Introduction
The chemotherapeutic agent doxorubicin (Dox), which
is a cytotoxic anthracycline antibiotic isolated from
Streptomyces peuxetus (1),
has been widely used in the clinical treatment of a broad spectrum
of cancers (2). The mechanism
underlying the antitumor effect of Dox has been associated with its
ability to induce the apoptosis of cancer cells (3). In the majority of cells,
caspase-dependent apoptosis is induced via the activation of either
the mitochondrial (intrinsic) signaling pathway or the death
receptor (extrinsic) signaling pathway (4). Unfortunately, Dox exhibits cytotoxic
effects on a wide range of cells, as well as cancer cells (5). In addition, Dox may be fatal in animals
as it damages several organs, including the heart, bones and
kidneys (6). The clinical
application of Dox has been limited due to association of
cardiomyopathy and heart failure with Dox usage (7). The severity of cardiac damage is
typically proportional to the cumulative dose of Dox in a patient
(8). Therefore, it is not possible
to increase the antitumor potency of Dox by increasing the dose of
Dox due to its adverse effects.
Breast cancer is one of the most common types of
cancer and is the fourth leading cause of cancer-associated
mortality worldwide (9). Since the
1970s, Dox has been considered one of the most effective agents for
the treatment of advanced breast cancer (10). However, recent studies have
demonstrated that numerous cancer cell types, including breast
cancer cells, are resistant to the apoptosis-inducing effects of
Dox (11–13). Therefore, the identification of
sensitizing agents that are able to increase the potency of Dox at
low doses with clinically acceptable adverse effects may help to
improve the treatment of breast cancer.
Laminaria japonica is a medicinal and dietary
brown algae plant, whose medicinal value has been recorded in
ancient Chinese books, including the Compendium of Materia Medica
and Jiayou Materia Medica (14).
Sulfated polysaccharide-protein complex (SPPC) is a cellular
interstitial proteoglycan complex produced by L. japonica.
Previous studies have demonstrated that SPPC has unique antitumor
biological activities (15,16), and that numerous sulfated
glycoproteins and sulfated polysaccharides are able to inhibit the
proliferation and metastasis of tumor cells (17–19). In
a study on the regulatory effect of sulfated polysaccharides on
macrophage-induced tumor cell apoptosis, it was demonstrated that
sulfated polysaccharides produced by L. japonica were able
to indirectly enhance the cytotoxic effect of macrophages on HepG2
cells (16). However, the majority
of previous studies have focused on the ability of SPPC to promote
tumor cell apoptosis and inhibit tumor cell proliferation and
metastasis when used alone (20,21); few
studies have addressed whether SPPC has a synergistic effect when
used simultaneously with other chemotherapeutic agents, such as
Dox, with a view to clinically alleviate their toxicities.
Therefore, the present study aimed to investigate the effect of
SPPC on Dox-induced apoptosis of the MDA-MB-231 breast cancer cell
line in vitro and in vivo.
Materials and methods
Reagents
Dox, Hoechst 33258, z-VAD-fmk and MTT were purchased
from Sigma-Aldrich (St. Louis, MO, USA). Molecular Probe JC-1 dye
was obtained from Thermo Fisher Scientific, Inc. (Waltham, MA,
USA). SPPC of 99% purity was purchased from Shanghai Traditional
Chinese Medicine Co., Ltd. (Shanghai, China) and was dissolved in
phosphate-buffered saline (PBS) to 10 mM as stock solution.
Cell lines and culture
The MDA-MB-231 human breast cancer cell line and the
Hs578Bst normal human mammary cell line were purchased from the
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). Cells were cultured to 5×105 cell
density in Minimum Essential Medium (MEM) supplemented with 10%
fetal bovine serum, penicillin and streptomycin (all Gibco; Thermo
Fisher Scientific, Inc.). Cells in culture flasks were incubated in
5% CO2 at 37°C.
MTT assay
Cells were plated on 96-well plates at a density of
5×103 cells/well and incubated at 37°C overnight,
followed by incubation at 37°C with various doses of SPPC (0–60 µM)
in complete MEM for 24 or 48 h. Following treatment, the medium was
replaced and the cells were incubated at 37°C with 0.5 mg/ml MTT in
complete MEM for 4 h. Hs578Bst normal human mammary cells incubated
with various doses of SPPC (0–6 µM) in complete MEM for 24 or 48 h
served as a control. Dimethyl sulfoxide (DMSO; 0.1%) was used as a
negative control. Viable cells converted MTT to formazan,
generating a blue-purple color when dissolved in DMSO. The
intensity was measured at 570 nm using a ELx800 Microplate Reader
(Bio-Tek Instruments, Inc., Winooski, VT, USA). The relative
percentage of viable cells was calculated by dividing the
absorbance of SPPC-treated cells by that of the control in each
experiment.
Annexin V/propidium iodide (PI)
staining assay
Apoptosis was assessed by measuring the membrane
redistribution of phosphatidylserine using an Annexin V-Fluorescein
Isothiocyanate (FITC) Apoptosis Detection kit (BD Pharmingen, San
Diego, CA, USA), according to the manufacturer's protocol. Briefly,
cells were incubated with 1 µM DOX, 5 µM SPPC or both for 24 h.
(22,23), and the cells were collected and
washed twice with PBS followed by resuspension in 500 µl staining
solution containing FITC-conjugated Annexin V antibody (5 µl) and
PI (250 µg/ml stock solution). Following incubation at 37°C in the
dark for 15 min, the cells were analyzed by flow cytometry. Basal
apoptosis and necrosis rates were identically determined for the
untreated cells. The percentage of cells undergoing apoptosis was
determined as an average of three independent experiments.
Hoechst 33258 staining
The MDA MB 231 and Hs578Bst cells following
incubation with 1 µM DOX, 5 µM SPPC or both for 24 h. Following
treatment, the cells were washed with isotonic PBS (pH 7.4) and
then fixed in 4% paraformaldehyde solution in PBS for 1 h at 37°C.
Subsequently, the nuclei were stained with 2 µg/ml Hoechst 33258
for 10 min at room temperature and morphological changes to the
nuclear chromatin were observed under a fluorescent microscope
(Nikon Eclipse TE2000-U; Nikon Corporation, Tokyo, Japan).
