Introduction
Human leukocyte antigen-G (HLA-G) is a molecule with
immunomodulatory activity that belongs to the non-classical HLA
class I family and its encoding gene is located at chromosome 6p21
(1,2). HLA-G may lead to immune tolerance by
interacting with receptors that are expressed in immune regulatory
cells, including immunoglobulin (Ig)-like transcript (ILT)2, ILT4,
killer cell Ig-like receptor, two Ig domains and long cytoplasmic
tail 4 and CD160 (3–5). Overexpression of HLA-G has been
identified in numerous types of human solid tumor and hematological
cancer (6), and high expression of
HLA-G was reported to be associated with primary carcinogenesis and
the metastatic capacity of breast invasive ductal carcinoma
(7,8). Overexpression of HLA-G may be a means
of tumor cells to avoid regulation by the immune system, by
inhibiting natural killer and T cell-mediated lysis (3).
Aberrant DNA methylation is one of the
characteristics of cancer cells (9,10). DNA
methylation is a covalent modification of DNA and is performed by
the DNA methyltransferase (DNMT) family, which mainly consists of
three members, DNMT1, DNMT3a and DNMT3b (11). The removal of methyl groups from DNA
is termed DNA demethylation. The ten-eleven translocation (TET)
family, which includes TET1, TET2 and TET3, has been indicated to
have important roles in DNA demethylation (12). DNA methylation has important roles in
a number of key genomic functions, including gene imprinting, X
chromosome inactivation, genome stability, retrotransposon
silencing and gene inactivation in cancer (13–15).
Dimethyloxallyl glycine (DMOG) is a small-molecule inhibitor of the
TET protein. In mice, treatment of embryos with 1 mM DMOG from the
germinal vesicle to the blastocyst stage effectively blocks the
activity of TET enzymes in vitro (16).
In general, the evasion of immune surveillance is
considered one of the emerging characteristics of cancer (17). High expression of HLA-G is essential
for tumor cells to avoid immune recognition and destruction
(18). DNA methylation modification
has important roles in regulating gene expression, and DNMTs and
TETs are responsible for the dynamic changes in DNA methylation
(19,20). However, whether the high expression
of HLA-G in tumor cells is induced by aberrant DNA methylation has
remained elusive. Therefore, in the present study, the expression
of HLA-G, DNMTs and TETs, as well as the DNA methylation levels of
HLA-G, were assessed in the HBL-100 breast cell line and the MCF-7
breast cancer cell line. The effects of TET activity on the
expression and DNA methylation levels of HLA-G in the MCF-7 cell
line were also assessed by treating the cells with DMOG.
Materials and methods
Cell culture
The HBL-100 and MCF-7 cell lines were purchased from
the Cell Bank of the Institute of Basic Medical Sciences, Chinese
Academy of Medical Sciences. The HBL-100 and MCF-7 cell lines were
cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal calf serum
(Bioind; Biological Industries), 100 IU/ml penicillin and 100 µg/ml
streptomycin (HyClone; GE Healthcare). The cells were cultured in a
humidified atmosphere with 5% CO2 at 37°C.
RNA isolation and reverse
transcription quantitative (RT-q) PCR
Cells were treated with 100, 200 or 400 µM DMOG for
48 h and untreated cells were used as controls. Trypsin/EDTA was
used to harvest the cultured cells, and RNA was isolated with
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. cDNA was synthesized using TransScript
One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech
Co., Ltd.). In brief, first-strand cDNA was synthesized in 20-µl
reactions from 2 µl total RNA using SuperMix and gDNA Remover, and
RT reaction steps consisted of 42°C for 15 min and 85°C for 5 sec.
qPCR was performed using SYBR Premix Ex Taq (Takara Bio, Inc.) on a
LightCycler 96 real-time PCR system (Roche). The 20-µl reaction
mixtures consisted of 8 µl water, 1 µl cDNA, 1 µl (10 µM) primers
(HLA-G, TET1, TET2, TET3, DNMT1, DNMT3a, DNMT3b and
GAPDH) and 10 µl SYBR Premix Ex Taq. GAPDH was used as an
internal control. The thermocycling program consisted of 95°C for
180 sec, followed by 50 cycles at 95°C for 10 sec, 60°C for 30 sec,
then 95°C for 30 sec, 65°C for 60 sec and 97°C for 1 sec, and a
final step at 37°C for 30 sec. Relative gene expression was
quantified by using the 2−ΔΔCq method (21). The primer sequences for HLA-G,
DNMT1, DNMT3a (22),
DNMT3b (22), TET1,
TET2 (23), TET3
(23) and GAPDH are listed in
Table I.
 | Table I.Primer sequences designed for PCR. |
Table I.
