Introduction
Hip fractures are one of the most common injuries in
elderly individuals and are associated with a high incidence of
complications and mortality (1–3). In the
USA, ~300,000 hip fractures occur annually, and this number is
expected to rise with the growth of elderly populations (4). Furthermore, mortality in the elderly
may reach 10% at 1 month, 20% at 4 months and 30% at 1 year
following hip fracture (5). The
majority of reports have indicated that early surgery improves
certain outcomes for the patient, including length of stay, the
incidence of pressure sores, lung infection and return to
independent living (6,7). Therefore, it would appear prudent to
surgically treat elderly patients with hip fractures within the
first 48 h of admission to hospital, and everything possible should
be done to ensure the majority of patients are surgically treated
within 1–2 days. However, the principle of early surgery may not be
feasible for high-risk and physiologically unstable patients
(8). It may be questioned whether
this principle of early surgery is applicable to all patients with
hip fracture. Limited studies have focused on the adverse effects
of early surgery in elderly patients with hip fracture and impaired
lung conditions.
It is understood that hip fracture and accompanying
early surgery has an adverse effect on the lungs of elderly
patients, and postoperative lung infections are associated with
impaired lung conditions (9).
Chronic lung disease is a compelling reason for delaying surgery
and patients often require additional treatments and tests that are
time-consuming. Chronic obstructive pulmonary disease (COPD),
including chronic bronchitis and emphysema, is a type of
obstructive lung disease characterized by chronically poor airflow
(10). It is typically progressive
in nature and not fully reversible. Individuals who smoke and have
COPD will have a 60–70% higher risk of mortality following proximal
femur fracture than those without (11). A growing body of evidence has
suggested that hip fracture induces substantial mitochondrial
(mt)DNA release, systemic inflammatory response and lung injury in
the elderly (12). Immediate
intramedullary nailing surgery could aggravate the above
pathological states (12).
Similarly, it was previously demonstrated by the current group that
hip fracture and surgery also elevate systemic proinflammatory
mediators in elderly patients, and the inflammatory response may
serve a key role in postoperative lung dysfunction (13).
Previous results have suggested that early surgery
increases mtDNA and cytokine release, which may subsequently
aggravate the systemic inflammatory response and lung injury
induced by elderly hip fracture (14–16). Hip
fracture and surgery in elderly patients may induce systemic
inflammatory responses and lung injury, which increases the risk of
pulmonary infection and mortality during the post-injury period
(12,13). Therefore, it may be hypothesized that
late surgery may be beneficial for survival by reducing the
magnitude of the inflammatory response. However, the approach of
reducing the inflammatory response is unknown as little research
has focused on the impact of surgery on elderly patients with
COPD.
We propose selective surgery as a solution for these
high-risk patients with severe COPD, in whom early fixation may be
associated with high inflammatory response and secondary injury to
the lungs. The aim of the present study was to evaluate the adverse
effect of early surgery in a model of elderly hip fracture with
COPD.
Materials and methods
Animal care
A total of 40 adult male Wistar rats (5 months old;
weight, 657±41 g) were obtained from the Animal Center of the
Chinese Academy of Medical Sciences (Beijing, China). The rats were
allowed to acclimatize to the laboratory conditions for 1 week
under a 12-h light/dark cycle at a constant temperature (22±2°C)
and a relative humidity of 50–70% with free access to rodent chow
and water. The model used in the present study mimics a person who
started smoking at 19 years old, with an expected life expenditure
of 80 years old (17). All rats were
maintained according to international guidelines on the ethical use
of animals in experiments and the present study was approved by the
Institutional Review Board of the Beijing Army General Hospital
(Beijing, China).
COPD model
A COPD model was created, as described in our
previous study (18), and rats were
exposed to cigarette smoke daily for 1–2 h for up to 37 weeks.
Commercial filtered cigarettes (Zhong Nan Hai, Beijing Cigarette
Factory, Beijing, China) containing 14 mg tar (equivalent to
1.5-fold of the tar quantity in the Kentucky Reference Cigarette
2R4F) and 1.4 mg nicotine (equivalent to 1.4-fold of the nicotine
quantity in the Kentucky Reference Cigarette 2R4F) per cigarette
were used in the present study (19).
The exposure system used was as previously described
(18) and had a volume of 2001.
Cigarette smoke was delivered into the exposure chamber through a
tube connected to a fan. The cigarette smoke was passed undiluted
through the tube into the chamber by a fan on top. A group of 10
rats were placed in the inhalation chamber. The COPD model was
evaluated through respiratory function and histological structure
of lungs, as previously described (18). The study progressed to the next step
only when the establishment of COPD was successful.