Caspase enzyme activity
Following treatment, the cells were washed with PBS
and lysed using cell lysis buffer provided in the caspase-3, −8 and
−9 Assay kits (Sigma-Aldrich). Samples were incubated on ice for 10
min and centrifuged in a microcentrifuge at 12,000 × g for 5 min at
4°C to precipitate the cellular debris. The activities of
caspase-3, −8 and −9 in the supernatant were measured using the
kits and a spectrophotometer at 450 nm, according to the
manufacturer's protocol. Briefly, the MDA MB 231 cells were treated
with 1 µM DOX and 5 µM SPPC, or SPPC only for 24 h before the
assay. The activities of caspase 3, −8 and −9 in the MDA MB 231
cells prior to and following co-treatment with SPPC were assessed
using colorimetric assay kits (K106-100, K113-100 and K119-200,
BioVision, Inc., Mountain View, CA, USA) according to the
manufacturer's instructions. Briefly, the cells were harvested and
incubated in ice-cold cell lysis buffer for 30 min on ice. The
supernatants were collected, and the proteins from each sample were
incubated with the appropriate caspase substrate. After 4 h
incubation at 37°C, the protease activity was determined at 405 nm
using a microplate spectrophotometer (xMark Microplate Absorbance
Spectrophotometer, Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Caspase activity was normalized to the cell lysate protein and
expressed as fold activation compared with the control.
Cell observations by scanning electron
microscopy
Following treatment, the cells were sequentially
fixed in 2.5% glutaraldehyde solution and 1% osmium solution,
dehydrated in graded alcohol, placed in tert-butanol for it to
infiltrated the samples, and were then frozen at 0°C for 12 h,
followed by observation under a scanning electron microscope
(S-2300; Hitachi High-Technologies Corporation, Tokyo, Japan). The
MDA MB 231 cells were treated with 1 µM Dox, 5 µM SPPC or SPPC only
for 24 h. The loss of the MMP was assayed by JC 1 staining. For
JC-1 staining, cells were stained with JC 1 (5 µg/m, T4069,
Sigma-Aldrich) in the dark for 10 min at 37°C, washed with culture
media and observed under a confocal laser scanning microscope.
Human tumor xenograft experiment
The present study was performed in strict accordance
with the recommendations outlined in the Guide for the Care and Use
of Laboratory Animals of Fudan University (Shanghai, China). The
protocol was approved by the Committee on the Ethics of Animal
Experiments of Fudan University. A total of 20 female nude athymic
BALB/c mice aged 4–6 weeks were obtained from the Fudan University
Animal Center (Shanghai, China). The mice were house under standard
and enriched conditions for six weeks at 21±1°C and 50±5% humidity
in controlled conventional colony rooms and under a reversed 12:12
h light:dark with water and standard rodent pellets ad
libitum. In vitro cultured MDA-MB-231 cells
(5×106 in 200 µl PBS volume) were injected
subcutaneously into the right supra scapula region of the mice. On
day 12 post-inoculation, when the tumors had grown to a mean volume
of 150 mm3, the mice were equally randomized into four
groups, as follows: i) Normal saline (NS) group (100 µl); ii) 40
mg/kg SPPC group; iii) 4 mg/kg Dox group; and iv) 4 mg/kg Dox plus
40 mg/kg SPPC group. SPCC was administered via intraperitoneal
injection three times a week, and Dox was administered via
intraperitoneal injection once a week for 3 weeks. Saline was
administered to the NS group, which was considered as the control
group, via intraperitoneal injection three times a week. The tumor
volume was determined using a caliper twice a week and was
estimated using the following formula: Tumor volume = length ×
width2/2. At the end of the experiment after 24 days,
the mice were sacrificed by CO2 asphyxiation and the
tumors were weighed to assess treatment efficacy. Tumor tissue
samples were fixed in 10% formal saline for 24 h, paraffin
embedded, sliced into 4 mm sections, stained with Harris
hematoxylin and eosin and evaluated for any structural changes
under a bright field microscope. Standard immunoperoxidase
procedures (24) were used to
visualize proliferating cell nuclear antigen (PCNA) in the tumor
samples.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analyses were performed using SPSS 19.0 software for
Windows (IBM SPSS, Armonk, NY, USA). The least significant
difference test for multiple comparisons was used to detect whether
there was any significant difference between the different
treatment groups. Student's two-tailed t-test was used to evaluate
the differences between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of SPPC on the growth of
breast cancer cells and normal mammary cells
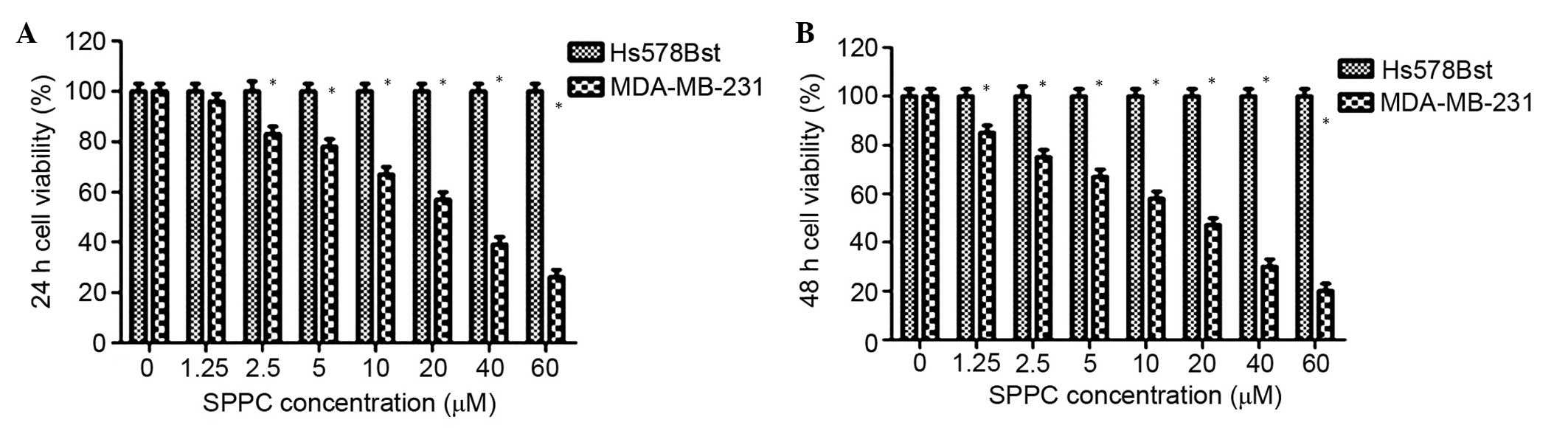
To determine the effects of SPPC on breast cancer
cells and normal mammary cells, MDA-MB-231 breast cancer cells and
Hs578Bst normal mammary cells were treated with various
concentrations of SPPC (0–60 µM) for 24 or 48 h. Cell viability was
assessed using MTT assays. As shown in Fig. 1, SPPC inhibited the growth of
MDA-MB-231 cells in a dose- and time-dependent manner, and the half
maximal inhibitory concentration (IC50) was 25.4 and
14.2 µM for 24 and 48 h treatment, respectively. Conversely, SPPC
did not affect the growth of Hs578Bst cells after 24 or 48 h, even
at high concentrations (>60 µM). These results suggest that SPPC
specifically induces cytotoxicity in breast cancer cells, without
affecting normal mammary cells.
