Introduction
Retinoblastoma (RB) is a malignant tumor of the
retina and generally affects children under the age of 6 years,
worldwide, with an incidence of 1:16,000-1:18,000 and 7,000-8,000
new cases every year (1). RB is a
threat to both the eyesight and life of children (2). Clinical treatments for RB include
chemotherapy, radiotherapy, surgery, laser treatment and freezing
(3-6).
Among them, chemotherapy serves an important role in the management
of RB, and vincristine (VCR) is one the most commonly used
chemotherapy drugs (7,8). However, the inherent or acquired
resistance of tumor cells to chemotherapeutic agents can lead to
treatment failure (9-11).
The SO-Rb50 cell line is a human RB cell line.
SO-Rb50 cells that are resistant to vincristine (SO-Rb50/VCR) can
be established by maintaining the cells in increasing
concentrations of vincristine over a period of nine months
(12). P-glycoprotein (P-gp) is an
important protein that indicates multidrug resistance (13). It is of particular relevance to the
treatment of RB to identify a compound that can effectively target
SO-Rb50/VCR cells.
Matrine is a type of alkaloid found in
Leguminosae plants, including Sophora flavescens Ait
(14,15). A number of studies have reported the
beneficial effects of matrine on the quality of life and immune
functions of patients with cancer (16,17).
Additionally, matrine has been reported to inhibit tumor cell
proliferation through a variety of mechanisms, including inducing
cancer cell differentiation and apoptosis, altering tumor cell
cycle and inhibiting telomerase activity (18-21).
Therefore, matrine may be a suitable compound for use in the
treatment of a number of cancer types or cancer-related conditions.
The present study demonstrated that matrine inhibited the
proliferation of immortalized RB cells, decreased the rate of
mitosis and increased apoptosis, which was also paralleled by
corresponding changes in levels of the proteins regulating the cell
cycle and apoptosis in the immortalized RB cells (22). However, to the best of our knowledge,
the effects of matrine on VCR-resistant RB cells have not
previously been reported. Therefore, the present study aimed to
identify the various effects of matrine on SO-Rb50/VCR cells.
Materials and methods
Establishment of drug-resistant cell
lines
SO-RB50 cells were purchased from The Cell Bank of
Type Culture Collection of the Chinese Academy of Sciences and
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Hyclone; GE Healthcare Life Sciences)
and 100 U/ml penicillin-streptomycin (Sigma-Aldrich; Merck KGaA) in
5% CO2 at 37˚C. Drug-resistance in SO-Rb50 cells was
induced by stimulation with increasing concentrations of VCR
(National Institutes for Food and Drug Control) at 37˚C, ranging
from 75 µg/l to 600 µg/l, over ~9 months, as previously described
(23,12). Cells receiving normal culture medium
without any treatment were utilized as controls. The wells without
cells and culture medium were used as blank controls.
Cell growth and proliferation
SO-Rb50/VCR cells were cultured in vitro as
aforementioned, and the cell growth curve was measured after
treatment with matrine at different concentrations. SO-Rb50/VCR
cells (3x103/ml) in the logarithmic growth phase were
inoculated onto 96-well plates (50 µl /well). The cells were
incubated with matrine at different concentrations at 37˚C (50 µl,
0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1, 1.1 and 1.3 mg/ml). CCK-8
assay (Dojindo Molecular Technologies, Inc.) was used to analyze
the cytotoxicity of matrine in accordance with the manufacturers
protocol, and half-maximal inhibitory concentration
(IC50) was calculated. The optimal drug concentration
(determined based on the IC50; 0.97 mg/ml) was
identified and selected for subsequent experiments.
Cell viability was calculated according to the
following formula. Cell viability (%)=[(As-Ab)/(Ac-Ab)] x 100%, in
which ‘As’ is the absorbance of the experimental group, Ac is the
absorbance of the control group (containing cell culture medium,
CCK-8), Ab is the blank group (without cell and matrine solution
culture medium, CCK-8). Inhibitory rate of cell proliferation
=100-cell viability.