Grouping and procedure of fracture and
fixation
A control group (n=10) and a COPD group (n=10) were
included in the present study. Control rats were not subjected to
tobacco smoke exposure, whereas COPD rats experienced persistent
tobacco exposure for 37 weeks. The control and COPD groups
experienced hip fracture and either early or late fixation (EF and
LF, respectively). According to our previous study (18), the peak of the inflammatory response
in remote organs was at 24–48 h post-fracture, followed by a
decrease of the inflammatory response. Surgery was implemented at
24 h after fracture (EF) or 72 h post-fracture (LF) in control rats
and rats with COPD.
Hip fracture models were created as previously
described (18), using a blunt
guillotine device with a weight of 500 g, and temporarily fixed by
plaster. The trochanteric fossa was prepared and the medullary
canal opened via a 2-cm skin incision. Sequential 1×1 mm nailing
was then inserted. All rats had to survive until the end of the
study period (24 h after stabilization). Each group contained 6
rats. Alternative rats were added in time if mortality occurred due
to various reasons (such as fighting).
Serum mtDNA isolation
Blood samples for mtDNA extraction were collected
into EDTA-containing tubes. To obtain cell-free plasma, EDTA-blood
samples were initially centrifuged at 900 × g for 10 min at room
temperature (RT), and the plasma was transferred into a clear
polypropylene tube. The plasma was centrifuged at 9,600 × g for 10
min at RT, and the upper portion of the plasma was transferred into
another clear tube and stored at −80°C. mtDNA was prepared from 200
µl plasma using a QIAamp DNA Blood Mini kit (Qiagen GmbH, Hilden,
Germany), according to the manufacturer's protocol. The same amount
of DNA was used for each quantitative polymerase chain reaction
(qPCR) using a SYBR Green Master mix (Applied Biosystems; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) on a Mastercycler EP
real lex (Eppendorf, Hamburg, Germany), as described below.
qPCR
According to a previous study (20), an mtDNA-230 primer set was used to
amplify a 230 bp long DNA fragment of non-apoptotic origin (forward
5′-CAGCCGCTATTAAAGGTTCG-3′ and reverse 5′-CCTGGATTACTCCGGTCTGA-3′).
The mtDNA plasmid was constructed using a TA cloning kit (Takara
Bio, Inc., Otsu, Japan) as follows: The purified PCR products were
linked into a pMD18-T vector (Takara Bio, Inc.), and the connective
product was transformed into DH5α competent Escherichia coli
(D9057A, Takara Bio, Inc.) according to the manufacturer's
protocol. The positive E. coli clones were screened and
enriched, and then mtDNA was extracted using the Qiagen Plasmid
Midi kit (Qiagen GmbH) from the plasmid and measured by NanoDrop™
One/OneC (Thermo Fisher Scientific, Inc.) when A260/280 ratio
limited from 1.8–2.0. A standard curve was generated by six
dilutions of DNA (range, 102–107 copies/µl) using an ABI 7500 ce
detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). qPCR reactions were conducted in 96-well plates within a
total volume of 20 µl/well containing the following reagents: 10 µl
SYBR Premix Ex Taq II (2X), 0.8 µl forward primer, 0.8 µl reverse
primer, 0.4 µl ROX reference Dye II (50X), 2 µl DNA and 6 µl
double-distilled H2O. Primer sequences were all
synthesized by Invitrogen (Thermo Fisher Scientific, Inc.). The
SYBR® Premix Ex Taq™ II PCR kit was purchased
from Takara Bio, Inc. The PCR reaction was conducted using the
following conditions: 95°C for 30 sec, followed by 95°C for 5 sec
and 60°C for 34 sec, repeated for 40 cycles. Each sample and DNA
standard was analyzed in duplicate, and the mean value was used for
quantification. A standard curve was created to quantify mtDNA
concentration using purified mtDNA with CytB as the target,
according to the protocol supplied with the SYBR® Premix
Ex Taq™ II PCR kit. Only standard curves with a
coefficient of correlation >0.96 were accepted.
Bronchoalveolar lavage fluid (BALF)
and lung permeability analysis
Bronchoalveolar lavage (BAL) was performed to remove
bronchoalveolar cells by instilling and withdrawing lavage solution
(1.0 ml of 0.9% saline) three times via the tracheal tube before
finally transferring it to a syringe. Recovery ranged from 70–90%
of the instilled fluid. The recovered BALF was centrifuged at RT,
900 × g for 10 min, and the supernatant was extracted. Serum
protein levels or BALF protein concentrations were quantified with
the bicinchoninic acid protein assay (Pierce; Thermo Fisher
Scientific, Inc.), and the BAL-serum-protein ratio was accepted as
a diagnostic procedure for pulmonary permeability changes and
pulmonary damage/dysfunction.
Analysis of cytokines and
myeloperoxidase (MPO) activity in lung tissue
In all rats, following the BAL procedure, pulmonary
tissues (100 mg) from the middle lobe of the right lung (100 mg)
were removed for homogenization, and were incubated at 4°C for 1 h.
