Introduction
Diabetes mellitus is associated with an increased
risk of ischemic cardiovascular diseases, including coronary heart
disease and stroke, which seriously jeopardize human health
(1). The mechanism of this
association is complicated. Vascular injury, occlusion or
degradation caused by endothelial dysfunction in hyperglycemia may
serve an important role. Accumulating evidence suggests that
circulating endothelial progenitor cells (EPCs) can promote
reendothelialization and restore endothelial function (2,3).
Nevertheless, the number of EPCs is decreased in type I and type II
diabetes (4,5). In addition, EPC functions, such as
proliferation, migration and angiogenesis, are severely suppressed
in diabetes (6,7). Therefore, ameliorating the impaired
functions of EPCs is urgent and critical for EPC-based cell
therapy, particularly with regard to ischemic cardiovascular
disease associated with diabetes.
Thymosin β4 (Tβ4) is a pleiotropic peptide serving
various roles in cell migration (8,9),
angiogenesis (10), apoptosis
(11) and inflammation (12). Recent studies have suggested that Tβ4
can exert cardiovascular protective effects by enhancing the
proliferation, migration and angiogenesis of a variety of stem or
progenitor cells (9,13–15).
Previous studies by the authors of the present study demonstrated
that Tβ4 could promote EPC migration, prevent EPCs from serum
deprivation-induced apoptosis and reduce the senescence of EPCs
in vitro (11,16,17).
However, whether Tβ4 can improve glucose-impaired EPC function, and
the underlying mechanism associated with this, remain unclear. In
there present study, the effect of Tβ4 on glucose-impaired EPC
function is investigated, and the potential signaling pathway
involved is discussed.
Materials and methods
Cell culture
Mononuclear cells were isolated from the peripheral
blood of healthy donors by density-gradient centrifugation using
Ficoll separating solution (Cedarlane Laboratories, Ltd., Hornby,
Canada). Then, 1×107 mononuclear cells were plated on
fibronectin (Chemicon, Temecula, CA, USA)-coated plates and
incubated with endothelial growth medium-2 (EGM-2 MV; Clonetics,
Walkersville, MD, USA). After 3 days of culturing, non-adherent
cells were removed by washing with phosphate buffered saline (PBS;
0.2 M, pH 7.4) and new medium was applied. A number of cells grew
into EPCs which exhibited a ‘cobblestone’ shape after 3–4 weeks.
The EPCs were detached by trypsin and collected for the following
experiments.
EPC migration assay
EPC migration was assessed using a transwell
migration assay (3422; Costar, Cambridge, MA, USA). A total of
5×104 cells were re-suspended in 100 µl serum-free
endothelial basal medium-2 (EBM-2; Clonetics) and placed in the
upper chambers of 24-well transwell plates with 8 µm-pore
membranes. Then, 600 µl EGM-2 supplemented with 10% bovine serum
albumin (Amresco LLC, Solon, OH, USA) was placed in lower chambers.
After 6 h of incubation at 37°C, the upper side of membrane was
wiped gently with cotton swabs to remove the cells that had not
migrated. The migrated cells were fixed in 4% paraformaldehyde and
stained using 4′6-diamidino-2-phenylindole (Roche Applied Science,
Indianapolis, IN, USA). Migrated cells were counted with a
fluorescence microscope at ×400 magnification and 5 random fields
were picked for counting. The average cell number of these 5 fields
was considered as the cell migration number for each group.
EPC tube formation assay
Tube formation was performed with Matrigel matrix
basement membrane (BD Biosciences, Franklin Lakes, NJ, USA) to
evaluate the ability of EPC angiogenesis. Matrigel solution was
thawed at 4°C overnight, diluted with EBM-2 and added in a 96-well
plate at 37°C for 1 h to solidify. EPCs were collected with trypsin
and re-suspended with EGM-2 MV medium, then 2×104 EPCs
were placed on the matrix for further incubation at 37°C for 6–8 h.
Tube formation was observed with a light microscope (magnification,
×100) and 5 random fields were chosen for each assay. The average
tube formation counted by branch point numbers was compared in
different groups by Image Pro Plus version 6.0 (Media Cybernetics,
Warrendale, PA, USA).