TUNEL assay
The level of cell apoptosis was evaluated before and
after administration of matrine (0.97 mg/ml; IC50) for
0, 12, 24 and 48 h. The cells were collected, fixed in 4%
paraformaldehyde at room temperature for 30 min and stained using
TUNEL (37˚C for 5 min)/DAPI (1 mg/ml for 5 min). Subsequently,
apoptosis was detected using flow cytometry (BD Biosciences) within
1 h and analyzed using FlowJo v7.6 software (FlowJo LLC).
Cell cycle analysis
The cultured SO-Rb50/VCR cells were treated with
matrine (0.97 mg/ml, IC50), and the cell cycle of cells
without matrine treatment (control) and after treatment was
assessed by flow cytometry (BD Biosciences) after PI (Merck KGaA)
staining for 5 min protected from light at room temperature. The
data were analyzed using FlowJo v7.6 software (FlowJo LLC).
Electron microscopic examination
To examine the cells using electron microscopy, the
cells treated with matrine or vehicle were centrifuged (1,000 x g
at 4˚C for 8 min), fixed with 3% glutaraldehyde at 4˚C for 2 h. For
the preparation of ultra-thin sections (1 µm), the cells were
washed with phosphate-buffered saline, fixed with 1% osmic acid for
1 h at 4˚C, dehydrated with acetone and embedded in epoxide resin.
After staining with uranyl acetate and lead citrate at room
temperature for 5 min, the sections were observed in at least least
five fields under a transmission electron microscope (model no.
EM208s; Koninklijke Philips N.V.). The images were processed using
Image J (v150-win-jre6; National Institutes of Health).
Western blot analysis
Cultured SO-Rb50/VCR cells were treated with matrine
(0.97 mg/ml; IC50) for 0, 12, 24 and 48 h and protein
was extracted from cell lines for western blot analysis using the
triplePrep kit (cat. no. 28-9425-44; GE Healthcare Life Sciences).
The 0 time point was set as control. Protein concentration was then
determined using the bicinchoninic acid method. Protein (25
µg/lane) was subsequently separated via 10% SDS-PAGE and
transferred onto nitrocellulose membranes. Thereafter, the
membranes were blocked in 5% skimmed milk for 2 h at room
temperature. The following antibodies were used for incubation for
24 h at 4˚C: Bcl-2 (1:200; cat. no. ab117115, Abcam), Bax (1:200;
cat. no. ab32503; Abcam) and β-actin (1:200; cat. no. ab179467;
Abcam). Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG
H&L (1:100; cat. no. ab205719; Abcam) or HRP-conjugated goat
anti-rabbit IgG H&L (1:100; cat. no. ab6721; Abcam) were added
and co-incubated for 1-2 h at room temperature. ECL Plus Western
Blotting Reagent (cat. no. RPN2133; GE Healthcare Life Sciences)
was added to the membrane. The bands were visualized utilizing a
gel imaging system (Bio-Rad Laboratories, Inc.). Grey density was
analyzed using Quantity One analysis software v1.4.6 (Bio-Rad
Laboratories, Inc.).
Statistical analysis
The data are presented as mean ± S.E.M (n=6 in each
group; analyses were performed in triplicate), and were analyzed
using SPSS 17.0 (SPSS, Inc.). Significant differences between two
groups were calculated by t-test. When three or more groups
were compared, one-way ANOVA was applied to the data, followed by
Tukey's post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Establishment and detection of
drug-resistant cell lines
SO-Rb50 cells grew in suspension with grape-like
clusters, displaying large-size and uniform pattern. Following
treatment with VCR, mass clusters of SO-Rb50 were disturbed and the
cells presented mostly in the form of small clusters or single
cells. The cell growth was significantly slower, and the cells
became uneven in size following VCR treatment. However, the
inhomogeneity of cell size became more obvious and there are more
degenerative cells and cell debris after 4 to 6 days. A few cells
lost their original shape and vacuolar structures appeared in the
cytoplasm and the nuclear structure became unclear. These cells
gradually increased with time, but there was no increase in the
number of these cells during the following three weeks. After
treatment for three weeks, the medium (with VCR) was washed away
and replaced with fresh medium for further cell growth. The growth
of SO-Rb50 cells was still slow at the beginning, but gradually
recovered over time (after ~2-3 weeks). At the same time, the
abnormal morphology was gradually decreased or even disappeared;
the cells were more uniform in size and the cell mass became
larger. The above changes occurred again after increasing the
concentration of drugs. SO-Rb50 cells could grow stably in the
medium containing 600 g/ml of VCR after an intermittent treatment
with different concentrations (data not shown).