The final homogenate was centrifuged at RT, 13,400 × g for 20 min.
Tissue supernatants were used for cytokine assays for tumor
necrosis factor (TNF)-α, interleukin (IL)-6 and IL-10, which were
determined by avidin-biotin complex (ABC) ELISA (custom kit,
R&D Systems, Inc., Minneapolis, MN, USA), according to the
manufacturer's protocol. Lowest limits of detection were 16 pg/mg
for TNF-a, IL-6 and IL-10.
MPO activity is a sensitive index of tissue
neutrophil infiltration, which was accepted as a marker of
polymorphonuclear neutrophil (PMN) infiltration in pulmonary
tissue. PMNs exert damaging effects through the release of
proteolytic enzymes, reactive oxygen species and vasoactive
substances when they have migrated into the lung tissue (21). According to the manufacturer's
protocol, the level of MPO was measured using ABC ELISA (custom
kit, R&D Systems, Inc. Minneapolis, MN, USA).
Statistical analysis
Data were presented as the mean ± standard error of
the mean. Data were analyzed using SPSS v.16.0 for Windows (SPSS,
Inc., Chicago, IL, USA). Continuous variables were compared using
the nonparametric Mann-Whitney test, while categorical variables
were analyzed using Fisher's exact test. The changes of variables
between groups were compared using analysis of variance with
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Changes to serum TNF-α, IL-6, IL-10
and mtDNA levels
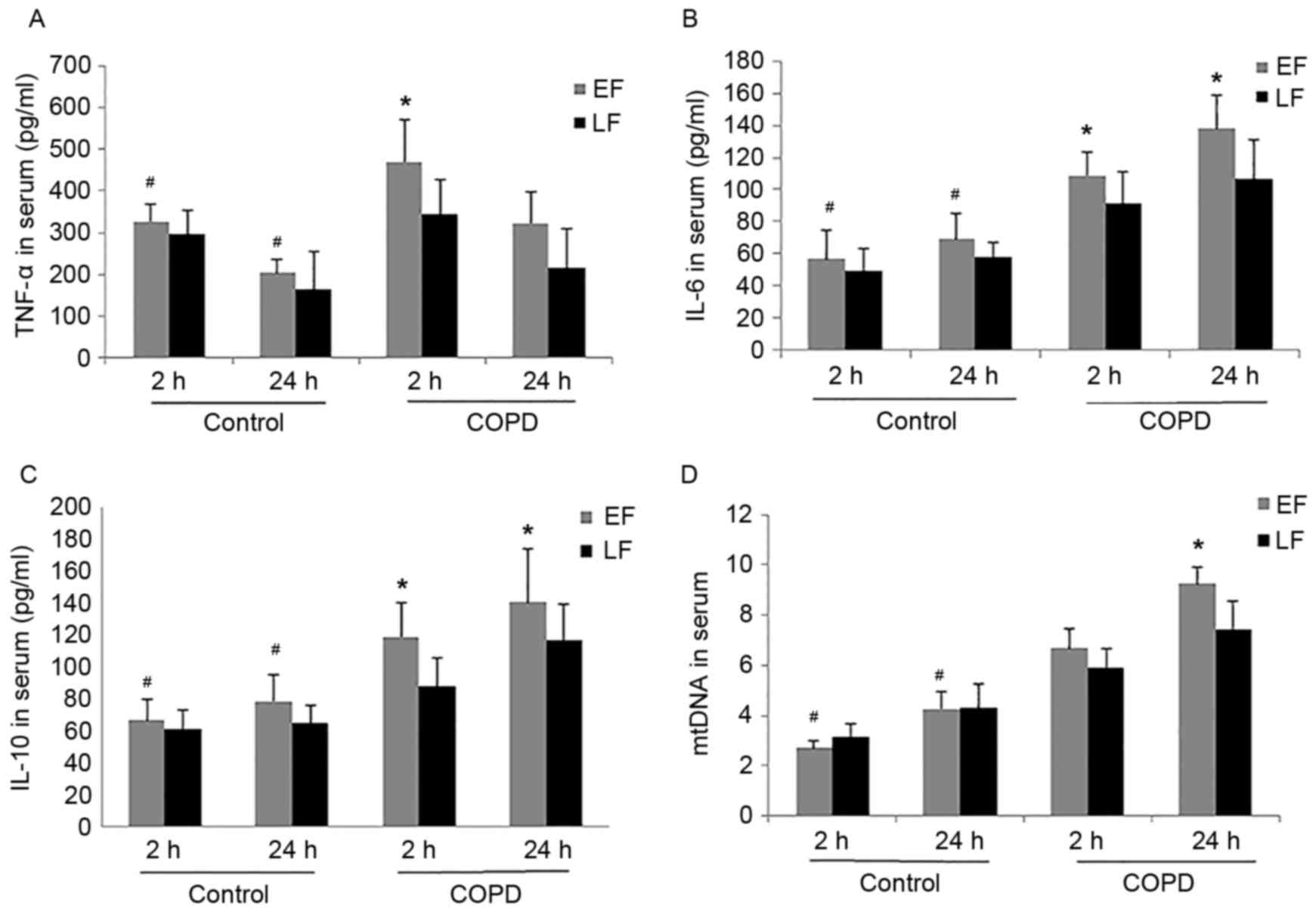
The present study examined the cytokines TNF-α, IL-6
and IL-10, and mtDNA levels in serum at 2 or 24 h after fixation
surgery. ELISA analysis of cytokines and qPCR for mtDNA
concentration from the experimental and control groups are
demonstrated in Fig. 1. Serum IL-6
and IL-10, and mtDNA levels in the control group and in the COPD
group increased rapidly at 2 h and peaked at 24 h; however, TNF-α
peaked at 2 h and then decreased at 24 h. The serum concentrations
of TNF-α, IL-6 and IL-10, and mtDNA in the COPD group increased
significantly compared with those in the control group at 2 and 24
h after fixation (P<0.05; Fig.