SPPC potentiates the anti-cancer
effect of Dox in human breast cancer cells
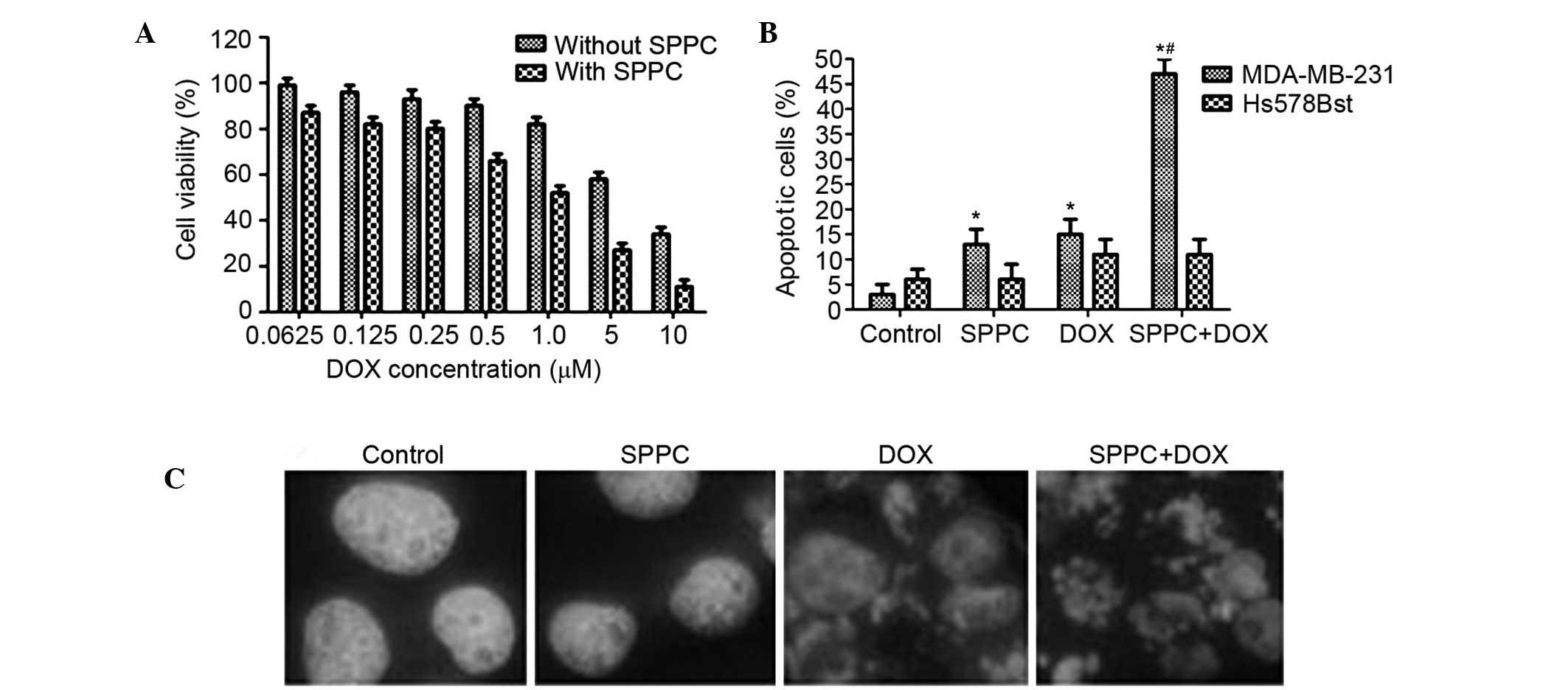
To determine whether low-dose SPPC is able to
enhance the anti-tumor effect of Dox, MDA-MB-231 cells were treated
with 5 µM SPPC plus various doses of Dox for 24 h. Dox treatment
was observed to induce breast cancer cell death, and this effect of
Dox was markedly increased by SPPC treatment. The IC50
of Dox against MDA-MB-231 cells was decreased from 5.3 to 1.5 µM
when Dox was used concomitantly with 5 µM SPPC (Fig. 2A). In a previous study, the peak and
the steady-state concentrations of Dox in human plasma were
reported to be 5 µM and 25–500 nM, respectively (7); thus, 1 µM Dox was selected in the
present study as representative of the plasma levels in patients
treated with Dox.
Whether SPPC-mediated anti-cancer activity was
associated with its ability to induce apoptosis was investigated.
Flow cytometric analysis showed that 1 µM Dox increased the
percentage of apoptotic MDA-MB-231 cells to 16% and co-treatment
with SPPC increased it to 48%. Conversely, the percentage of
apoptotic Hs578Bst cells was 11.6% following Dox treatment and
10.9% following co-treatment with SPPC (Fig. 2B).
Hoechst 33258 staining was used to assess
morphological changes to the MDA-MB-231 and Hs578Bst cells
following treatment. Following co-treatment with 5 µM SPPC and 1 µM
Dox for 24 h, morphological changes that are characteristic of
apoptosis, including chromatin condensation, nuclear fragmentation
and apoptotic body formation, were observed in MDA-MB-231 cells. At
the same time, some apoptotic nuclei were observed in cells treated
with SPPC or Dox alone (Fig. 2C).
These results suggest a preferential potentiation effect of SPPC on
Dox-mediated cytotoxicity in breast cancer cells.