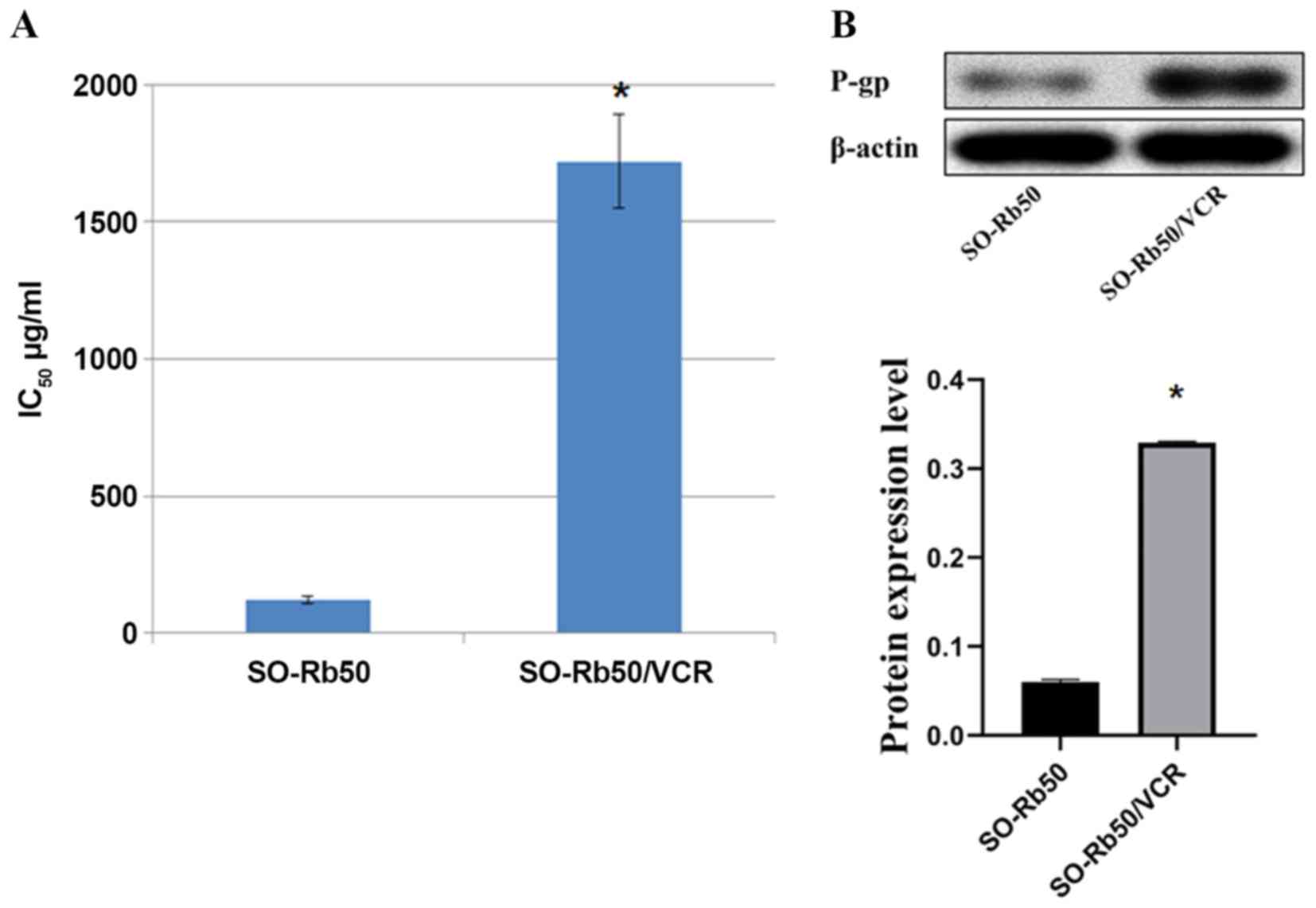
The IC50 values of VCR on SO-Rb50 and
SO-Rb50/VCR cells for 48 h were 122±17.1 µg/ml and 1720±77.4 µg/ml,
respectively. The tolerance of SO-Rb50/VCR cells to VCR was ~14.1
times higher than that of SO-Rb50 (Fig.