Primer sequences designed for PCR.
| Gene | Primer pair sequences
(5′ to 3′) | Product size
(bp) | Reference |
|---|
| RT-qPCR |
|
HLA-G | F:
AGAGGAGACACGGAACACCAAGG | 127 | NC_000006.12 |
|
| R:
CAGGTCGCAGCCAATCATCCAC |
|
|
|
DNMT1 | F:
CCTCCAAAAACCCAGCCAAC | 101 | NC_000019.10 |
|
| R:
TCCAGGACCCTGGGGATTTC |
|
|
|
DNMT3a | F:
CCAACATCGAATCCATGAAA | 140 | (22) |
|
| R:
CTTGCGCTTGCTGATGTAGT |
|
|
|
DNMT3b | F:
CGAATTTTACCACCTGCTGAATT | 59 | (22) |
|
| R:
AGAACGGCCGGTCATCAC |
|
|
|
TET1 | F:
ACCTATTCCCCGAATCAAGC | 100 | NC_000010.11 |
|
| R:
TTGCACGGTCTCAGTGTTACTC |
|
|
|
TET2 | F:
AGCCCCATCACGTACAAAAC | 129 | (23) |
|
| R:
TGTGGTGGCTGCTTCTGTAG |
|
|
|
TET3 | F:
CAGCAGCCGAGAAGAAGAAG | 125 | (23) |
|
| R:
GGACAATCCACCCTTCAGAG |
|
|
|
GAPDH | F:
CAGGAGGCATTGCTGATGAT | 138 | NC_000012.12 |
|
| R:
GAAGGCTGGGGCTCATTTT |
|
|
|
Bisulfite-sequencing PCR |
|
|
|
|
HLA-G | F:
TGGGTTAAGATTTAGGGAGATA | 249 | (25) |
|
| R:
TAACTTCTCTAAAAACCTATCACCTAA |
|
|
Bisulfite genomic sequencing
DNA was isolated using a TIANamp Genomic DNA kit
[Tiangen Biotech (Beijing) Co., Ltd.] after the cells had been
treated with DMOG or left untreated (control) for 48 h, followed by
analysis using bisulfite sequencing. In brief, DNA was extracted
from breast cancer cells treated as mentioned above and boiled in a
water bath for 5 min, followed by chilling on ice. Subsequently, 4
µl of 2 M NaOH (final concentration, 0.3 M NaOH) was added to the
DNA, and the mixture was incubated for 15 min at 50°C. Next, the
solution was mixed with two volumes of 2% low-melting-point agarose
(Sigma-Aldrich, Merck KGaA) and seven 10 µl-aliquotes of the
DNA-agarose mixture were pipetted into ice-cold mineral oil to form
beads. Then, at least seven beads were immersed in the fresh
bisulfite solution (2.5 M sodium metabisulfite and 125 mM
hydroquinone, pH 5) (24). These
beads were incubated for 3–5 h in the dark and covered with mineral
oil at 50°C. Next, the supernatant was discarded and the seven
beads were washed four times in 1 ml Tris-EDTA buffer (pH 8.0) 15
min each time. After desulfonation in 0.5 ml of 0.2 M NaOH 2 times
for 15 min each, the beads were washed with 1 ml Tris-EDTA buffer 3
times for 10 min each and with H2O 2 times for 15 min
each, and then used as the input for PCR. The PCR primer sequence
for HLA-G (25) is listed in
Table I.
Statistical analysis
All experiments were performed at least three times.
Statistical analysis was performed by one-way analysis of variance
using SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA) followed
the LSD method to assess the differences between more than two
groups. Differences were considered statistically significant at
P<0.05.
Results
HLA-G expression and promoter DNA
methylation levels in HBL-100 and MCF-7 cells
HLA-G expression was analyzed in HBL-100 and MCF-7
cells by RT-qPCR analysis. As presented in Fig. 1A, the expression of HLA-G was
significantly greater in MCF-7 cells than in HBL-100 cells
(P<0.01). Subsequently, the DNA methylation level of the HLA-G
promoter region was compared between the two cell lines. As
presented in Fig. 1B, the DNA
methylation level of the HLA-G promoter region was 96.7% in HBL-100
cells, but only 56.9% in MCF-7 cells.
