Introduction
Cerebral ischemia is a life-threatening disease with
high morbidity and mortality rates worldwide. As the pathogenesis
of stroke is very complex, there are currently no clinically
effective therapies, making the need to develop new therapies ever
more urgent. Endoplasmic reticulum stress (ERS) and autophagy
activation play important roles in cerebral ischemia/reperfusion
(I/R) injury, which is a double-edged sword as their activation
will affect the fate of neurons following I/R injury (1). These mechanisms both help to clean up
damaged organelles and promote energy and molecule recycling.
However, their activation can also induce cell death, for example
by apoptosis, which is a key mechanism that leads to cell death
following cerebral ischemia (2).
Several lines of evidence suggest that there is communication
between ER stress and autophagy (3).
They are regarded as new therapeutic targets for ischemic stroke
and other central nervous system diseases.
The neuroprotective effects of Jingzhi Qingkailing
(JZQKL) injection, which consists of cholic acid, hyodeoxycholic
acid, baicalin, and jasminoidin, and was developed from a famous
anti-cerebral ischemia Chinese medicine, Qingkailing (QKL), are
well established (4). Our previous
studies have highlighted the neuroprotective effects of Geniposide
and cholic acid compositions, especially Tauroursodeoxycholic acid
(TUDCA) (5). Geniposide and TUDCA
are pharmacologically active compounds purified from the two
Chinese herbs Gardenia jasminoides Ellis and Bezoar,
respectively, which have been used for the treatment of stroke for
thousands of years (6,7). Recent data suggest that Geniposide may
be induce a wide range of biological processes, and thus likely
exerts its neuroprotective effects through a variety of mechanisms.
For example, Geniposide has been shown to protect neurons from
oxidative damage by increasing the expression of anti-apoptotic
proteins, including Bcl-2 and heme oxygenase-1 (HO-1) (8). Geniposide has also been demonstrated to
increase adenosine triphosphate (ATP) generation, mitochondrial
membrane potential (MMP), cytochrome c oxidase (CcO) and caspase-3
and −9 activity, reduce reactive oxygen species (ROS) production
and cytochrome c leakage, as well as inhibit apoptosis (9). Finally, some studies have shown that
Geniposide exerts its neuroprotective effects in vivo by
inhibiting inflammation and ameliorating amyloid pathology
(10). Moreover, it has been shown
to improve cognition (10). TUDCA,
an endogenous bile acid, is formed by the conjugation of
ursodeoxycholic acid (UDCA) with taurine. TUDCA has been shown to
have neuroprotective effects in a variety of experimental systems,
including models of neurodegenerative disorders such as Alzheimer's
disease and Huntington's disease (11–13). In
addition, it has been shown to protect against damage induced by
ischemia and hemorrhagic stroke (12,14). The
molecular mechanisms underlying the neuroprotective effects of
TUDCA appear to be complex and may engage a number of different
molecular targets, possibly involved in gene regulation, resulting
in robust anti-apoptotic, anti-inflammatory, immunomodulatory, and
antioxidant effects (15,16).
In the clinical practice of Traditional Chinese
Medicine (TCM), Gardenia jasminoides Ellis and Bezoar are
always used together in one formula, such as in Niuhuang Shangqing
or Angong Niuhuang pills, to treat neurovascular diseases (17,18). The
neuroprotective effects of Geniposide have been well established,
and our previous studies have shown that the effective components
of Bezoar alleviate cerebral ischemic injury by inhibiting ERS
(5). Moreover, TUDCA has been shown
to be more effective than taurine and UDCA both in vivo and
in vitro (19). However, the
synergistic effects of the two components combined remains to be
fully elucidated. In the present study, we assessed the
cytoprotective potential of Geniposide alone or in combination with
TUDCA against OGD/R-induced injury to SH-SY5Y cells.