SPPC enhances Dox-induced caspase
activation
The activities of caspase-3, caspase-8 and caspase-9
in MDA-MB-231 cells prior to and following co-treatment with SPPC
were assessed using colorimetric assays. As shown in Fig. 3A-C, the activities of caspase-3/8/9
were increased in MDA-MB-231 cells exposed to 5 µM SPPC for 24 h.
Dox-induced apoptosis has been associated with two distinct
apoptosis pathways: The death receptor pathway via activation of
caspase-8; and the mitochondrial pathway via activation of
caspase-9 (25,26). Dox treatment increased the activities
of caspase-3/8/9 to 7.3, 3.2 and 5.4-fold above control cells,
respectively. SPPC-induced apoptosis has been associated with the
mitochondrial signaling pathway via activation of caspase-9
(27,28). Co-treatment with Dox and SPPC
resulted in markedly higher ratios of caspase-3 and caspase-9
activity, which were 13.1 and 8.9-fold that of controls. However,
SPPC was not shown to increase Dox-induced caspase-8 activation. To
further define the role of caspases in SPPC potentiated Dox-induced
apoptosis, cells were treated with the broad-spectrum caspase
inhibitor, z-VADfmk (100 µM), for 2 h prior to treatment with SPCC
and/or Dox and apoptosis was detected 24 h following treatment. As
shown in Fig. 3D, apoptosis induced
by SPPC and Dox was markedly attenuated by pretreatment with
z-VAD-fmk. These results suggest that SPPC sensitizes Dox-induced
apoptosis via a caspase-dependent signaling pathway.
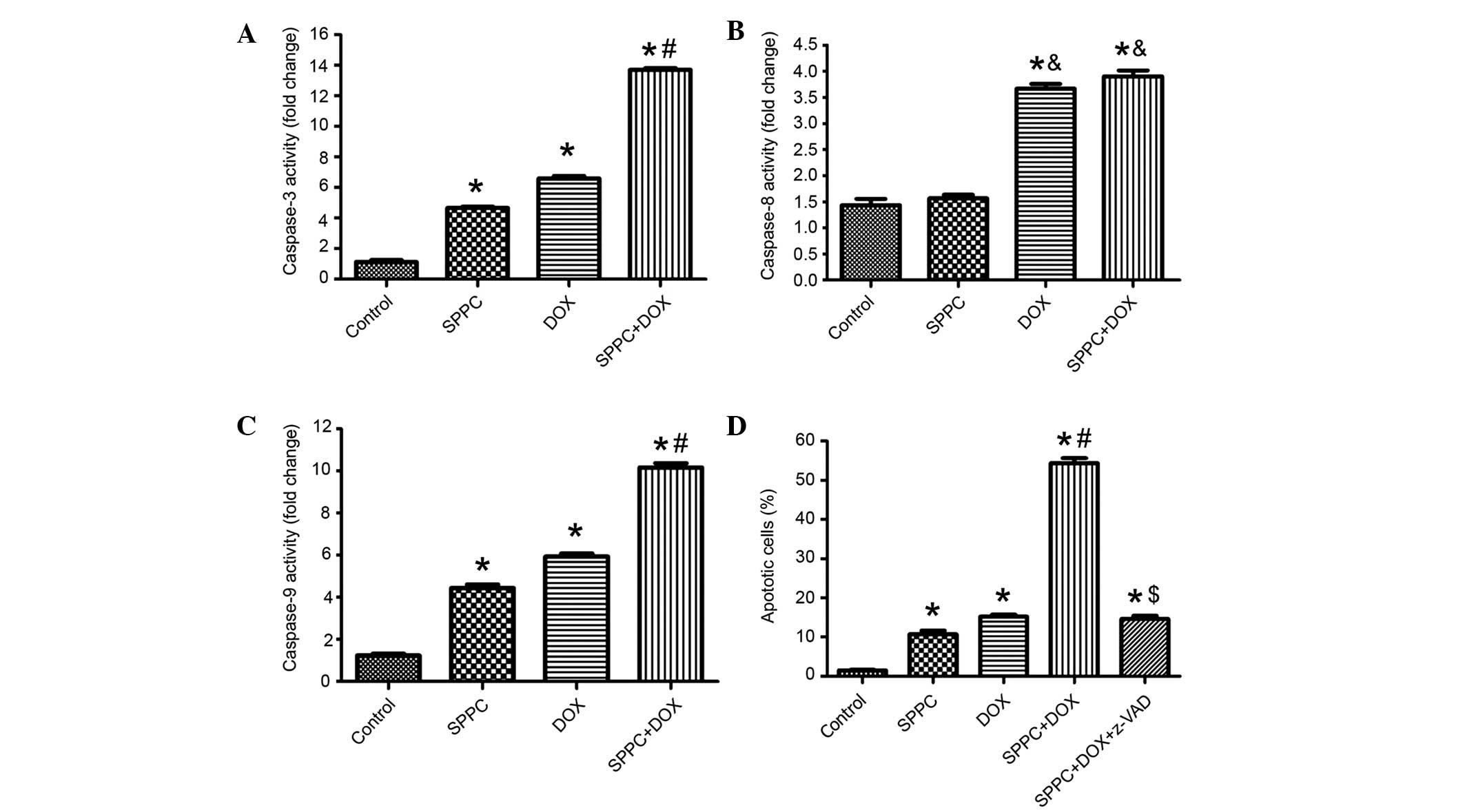 | Figure 3.SPPC enhances Dox-induced activation
of caspases. The activities of (A) caspase-3, (B) caspase-8 and (C)
caspase-9 in MDA-MB-231 cells treated with 1 µM DOX and/or 5 µM
SPPC for 24 h were assayed. Caspase activity was normalized to cell
lysate protein and expressed as fold activation as compared with
the control. (D) The caspase inhibitor, Z-VAD-fmk (100 µM), reduced
the effect of SPPC on Dox-induced apoptosis in MDA-MB-231 cells, as
determined by Annexin-V/propidium iodide staining and flow
cytometry. Data are presented as the mean ± standard deviation of
three independent experiments. *P<0.05, vs. the control group.
&P<0.05, vs. SPPC alone group.
#P<0.05, vs. the SPPC alone and DOX alone groups.