Expression of DNMTs in HBL-100 and
MCF-7 cells
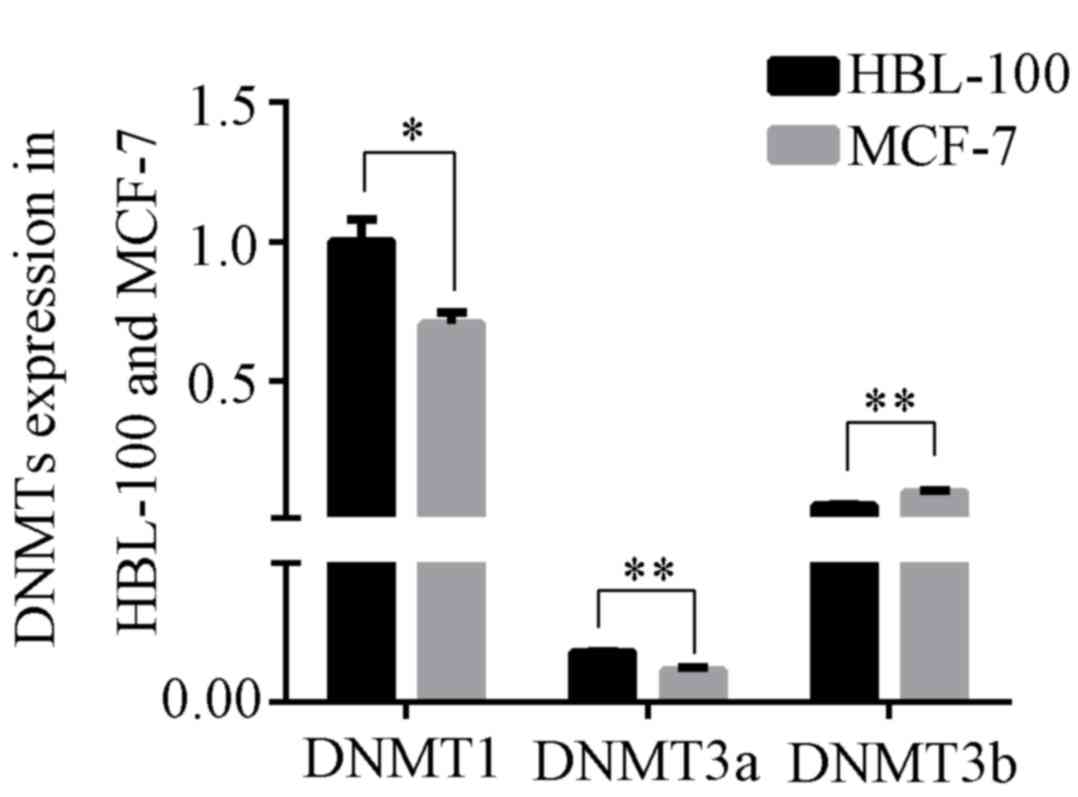
DNA methylation is catalyzed by DNMTs. The
expression of various DNMTs was investigated by RT-qPCR. The
expression of DNMT1, DNMT3a and DNMT3b was compared between HBL-100
and MCF-7 cells. The expression levels of DNMT1 and DNMT3a in
HBL-100 cells were significantly greater than those in MCF-7 cells
(DNMT1: P<0.05; DNMT3a: P<0.01; Fig. 2), but the expression levels of DNMT3b
in HBL-100 cells were lower than those in MCF-7 cells
(P<0.01).