Small interfering RNA (siRNA)
transfection
siRNA against Akt and eNOS were synthesized by
GenePharma Co., Ltd. (Shanghai, China) and a nonsense sequence was
designed as a negative control. The effective Akt siRNA sequence
was as follows: Sense, 5′-GCACUUUCGGCAAGGUGAUTT-3′ and antisense,
5′-AUCACCUUGCCGAAAGUGCTT-3′. The effective eNOS siRNA sequence was
as follows: Sense, 5′-CAGUACUACAGCUCCAUUATT-3′ and antisense,
5′-UAAUGGAGCUGUAGUACUGTT-3′. Then, 4×105 EPCs were
placed in 6-well plates, and when cells reached 60–80% confluence,
they were transfected with Akt and eNOS siRNA and cultured for 24
h, then incubated with a high concentration of glucose (25 mM) for
4 days (except for the control group), and with or without Tβ4
(ProSpec-Tany TechnoGene, Ltd., Rehovot, Israel) for the last 24 h.
The effectiveness of Akt and eNOS knock-down was determined by
western blotting.
Western blots analysis and measurement
of nitric oxide (NO)
Total protein from EPCs was extracted with
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China). Protein concentration was measured
using a BCA assay kit (P0012; Beyotime Institute of Biotechnology).
Equal quantities of protein (20 µg) were run on a 10% tris-glycine
gradient gel, then transferred to polyvinylidene difluoride
membranes and blocked with 5% non-fat milk in Tris-buffered
solution containing 0.1% Tween 20 (TBST) for 1 h at 25°C. Membranes
were then incubated with primary antibodies at 4°C overnight and
with secondary antibodies at room temperature for 1–2 h. Membranes
were washed three times with TBST for 15 min before and after the
incubations. Finally, proteins were visualized with enhanced
chemiluminescence reagent (Lianke Biotechnology, Hangzhou, China)
and exposed to Image Quant LAS-4000 (Fujifilm, Tokyo, Japan).
Grayscale of the bands were measured by Multi-Gauge 3.0 image
analysis software (Fujifilm). Antibodies including
anti-phospho-Akt-Ser473 (1:1,000; 3787), anti-Akt
(1:1,000; 9272), anti-phospho-endothelial nitric oxide synthase
(eNOS)-Ser1177 (1:1,000; 9571), anti-eNOS (1:1,000;
9572) and horseradish peroxidase-conjugated goat anti-rabbit IgG
(1:5,000; 7074) were purchased from Cell Signaling Technology, Inc.
(Beverly, MA, USA). After incubation of EPCs with or without Tβ4 in
glucose for 4 days, the conditioned medium was examined for NO
using Griess regent (Beyotime Institute of Biotechnology).
Statistical analysis
All experiments were performed for at least three
individual experiments. Statistical analysis was performed using
SPSS version 19.0 software (IBM SPSS, Amronk, NY, USA), using
one-way analysis of variance nad post-hoc least standard difference
analysis. Results are presented as the mean ± standard error.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Tβ4 ameliorates glucose-impaired EPC
migration and angiogenesis
It was investigated whether the administration of
Tβ4 could improve glucose-impaired EPC functions. As shown in
Fig. 1A, incubation of EPCs with
medium with a high concentration of glucose (25 mM) for 4 days
could significantly suppress EPC migration (P<0.01 vs. the
control group). However, treatment with Tβ4 for 24 h after the
exposure of EPCs to high glucose conditions could improve
glucose-suppressed EPC migration in a concentration-dependent
manner; the maximal pro-migratory dose was observed at 1,000 ng/ml
(P<0.01 vs. the glucose group; Fig.
1A). In addition, high concentrations of glucose significantly
impaired EPC tube formation ability (P<0.01 vs. the control
group; Fig. 1B). Treatment with Tβ4
for 24 h after glucose exposure ameliorated glucose-suppressed EPC
tube formation ability in a concentration-dependent manner; the
maximal pro-angiogenic dose was observed at 1,000 ng/ml (P<0.01
vs. the glucose group; Fig. 1B).