1A). P-gp protein expression was relatively low in untreated
SO-Rb50 cells, but the level of P-gp protein increased
significantly after an increase in VCR concentration (Fig. 1B).
Effect of matrine on cell
proliferation
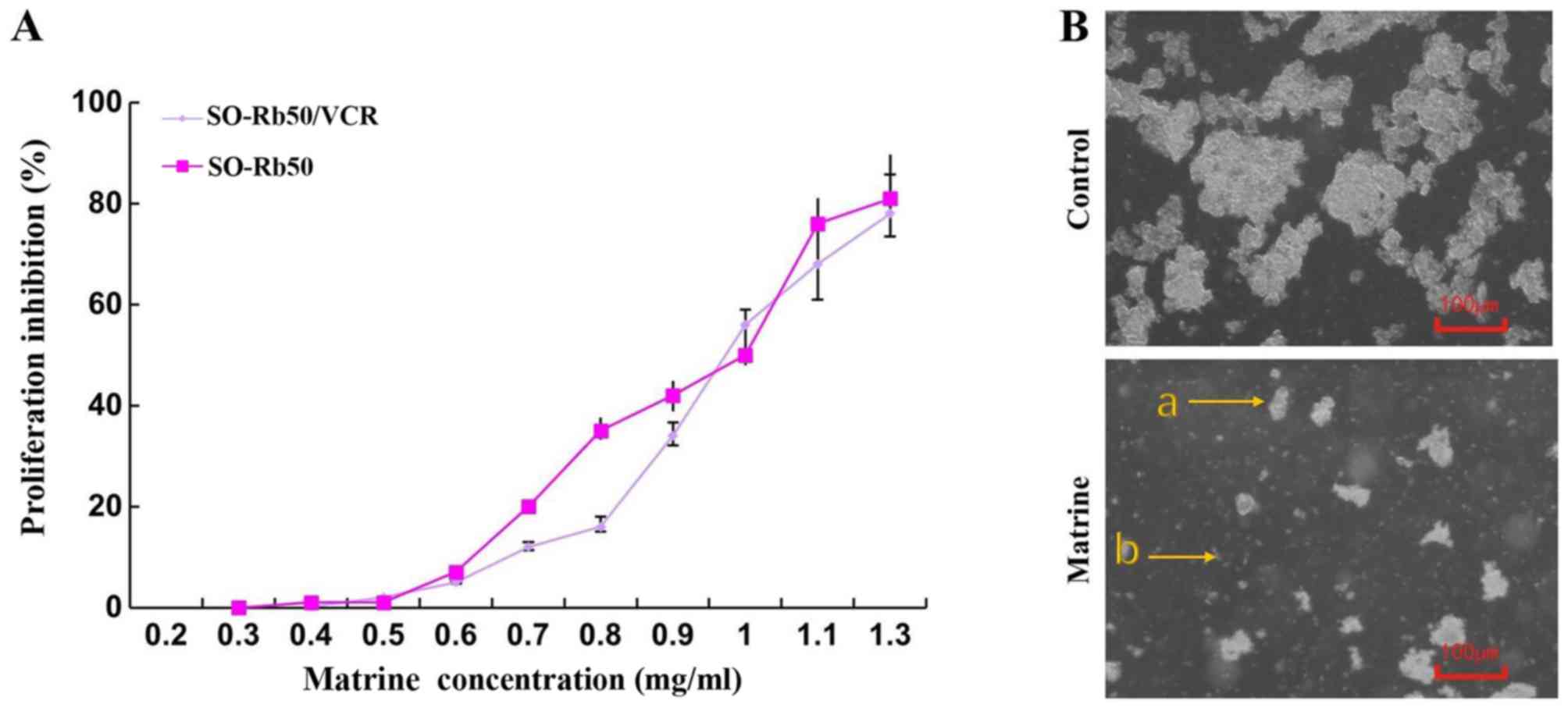
As indicated in Fig.