Expression of TETs in HBL-100 and
MCF-7 cells
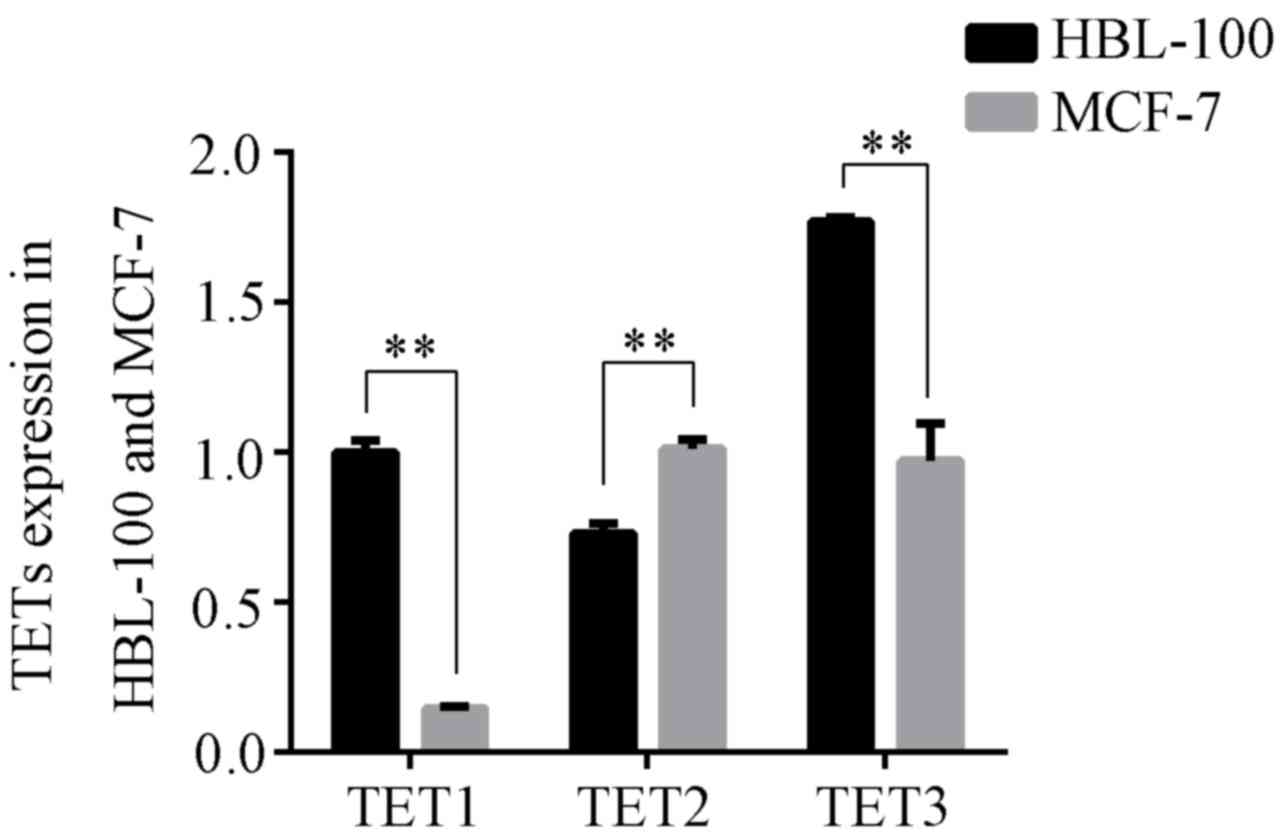
The ten-eleven tanslocation (TET) family, which
includes TET1, TET2 and TET3, is generally thought to be
responsible for DNA demethylation. As presented in Fig. 3, the expression levels of TET1 and
TET3 in MCF-7 cells were significantly lower than those in HBL-100
cells (P<0.01); however, TET2 expression in MCF-7 cells was
greater than that in HBL-100 cells (P<0.01).
Effects of inhibition of TET on the
expression and promoter DNA methylation of HLA-G in MCF-7
cells
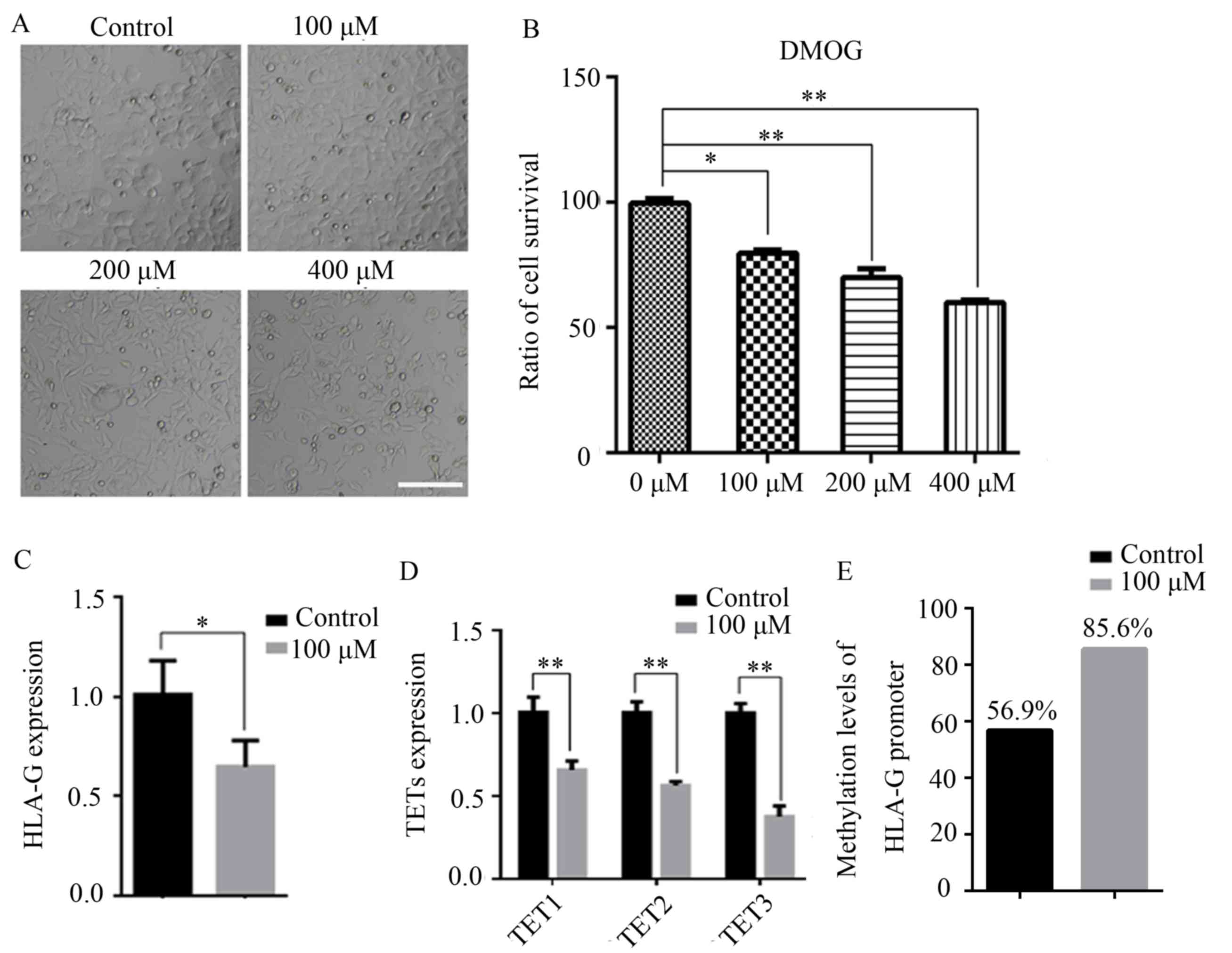
MCF-7 cells were treated with 100, 200 or 400 µM
DMOG for 48 h, and untreated cells were used as controls. As
presented in Fig. 4A, treatment with
200 and 400 µM DMOG changed the morphology of MCF-7 cells,
indicating that the 200 and 400 µM DMOG treatments were cytotoxic.
An MTT assay also indicated that DMOG had inhibitory effects on
MCF-7 cells (Fig. 4B). Therefore,
100 µM DMOG was used as the final concentration for the subsequent
experiments. It was indicated that treatment with DMOG
significantly decreased the expression of HLA-G in MCF-7 cells
(P<0.05; Fig. 4C). Furthermore,
the expression levels of TET1, TET2 and TET3 were all significantly
decreased (P<0.01; Fig. 4D). The
promoter DNA methylation level of HLA-G in MCF-7 cells after DMOG
treatment was then assessed, revealing that DMOG treatment
significantly increased the promoter DNA methylation level of HLA-G
in MCF-7 cells compared with that in the control cells (Fig. 4E).
Discussion
HLA-G was first reported to allow tumors to avoid
immunosurveillance in 1998 (26).
Since then, numerous studies have been performed to support this
hypothesis. HLA-G-induced suppression of T-cell responses has
indicated the presence of an immune escape pathway in human
glioblastoma (27). HLA-G has been
reported to be overexpressed in a number of cancer types, including
melanoma (28), primary cutaneous
lymphomas (29), lung cancer
(30) and breast cancer (31). However, the molecular mechanisms of
the induction of HLA-G overexpression in cancer remain to be fully
elucidated. Therefore, in the present study, the possible
association between the expression of HLA-G and DNA methylation of
its promoter region was determined in the MCF-7 breast cancer cell
line as an underlying molecular mechanism that induces high HLA-G
expression in cancer.
The present results indicated that HLA-G expression
in MCF-7 cells was significantly greater than that in HBL-100
cells, which was in line with previously reported results in a
variety of cancer types (28–31).