$P<0.05, vs. the SPPC + DOX group. SPPC, sulfated
polysaccharide-protein complex; DOX, doxorubicin. |
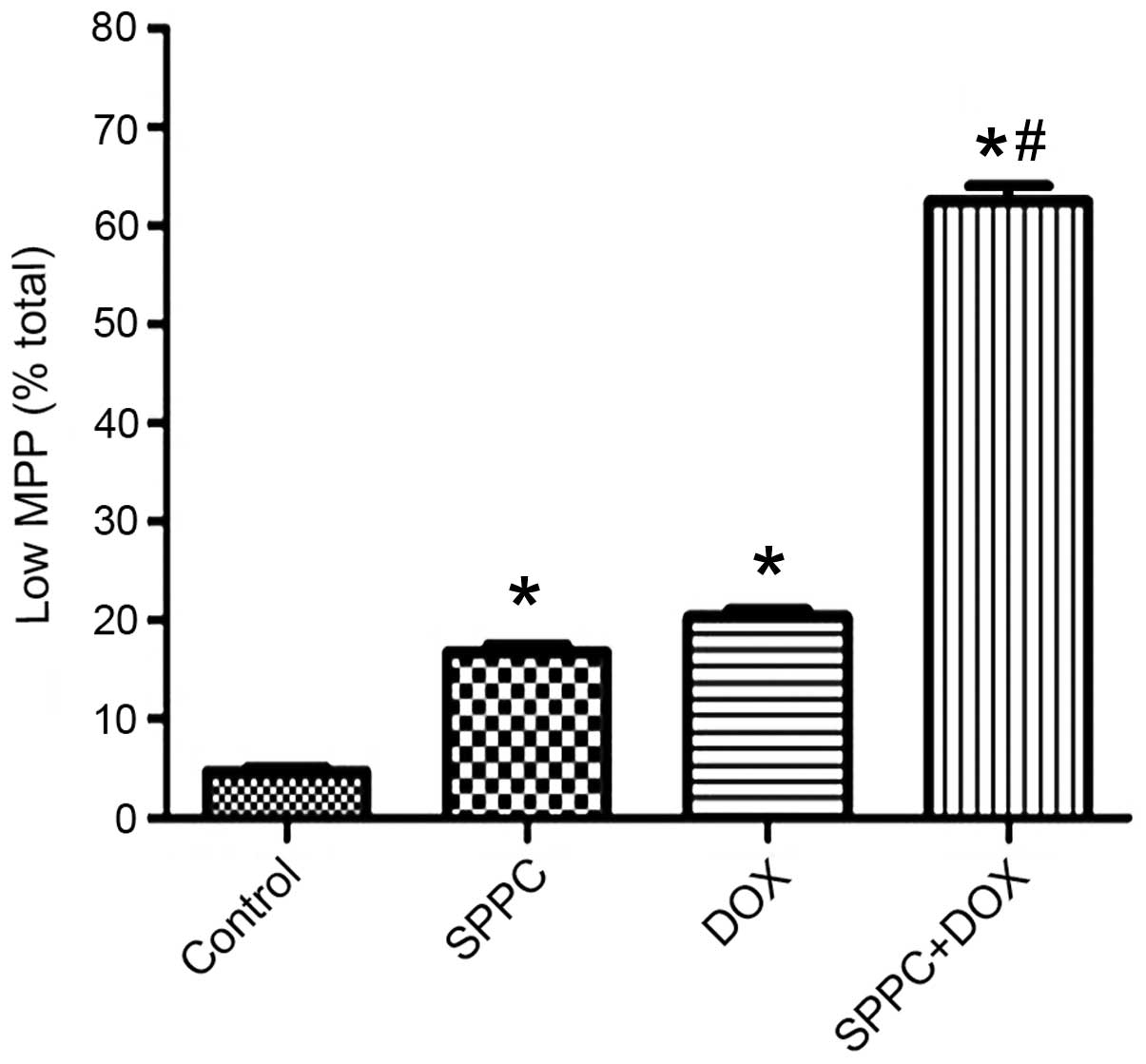
SPPC enhances the Dox-induced loss of
the mitochondrial membrane potential (MMP)
In the present study, Dox and SPPC co-treatment
markedly enhanced caspase-9 activation but not caspase-8, thus
suggesting that SPPC may sensitize Dox-induced apoptosis
predominantly via the mitochondrial pathway. In the mitochondrial
pathway, a loss of MMP precedes caspase-9 activation (29). Therefore, the present study assessed
whether there was a loss of the MMP during combined treatment using
the cationic lipophilic probe, JC-1. Quantitative measurement
demonstrated that MDA-MB-231 cells treated with SPPC alone
underwent significant dissolution, as compared with the control.
Dox treatment resulted in a rapid dissipation of the MMP (Fig. 4); however, a significant decrease in
the fluorescence was observed following co-treatment with SPPC.
These results suggest that SPPC sensitizes breast cancer cells to
Dox-induced apoptosis via the mitochondrial apoptosis pathway.