2A, matrine (0.3-1.3 mg/ml over 24 h) exhibited significantly
suppressive effects on cellular growth of SO-Rb50 and SO-Rb50/VCR
cells in a dose-dependent manner, and the IC50 values of
matrine on SO-Rb50 and SO-Rb50/VCR cells for 24 h were 0.96±0.04
mg/ml and 0.97±0.08 mg/ml, respectively (Fig. 2A). There was no significant
difference indicated in the proliferation-inhibition of matrine in
SO-Rb50 and SO-Rb50/VCR cells (Fig.
2A). SO-Rb50/VCR cells grew as suspended aggregates in a
uniform pattern with large nuclei. After treatment with matrine
(IC50) for 24 h, the cell mass was reduced, the cells
became uneven in size and cytoplasmic debris and vacuolated cells
were observed (Fig. 2B).
Effect of matrine on apoptosis and the
cell cycle
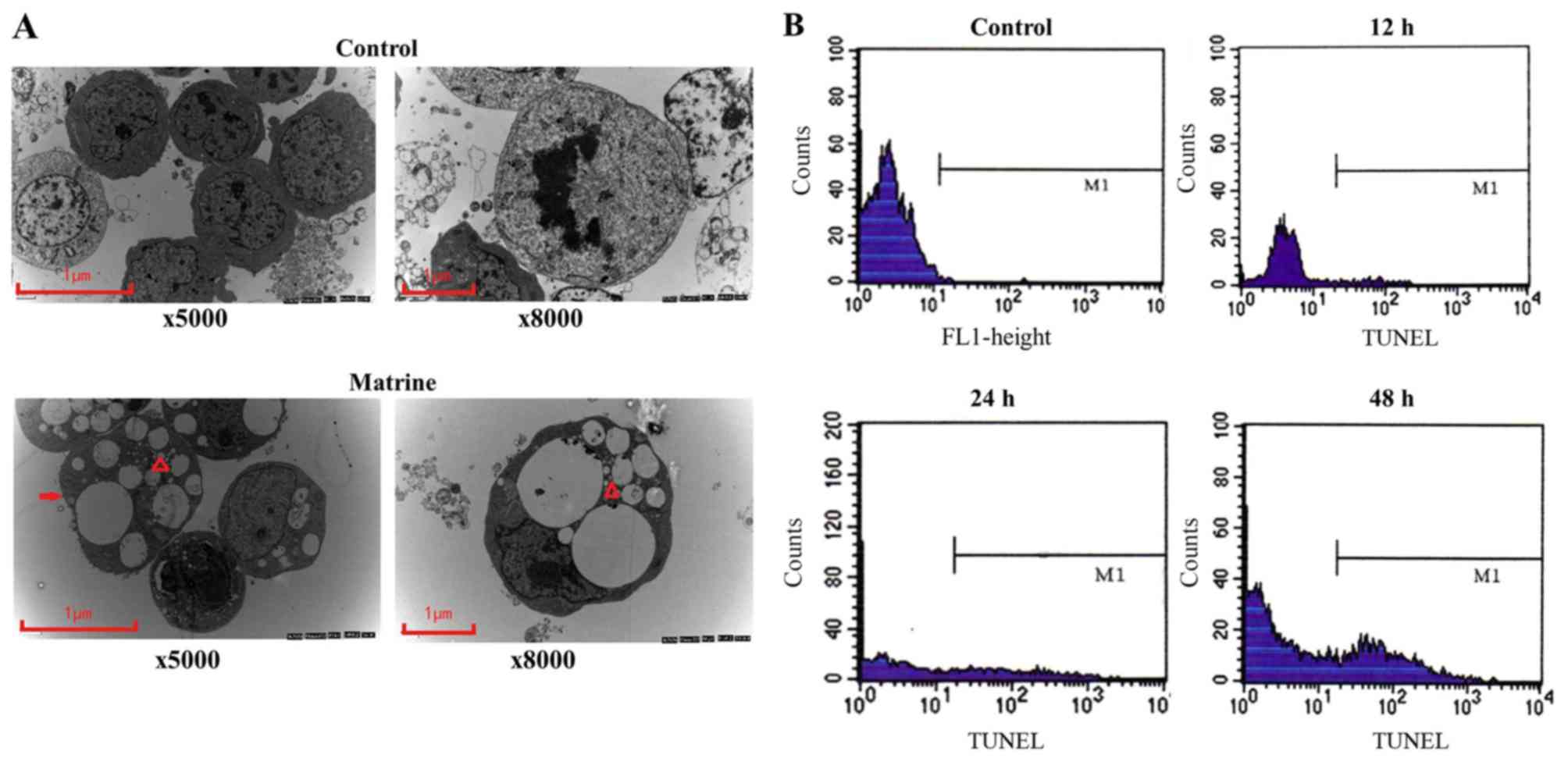
The ultrastructures of SO-Rb50/VCR cells in the
control group and the matrine-treated group was observed using
electron microscopy, and the cells exhibited significant
degeneration, demonstrating features of apoptosis (chromatin
condensation and vacuolar accumulation) after drug treatment
compared with control group (Fig.