High HLA-G expression may be responsible for the avoidance of
immunosurveillance by MCF-7 cells. However, the mechanisms that
cause high expression of HLA-G in MCF-7 cells remain elusive. DNA
methylation is a means of gene expression regulation, and an
aberrant DNA methylation pattern is one of the characteristics of
cancer cells (9,10). Furthermore, previous studies have
indicated the activation of HLA-G transcription after incubation
with a DNMT inhibitor (5-aza-2′-deoxycytidine) in a broad panel of
human leukemia cell lines (32),
suggesting that DNA methylation regulates HLA-G expression in those
cells (33). Therefore, it may be
inferred that the differing DNA methylation in the HLA-G promoter
region was responsible for the differences in HLA-G expression
between HBL-100 and MCF-7 cells. In most cases, the DNA methylation
level is negatively correlated with gene expression, e.g. the
expression of the NANOG gene (34).
The present results indicated that the DNA methylation level of the
promoter region of the HLA-G gene in MCF-7 cells was lower than
that in HBL-100 cells. Thus, similar to other genes, DNA
methylation of the promoter region of the HLA-G gene negatively
regulates its expression. Aberrant DNA methylation modification
causes abnormally high expression of HLA-G in MCF-7 cells. DNMT and
TET activities have important roles in dynamic changes in DNA
methylation. The present results indicated that DNMT1 and DNMT3a
were expressed at lower levels and that TET2 was expressed at
higher levels in MCF-7 cells than in HBL-100 cells. However, DNMT3b
expression was greater and TET1 and TET3 expression was lower in
MCF-7 cells than in HBL-100 cells. The different downstream targets
of DNMT1, DNMT3a and DNMT3b may be the reason for the different
expression patterns of the members of the DNMT or TET families
between HBL-100 and MCF-7 cells. Accumulating evidence suggests
that somatic mutations in DNA methyltransferases and 5mC-modifying
enzymes, including TET proteins, are associated with oncogenic
transformation (12). Therefore, it
may be inferred that the lower DNMT1 and DNMT3a expression levels
and greater TET2 expressions levels were the reason for abnormal
DNA methylation modification of the HLA-G gene in MCF-7 cells.
Treatment with small-molecule inhibitors of DNMT or
TET may change the extent of DNA methylation and thereby gene
expression (35,36). DMOG is a non-specific 2-OG-dependent
dioxygenase inhibitor (37). In
cows, treatment of parthenogenetic embryos with 1 mM DMOG
effectively blocked the activity of TET enzymes and impeded
parthenogenetic embryo development in vitro by disturbing
the DNA demethylation progress (38). Therefore, it was assessed whether
treatment with the TET inhibitor DMOG increases the DNA methylation
level of HLA-G, and whether this decreases HLA-G expression in
MCF-7 cells. The results suggested that treatment with 100 µM DMOG
for 48 h significantly increased the DNA methylation level of the
HLA-G promoter region in MCF-7 cells. More importantly, a negative
association between the promoter region DNA methylation level and
gene expression was observed, as treatment with DMOG significantly
decreased HLA-G expression in MCF-7 cells. Unexpectedly, DMOG also
significantly decreased TETs expression in MCF-7 cells, which
indicated that TETs may regulate self-expression in MCF-7 cells.
Overall, the results indicated that TETs are, at least in part,
responsible for the lower DNA methylation level of the HLA-G
promoter in MCF-7 cells.
In conclusion, the present study indicated that
HLA-G was highly expressed and that the DNA methylation level of
its gene promoter region was low in the MCF-7 breast cancer cell
line. DNMTs and TETs were aberrantly expressed in MCF-7 cells,
which may be the reason for the low DNA methylation level of the
HLA-G promoter region. Inhibition of TET activity increased HLA-G
promoter region DNA methylation levels and decreased the expression
of HLA-G in MCF-7 cells. These results indicated that TETs are, at
least in part, responsible for the lower DNA methylation level of
the HLA-G promoter and overexpression of HLA-G in MCF-7 cells,
which may provide potential targets for novel anti-cancer drugs.
The exact upstream mechanisms that regulate the overexpression of
HLA-G in cancers may require further investigation.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Key R&D
Program of China (grant no. 2017YFA0104400) and the Program for
Changjiang Scholars and Innovative Research Team in University
(grant no. IRT_16R32).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
DZ and XA mainly performed the experiments. ZL and
SZ conceived the project, supervised the experiments and revised
the manuscript. All authors agreed to be accountable for the
content of the work. All authors read and approved the final
manuscript.