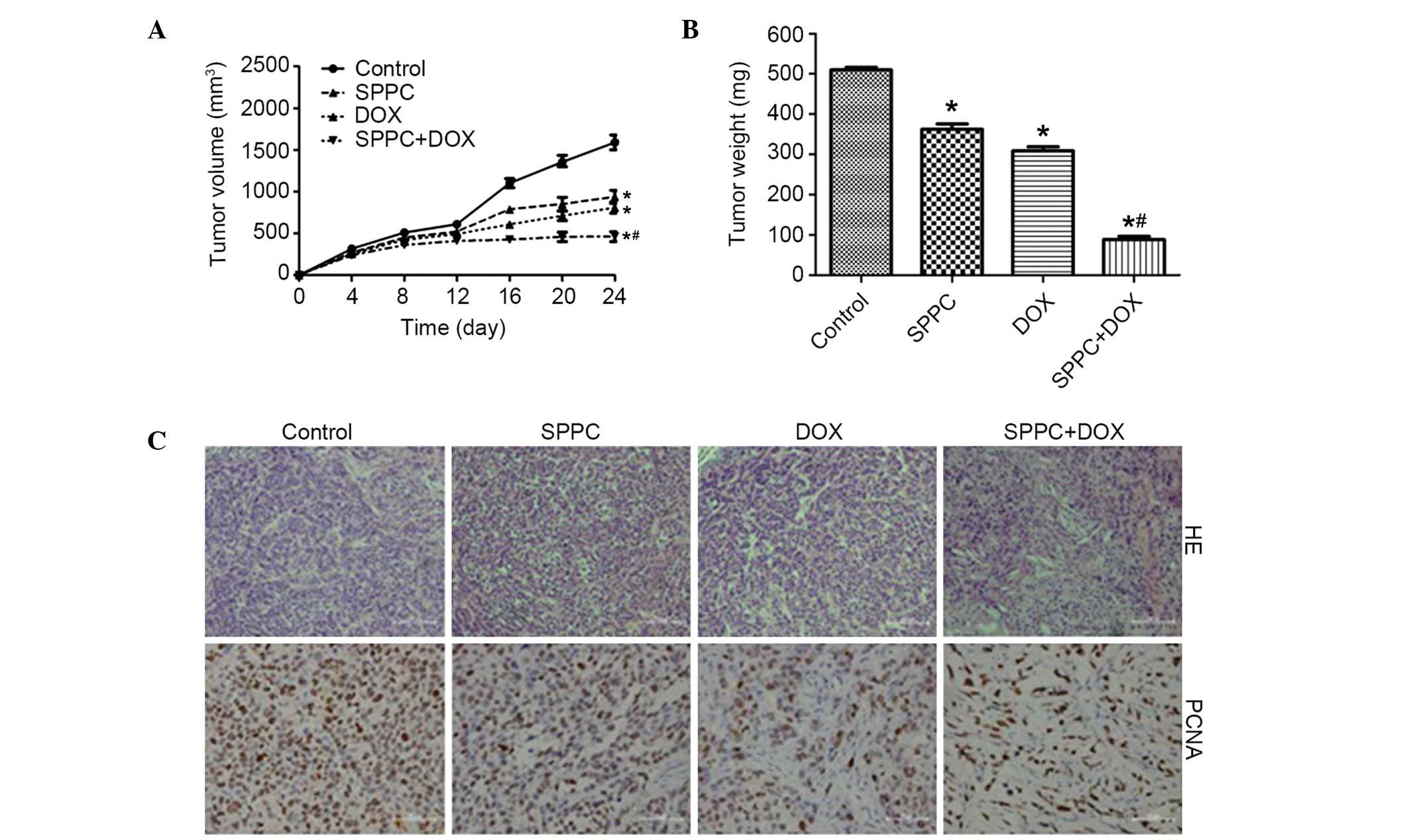
In vivo antitumor activity of Dox and
SPPC
In order to confirm the anticancer activity of
combined SPPC and Dox treatment on human breast cancer cells, an
in vivo experiment was performed using an MDA-MB-231
cell-xenografted mouse model. Male nude mice inoculated with human
MDA-MB-231 cells were divided into four groups 12 days post-tumor
cell inoculation. At the end of the experiment, the mean tumor
volume was 1,687 mm3 for the control group and 661
mm3 for the Dox-treated group, which was significantly
reduced (P<0.05; Fig. 5A).
Furthermore, SPPC induced regression of MDA-MB-231 tumors. The
tumor growth inhibitory rate was 58.8% in the 40 mg/kg SPPC-treated
mice, as compared with the control group (P<0.05). Notably, our
results demonstrated a significant reduction in tumor volume from
day 4 post-treatment to the end of the experiment in the mice
co-treated with SPPC and Dox, as compared with the control group.
The tumors in the co-treatment group grew at a slower rate after
co-treatment, from the mean volume of 201 mm3 in the
beginning to 399 mm3 in the end. The mean tumor weight
was 523 mg for the control group versus 315 mg for the Dox only
group, 358 mg for the SPPC only group and 89 mg for the Dox plus
SPPC group (Fig. 5B). These results
suggest that SPPC is able to augment the antitumor activity of Dox
in vivo.
Histologically, the tumors from the mice in the SPPC
plus Dox-treated group contained markedly fewer cells and were
predominantly composed of acellular material. Conversely, the tumor
cells in the mice administered vehicle control were arranged as
nests separated by bundles of extracellular matrix (Fig. 5C). To investigate the effect of
combined Dox and SPPC treatment on tumor cell proliferation, tumor
sections from nude mice were assessed for PCNA expression. At the
end of the experiment, the percentage of PCNA+ tumor
cells in the combination treatment group was 34%, versus 70% in the
Dox only group, 78% in the SPPC only group and 95% in the vehicle
control group. These in vivo results suggest that SPPC is
able to potentiate the Dox-mediated inhibition of tumor growth.
Discussion
Chemotherapy is the most frequently used treatment
for breast cancer and other cancer types (30). Dox is an efficacious chemotherapeutic
agent with a wide anti-cancer spectrum; however, it has been
associated with severe dose-dependent cardiotoxicities once the
cumulative dose has reached >500 mg/m2 (31). Therefore, it is important to identify
novel compounds that are able to enhance the anticancer effect of
Dox and reduce Dox-associated side effects.
Various natural products have been shown to exhibit
synergistic antitumor effects with chemotherapeutics (32). In a previous study, SPPC was able to
induce apoptosis and inhibit the growth of the HL-60 and K562 human
leukemia cell lines without causing cytotoxicity to normal
hematopoietic cells (33).
Furthermore, SPPC has been shown to induce the caspase-3-dependent
apoptosis of NB4 leukemia cells via the survivin-mediated apoptotic
pathway (1). Previous studies used
the trypan blue exclusion assay to demonstrate that SPPC at 25 and
35 µM was able to inhibit the proliferation of SMMC-7721 cells
(15,34). Sulfated polysaccharides have
previously been reported to improve antitumor activity (35). In addition, SPPC was able to promote
apoptosis and cell cycle arrest in human breast cancer cells,
leading to loss of the MMP and activation of caspase-3 and
caspase-9 (36). However, whether or
not SPPC has a synergistic effect on the activity of Dox against
human breast cancer cells is currently unclear. The present study
aimed to investigate the in vitro and in vivo effects
of co-treatment with SPPC and DOX on the growth of the MDA-MB-231
human breast cancer cell line. It was demonstrated that SPPC
significantly enhanced the anticancer activity of Dox by
significantly reducing the viability of MDA-MB-231 cells. In
addition, SPPC was shown to sensitize breast cancer cells to
Dox-induced apoptosis. These results suggested that SPPC may be
used to reduce the dose of Dox and, in turn, its associated
toxicities, thereby presenting a novel therapeutic strategy for
breast cancer with fewer side effects.