3A).
The results of a TUNEL assay indicated that the
apoptosis rates of SO-Rb50/VCR cells in the control group and the
matrine-treated (at IC50 concentration) group at 12, 24
and 48 were 0.13, 3.2, 20.2 and 28.8%, respectively (Fig. 3B).
In the control group, the cell cycle of SO-Rb50/VCR
cells was 50±2.44% in the G0/G1 phase, 28±2.4% in the G2/M phase,
and 32±2.41% in the S phase. After treatment with matrine, the
proportion of cells in G0/G1 phase was elevated in a time-dependent
manner between 12 and 48 h. After treatment for 12 h, the cells the
proportions of cells were 54±3.77% in the G0/G1 phase, 16±0.16% in
the G2/M phase and 30±1.76% in the S phase. Following treatment
with matrine for 24 h, cells were 68±4.13% in the G0/G1 phase,
7±0.87% in the G2/M phase and 22±1.4% in the S phase. A period of
48 h after treatment, cells were 70±5.08% in the G0/G1 phase,
7±0.65% in the G2/M phase and 19±0.89% in the S phase (Fig. 4).
Effect of matrine on Bax, Bcl-2 and
cyclin D1 expression
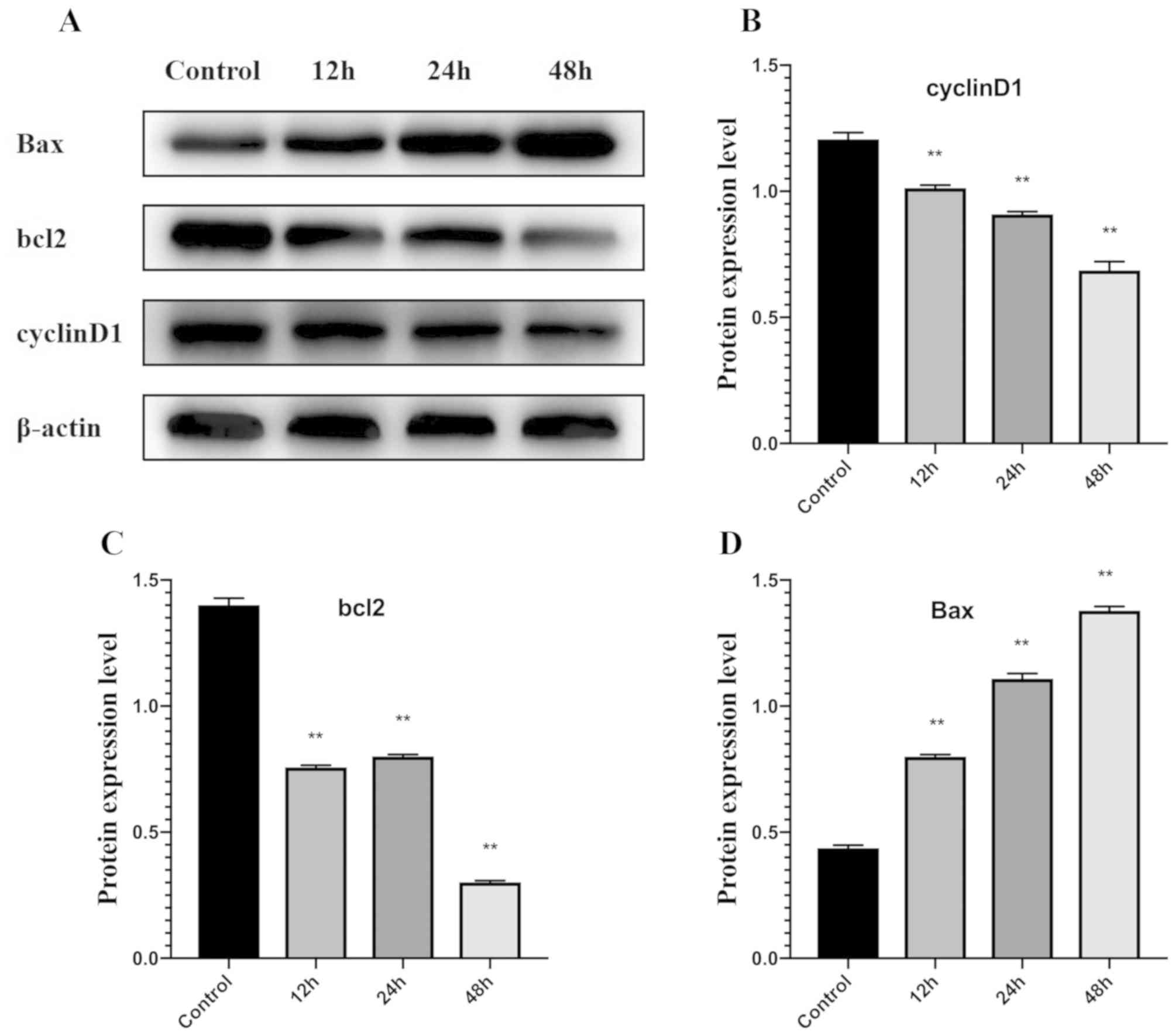
Western blot analysis demonstrated that expression
of Bcl-2 protein was significantly downregulated in the
matrine-treated group (at IC50 concentration) compared
with the control group at 12, 24 and 48 h, whereas Bax protein was
significantly upregulated at all time-points compared with the
control (Fig. 5A-C).
The expression of Cyclin D1 was significantly
downregulated after cells were treated with matrine for 12, 24 and
48 h, compared with the control group (Fig. 5D).
Effect of matrine on drug resistance
of SO-Rb50/VCR cells
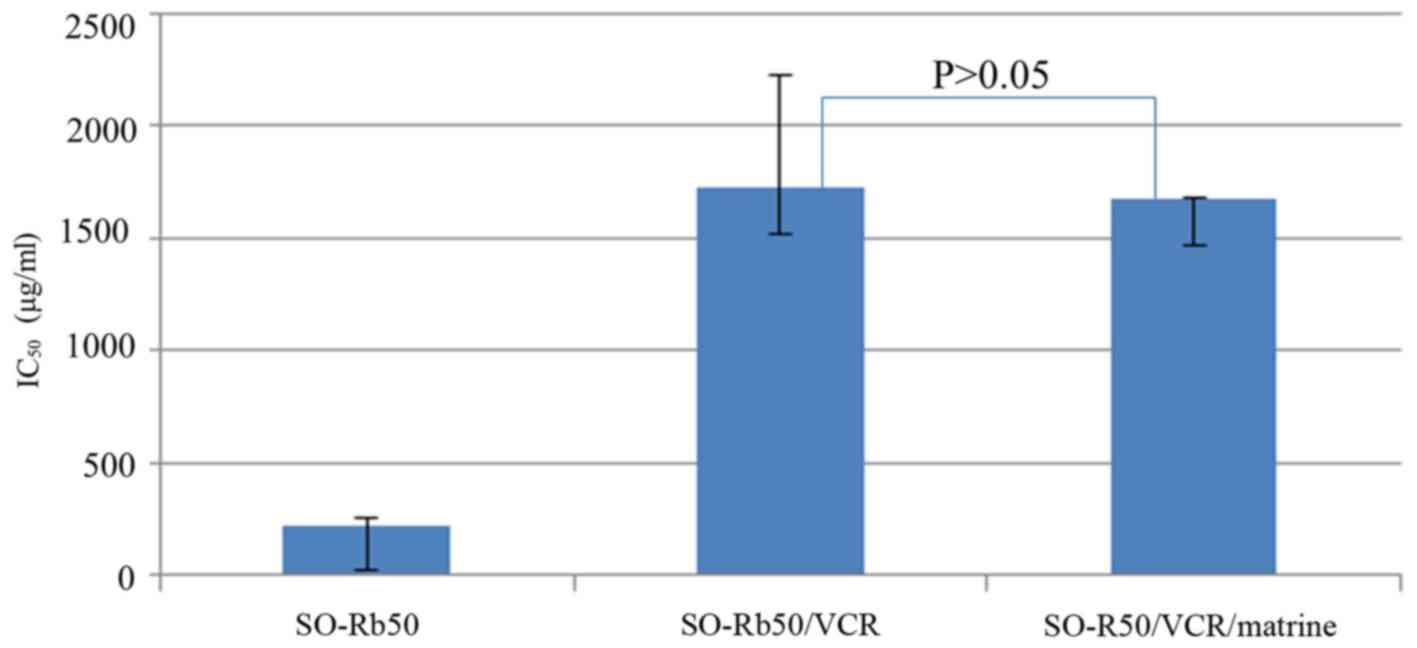