Materials and methods
Cell culture
SH-SY5Y cells were were obtained Shanghai Institute
of Biochemistry and Cell Biology (Shanghai, China), cultured in
Dulbecco's Modified Eagle's Medium (DMEM) containing 10% (v/v)
fetal bovine serum (Invitrogen, Carlsbad, CA, USA), and maintained
in 5% (v/v) CO2/90% (v/v) humidity at 37°C. Cells were
plated onto 96-well culture plates at a density of 1×104
cells/well for the cytotoxicity assays, or 60-mm culture dishes at
a density of 1×106 cells/well for western blot, qPCR and
flow cytometry. After 24 h, the culture medium was replaced with
serum-free DMEM supplemented with 0.12 mM formaldehyde, and
different concentrations of drugs were used to treat the cells as
indicated in the results section.
Oxygen-glucose deprivation and
reoxygenation procedure
OGD/R was performed with the AnaeroPack system,
which includes a rectangular container (9.5×6.75×3.25 in; 2.5 l)
and one AnaeroPack sachet. Briefly, SH-SY5Y cells were plated in
DMEM. After treatment, the cells were incubated in the sachet with
a BBL disposable anaerobic indicator strip and placed into the
container. After 60 min of incubation, the oxygen concentration was
less than 1% and the CO2 concentration was approximately
18%. After 12 h, the cells were incubated for 2 h for reoxygenation
and glucose restoration. In the normoxia control group, the cells
were cultured with DMEM containing glucose under normal
conditions.
Cell viability assessment
The viability of SH-SY5Y cells was determined using
a CCK-8 cell viability test Kit (CK04; Dojindo Laboratories,
Kumamoto, Japan). Briefly, 10 µl per well CCK-8 solution was added
to the culture medium. The absorbance value (A) was measured at 450
nm using a spectrophotometer (Multiskan FC; Thermo Fisher
Scientific, Waltham, MA, USA). The percentage of viable cells was
calculated using the following formula: cell viability (%)=(A of
experiment well/A of control well) ×100%.
LDH assay
SH-SY5Y cells were cultured in a 96-well culture
plate at a density of 4.0×103 cells/cm2 for
24 h. Each treatment group consisted of a set of six wells. The
medium was removed from each well post-treatment. The media samples
were centrifuged at 1,000 rpm for 1 min, and the supernatants were
collected and subsequently used in the LDH assay, which was
performed according to the manufacturer's instructions.
Quantitative PCR analysis
Total RNA was extracted from cells lysed using
TRizol reagent (Takara Bio, Dalian, China) per the manufacturer's
instructions (19). The quality and
quantity of the RNA purity were assessed using a spectrophotometer
and standard electrophoresis. cDNA was synthesized from 1 µg RNA
and reverse transcribed using a PrimerScript™ RT reagent kit
(Takara Bio). The mRNA expression levels were quantified using a
TaqMan™ Assay kit (Applied Biosystems Life Technologies, Foster
City, CA, USA) in accordance with the manufacturer's instructions.
The primer sequences used for CHOP were as follows:
5′-CACTCTTGACCTGCTTC-3′ (forward) and 5′-AGTCGCCTCTACTTCCCT-3′
(reverse). The product size was approximated at 307 base pairs
(bp). The primers for beclin-1 were as follows:
5′-CGTGGAGAAAGGCAAGATT-3′ (forward) and 5′-AGAACTGTGAGGACACCCAAG-3′
(reverse). The product size was approximated at 152 bp. The primers
for glyceraldehyde-3-phosphatedehydrogenase (GAPDH), the internal
control, were as follows: 5′-CCATGGAGAAGGCTGGGG−3′ (forward) and
5′-GTCATCCATGACAACTTTG-3′ (reverse). The product size was
approximated at 195 bp. Each reaction was performed in triplicate
using an ABI 7500 fast RT- PCR system. Gene expression levels were
calculated based on the threshold cycle (Cq) value. The relative
mRNA expression levels in the samples were assessed using the
comparative delta-delta Cq method (TaqMan Relative Quantification
Assay software), adjusted to the mRNA expression level of
GAPDH.
Flow cytometry analysis
Flow cytometry was used to assess the percentage of
apoptotic cells in the different treatment groups. The cells were
stained using an Annexin V-FITC/PI double staining kit (AD10;
Dojindo Laboratories) per the manufacturer's instructions.
Detection of ROSusing the
dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay and flow
cytometry
SH-SY5Y cells were seeded into 6-well culture plates
(4.0×103 cells/cm2) and cultured for 24 h.