Tβ4 restores glucose-inhibited
Akt/eNOS phosphorylation and NO production
High concentrations of glucose can inhibit Akt and
eNOS activation and reduce NO production in EPCs (18). A previous study indicated that Tβ4
may activate the phosphorylation of Akt and eNOS (16,17).
Therefore, the effects of Tβ4 on glucose-exposed EPCs were
investigated in order to determine whether Tβ4 can restore
glucose-inhibited Akt and eNOS phosphorylation in EPCs. As shown in
Fig. 2A, high concentrations of
glucose (25 mM) dramatically down-regulated the phosphorylation of
Akt at Ser473 and eNOS at Ser1177 in EPCs,
while there was no significant change in total Akt and eNOS
expression. The inhibition of eNOS phosphorylation led to reduced
NO production of EPCs (Fig. 2B).
However, administration of Tβ4 in the glucose medium for 24 h
concentration-dependently upregulated the phosphorylation of Akt
and eNOS, and augmented NO production (Fig. 2).
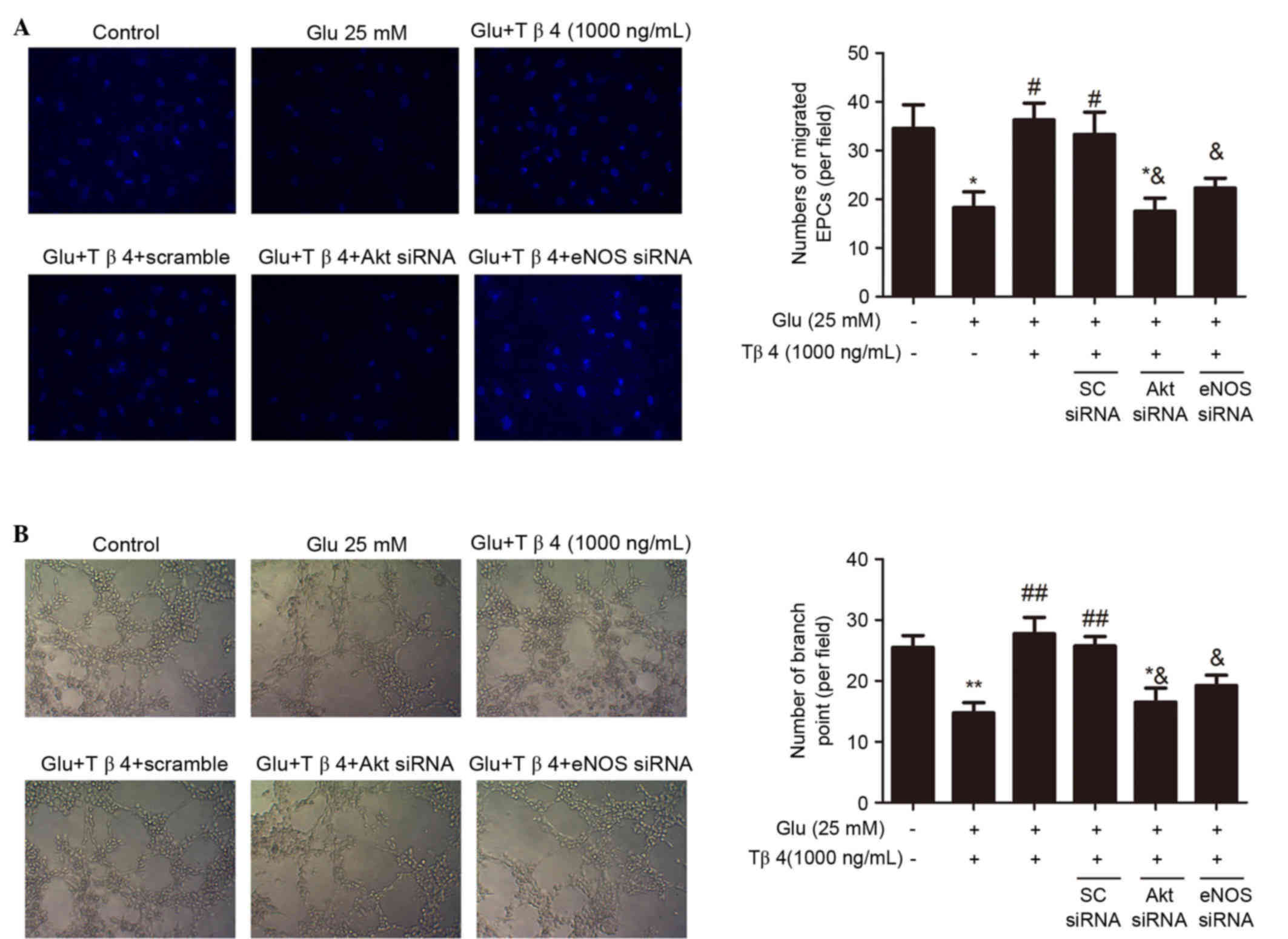
Akt/eNOS signaling pathway is involved
in the amelioration of glucose-impaired EPC functions by Tβ4
To identify the role of the Akt/eNOS signaling
pathway in the beneficial effect of Tβ4 on glucose-impaired EPC
functions, EPCs were transfected with Akt siRNA and eNOS siRNA to
decrease Akt and eNOS activity, respectively. As shown in Fig. 3, administration of Akt and eNOS siRNA
(50 nM) markedly inhibited the benefit of Tβ4 in improving EPC
migration and tube formation, suggesting that the Akt/eNOS pathway
accounts for Tβ4-induced improvement of glucose-suppressed EPC
functions.
Discussion
Previous studies performed by the authors of the
present study have demonstrated that Tβ4 may exert an important
role in enhancing EPC functions, including promoting migration,
inhibiting apoptosis and senescence (11,16,17).
However, the capacity of Tβ4 to reverse impaired EPC functions in
some pathological conditions remains unclear. The present study
demonstrated that Tβ4 could reverse glucose-impaired EPC functions
in a dose-dependent manner and elucidated that the beneficial
effect of Tβ4 may be associated with the Akt/eNOS signaling pathway
and NO-related mechanisms.