CCK-8 assay was performed to determine the reversal
effect of matrine on drug resistance in SO-Rb50/VCR, the
IC50 value of VCR of SO-Rb50/VCR cells treated with or
without matrine for 24 h was 1720±77.4 µg/ml and 1670±103.6 µg/ml,
respectively, which indicated that matrine did not affect drug
sensitivity after short-term treatment (Fig. 6).
Discussion
The treatment of retinoblastoma with
chemotherapeutic drugs serves an important role in improved
clinical outcomes for patients (24,25).
However, one of the biggest problems clinicians face is that
patients are prone to drug resistance, leading to treatment failure
(26,27). In the present study, matrine
treatment was indicated to reduce proliferation, induced apoptosis
and arrested SO-Rb50/VCR cells cell cycle in the G0/G1 phase.
However, matrine did not affect drug sensitivity after short-term
treatment. These data suggest matrine may be a potential treatment
for VCR-resistant retinoblastoma.
The results of the present study indicated that
matrine reduces the proliferation rate of tumor cells SO-Rb50 and
SO-Rb50/VCR in a dose-dependent manner. The IC50 values
of matrine in SO-Rb50 and SO-Rb50/VCR cells after 24 h were
0.96±0.04 mg/ml and 0.97±0.08 mg/ml, respectively. The present
study is in agreement with previous studies indicating the
anticancer effects of matrine on liver cancer cells, leukemia cells
and other tumor cells (28-31).
This suggests that matrine treatment may exhibit broad antitumor
activity, particularly against VCR-resistant tumor cells.
Apoptosis is not just a simple phenomenon of cell
