Introduction
VanWyk-Grumbach syndrome (VWGS) is characterized by
delayed bone age, juvenile hypothyroidism and isosexual precocious
puberty (1). VWGS patients usually
show decreased free thyroxine (T4), together with elevated
prolactin, estradiol and thyroid-stimulating hormone (TSH). At
present, VWGS is well acknowledged as a prepubertal response
mediated by follicle-stimulating hormone (FSH). Besides, the
expression of luteinizing hormone (LH) is suppressed as revealed by
the elevation of LH-releasing hormone (LHRH). All of these results
confirmed that VWGS is a gonadotropin-releasing hormone
(GnRH)-independent type of precocious pseudopuberty (2–9).
Phenotypically, female patients with VWGS show
breast enlargement, early onset of menstrual bleeding and enlarged
multicystic ovaries. In male patients, the only symptom is
testicular enlargement without substantial Leydig cell stimulation
or testosterone secretion (5). To
the best of our knowledge, the incidence of VWGS has been more
commonly reported in females (3,4,10,11),
with very few case studies on males (9,12). The
present case report presented a boy with features of VWGS and
described the pathophysiology, clinical manifestation and
treatment. Furthermore, a literature review was performed regarding
the treatment of VWGS in male patients and its outcome.
Case report
A 14-year-old male patient of non-consanguineous
parents was referred to the Department of Pediatrics of the General
Hospital of Tianjin Medical University (Tianjin, China) due to
obesity, short stature and muscle weakness in February, 2014. He
had shown progressive weight gain, delay in growth, constipation,
muscle weakness and poor academic scores over the past 3–4 years.
Physical examination results were as follows: Body temperature,
36.5°C; heart rate, 60 beats per min; respiratory rate, 20/min;
blood pressure, 100/70 mmHg; body weight, 59 kg (>97th
percentile); height, 139 cm (<3rd percentile); and body mass
index, 30.5 (>97th percentile). The thyroid gland was enlarged.
Abdominal examination indicated hepatomegaly and cardiac
examination indicated a slightly distant heart sound without
murmurs. The bilateral testicular volume was 25 ml as measured by a
Prader orchidometer (Creative Health Products, Ann Arbor, MI, USA)
and stretched penile length was 5 cm. No pubic or axillary hair was
observed. Laboratory results are summarized in Table I. Laboratory parameters associated
with renal function were normal. Thyromegaly accompanied with