1). There was no significant difference observed in the control
group between EF and LF for TNF-α (P=0.203 and P=0.974), IL-6
(P=0.210 and P=0.103), IL-10 (P=0.554 and P=0.231) and mtDNA
(P=0.247 and P=0.108) at 2 or 24 h, respectively. EF in rats with
COPD induced a significant increase of TNF-α (P<0.001 at 2 h),
IL-6 (P<0.001 at 2 and 24 h), IL-10 (P=0.010 at 2 h and P=0.001
at 24 h) and mtDNA (P<0.001 for 24 h) compared with the LF group
(Fig. 1).
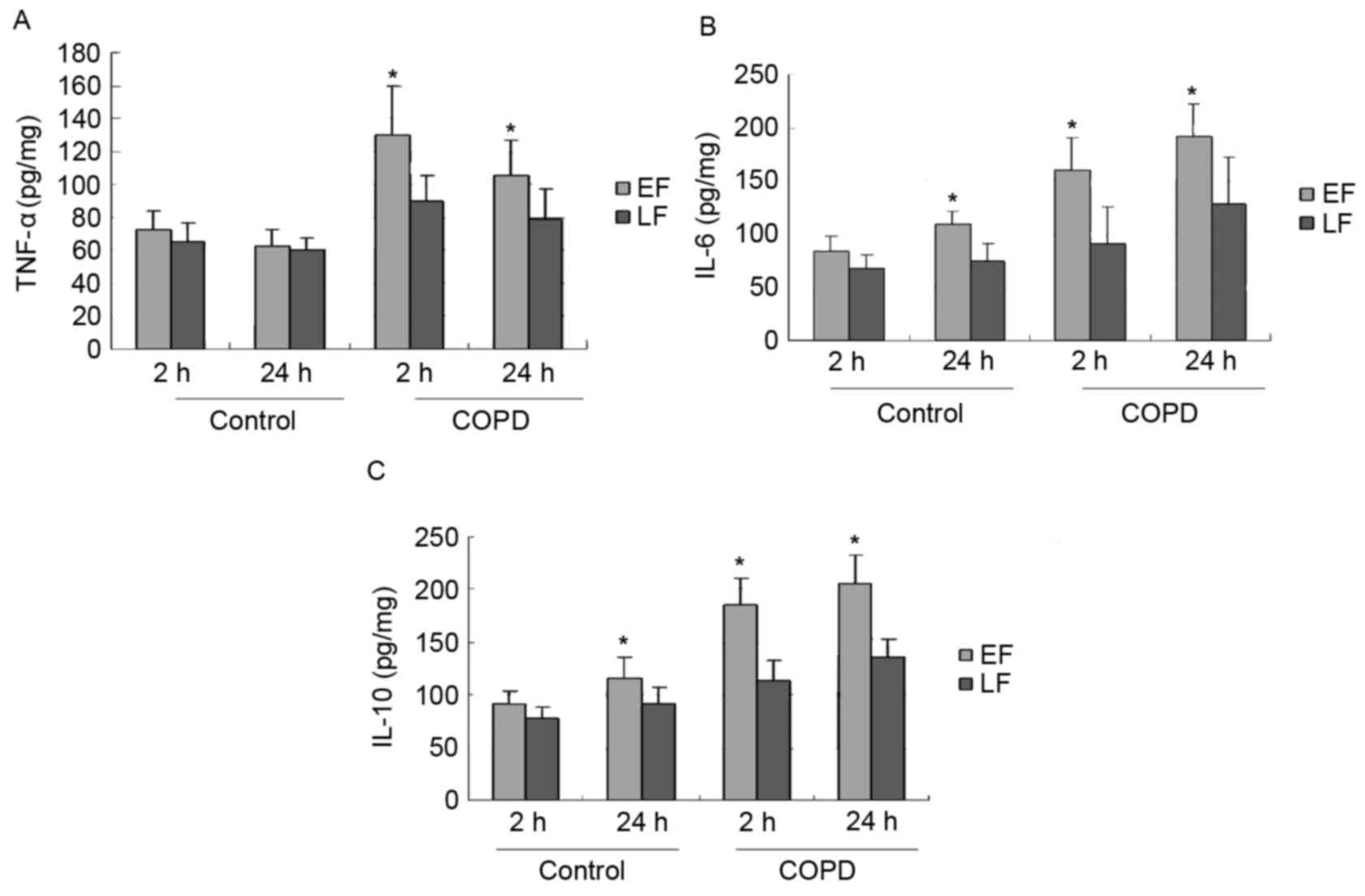
Inflammatory mediators in pulmonary
extracts
The levels of TNF-α, IL-6 and IL-10 in lung tissue
extracts of experimental and control aged rats are demonstrated in