At present, diabetes mellitus has become a common
diseases worldwide, which is associated with poor clinical outcomes
and premature morality due to its cardiovascular complications
(19,20). Several studies have suggested that
the imbalance between endothelial injury and repair is a key
process of diabetes-associated cardiovascular complications
(21,22). Endothelial function is impaired in
diabetes and the complication of vascular occlusion in diabetic
patients has been attributed to compromised collateral vessel
formation due to diminished capacity of mature endothelial cells
(1). EPCs have proliferating
potential, differentiating into endothelial cells and promoting
regeneration and angiogenesis of endothelial cells, which serves an
important role in maintaining endothelial function and integrity,
as well as post-natal neovascularization (23). Interestingly, emerging evidence has
showed that post-natal neovascularization in adults may be involved
with EPCs directly (24). Hence,
EPCs could be a potential method for cardiovascular therapy.
However, it has been revealed that the number and function of EPCs
may be reduced by several cardiovascular risk factors, such as
hypertension, hyperglycemia and hypercholesterolemia (25). Reduced numbers and suppressed
function of EPCs can be associated with endothelial dysfunction,
impaired angiogenesis and decreased compensational
collateralization in occlusive vascular diseases (26). Type I and type II diabetes are both
associated with decreased numbers and impaired function of EPCs.
Therefore, strategies to improve the suppressed functions of EPCs
is critical and beneficial for EPC-based cell therapy, particularly
in treating diabetic patients.
In our present study, the functions of circulating
EPCs is suppressed in high concentrations of glucose, and Tβ4 was
demonstrated to reverse the dysfunction of EPCs. Tβ4 is a
polypeptide originally isolated from calf thymus (27). Previous studies have observed that
Tβ4 can modulate various physiological and pathological process,
such as tissue development, wound healing, cell survival and vessel
formation (10,28–30).