The present study demonstrated that z-VAD-fmk, a
caspase inhibitor, was able to inhibit the apoptosis of breast
cancer cells induced by treatment with Dox alone or Dox plus SPPC.
In addition, it was able to reduce the difference in the apoptotic
rates observed between the Dox alone and Dox plus SPPC groups, thus
suggesting that the SPPC potentiation of Dox-induced apoptosis of
breast cancer cells was caspase-dependent. Since it has been
demonstrated that two caspase-dependent signaling pathways are
involved in Dox-induced apoptosis (37), the present study aimed to determine
the apoptotic signaling pathway affected by SPPC. It was
demonstrated that SPPC significantly promoted the loss of the MMP
in MDA-MB-231 cells, which was consistent with the observed
elevation of mitochondrial breakdown and subsequent increase in the
activities of caspase-9 and caspase-3, although there was no
significant difference in the activity of caspase-8 between the
SPPC and control groups. These results suggested that SPPC may
sensitize breast cancer cells to Dox-induced apoptosis
predominantly via the mitochondrial signaling pathway.
One mechanism by which cancer cells may develop
resistance to chemotherapeutic agents is by expelling intracellular
anticancer drugs out of cells via IL-8 (38). Cancer cells require oxygen, food and
growth proteins to grow and spread. The transport of these
essential nutrients to the cancer cells is performed by blood
vessels. Angiogenesis is the process of creating new blood vessels
necessary to transport this food to the cancer cells, and IL-8 is
involved in the process of angiogenesis. SPPC has been observed to
exhibit negligible cytotoxicity against normal cells; however, it
markedly inhibited the proliferation of SMMC-7721 cells and blocked
the cells in the S phase in a previous study (15). In addition, SPPC was shown to inhibit
human SMMC-7721 cell growth in a dose-dependent manner (20), and it induced cell apoptosis,
increased the protein expression levels of caspase-3 and the drug
excretion capacity of cells, and reduced the mRNA and protein
expression levels of IL-8 (16). The
present study demonstrated that co-treatment with SPPC and Dox
markedly potentiated the antitumor activity of DOX against
MDA-MB-231 human breast cancer cells. These results suggested that
the potentiation of Dox-induced apoptosis of breast cancer cells by
SPPC may be due to the inhibitory effect of SPPC on IL-8 and the
increased concentration of Dox within tumor cells. However, this
hypothesis requires confirmation by further studies.
The adverse effects of Dox include dilated
cardiomyopathy and congestive heart failure due to the induction of
apoptosis in cardiomyocytes. It has previously been reported that
the Dox-induced apoptotic mechanism is reactive oxygen
species-dependent in normal cells and p53-dependent in cancer cells
(39). In addition, SPPC, the
extract of L. japonica, was shown to promote apoptosis by
downregulating IL-10 expression (40). The present study demonstrated that
SPPC reduced the viability of breast cancer cells and that, when
used in combination with DOX, it was able to promote the apoptosis
of breast cancer cells, while simultaneously reducing the dose and
adverse effects of Dox. Whether SPPC may also reduce the toxicities
associated with other anticancer agents requires investigation in
further studies.
References
|
1
|
Gabius S and Gabius HJ: Sugar receptors of
the stromal cell layer in human long-term bone marrow cultures:
Their presence, modulatory responses to changes in the
microenvironment and potential role in cellular adhesion. Blut.
61:232–239. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanušová V, Boušová I and Skálová L:
Possibilities to increase the effectiveness of doxorubicin in
cancer cells killing. Drug Metab Rev. 43:540–557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harashima HI, Iida S, Urakami Y,
Tsuchihashi M and Kiwada H: Optimization of antitumor effect of
liposomally encapsulated doxorubicin based on simulations by
pharmacokinetic/pharmacodynamic modeling. J Control Release.
61:93–106. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Green DR: Apoptotic pathways: Paper wraps
stone blunts scissors. Cell. 102:1–4. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang S, Konorev EA, Kotamraju S, Joseph J,
Kalivendi S and Kalyanaraman B: Doxorubicin induces apoptosis in
normal and tumor cells via distinctly different mechanisms.
intermediacy of H(2)O(2)- and p53-dependent pathways. J Biol Chem.
279:25535–25543. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singal PK and Iliskovic N:
Doxorubicin-induced cardiomyopathy. N Engl J Med. 339:900–905.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Minotti G, Menna P, Salvatorelli E, Cairo
G and Gianni L: Anthracyclines: Molecular advances and
pharmacologic developments in antitumor activity and
cardiotoxicity. Pharmacol Rev. 56:185–229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiong Y, Liu X, Lee CP, Chua BH and Ho YS:
Attenuation of doxorubicin-induced contractile and mitochondrial
dysfunction in mouse heart by cellular glutathione peroxidase. Free
Radic Biol Med. 41:46–55. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nickels S, Truong T, Hein R, Stevens K,
Buck K, Behrens S, Eilber U, Schmidt M, Häberle L, Vrieling A, et
al: Evidence of gene-environment interactions between common breast
cancer susceptibility loci and established environmental risk
factors. PLoS Genet. 9:e10032842013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Glasziou P and Houssami N: The evidence
base for breast cancer screening. Prev Med. 53:100–102. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Febriansah R, Putri DD, Sarmoko, Nurulita
NA, Meiyanto E and Nugroho AE: Hesperidin as a preventive
resistance agent in MCF-7 breast cancer cells line resistance to
doxorubicin. Asian Pac J Trop Biomed. 4:228–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang S, Chen R, Zhong Z, Shi Z, Chen M and
Wang Y: Epigallocatechin-3-gallate potentiates the effect of
curcumin in inducing growth inhibition and apoptosis of resistant
breast cancer cells. Am J Chin Med. 42:1279–1300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shirosaki M and Koyama T: Laminaria
japonica as a food for the prevention of obesity and diabetes. Adv
Food Nutr Res. 64:199–212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen D, Yao WJ, Zhang XL, Han XQ, Qu XY,
Ka WB, Sun DG, Wu XZ and Wen ZY: Effects of Gekko sulfated
polysaccharide-protein complex on human hepatoma SMMC-7721 cells:
Inhibition of proliferation and migration. J Ethnopharmacol.