Fig. 2. LF in the COPD group induced
a significant decrease of TNF-α (P=0.001 and P=0.006), IL-6
(P<0.001 and P=0.001) and IL-10 (P=0.035 and P<0.001) at 2 or
24 h, respectively, compared to the levels following EF. Although
the levels of TNF-α (P=0.487), IL-6 (P=0.284) and IL-10 (P=0.220)
following EF in the control group were higher at 2 h compared with
those following LF, the differences were not statistically
significant. At 24 h after fixation, IL-6 (P=0.043) and IL-10
(P=0.045) levels were significantly decreased following LF compared
with EF in the control group.
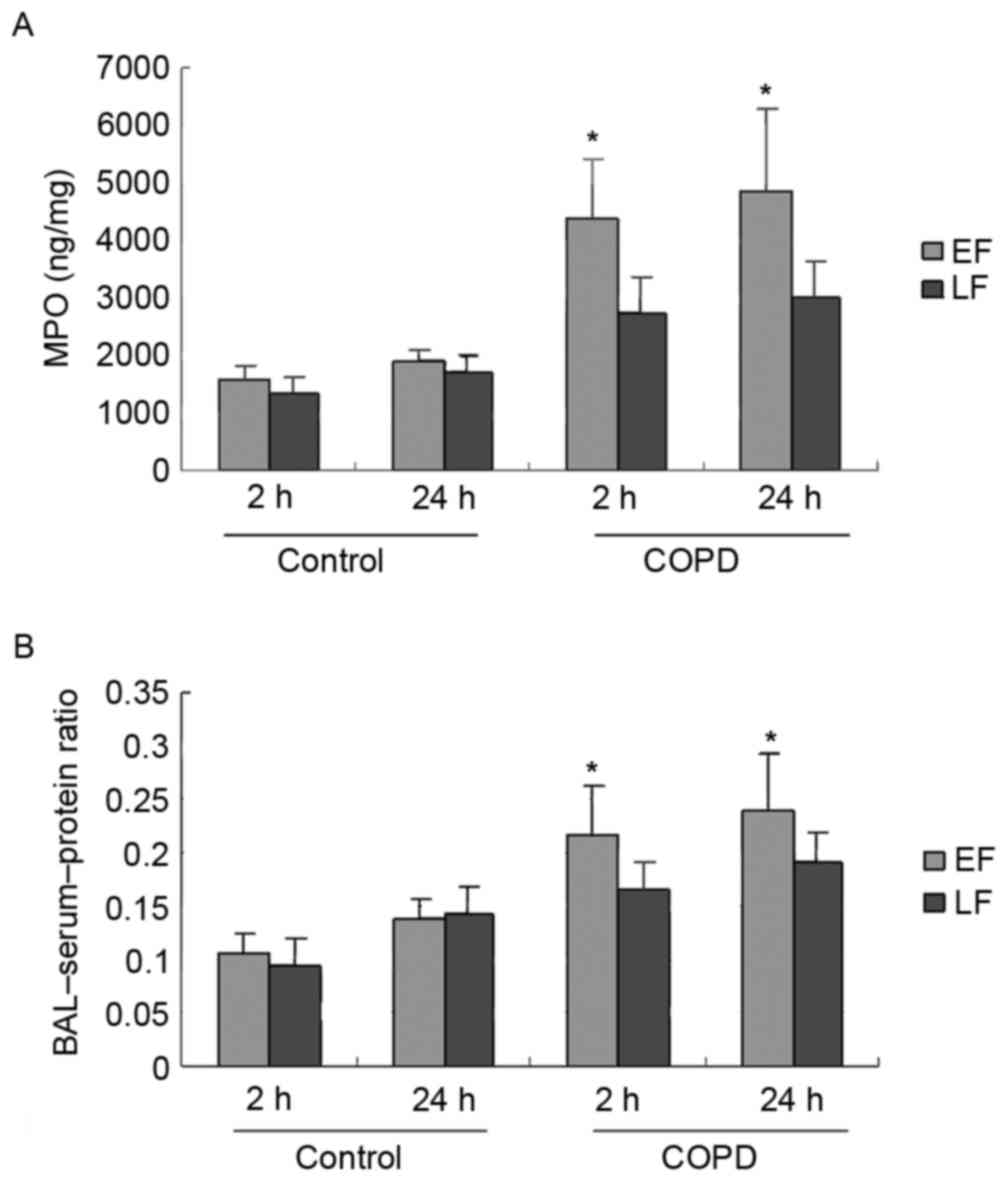
Neutrophil infiltration and lung
permeability
As demonstrated in Fig.