The medium was removed from the wells after treatment. The cells
were rinsed once with 0.1 M phosphate buffered saline (PBS) and
passaged using 0.25% trypsinization for 2 min. Cells were collected
in centrifuge tubes and centrifuged at 1,500 rpm for 5 min, and the
supernatants were discarded. DCFH-DA (200 µl) was added to each
tube for 20 min at 37°C in the absence of light. A keratinocyte
serum-free medium (500 µl) was added to rinse the cells, and cells
were centrifuged at 1,000 rpm for 5 min. The supernatants were
discarded, and 200 µl keratinocyte serum-free medium were added to
the cells for testing.
Western blot analysis
Total protein was extracted using a kit (KGP250;
Nanjing KeyGen Biotech Co., Ltd., Nanjing, China). Whole cell
lysates were separated by 10–15% sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred
to a nitrocellulose membrane. The membrane was blocked with 5%
skimmed milk powder in Tris-buffered saline-Tween-20 (TBS-T; 0.1%
Tween-20 in TBS) for 1 h at room temperature and incubated
overnight at 4°C with antibodies against Beclin-1 (1:1,000,
ab62557; Abcam, Cambridge, UK), CHOP (1:1,000, ab11419; Abcam), and
β-actin (1:2,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA)
followed by incubation with horseradish peroxidase-conjugated goat
anti-rabbit IgG antibody (1:3,000; Proteintech Group Inc., Hubei,
China). Immunoreactive bands were visualized using a
chemiluminescence kit (ECL kit; Santa Cruz Biotechnology, Inc.),
and protein bands were scanned using Chemi Imager 5500 V2.03
software. The integrated density value (IDV) for each band was
calculated with a computer-aided image analysis system (Fluor Chen
2.0).
Statistical analysis
All data were expressed as means ± standard
deviation (SD). Data were analyzed using a one-way analysis of
variance (ANOVA) followed by a Bonferroni correction for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
The synergistic effects of geniposide
and TUDCA on SH-SY5Y cell viability
To investigate the synergistic protective effects of
Geniposide and TUDCA against OGD/R (oxygen glucose
deprivation-reoxygenation)-induced cell death, a Cell Count Kit-8
(CCK-8) assay was used to assess cellular viability in SH-SY5Y
cells. We observed a concentration-dependent reduction in
OGD/R-induced injury with increasing concentrations of Geniposide
(Fig. 1A). A neuroprotective effect
was observed following treatment with TUDCA at a concentration of
25 µg/ml only (P<0.05; Fig. 1B).
Treatment with a combination of Geniposide and TUDCA (GT) at a
constant 20:1 Geniposide:TUDCA ratio increased the proliferation of
SH-SY5Y cells (P<0.001) more than Geniposide alone following
OGD/R treatment (Fig. 1C).
Therefore, these concentrations were used in further experiments in
combination. However, no statistically significant decreases in
lactate dehydrogenase (LDH) activity were observed with these
agents when compared to cells treated with OGD/R alone (data not
shown).
Synergistic anti-apoptotic effects of
geniposide and TUDCA
To investigate the synergistic effects of combined
treatment with GT on OGD/R-induced SH-SY5Y cell injury, we examined
apoptosis in SH-SY5Y cells treated with Annexin V-FITC/PI using
flow cytometry (Fig. 2). As
expected, the Annexin V/PI flow cytometric apoptosis assay showed
that early apoptotic cells were PI−/Annexin
V+, and late apoptotic cells were PI+/Annexin
V+. Most of the cells in the control group were
surviving cells. However, following OGD/R treatment, the number of
surviving cells decreased remarkably, and more apoptotic cells were
detected. As shown in Fig. 3B, the
percentage of SH-SY5Y cells at an early stage of apoptosis
increased from 8.10±1.27 to 11.60±1.56% (P<0.01), and the
percentage at a late stage increased from 12.80±7.35 to 29.30±2.26%
(P<0.001) in the OGD/R treated group when compared with those in
the control group. In contrast, pretreatment with 100 nl/ml JZQKL
suppressed early and late stage apoptosis rates to 10.45±2.45%
(P<0.05) and 25±9.76% (P<0.01), respectively. Pretreatment
with 100 µg/ml Geniposide, or a combination of 100 µg/ml Geniposide
and 5 µg/ml TUDCA (GT) suppressed the early stage apoptosis rates
to 11.1±0.71% (P<0.05) and 9.5±2.12% (P<0.001), respectively.