Previous studies by the authors of the present study have
demonstrated that Tβ4 was able to enhance EPC migration, inhibit
EPC apoptosis and senescence in vitro (11,16). In
addition, Tβ4 has been reported to enhance the angiogenesis of
endothelial cells (31).
Furthermore, Tβ4 exerts excellent therapeutic effects against
glucose damage. Tβ4 is able to abolish glucose-suppressed
capillary-like tube formation of endothelial cells and promote the
recovery of neurological function in diabetic peripheral neuropathy
(32). Kim and Kwon found that Tβ4
could ameliorate glucose-injured high-glucose-injured human
umbilical vein endothelial cell function through the insulin-like
growth factor-1 signaling pathway (33). According to the evidence above, the
present study further revealed the beneficial effects of Tβ4 on
human EPCs in high concentrations of glucose, which may provide
novel insights into its potential application for cardiovascular
protection and therapy in clinical cases.
The Akt/eNOS signaling pathway has been reported to
be a critical pathway that regulates cell migration and
angiogenesis (34). As for EPCs,
previous research has demonstrated that exercise and some
medications, such as statins, could increase EPC number,
proliferation and migration through the activation of the Akt
protein (35). The stimulation of
Akt protein kinase can further activate eNOS (36), which is essential and pivotal for the
mobilization of stem and progenitor cells (37). A number of studies documented that
hyperglycemia could affect various functions of different kinds of
cells through the Akt/eNOS signaling pathway. Sun et al
(38) identified that vaspin, a type
of adipose factor, could alleviate EPC dysfunction caused by high
concentrations of glucose via the PI3K/Akt/eNOS signaling pathway.
Chen et al (18) indicated
that high concentrations of glucose impaired EPC proliferation,
migration and angiogenesis by down-regulating the phosphorylation
Akt and eNOS, and NO production (18). The authors of the present study have
previously revealed that Tβ4 promotes EPC migration and inhibits
EPC apoptosis through the PI3K/Akt/eNOS signaling pathway (11,16),
therefore, it was postulated that this pathway may account for the
Tβ4-mediated improvement of glucose-suppressed EPC functions.
In present study, it was demonstrated that treatment
with Tβ4 could provoke Akt and eNOS activity, and enhance the
migration and tube formation of EPCs in high concentrations of
glucose. These beneficial effects were blocked by the siRNA of Akt
and eNOS. These findings suggest that Tβ4 can ameliorate
glucose-impaired EPC functions through the Akt/eNOS signaling
pathway. According the findings above, Tβ4 may be beneficial for
maintaining EPC functions in high concentrations of glucose, which
is helpful for understanding the therapeutic potential of Tβ4 for
patients with diabetes and cardiovascular complications.
Acknowledgements
The present study was supported by the Special Major
Projects of Zhejiang Province (grant no. 2010C13027) and the
National Natural Sciences Foundation of China (grant nos. 81200112
and 81200114).
References
|
1
|
Abaci A, Oğuzhan A, Kahraman S, Eryol NK,
Unal S, Arinç H and Ergin A: Effect of diabetes mellitus on
formation of coronary collateral vessels. Circulation.
99:2239–2242. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fujiyama S, Amano K, Uehira K, Yoshida M,
Nishiwaki Y, Nozawa Y, Jin D, Takai S, Miyazaki M, Egashira K, et
al: Bone marrow monocyte lineage cells adhere on injured
endothelium in a monocyte chemoattractant protein-1-dependent
manner and accelerate reendothelialization as endothelial
progenitor cells. Circ Res. 93:980–989. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Werner N, Priller J, Laufs U, Endres M,
Böhm M, Dirnagl U and Nickenig G: Bone marrow-derived progenitor
cells modulate vascular reendothelialization and neointimal
formation: Effect of 3-hydroxy-3-methylglutaryl coenzyme a
reductase inhibition. Arterioscler Thromb Vasc Biol. 22:1567–1572.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Loomans CJ, de Koning EJ, Staal FJ,
Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B,
Rabelink TJ and van Zonneveld AJ: Endothelial progenitor cell
dysfunction: A novel concept in the pathogenesis of vascular
complications of type 1 diabetes. Diabetes. 53:195–199. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fadini GP, Miorin M, Facco M, Bonamico S,
Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A,
Agostini C and Avogaro A: Circulating endothelial progenitor cells
are reduced in peripheral vascular complications of type 2 diabetes
mellitus. J Am Coll Cardiol. 45:1449–1457. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ingram DA, Lien IZ, Mead LE, Estes M,
Prater DN, Derr-Yellin E, DiMeglio LA and Haneline LS: In vitro
hyperglycemia or a diabetic intrauterine environment reduces
neonatal endothelial colony-forming cell numbers and function.