low-intensity echoes were identified by thyroid ultrasound.
Abdominal ultrasound revealed hepatomegaly. Delayed bone age was
confirmed according to X-ray imaging of the left wrist and hand
(Fig. 1) with an estimated bone age
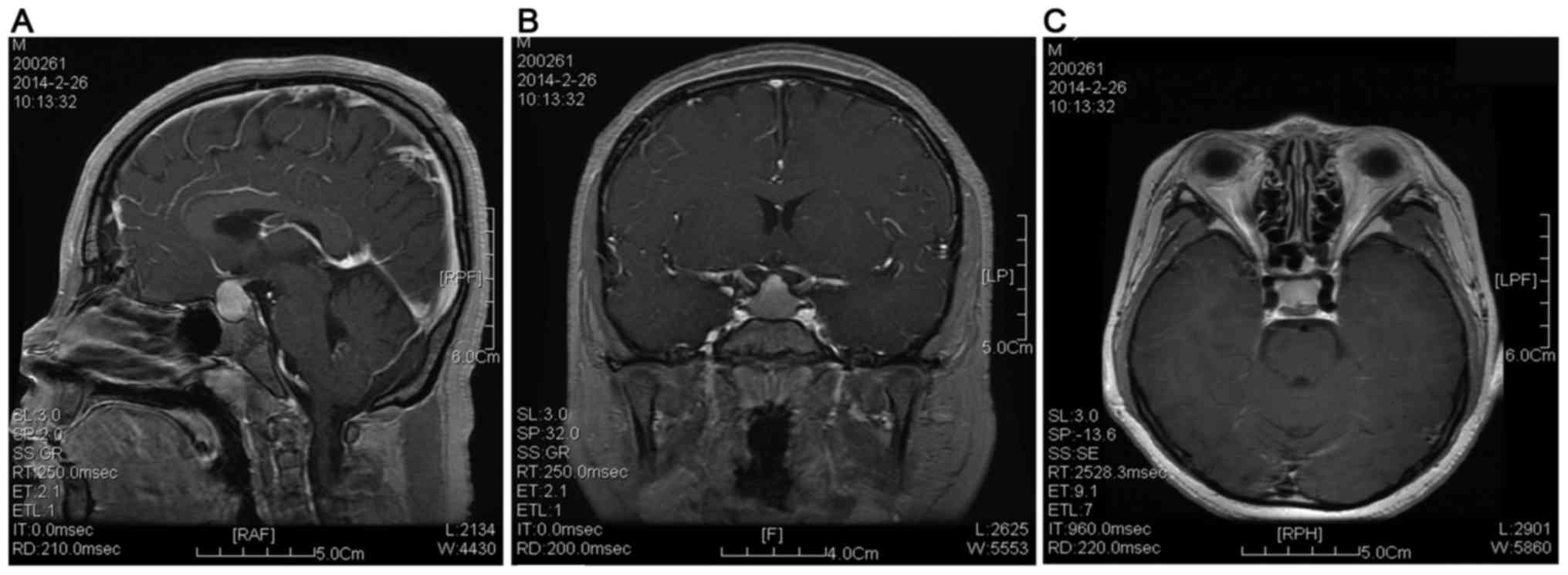
of 10 years. Cranial magnetic resonance imaging (MRI) indicated
enlargement of the pituitary gland (Fig.
2), and pituitary hyperplasia was suspected. Based on these
results, the patient was finally diagnosed as VWGS. As a treatment,
replacement therapy was given using levothyroxine with an initial
dose of 25 µg/m2/day, which was gradually increased to
100 µg/m2/day. The patient was followed up for 6 months
and the levels of free triiodothyronine (T3), T4 and TSH were 4.35
pmol/l, 18.65 pmol/l and 3.31 µIU/ml, respectively. The body height
showed an increase of 5 cm after the treatment.
 | Table I.Laboratory results of the patient. |
Table I.
Laboratory results of the patient.
| Parameter | Value | Normal range |
|---|
| Hemoglobin, g/l | 87 | 120–150 |
| Thyroid-stimulating
hormone, µIU/ml | >150 | 0.3–5 |
| T3, pmol/l | 1.19 | 3.5–6.5 |
| Free thyroxine,
pmol/l | 4.78 | 11.5–23.5 |
| Thyroglobulin
antibody, IU/ml | 164 | 0–40 |
|
Anti-thyroidperoxidase antibody,
IU/ml | >1,000 | 0–35 |
| Antibodies against
TSH receptor, IU/ml | 0.01 | 0–1.5 |
| Follicle-stimulating
hormone, IU/ml | 26.7 | 1.4–18 I |
| Luteinizing hormone,
IU/ml | 0.37 | 1.5–34.6 |
| Progesterone,
ng/ml | <0.15 | 0.28–1.22 |
| Estradiol, pg/ml | 12.11 | 0–40 |
| Prolactin, pg/ml | 21.94 | 2.1–17.7 |
| Testosterone,
ng/dl | 72.5 | 241–827 |
| Adrenocorticotropic
hormone, pg/ml | 23.6 | 0–46 |
| Serum cortisol,
µg/dl | 11.5 | 5–25 |
| Cholesterol,
mmol/l |
12.17 | <5.20 |
| Triglyceride,
mmol/l | 1.12 | 0.56–1.70 |
| Alanine
aminotransferase, U/l | 87 | 5–40 |
| Aspartate
aminotransferase, U/l | 82 | 8–40 |
| Alkaline phosphatase,
U/l | 122 | 40–150 |
Discussion
Primary hypothyroidism has been well acknowledged to
be associated with growth and pubertal delay. In rare cases,
hypothyroidism was reported to induce precocious puberty and
delayed bone age. These symptoms were initially described in 1905;
however, VWGS was only defined in 1960 (1). A literature review revealed that VWGS
has been rarely reported in male patients (13,14). The
present study presented a rare case of a male pediatric patient
with VWGS.
The pathogenesis of VWGS is closely associated with
the complex interactions between the hypothalamic-pituitary axis.