Ethical approval and informed consent
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors have no competing interests to
declare.
References
|
1
|
Carosella ED, Moreau P, Lemaoult J and
Rouas-Freiss N: HLA-G: From biology to clinical benefits. Trends
Immunol. 29:125–132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jeong S, Park S, Park BW, Park Y, Kwon OJ
and Kim HS: Human leukocyte antigen-G (HLA-G) polymorphism and
expression in breast cancer patients. PLoS One. 9:e982842014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pistoia V, Morandi F, Wang X and Ferrone
S: Soluble HLA-G: Are they clinically relevant? Semin Cancer Biol.
17:469–479. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baudhuin J, Migraine J, Faivre V, Loumagne
L, Lukaszewicz AC, Payen D and Favier B: Exocytosis acts as a
modulator of the ILT4-mediated inhibition of neutrophil functions.
Proc Natl Acad Sci USA. 110:17957–17962. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Le Page ME, Goodridge JP, John E,
Christiansen FT and Witt CS: Killer Ig-like receptor 2DL4 does not
mediate NK cell IFN-gamma responses to soluble HLA-G preparations.
J Immunol. 192:732–740. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morandi F, Rizzo R, Fainardi E,
Rouas-Freiss N and Pistoia V: Recent advances in our understanding
of HLA-G biology: Lessons from a wide spectrum of human diseases. J
Immunol Res. 2016:43264952016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
da Silva GB, Silva TG, Duarte RA, Neto NL,
Carrara HH, Donadi EA, Gonçalves MA, Soares EG and Soares CP:
Expression of the classical and Nonclassical HLA molecules in
breast cancer. Int J Breast Cancer. 2013:2504352013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Elliott RL, Jiang XP, Phillips JT, Barnett
BG and Head JF: Human leukocyte antigen G expression in breast
cancer: Role in immunosuppression. Cancer Biother Radiopharm.
26:153–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Esteller M: Epigenetics in cancer. N Engl
J Med. 358:1148–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gal-Yam EN, Saito Y, Egger G and Jones PA:
Cancer epigenetics: Modifications, screening, and therapy. Annu Rev
Med. 59:267–280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang S, Chen X, Wang F, An X, Tang B,
Zhang X, Sun L and Li Z: Aberrant DNA methylation reprogramming in
bovine SCNT preimplantation embryos. Sci Rep. 6:303452016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu H and Zhang Y: Mechanisms and functions
of Tet protein-mediated 5-methylcytosine oxidation. Genes Dev.
25:2436–2452. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bird AP and Wolffe AP: Methylation-induced
repression-belts, braces, and chromatin. Cell. 99:451–454. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bestor TH: The DNA methyltransferases of
mammals. Hum Mol Genet. 9:2395–2402. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bird A: DNA methylation patterns and
epigenetic memory. Genes Dev. 16:6–21. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amouroux R, Nashun B, Shirane K, Nakagawa
S, Hill PW, D'Souza Z, Nakayama M, Matsuda M, Turp A, Ndjetehe E,
et al: De novo DNA methylation drives 5hmC accumulation in mouse
zygotes. Nat Cell Biol. 18:225–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schreiber RD, Old LJ and Smyth MJ: Cancer
immunoediting: Integrating immunity's roles in cancer suppression
and promotion. Science. 331:1565–1570. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Kruijf EM, Sajet A, van Nes JG, Natanov
R, Putter H, Smit VT, Liefers GJ, van den Elsen PJ, van de Velde CJ
and Kuppen PJ: HLA-E and HLA-G expression in classical HLA class
I-negative tumors is of prognostic value for clinical outcome of
early breast cancer patients. J Immunol. 185:7452–7459. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Okano M, Bell DW, Haber DA and Li E: DNA
methyltransferases Dnmt3a and Dnmt3b are essential for de novo
methylation and mammalian development. Cell. 99:247–257. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gu TP, Guo F, Yang H, Wu HP, Xu GF, Liu W,
Xie ZG, Shi L, He X, Jin SG, et al: The role of Tet3 DNA
dioxygenase in epigenetic reprogramming by oocytes. Nature.