Meanwhile, the percentage of late stage apoptotic cells was reduced
to 24.6±0.85% (P<0.01) and 22.95±0.21% (P<0.001),
respectively. Thus, the results from the flow cytometry analyses
indicate that GT inhibits OGD/R-induced apoptosis better than
Geniposide alone although no statistic difference was shown.
Effect of GT on intracellular ROS in
SH-SY5Y cells
The levels of ROS were analyzed using flow cytometry
by incubating SH-SY5Y cells with the fluorescent dye CM-H2DCFDA
(Fig. 3). OGD/R-induced injury
significantly increased intracellular ROS levels in SH-SY5Y cells
relative to the levels observed in control cells (P<0.001). The
OGD/R-induced increase in ROS was suppressed by GT (P<0.01). In
this experiment 100 µM NAC was used as a positive control.
Effect of GT on ER stress and
autophagy-related apoptosis
To examine ER stress and autophagy-related protein
changes, western blot and real-time polymerase chain reaction
(RT-PCR) analyses were carried out to determine the expression
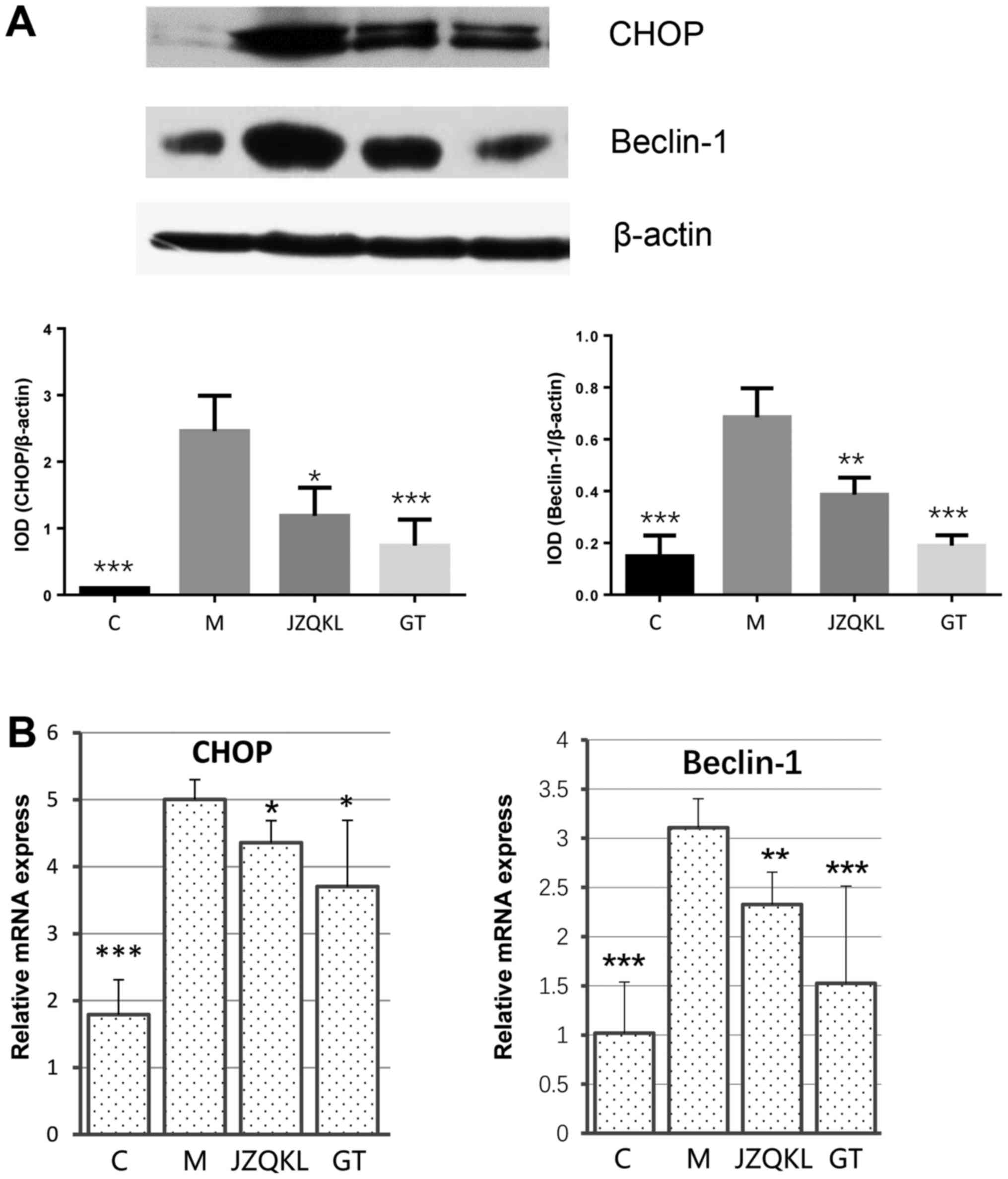
levels of C/EBP homology protein (CHOP) and beclin 1. As shown in
Fig. 4, ODG/R altered CHOP and
beclin 1 mRNA levels. JZQKL and GT both dramatically reduced
OGD/R-induced CHOP (P<0.05, P<0.05) and beclin 1 (P<0.01,
P<0.001) mRNA levels. The protein levels of CHOP (P<0.001)
and beclin 1 (P<0.001) were reduced in the presence of GT. These
results suggest a mechanism by which combined treatment with
Geniposide and TUDCA might inhibit OGD/R-induced changes in CHOP
and beclin 1 levels.
Discussion
Pharmacodynamic constituents from natural medicines
have been investigated for the treatment of ischemic stroke.
Multi-component treatments, characterized by the combination of two
or more agents that interact with multiple targets simultaneously,
are considered to be rational and efficient forms of therapy for