3A, MPO activity, a marker of PMN infiltration, following LF in
the COPD group was significantly decreased at 2 (P<0.001) and 24
h (P=0.001) compared to EF in the COPD group. No significant
differences were observed between EF and LF at 2 (P=0.504) or 24 h
(P=0.668) for PMN infiltration in the control group.
The results demonstrated that the BAL-serum-protein
ratio following EF in the COPD group was significantly elevated
compared to LF at 2 (P=0.009) and 24 h (P=0.018; Fig. 3B). LF in the control group
demonstrated a decrease in the BAL-serum-protein ratio at 2 h
(P=0.572) and an increase at 24 h (P=0.866) compared to EF;
however, these results were not statistically significant.
Discussion
It has been reported that COPD is the fourth most
common cause of mortality in adults, responsible for ~2 million
mortalities per year (22). Certain
therapies used in COPD, such as oral and inhaled corticosteroids,
have been demonstrated to increase the risk of osteoporosis, making
elderly patients more prone to hip fracture (23). Two previous studies examined the
contribution of a prior history of COPD on hip fracture prognosis.
In a multicenter, retrospective study of 390 Medicare beneficiaries
with hip fracture, a history of COPD was an independent predictor
of 30-day mortality (24). In
another retrospective study, individuals with COPD were found to
have a 60–70% higher risk of mortality following hip fracture than
those without COPD (25). COPD
produced a marked increase of the inflammatory response (TNF-α,
IL-6 and IL-10) following proximal femur fracture, which resulted
in organ injury (18). Hip fractures
in elderly patients are known to have a reduced physiological
reserve, have weaker connective tissue and often multiple
additional pre-existing co-morbidities that make management more
complicated (26). The dilemma of
optimal timing of surgery for these high-risk patients in hip
fracture is a challenge for all trauma surgeons.
Damage control orthopedics (DCO) is an approach that
contains and stabilizes orthopedic injuries to enable improvement
of the patient's overall physiology (21,27,28). Its
purpose is to avoid worsening of the patient's condition by the
‘second hit’ of a major orthopedic procedure and to delay
definitive fracture repair until a time when the overall condition
of the patient is optimized (28).
DCO has been suggested for the management of femoral shaft
fractures in severely injured patients because patients were unable
to tolerate the burden of early determinative surgery (21,29). A
clinical study investigated the type and timing of surgery and
demonstrated that improvements in the clinical status coincided
with a less sustained inflammatory response if DCO was followed
(30).
The results of the present study demonstrated that
LF in the COPD group (surgery followed by peak of inflammatory
response to trauma) resulted in significant decreases of cytokines
(TNF-α, IL-6 and IL-10) and mtDNA in the circulation and pulmonary
samples, and reduced the severity of injury to pulmonary tissues
(MPO activity and permeability) compared to EF (surgery performed
at the peak of the inflammatory response induced by trauma; early
definitive fixation). The present results indicated that
inflammatory infiltration may be reduced by modifying the timing of
surgery, as demonstrated in an elderly rat model of COPD. It
remains to be further determined whether this strategy may be
introduced in clinical practice for elderly patients with hip
fracture, in particular in those with COPD.
Notably, although LF in the control group was
associated with reduced levels of cytokines (TNF-α, IL-6 and IL-10)
in pulmonary samples at 24 h after surgery compared to EF, the
difference of the level of MPO and permeability between these
groups did not reach significance. This suggested that injury to
pulmonary tissue induced by early surgery was similar to that of
late surgery in normal elderly patients.
To the best of our knowledge, the present study was
the first to evaluate the adverse effects of early surgery in a
model of elderly hip fracture with COPD. Various studies have
demonstrated that mtDNA is a critical activator of inflammation and
the innate immune system (14,31).