Van Wyk and Grumbach postulated that a hormonal overlap occurred in
the pituitary feedback mechanism. Given that gonadotropins as well
as TSH are glycoproteins, their overlap at the hormonal level is
partly associated with the lack of specificity at the hypothalamic
level (1). Moreover, excess TSH
induced by thyrotropin-releasing hormone (TRH) may act as an
agonist of FSH receptor (FSH-R) and other G protein-coupled
receptors (GPCRs). In the peripubertal phase, where FSH levels are
low, FSH-R is more apt to be stimulated by TSH (2,15,16). In
addition, FSH-R responds to TSH in a dose-dependent manner
(15,16). Furthermore, human chorionic
gonadotropin (hHCG), LH, FSH and TSH share the same α-subunit,
while a unique β-subunit specific to each hormone was identified.
These molecules activate adenylate cyclase and stimulate cyclic
adenosine monophosphate (cAMP) production by interacting with GPCRs
(17).
In a previous study, Anasti et al (15) indicated that recombinant human TSH
elicited a dose-dependent cAMP response in an in vitro
hFSH-R bioassay. However, the concentration of recombinant hTSH
required for half-maximal stimulation was several logs greater than
that of hFSH. These results suggested that hTSH and hFSH act
through the same receptor. The overlap between the glycoprotein
hormones is not unprecedented in the presence of excessive
secretion of hormones. For instance, with the homologous β-subunits
of LH, HCG is able to stimulate LH receptors and serve as an
evaluation method for testicular function in male pediatric
patients through determination of the binding of HCG and LH
receptor. To investigate the exact activation of TSH on FSH-R, Ryan
et al (16) sequenced the
FSH-R gene in 8 patients with overexpression of sex hormone
secondary to primary hypothyroidism, which revealed no hFSH-R
mutations in the patient population; however, two known
polymorphisms were identified. Besides, the gonadal
hyperstimulation associated with severe primary hypothyroidism is
likely due to the actions of the elevated concentrations of TSH on
the wild-type hFSHR, which is not dependent on the hFSH-R isoform
(18).
Severe hypothyroidism is one of the major causes for
the changes of gonadotrophins, such as elevation of FSH or decrease
of LH (19,20). In primary hypothyroidism patients,
the level of TRH was elevated, which resulted in a decrease of
pulse frequency of GnRH and the downregulation of GnRH secretion
(21–23). Under these conditions, the expression
of FSH was elevated and the elevation of TRH induced
hyperprolactinemia, which induced a decrease of LH (24). Such aspects may explain for the
discordance between LH and FSH in VWGS. In a previous study,
Francavilla et al (25)
indicated that hypothyroidism directly affected the function of
testis prior to puberty, resulting in excess proliferation of
Sertoli cells and testicle enlargement. Male patients with VWGS
have macroorchidism without obvious virilisation, and testicular
histology reveals a predominance of tubular structures without
increased Leydig cells, in line with an FSH-dominated response
(15,26). However, enlargement of gonads and
formation of ovarian cysts are usually associated with malignant
conditions (3,4,27). Thus,
early diagnosis of the disease may help to identify cases that
require surgery (5,6).
Weight gain has been regarded as a symptom of
hypothyroidism. In the complete absence of thyroid hormone, basal
metabolic rate or resting energy expenditure was reduced by 30% or
even 59% (28). In the present case,
the patient showed elevation of cholesterol liver enzymes and
hepatomegaly, which may be associated with hypercholesterolemia
induced by hypothyroidism. Besides, hypothyroidism has been
reported to have an important role in the pathogenesis of
nonalcoholic fatty liver disease (NAFLD) (29). Specifically, Chung et al
(30) reported that NAFLD was more
severe and liver enzyme was significantly elevated in patients with
hypothyroidism compared to those in normal subjects. In the present
case, pituitary enlargement was revealed by cranial MRI, which may
result in long-term hypothyroidism. In addition, the thyrotroph
hyperplasia may lead to sella turcica expansion and pituitary
hyperplasia.
Certain uncommon features, including PTH
suppression, streaky hyperpigmentation and severe anemia, have been
reported in certain patients (10).