the treatment of complex diseases (19). According to the results obtained from
the CCK-8 and LDH assays, proteins secreted from OGD/R-activated
SH-SY5Y cells are involved in the neuronal cell damage observed
following treatment, indicating that OGD/R induces neurotoxicity in
SH-SY5Y cells. Here, Geniposide had neuroprotective effects at all
concentrations examined, while TUDCA was found to have
neuroprotective effects at 25 µg/ml only. Other groups have found
geniposide inhibits H/R-induced myocardial apoptosis by reversing
mitochondrial dysfunction (20),
protects rat insulinoma cells from apoptosis in high-glucose
concentrations (21) and attenuate
Aβ-induced and rotenone-induced neuronal injury by inhibiting
mitochondrial dysfunction and oxidative stress (9,22).
Interestingly, a synergistic protective effect of Geniposide and
TUDCA (GT) was observed following OGD/R-induced SH-SY5Y cell
injury, especially at a constant 20:1 Geniposide:TUDCA ratio.
However, GT had no effect on LDH leakage, suggesting that GT
improved SH-SY5Y cell survival through pathways other than those
involving LDH.
Apoptosis occurs in many multicellular organisms and
arises as part of the normal physiological process in the body
(23). Our data show that GT
inhibited both early and late stage apoptosis, and was more
effective than JZQKL or Geniposide alone. Oxidative stress during
neuronal I/R occurs because of the excessive generation or
accumulation of free radicals or their oxidation products (24). Excessive ROS destroys mitochondrial
membrane integrity, leading to cytochrome c and apoptosis-inducing
factor (AIF) release, caspase activation, and ultimately apoptosis
(25). Our data showed that combined
treatment with Geniposide and TUDCA decreases OGD/R-induced ROS
levels, which means ROS may be a potential target for this
combination.
