradiol J. 26:133–142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wingerchuk DM, Banwell B, Bennett JL,
Cabre P, Carroll W, Chitnis T, de Seze J, Fujihara K, Greenberg B,
Jacob A, et al: International Panel for NMO Diagnosis:
International consensus diagnostic criteria for neuromyelitis
optica spectrum disorders. Neurology. 85:177–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khanna S, Sharma A, Huecker J, Gordon M,
Naismith RT and Van Stavern GP: Magnetic resonance imaging of optic
neuritis in patients with neuromyelitis optica versus multiple
sclerosis. J Neuroophthalmol. 32:216–220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tackley G, Kuker W and Palace J: Tackle
yG, Kuker W and Palace J: Magnetic resonance imaging in
neuromyelitis optica. Mult Scler. 20:1153–1164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schneider E, Zimmermann H, Oberwahrenbrock
T, Kaufhold F, Kadas EM, Petzold A, Bilger F, Borisow N, Jarius S,
Wildemann B, et al: Optical coherence tomography reveals distinct
patterns of retinal damage in neuromyelitis optica and multiple
sclerosis. PLoS One. 8:e661512013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sinnecker T, Dörr J, Pfueller CF, Harms L,
Ruprecht K, Jarius S, Brück W, Niendorf T, Wuerfel J and Paul F:
Distinct lesion morphology at 7-T MRI differentiates neuromyelitis
optica from multiple sclerosis. Neurology. 79:708–714. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iyer A, Elsone L, Appleton R and Jacob A:
A review of the current literature and a guide to the early
diagnosis of autoimmune disorders associated with neuromyelitis
optica. Autoimmunity. 47:154–161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kolber P, Montag S, Fleischer V, Luessi F,
Wilting J, Gawehn J, Gröger A and Zipp F: Identification of
cortical lesions using DIR and FLAIR in early stages of multiple
sclerosis. J Neurol. 262:1473–1482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Coebergh JA, Roosendaal SD, Polman CH,
Geurts JJ and van Woerkom TC: Acute severe memory impairment as a
presenting symptom of multiple sclerosis: a clinical case study
with 3D double inversion recovery MR imaging. Mult Scler.
16:1521–1524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Calabrese M and De Stefano N: Cortical
lesion counts by double inversion recovery should be part of the
MRI monitoring process for all MS patients: yes. Mult Scler.
20:537–538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Q, Zhang Q, Sun H, Zhang Y and Bai R:
Double inversion recovery magnetic resonance imaging at 3 T:
diagnostic value in hippocampal sclerosis. J Comput Assist Tomogr.
35:290–293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seewann A, Kooi EJ, Roosendaal SD, Pouwels
PJ, Wattjes MP, van der Valk P, Barkhof F, Polman CH and Geurts JJ:
Postmortem verification of MS cortical lesion detection with 3D
DIR. Neurology. 78:302–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pittock SJ, Weinshenker BG, Lucchinetti
CF, Wingerchuk DM, Corboy JR and Lennon VA: Neuromyelitis optica
brain lesions localized at sites of high aquaporin 4 expression.
Arch Neurol. 63:964–968. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ito S, Mori M, Makino T, Hayakawa S and
Kuwabara S: ‘Cloud-like enhancement’ is a magnetic resonance
imaging abnormality specific to neuromyelitis optica. Ann Neurol.
66:425–428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hodel J, Outteryck O, Bocher AL, Zéphir H,
Lambert O, Benadjaoud MA, Chechin D, Pruvo JP, Vermersch P and
Leclerc X: Comparison of 3D double inversion recovery and 2D STIR
FLAIR MR sequences for the imaging of optic neuritis: pilot study.
Eur Radiol. 24:3069–3075. 2014. View Article : Google Scholar : PubMed/NCBI
|