127:702–708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen D, Zhang X, Du Y, Jia B, Ka W, Sun D,
Yao W and Wen Z: Effects of Gekko sulfated polysaccharide-protein
complex on the defective biorheological characters of dendritic
cells under tumor microenvironment. Cell Biochem Biophys.
62:193–201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parish CR, Coombe DR, Jakobsen KB, Bennett
FA and Underwood PA: Evidence that sulphated polysaccharides
inhibit tumour metastasis by blocking tumour-cell-derived
heparanases. Int J Cancer. 40:511–518. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Parish CR, Freeman C, Brown KJ, Francis DJ
and Cowden WB: Identification of sulfated oligosaccharide-based
inhibitors of tumor growth and metastasis using novel in vitro
assays for angiogenesis and heparanase activity. Cancer Res.
59:3433–3441. 1999.PubMed/NCBI
|
|
19
|
Wu XZ, Chen D and Han XQ: Anti-migration
effects of Gekko sulfated glycopeptide on human hepatoma SMMC-7721
cells. Molecules. 16:4958–4970. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ding XL, Man YN, Hao J, Zhu CH, Liu C,
Yang X and Wu XZ: The antitumor effect of Gekko sulfated
glycopeptide by inhibiting bFGF-induced lymphangiogenesis. Biomed
Res Int. Apr 12–2016.(Epub ahead of print) doi:
10.1155/2016/7396392. View Article : Google Scholar
|
|
21
|
Atashrazm F, Lowenthal RM, Woods GM,
Holloway AF and Dickinson JL: Fucoidan and cancer: A
multifunctional molecule with anti-tumor potential. Mar Drugs.
13:2327–2346. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nair KL, Jagadeeshan S, Nair SA and Kumar
GS: Folic acid conjugated δ-valerolactone-poly(ethylene glycol)
based triblock copolymer as a promising carrier for targeted
doxorubicin delivery. PLoS One. 8:e706972013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao H, Li Y, Wang Y, Zhang J, Ouyang X,
Peng R and Yang J: Antitumor and immunostimulatory activity of a
polysaccharide-protein complex from Scolopendra subspinipes
mutilans L. Koch in tumor-bearing mice. Food Chem Toxicol.
50:2648–2655. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jafari M, MonsefEsfahani A and Solimani B:
Diagnostic value of immunoperoxidase staining and
immunofluorescence in the study of kidney biopsy specimens. Iran J
Kidney Dis. 9:286–290. 2015.PubMed/NCBI
|
|
25
|
Thorburn J, Bender LM, Morgan MJ and
Thorburn A: Caspase- and serine protease-dependent apoptosis by the
death domain of FADD in normal epithelial cells. Mol Biol Cell.
14:67–77. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang L, Wang P, Wang H, Li Q, Teng H, Liu
Z, Yang W, Hou L and Zou X: Fucoidan derived from Undaria
pinnatifida unduces apoptosis in human hepatocellular carcinoma
SMMC-7721 cells via the ROS-mediated mitochondrial pathway. Mar
Drugs. 11:1961–1976. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu G, Kuang S, Wu S, Jin W and Sun C: A
novel polysaccharide from Sargassum integerrimum induces apoptosis
in A549 cells and prevents angiogenesis in vitro and in vivo. Sci
Rep. May 24–2016.(Epub ahead of print) doi: 10.1038/srep26722.
|
|
29
|
Kim JY and Park JH: ROS-dependent
caspase-9 activation in hypoxic cell death. FEBS Lett. 549:94–98.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Diaby V, Tawk R, Sanogo V, Xiao H and
Montero AJ: A review of systematic reviews of the
cost-effectiveness of hormone therapy, chemotherapy, and targeted
therapy for breast cancer. Breast Cancer Res Treat. 151:27–40.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yeung TK, Chakrabarti K, Wilding D and
Hopewell JW: Modification of doxorubicin-induced cardiotoxicity:
Manipulation of the dosage schedule. Hum Exp Toxicol. 21:607–614.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dayton A, Selvendiran K, Meduru S, Khan M,
Kuppusamy ML, Naidu S, Kálai T, Hideg K and Kuppusamy P:
Amelioration of doxorubicin-induced cardiotoxicity by an
anticancer-antioxidant dual-function compound, HO-3867. J Pharmacol
Exp Ther. 339:350–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu YY, Dong Q, Zhao X, Gan X, Lu Q and Xu
X: SPPC: A novel inhibitor of bcr fusion gene with potent
anti-leukemia activity. Zhong Yi Za Zhi. 30:17–23. 2010.
|
|
34
|
Wu X, Chen D and Xie GR: Effects of Gekko
sulfated polysaccharide on the proliferation and differentiation of
hepatic cancer cell line. Cell Biol Int. 30:659–664. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang L, Zhang M, Zhou Q, Chen J and Zeng
F: Solution properties of antitumor sulfated derivative of
alpha-(1->3)-D-glucan from Ganoderma lucidum. Biosci Biotechnol
Biochem. 64:2172–2178. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang GY, Lu QH, Xu RZ and Dong QH: SPPC
induces apoptosis in human osteosarcoma cell line MG-63 by loss in
mitochondrial transmembrane potential and caspase activation.
Zhejiang Da Xue Xue Bao. 8:248–255. 2007.(In Chinese).
|
|
37
|
Liu J, Mao W, Ding B and Liang CS:
ERKs/p53 signal transduction pathway is involved in
doxorubicin-induced apoptosis in H9c2 cells and cardiomyocytes. Am
J Physiol Heart Circ Physiol. 295:H1956–H1965. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zigler M, Villares GJ, Lev DC, Melnikova
VO and Bar-Eli M: Tumor immunotherapy in melanoma: Strategies for
overcoming mechanisms of resistance and escape. Am J Clin Dermatol.
9:307–311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Postescu ID, Chereches G, Tatomir C,
Daicoviciu D and Filip GA: Modulation of doxorubicin-induced
oxidative stress by a grape (Vitis vinifera L.) seed extract in
normal and tumor cells. J Med Food. 15:639–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu B, Wang SS and Du Q: Traditional
Chinese medicine for prevention and treatment of hepatocarcinoma:
From bench to bedside. World J Hepatol. 7:1209–1232. 2015.
View Article : Google Scholar : PubMed/NCBI
|