Some research reported that cellular injury released mtDNA into
blood, which is a key link between trauma, inflammation, soluble
immune response suppressor (SIRS) and acute lung injury in
polytrauma patients and the elderly hip fracture model (12,13,16).
mtDNA contains unmethylated CpG motifs that exhibit immune
stimulatory capacities (31).
Recently, our previous studies have demonstrated the mtDNA release
induced by hip fracture induces the systemic inflammatory response
and lung injury by activating the Toll-like receptor 9
(TLR9)/nuclear factor (NF)-κB pathway in rats (12,30), and
may induce systemic inflammation through TLR9/NF-κB and
p38/mitogen-activated protein kinase signaling, thereby initiating
an immunological response characteristic of SIRS and acute
respiratory distress syndrome (12,15,30). In
the present study, the serum mtDNA concentration was increased
rapidly and considerably when fixed at 24 h in EF and LF groups.
Furthermore, the serum mtDNA concentration following EF was
significantly higher at 2 and 24 h compared with those following LF
in the COPD group.
Several limitations should be considered when
interpreting the present results. Firstly, no continuous
measurement in the same rat was obtained and the individual
variability in cytokine release must therefore be acknowledged
because a different set of rats was sacrificed at each time point.
Secondly, the applicability of the present method of bone fracture
and fixation to the clinical setting of hip fracture was unknown.
The present method was used to induce injury due to its
reproducibility and to maintain compliance with animal research
guidelines.
In conclusion, the present study demonstrated that
the inflammatory response was notably present in pulmonary samples
and blood in the COPD model following surgery, and this was
associated with an increase in MPO activity and permeability. LF in
the COPD group significantly reduced severity of inflammatory
infiltration, MPO activity and permeability in pulmonary samples
compared to EF, suggesting the validity of DCO to reduce damage of
organs in elderly patients with hip fracture and COPD.
Acknowledgements
The present study was funded by the Capital Training
Program of Beijing Municipal Commission of Science and Technology
(grant no. Z141100002114030).
References
|
1
|
Buecking B, Bohl K, Eschbach D, Bliemel C,
Aigner R, Balzer-Geldsetzer M, Dodel R, Ruchholtz S and Debus F:
Factors influencing the progress of mobilization in hip fracture
patients during the early postsurgical period? A prospective
observational study. Arch Gerontol Geriatr. 60:457–463. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Caillet P, Klemm S, Ducher M, Aussem A and
Schott AM: Hip fracture in the elderly: A re-analysis of the EPIDOS
study with causal Bayesian networks. PLoS One. 10:e01201252015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cook WL, Schiller C, McAllister MM, Hanson
HM, Brasher PM, Donaldson MG, Macri E, Preto R, Guy P and Ashe MC:
Feasibility of a follow-up hip fracture clinic. J Am Geriatr Soc.
63:598–599. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hawkes D, Baxter J, Bailey C, Holland G,
Ruddlesdin J, Wall A and Wykes P: Improving the care of patients
with a hip fracture: A quality improvement report. BMJ Qual Saf.
24:532–538. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heyes GJ, Tucker A, Marley D and Foster A:
Predictors for 1-year mortality following hip fracture: A
retrospective review of 465 consecutive patients. Eur J Trauma
Emerg Surg. 43:113–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heyes GJ, Tucker A, Marley D and Foster A:
Predictors for readmission up to 1 year following hip fracture.
Arch Trauma Res. 4:e271232015.PubMed/NCBI
|
|
7
|
Boylan MR, Rosenbaum J, Adler A, Naziri Q
and Paulino CB: Hip fracture and the weekend effect: Does weekend
admission affect patient outcomes? Am J Orthop (Belle Mead NJ).
44:458–464. 2015.PubMed/NCBI
|
|
8
|
Clague JE, Craddock E, Andrew G, Horan MA
and Pendleton N: Predictors of outcome following hip fracture.
Admission time predicts length of stay and in-hospital mortality.
Injury. 33:1–6. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lo IL, Siu CW, Tse HF, Lau TW, Leung F and
Wong M: Pre-operative pulmonary assessment for patients with hip
fracture. Osteoporos Int. 21 Suppl 4:S579–S586. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vogiatzis I and Zakynthinos S: Factors
limiting exercise tolerance in chronic lung diseases. Compr
Physiol. 2:1779–1817. 2012.PubMed/NCBI
|
|
11
|
Sathiyakumar V, Avilucea FR, Whiting PS,
Jahangir AA, Mir HR, Obremskey WT and Sethi MK: Risk factors for
adverse cardiac events in hip fracture patients: An analysis of
NSQIP data. Int Orthop. 40:439–445. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gan L, Zhong J, Zhang R, Sun T, Li Q, Chen
X and Zhang J: The immediate intramedullary nailing surgery
increased the mitochondrial DNA release that aggravated systemic
inflammatory response and lung injury induced by elderly hip
fracture. Mediators Inflamm. 2015:5873782015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gan L, Chen X, Sun T, Li Q, Zhang R, Zhang
J and Zhong J: Significance of serum mtDNA concentration in lung
injury induced by hip fracture. Shock. 44:52–57. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McIlroy DJ, Jarnicki AG, Au GG, Lott N,
Smith DW, Hansbro PM and Balogh ZJ: Mitochondrial DNA neutrophil
extracellular traps are formed after trauma and subsequent surgery.