Diabetes. 57:724–731. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tepper OM, Galiano RD, Capla JM, Kalka C,
Gagne PJ, Jacobowitz GR, Levine JP and Gurtner GC: Human
endothelial progenitor cells from type II diabetics exhibit
impaired proliferation, adhesion, and incorporation into vascular
structures. Circulation. 106:2781–2786. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Malinda KM, Goldstein AL and Kleinman HK:
Thymosin beta 4 stimulates directional migration of human umbilical
vein endothelial cells. FASEB J. 11:474–481. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smart N, Risebro CA, Melville AA, Moses K,
Schwartz RJ, Chien KR and Riley PR: Thymosin beta4 induces adult
epicardial progenitor mobilization and neovascularization. Nature.
445:177–182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grant DS, Rose W, Yaen C, Goldstein A,
Martinez J and Kleinman H: Thymosin beta4 enhances endothelial cell
differentiation and angiogenesis. Angiogenesis. 3:125–135. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao Y, Qiu F, Xu S, Yu L and Fu G:
Thymosin β4 activates integrin-linked kinase and decreases
endothelial progenitor cells apoptosis under serum deprivation. J
Cell Physiol. 226:2798–2806. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sosne G, Szliter EA, Barrett R, Kernacki
KA, Kleinman H and Hazlett LD: Thymosin beta 4 promotes corneal
wound healing and decreases inflammation in vivo following alkali
injury. Exp Eye Res. 74:293–299. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Riley PR and Smart N: Thymosin beta4
induces epicardium-derived neovascularization in the adult heart.
Biochem Soc Trans. 37:1218–1220. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bock-Marquette I, Saxena A, White MD,
Dimaio JM and Srivastava D: Thymosin beta4 activates
integrin-linked kinase and promotes cardiac cell migration,
survival and cardiac repair. Nature. 432:466–472. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bock-Marquette I, Shrivastava S, Pipes GC,
Thatcher JE, Blystone A, Shelton JM, Galindo CL, Melegh B,
Srivastava D, Olson EN and DiMaio JM: Thymosin beta4 mediated PKC
activation is essential to initiate the embryonic coronary
developmental program and epicardial progenitor cell activation in
adult mice in vivo. J Mol Cell Cardiol. 46:728–738. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiu FY, Song XX, Zheng H, Zhao YB and Fu
GS: Thymosin beta4 induces endothelial progenitor cell migration
via PI3K/Akt/eNOS signal transduction pathway. J Cardiovasc
Pharmacol. 53:209–214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Yu L, Zhao Y, Fu G and Zhou B:
Thymosin β4 reduces senescence of endothelial progenitor cells via
the PI3K/Akt/eNOS signal transduction pathway. Mol Med Rep.
7:598–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen YH, Lin SJ, Lin FY, Wu TC, Tsao CR,
Huang PH, Liu PL, Chen YL and Chen JW: High glucose impairs early
and late endothelial progenitor cells by modifying nitric
oxide-related but not oxidative stress-mediated mechanisms.
Diabetes. 56:1559–1568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bitzur R: Diabetes and cardiovascular
disease: When it comes to lipids, statins are all you need.
Diabetes Care. 34 Suppl 2:S380–S382. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bloomgarden ZT: Diabetes and
cardiovascular disease. Diabetes care. 34:e24–e30. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tabit CE, Chung WB, Hamburg NM and Vita
JA: Endothelial dysfunction in diabetes mellitus: Molecular
mechanisms and clinical implications. Rev Endocr Metab Disord.