The ER performs several functions, including protein
folding and transport, and regulation of intracellular calcium
concentrations (26). Cells trigger
the unfolded protein response (UPR) as a self-protective mechanism
upon disruption of ER functions via the accumulation of
unfolded/misfolded proteins in the ER. ER stress-mediated apoptosis
is triggered by the induction of CHOP (27). In the present study, GT induced the
protein and gene expression of CHOP better than JZQKL, suggesting
an anti-ER stress-related role in conditions of OGD/R. Autophagy is
a cellular defense mechanism that involves the degradation and
recycling of cytoplasmic constituents (28). Beclin 1 is an important marker of the
stage of the autophagy process (29). Our data show that GT induces the
protein and gene expression of beclin 1, suggesting a potential
anti-autophagy effect of GT. In conclusion, our results showcase
the potential of the combined use of Geniposide and TUDCA to
modulate the toxic effects resulting from OGD/R injury. GT
treatment may have potential anti-apoptotic, anti-oxidative,
anti-ER stress, and anti-autophagy effects. Thus, the use of a
multi-targeted treatment approach may be beneficial for the
treatment of complex diseases.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (81430102, 81373886 and
81303260) and the Classical Prescription Basic Research Team of the
Beijing University of Chinese Medicine. The authors thank Elsevier
Language Services for providing language assistance and for
proofreading the manuscript.
References
|
1
|
Fan YY, Hu WW, Nan F and Chen Z:
Postconditioning-induced neuroprotection, mechanisms and
applications in cerebral ischemia. Neurochem Int. 107:43–56. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Portt L, Norman G, Clapp C, Greenwood M
and Greenwood MT: Anti-apoptosis and cell survival: A review.
Biochim Biophys Acta. 1813:238–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kong FJ, Wu JH, Sun SY and Zhou JQ: The
endoplasmic reticulum stress/autophagy pathway is involved in
cholesterol-induced pancreatic β-cell injury. Sci Rep. 7:447462017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng F, Zhong X, Lu Y, Wang X, Song W,
Guo S, Wang X, Liu D and Wang Q: Refined qingkailing protects MCAO
mice from endoplasmic reticulum stress-induced apoptosis with a
broad time window. Evid Based Complement Alternat Med.
2012:5678722012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu XL, Ma CY, Wang XQ, Wang GL, Zhai CM,
Yue WC, Li CX, Zhang XY, Shen XD, Mu J, et al: Comparative study of
cholic acid compounds of bezoar on anti-cerebral infarction and
regulating endoplasmic reticulum stress. Drug Evaluation Res.
40:11–19. 2017.(In Chinese).
|
|
6
|
Cao K and Tong CW: Research progress of
pharmacological effects and application of pine needle. J Mianyang
Norm Univ. 2015.(In Chinese).
|
|
7
|
Li C, Pan Y and Jia X: Effects of Huangqin
(dried root of scutellaria baicalensis) and Zhizi (dried fruit of
Gardenia jasminoides) used in combination on ischemic cascade
reaction in the rat model of focal cerebral ischemia and
reperfusion. J Beijing Univ Tradit Chin Med. 2002.
|
|
8
|
Liu J, Yin F, Zheng X, Jing J and Hu Y:
Geniposide, a novel agonist for GLP-1 receptor, prevents PC12 cells
from oxidative damage via MAP kinase pathway. Neurochem Int.
51:361–369. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao C, Lv C, Li H, Du S, Liu X, Li Z, Xin
W and Zhang W: Geniposide protects primary cortical neurons against
oligomeric Aβ1-42-induced neurotoxicity through a mitochondrial
pathway. PLoS One. 11:e01525512016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lv C, Wang L, Liu X, Yan S, Yan SS, Wang Y
and Zhang W: Multi-faced neuroprotective effects of geniposide
depending on the RAGE-mediated signaling in an Alzheimer mouse
model. Neuropharmacology. 89:175–184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Keene CD, Rodrigues CM, Eich T, Chhabra
MS, Steer CJ and Low WC: Tauroursodeoxycholic acid, a bile acid, is
neuroprotective in a transgenic animal model of Huntington's
disease. Proc Natl Acad Sci USA. 99:pp. 10671–10676. 2002;
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rodrigues CM, Sola S, Nan Z, Castro RE,
Ribeiro PS, Low WC and Steer CJ: Tauroursodeoxycholic acid reduces
apoptosis and protects against neurological injury after acute
hemorrhagic stroke in rats. Proc Natl Acad Sci USA. 100:pp.