It has been well acknowledged that melanocyte stimulating hormone
(MSH) may be involved in the pigmentation of local skin tissues
(1). As MSH acts via GPCRs, it is
reasonable to speculate that streaky hyperpigmentation may be
induced by differences in receptor distribution and activities in
the presence of homologies and cross reaction between MSH and TSH.
In the present case, the patient presented with anemia, which is,
to the best of our knowledge, not common in hypothyroidism
(31), while it has been noted in
certain cases of VWGS (2–5,7,32). It was speculated that the anemia may
be associated with the reduced red cells and decreased metabolic
oxygen requirement in tissues of patients with hypothyroidism
(31).
In conclusion, the pathogenesis of VWGS involves a
complex interaction, which is, at least in part, directly mediated
by FSH and TSH receptors. It is suggested that the ‘overlap’ of
hormone actions, postulated by Van Wyk and Grumbach (1), may be reflected in receptor levels. In
particular, all hormones involved may act through GPCRs and common
intracellular signaling pathways in the presence of elevated TSH.
Early diagnosis and thyroxine replacement therapy are recommended
for the treatment of VWGS.
References
|
1
|
Van Wyk JJ and Grumbach MM: Syndrome of
precocious menstruation and galactorrhea in juvenile
hypothyroidism: An example of hormonal overlap in pituitary
feedback. J Pediatrics. 57:416–435. 1960. View Article : Google Scholar
|
|
2
|
Shu J, Xing L, Zhang L, Fang S and Huang
H: Ignored adult primary hypothyroidism presenting chiefly with
persistent ovarian cysts: A need for increased awareness. Reprod
Biol Endocrinol. 9:1192011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Browne LP, Boswell HB, Crotty EJ, O'Hara
SM, Birkemeier KL and Guillerman RP: Van Wyk and Grumbach syndrome
revisited: Imaging and clinical findings in pre-and postpubertal
girls. Pediatr Radiol. 38:538–542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Panico A, Lupoli GA, Fonderico F,
Colarusso S, Marciello F, Poggiano MR, Del Prete M, Magliulo R,
Iervolino P and Lupoli G: Multiple ovarian cysts in a young girl
with severe hypothyroidism. Thyroid. 17:1289–1293. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Indumathi C, Bantwal G and Patil M:
Primary hypothyroidism with precocious puberty and bilateral cystic
ovaries. Indian J Pediatr. 74:781–783. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chattopadhyay A, Kumar V and Marulaiah M:
Polycystic ovaries, precocious puberty and acquired hypothyroidism:
The Van Wyk and Grumbach syndrome. J Pediatr Surg. 38:1390–1392.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ozgen T, Güven A and Aydin M: Precocious
puberty in a girl with Down syndrome due to primary hypothyroidism.
Turk J Pediatr. 51:381–383. 2009.PubMed/NCBI
|
|
8
|
Zhang H, Geng N, Wang Y, Tian W and Xue F:
Van Wyk and Grumbach syndrome: Two case reports and review of the
published work. J Obstet Gynaecol Res. 40:607–610. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Esen I and Demirel F:
Hypothyroidism-associated testicular enlargement: Is it a form of
precocious puberty or not? A case report. Turk J Pediatr.
53:210–212. 2011.PubMed/NCBI
|
|
10
|
Baranowski E and Högler W: An unusual
presentation of acquired hypothyroidism: The Van Wyk-Grumbach
syndrome. Eur J Endocrinol. 166:537–542. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rastogi A, Bhadada SK and Bhansali A: An
unusual presentation of a usual disorder: Van Wyk-Grumbach
syndrome. Indian J Endocrinol Metab. 15:(Suppl 2). S141–S143. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Castro-Magaña M, Angulo M, Cañas A, Sharp
A and Fuentes B: Hypothalamic-pituitary gonadal axis in boys with
primary hypothyroidism and macroorchidism. J Pediatr. 112:397–402.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Omran A, Peng J, Shrestha B, Ashhab MU and
Yin F: Male child with Van Wyk-Grumbach's syndrome and other
complications of long-standing primary hypothyroidism: A case
report. Case Rep Pediatr. 2012:3527512012.PubMed/NCBI
|
|
14
|
Nebesio TD and Eugster EA: Current
concepts in normal and abnormal puberty. Curr Probl Pediatr Adolesc
Health Care. 37:50–72. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anasti J, Flack M, Froehlich J, Nelson L
and Nisula B: A potential novel mechanism for precocious puberty in
juvenile hypothyroidism. J Clin Endocrinol Metab. 80:276–279. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ryan GL, Feng X, d'Alva CB, Zhang M, Van
Voorhis BJ, Pinto EM, Kubias AE, Antonini SR, Latronico AC and
Segaloff DL: Evaluating the roles of follicle-stimulating hormone
receptor polymorphisms in gonadal hyperstimulation associated with
severe juvenile primary hypothyroidism. J Clin Endocrinol Metab.