11:61–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hill JM, Zalos G, Halcox JP, Schenke WH,
Waclawiw MA, Quyyumi AA and Finkel T: Circulating endothelial
progenitor cells, vascular function, and cardiovascular risk. N
Engl J Med. 348:593–600. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rafii S and Lyden D: Therapeutic stem and
progenitor cell transplantation for organ vascularization and
regeneration. Nat Med. 9:702–712. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vasa M, Fichtlscherer S, Aicher A, Adler
K, Urbich C, Martin H, Zeiher AM and Dimmeler S: Number and
migratory activity of circulating endothelial progenitor cells
inversely correlate with risk factors for coronary artery disease.
Circ Res. 89:E1–E7. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Landmesser U, Engberding N, Bahlmann FH,
Schaefer A, Wiencke A, Heineke A, Spiekermann S, Hilfiker-Kleiner
D, Templin C, Kotlarz D, et al: Statin-induced improvement of
endothelial progenitor cell mobilization, myocardial
neovascularization, left ventricular function, and survival after
experimental myocardial infarction requires endothelial nitric
oxide synthase. Circulation. 110:1933–1939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Klein JJ, Goldstein AL and White A:
Enhancement of in vivo incorporation of labeled precursors into DNA
and Total protein of mouse lymph nodes after administration of
thymic extracts. Proc Natl Acad Sci USA. 53:812–817. 1965.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rossdeutsch A, Smart N, Dube KN, Turner M
and Riley PR: Essential role for thymosin beta4 in regulating
vascular smooth muscle cell development and vessel wall stability.
Circ Res. 111:e89–e102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kaur H and Mutus B: Platelet function and
thymosin beta 4. Biol Chem. 393:595–598. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Malinda KM, Sidhu GS, Mani H, Banaudha K,
Maheshwari RK, Goldstein AL and Kleinman HK: Thymosin beta 4
accelerates wound healing. J Invest Dermatol. 113:364–368. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lv S, Cheng G, Zhou Y and Xu G: Thymosin
beta4 induces angiogenesis through Notch signaling in endothelial
cells. Mol Cell Biochem. 381:283–290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang L, Chopp M, Szalad A, Liu Z, Lu M,
Zhang L, Zhang J, Zhang RL, Morris D and Zhang ZG: Thymosin beta4
promotes the recovery of peripheral neuropathy in type II diabetic
mice. Neurobiol Dis. 48:546–555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim S and Kwon J: Effect of thymosin beta
4 in the presence of up-regulation of the insulin-like growth
factor-1 signaling pathway on high-glucose-exposed vascular
endothelial cells. Mol Cell Endocrinol. 401:238–247. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Everaert BR, Van Craenenbroeck EM, Hoymans
VY, Haine SE, Van Nassauw L, Conraads VM, Timmermans JP and Vrints
CJ: Current perspective of pathophysiological and interventional
effects on endothelial progenitor cell biology: Focus on
PI3K/AKT/eNOS pathway. Int J Cardiol. 144:350–366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dimmeler S, Aicher A, Vasa M, Mildner-Rihm
C, Adler K, Tiemann M, Rütten H, Fichtlscherer S, Martin H and
Zeiher AM: HMG-CoA reductase inhibitors (statins) increase
endothelial progenitor cells via the PI 3-kinase/Akt pathway. J
Clin Invest. 108:391–397. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fulton D, Gratton JP, McCabe TJ, Fontana
J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A and Sessa WC:
Regulation of endothelium-derived nitric oxide production by the
protein kinase Akt. Nature. 399:597–601. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aicher A, Heeschen C, Mildner-Rihm C,
Urbich C, Ihling C, Technau-Ihling K, Zeiher AM and Dimmeler S:
Essential role of endothelial nitric oxide synthase for
mobilization of stem and progenitor cells. Nat Med. 9:1370–1376.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun N, Wang H and Wang L: Vaspin
alleviates dysfunction of endothelial progenitor cells induced by
high glucose via pi3k/Akt/eNOS pathway. Int J Clin Exp Pathol.
8:482–489. 2015.PubMed/NCBI
|