6087–6092. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fernández-Sánchez L, Lax P, Pinilla I,
Martín-Nieto J and Cuenca N: Tauroursodeoxycholic acid prevents
retinal degeneration in transgenic P23H rats. Invest Ophthalmol Vis
Sci. 52:4998–5008. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rodrigues CM, Spellman SR, Solá S, Grande
AW, Linehan-Stieers C, Low WC and Steer CJ: Neuroprotection by a
bile acid in an acute stroke model in the rat. J Cereb Blood Flow
Metab. 22:463–471. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amaral JD, Viana RJ, Ramalho RM, Steer CJ
and Rodrigues CM: Bile acids: Regulation of apoptosis by
ursodeoxycholic acid. J Lipid Res. 50:1721–1734. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gaspar JM, Martins A, Cruz R, Rodrigues
CM, Ambrósio AF and Santiago AR: Tauroursodeoxycholic acid protects
retinal neural cells from cell death induced by prolonged exposure
to elevated glucose. Neuroscience. 253:380–388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong XM, Ren XC, Lou YL, Chen MJ, Li GZ,
Gong XY and Huang Z: Effects of in-vitro cultured calculus bovis on
learning and memory impairments of hyperlipemia vascular dementia
rats. J Ethnopharmacol. 192:390–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fu WJ, Lei T, Yin Z, Pan JH, Chai YS, Xu
XY, Yan YX, Wang ZH, Ke J, Wu G, et al: Anti-atherosclerosis and
cardio-protective effects of the Angong Niuhuang Pill on a high fat
and vitamin D3 induced rodent model of atherosclerosis. J
Ethnopharmacol. 195:118–126. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Hou J, Lei H, Fu J, Pan Y and Liu
J: Synergistic neuroprotective effect of microglial-conditioned
media treated with geniposide and ginsenoside Rg1 on hypoxia
injured neurons. Mol Med Rep. 12:5328–5334. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang YQ, Chang GL, Wang Y, Zhang DY, Cao
L and Liu J: Geniposide prevents hypoxia/reoxygenation-induced
apoptosis in H9c2 cells: Improvement of mitochondrial dysfunction
and activation of GLP-1R and the PI3K/AKT signaling pathway. Cell
Physiol Biochem. 39:407–421. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo LX, Liu JH, Zheng XX, Yin ZY, Kosaraju
J and Tam KY: Geniposide improves insulin production and reduces
apoptosis in high glucose-induced glucotoxic insulinoma cells. Eur
J Pharm Sci. pii:S0928-0987(17)30173-2. 2017.
|
|
22
|
Li L, Zhao J, Liu K, Li GL, Han YQ and Liu
YZ: Geniposide prevents rotenone-induced apoptosis in primary
cultured neurons. Neural Regen Res. 10:1617–1621. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gordeziani M, Adamia G, Khatisashvili G
and Gigolashvili G: Programmed cell self-liquidation (apoptosis).
Ann Agrarian Sci. 15:148–154. 2017. View Article : Google Scholar
|
|
24
|
Narne P, Pandey V and Phanithi PB:
Interplay between mitochondrial metabolism and oxidative stress in
ischemic stroke: An epigenetic connection. Mol Cell Neurosci.
82:176–194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu TS, Liao YC, Yu FY, Chang CH and Liu
BH: Mechanism of patulin-induced apoptosis in human leukemia cells
(HL-60). Toxicol Lett. 183:105–111. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ogen-Shtern N, Ben David T and Lederkremer
GZ: Protein aggregation and ER stress. Brain Res. 1648:658–666.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Senft D and Ronai ZA: UPR, autophagy, and
mitochondria crosstalk underlies the ER stress response. Trends
Biochem Sci. 40:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Towers CG and Thorburn A: Therapeutic
targeting of autophagy. EBioMedicine. 14:15–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maejima Y, Isobe M and Sadoshima J:
Regulation of autophagy by beclin 1 in the heart. J Mol Cell
Cardiol. 95:19–25. 2016. View Article : Google Scholar : PubMed/NCBI
|