J Crit Care. 29(1133): e1–e5. 2014.
|
|
15
|
Tranah GJ, Nalls MA, Katzman SM, Yokoyama
JS, Lam ET, Zhao Y, Mooney S, Thomas F, Newman AB, Liu Y, et al:
Mitochondrial DNA sequence variation associated with dementia and
cognitive function in the elderly. J Alzheimers Dis. 32:357–372.
2012.PubMed/NCBI
|
|
16
|
Zhong N, Zhang Y, Zhu HF and Zhou ZN:
Intermittent hypoxia exposure prevents mtDNA deletion and
mitochondrial structure damage produced by ischemia/reperfusion
injury. Sheng Li Xue Bao. 52:375–380. 2000.PubMed/NCBI
|
|
17
|
Zheng H, Liu Y, Huang T, Fang Z, Li G and
He S: Development and characterization of a rat model of chronic
obstructive pulmonary disease (COPD) induced by sidestream
cigarette smoke. Toxicol Lett. 189:225–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun T, Wang X, Liu Z, Liu S and Zhang J:
Patterns of cytokine release and evolution of remote organs from
proximal femur fracture in COPD rats. Injury. 42:825–832. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen PX and Moldoveanu SC: Mainstream
smoke chemical analyses for 2R4F kentucky reference cigarette.
Beiträge Zur Tabakforschung International/Contributions to Tobacco
Research. 20:448–458. 2014.
|
|
20
|
Ellinger J, Müller DC, Müller SC, Hauser
S, Heukamp LC, von Ruecker A, Bastian PJ and Walgenbach-Brunagel G:
Circulating mitochondrial DNA in serum: A universal diagnostic
biomarker for patients with urological malignancies. Urol Oncol.
30:509–515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pape HC: Effects of changing strategies of
fracture fixation on immunologic changes and systemic complications
after multiple trauma: damage control orthopedic surgery. J Orthop
Res. 26:1478–1484. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin HH, Murray M, Cohen T, Colijn C and
Ezzati M: Effects of smoking and solid-fuel use on COPD, lung
cancer, and tuberculosis in China: A time-based, multiple risk
factor, modelling study. Lancet. 372:1473–1483. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rossini M, Viapiana O, Adami S, Idolazzi
L, Buda S, Veronesi C, Esposti Degli L and Gatti D: Medication use
before and after hip fracture: A population-based cohort and
case-control study. Drugs Aging. 31:547–553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nettleman MD, Alsip J, Schrader M and
Schulte M: Predictors of mortality after acute hip fracture. J Gen
Intern Med. 11:765–767. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Luise C, Brimacombe M, Pedersen L and
Sørensen HT: Chronic obstructive pulmonary disease and mortality
following hip fracture: A population-based cohort study. Eur J
Epidemiol. 23:115–122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hirose J, Mizuta H, Ide J and Nomura K:
Evaluation of estimation of physiologic ability and surgical stress
(E-PASS) to predict the postoperative risk for hip fracture in
elder patients. Arch Orthop Trauma Surg. 128:1447–1452. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pape HC, van Griensven M, Rice J, Gänsslen
A, Hildebrand F, Zech S, Winny M, Lichtinghagen R and Krettek C:
Major secondary surgery in blunt trauma patients and perioperative
cytokine liberation: Determination of the clinical relevance of
biochemical markers. J Trauma. 50:989–1000. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pape HC, Grimme K, Van Griensven M, Sott
AH, Giannoudis P, Morley J, Roise O, Ellingsen E, Hildebrand F,
Wiese B, et al: Impact of intramedullary instrumentation versus
damage control for femoral fractures on immunoinflammatory
parameters: Prospective randomized analysis by the EPOFF Study
Group. J Trauma. 55:7–13. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scalea TM: Optimal timing of fracture
fixation: Have we learned anything in the past 20 years? J Trauma.
65:253–260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Harwood PJ, Giannoudis PV, van Griensven
M, Krettek C and Pape HC: Alterations in the systemic inflammatory
response after early total care and damage control procedures for
femoral shaft fracture in severely injured patients. J Trauma.
58:446–454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang JZ, Liu Z, Liu J, Ren JX and Sun TS:
Mitochondrial DNA induces inflammation and increases TLR9/NF-κB
expression in lung tissue. Int J Mol Med. 33:817–824. 2014.
View Article : Google Scholar : PubMed/NCBI
|