477:606–610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deivendran S, Marzook H, Santhoshkumar TR,
Kumar R and Pillai MR: Metastasis-associated protein 1 is an
upstream regulator of DNMT3a and stimulator of insulin-growth
factor binding protein-3 in breast cancer. Sci Rep. 7:442252017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Collignon E, Canale A, Al Wardi C, Bizet
M, Calonne E, Dedeurwaerder S, Garaud S, Naveaux C, Barham W,
Wilson A, et al: Immunity drives TET1 regulation in cancer through
NF-κB. Sci Adv. 4:eaap73092018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu C, Zhang YL, Pan WW, Li XM, Wang ZW, Ge
ZJ, Zhou JJ, Cang Y, Tong C, Sun QY and Fan HY: CRL4 complex
regulates mammalian oocyte survival and reprogramming by activation
of TET proteins. Science. 342:1518–1521. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Verloes A, Spits C, Vercammen M, Geens M,
LeMaoult J, Sermon K, Coucke W and Van de Velde H: The role of
methylation, DNA polymorphisms and microRNAs on HLA-G expression in
human embryonic stem cells. Stem Cell Res. 19:118–127. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Paul P, Rouas-Freiss N, Khalil-Daher I,
Moreau P, Riteau B, Le Gal FA, Avril MF, Dausset J, Guillet JG and
Carosella ED: HLA-G expression in melanoma: A way for tumor cells
to escape from immunosurveillance. Proc Natl Acad Sci USA.
95:4510–4515. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wiendl H, Mitsdoerffer M, Hofmeister V,
Wischhusen J, Bornemann A, Meyermann R, Weiss EH, Melms A and
Weller M: A functional role of HLA-G expression in human gliomas:
An alternative strategy of immune escape. J Immunol. 168:4772–4780.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ugurel S, Rebmann V, Ferrone S, Tilgen W,
Grosse-Wilde H and Reinhold U: Soluble human leukocyte antigen-G
serum level is elevated in melanoma patients and is further
increased by interferon-alpha immunotherapy. Cancer. 92:369–376.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Urosevic M, Willers J, Mueller B, Kempf W,
Burg G and Dummer R: HLA-G protein up-regulation in primary
cutaneous lymphomas is associated with interleukin-10 expression in
large cell T-cell lymphomas and indolent B-cell lymphomas. Blood.
99:609–617. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Urosevic M, Kurrer MO, Kamarashev J,
Mueller B, Weder W, Burg G, Stahel RA, Dummer R and Trojan A: Human
leukocyte antigen G up-regulation in lung cancer associates with
high-grade histology, human leukocyte antigen class I loss and
interleukin-10 production. Am J Pathol. 159:817–824. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lefebvre S, Antoine M, Uzan S, McMaster M,
Dausset J, Carosella ED and Paul P: Specific activation of the
non-classical class I histocompatibility HLA-G antigen and
expression of the ILT2 inhibitory receptor in human breast cancer.
J Pathol. 196:266–274. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Poláková K, Bandzuchová E, Kuba D and Russ
G: Demethylating agent 5-aza-2′-deoxycytidine activates HLA-G
expression in human leukemia cell lines. Leuk Res. 33:518–524.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moreau P, Mouillot G, Rousseau P, Marcou
C, Dausset J and Carosella ED: HLA-G gene repression is reversed by
demethylation. Proc Natl Acad Sci USA. 100:1191–1196. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang S, Tang B, Fan C, Shi L, Zhang X,
Sun L and Li Z: Effect of DNMT inhibitor on bovine parthenogenetic
embryo development. Biochem Biophys Res Commun. 466:505–511. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brueckner B, Garcia Boy R, Siedlecki P,
Musch T, Kliem HC, Zielenkiewicz P, Suhai S, Wiessler M and Lyko F:
Epigenetic reactivation of tumor suppressor genes by a novel
small-molecule inhibitor of human DNA methyltransferases. Cancer
Res. 65:6305–6311. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stresemann C, Brueckner B, Musch T,
Stopper H and Lyko F: Functional diversity of DNA methyltransferase
inhibitors in human cancer cell lines. Cancer Res. 66:2794–2800.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Elvidge GP, Glenny L, Appelhoff RJ,
Ratcliffe PJ, Ragoussis J and Gleadle JM: Concordant regulation of
gene expression by hypoxia and 2-oxoglutarate-dependent dioxygenase
inhibition: The role of HIF-1alpha, HIF-2alpha, and other pathways.
J Biol Chem. 281:15215–15226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang J, Zhang S, Wang Y, Cheng H, Hao L,
Zhai Y, Zhang Z, An X, Ma X, Zhang X, et al: Effect of TET
inhibitor on bovine parthenogenetic embryo development. PLoS One.
12:e01895422017. View Article : Google Scholar : PubMed/NCBI
|