92:2312–2317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kroeze WK, Sheffler DJ and Roth BL:
G-protein-coupled receptors at a glance. J Cell Sci. 116:4867–4869.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De Leener A, Montanelli L, Van Durme J,
Chae H, Smits G, Vassart G and Costagliola S: Presence and absence
of follicle-stimulating hormone receptor mutations provide some
insights into spontaneous ovarian hyperstimulation syndrome
physiopathology. J Clin Endocrinol Metab. 91:555–562. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takeuchi K, Deguchi M, Takeshima Y and
Maruo T: A case of multiple ovarian cysts in a prepubertal girl
with severe hypothyroidism due to autoimmune thyroiditis. Int J
Gynecol Cancer. 14:543–545. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rosenfield R, Cooke D and Radovick S:
Puberty and its disorders in the female. Pediatr Endocrinol.
3:573–590. 2008.
|
|
21
|
Denef C: Paracrinicity: The story of 30
years of cellular pituitary crosstalk. J Neuroendocrinol. 20:1–70.
2008.PubMed/NCBI
|
|
22
|
Krassas G, Poppe K and Glinoer D: Thyroid
function and human reproductive health. Endocr Rev. 31:702–755.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grattan DR, Jasoni CL, Liu X, Anderson GM
and Herbison AE: Prolactin regulation of gonadotropin-releasing
hormone neurons to suppress luteinizing hormone secretion in mice.
Endocrinology. 148:4344–4351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thackray VG, Mellon PL and Coss D:
Hormones in synergy: Regulation of the pituitary gonadotropin
genes. Mol Cell Endocrinol. 314:192–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Francavilla S, Cordeschi G, Properzi G, Di
Cicco L, Jannini EA, Palmero S, Fugassa E, Loras B and D'Armiento
M: Effect of thyroid hormone on the pre- and post-natal development
of the rat testis. J Endocrinol. 129:35–42. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bruder J, Samuels M, Bremner W, Ridgway E
and Wierman M: Hypothyroidism-induced macroorchidism: Use of a
gonadotropin-releasing hormone agonist to understand its mechanism
and augment adult stature. J Clin Endocrinol Metab. 80:11–16. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Niedziela M and Korman E: Severe
hypothyroidism due to autoimmune atrophic thyroiditis-predicted
target height and a plausible mechanism for sexual precocity. J
Pediatr Endocrinol Metab. 14:901–907. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Silva JE: The thermogenic effect of
thyroid hormone and its clinical implications. Ann Intern Med.
139:205–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Silveira MG, Mendes FD, Diehl NN, Enders
FT and Lindor KD: Thyroid dysfunction in primary biliary cirrhosis,
primary sclerosing cholangitis and non-alcoholic fatty liver
disease. Liver Int. 29:1094–1100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chung GE, Kim D, Kim W, Kim W, Yim JY,
Park MJ, Kim YJ, Yoon JH and Lee HS: Non-alcoholic fatty liver
disease across the spectrum of hypothyroidism. J Hepatol.
57:150–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chu JY, Monteleone JA, Peden VH, Graviss
ER and Vernava AM: Anemia in children and adolescents with
hypothyroidism. Clin Pediatr (Phila). 20:696–699. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hunold A, Alzen G, Wudy SA,
Bluetters-Sawatzki R, Landmann E, Reiter A and Wagner HJ: Ovarian
tumor in a 12-year old female with severe hypothyroidism: A case of
Van Wyk and Grumbach syndrome. Pediatr Blood Cancer. 52:677–679.
2009. View Article : Google Scholar : PubMed/NCBI
|