disintegration, but is also closely associated with the onset of a
variety of diseases (32). Apoptosis
is one of the main outcomes induced by different treatments on
tumor cells (33,34). The apoptosis induced by matrine
occurred in a time-dependent manner. In the present study, the
ultrastructures of RB cells were examined using transmission
electron microscopy. Before treatment with matrine, SO-Rb50/VCR
cells were characterized by a round or ellitical shape with a large
nucleus and minimal cytoplasm. Karyokinesis and multinucleated
cells were common. After treatment with matrine, apoptosis
increased. The findings of the TUNEL assay further demonstrated
that matrine treatment could markedly increase the apoptosis rate
of SO-Rb50/VCR cells. The Bcl-2-related family of proteins
constitutes an important class of either anti-apoptotic (e.g.,
Bcl-2, Bcl-XL) or pro-apoptotic gene products (including Bax and
Bak) (35). Bcl-2 directly or
indirectly preserves the integrity of the outer mitochondrial
membrane, thus promoting cellular survival (36). In contrast, the pro-apoptotic Bcl-2
family member Bax, promotes permeabilization and the release of
cytochrome C and reactive oxygen species, which are important
signals in the apoptosis cascade (37). In the present study, the
apoptosis-associated protein Bcl-2 was downregulated and the Bax
protein was upregulated in SO-Rb50/VCR cells after treatment with
matrine.
In addition, the results of the present study showed
that the presence of matrine exerted a function in cell cycle
compared with control cells. The treated cells accumulated in the
G0/G1 phase, and correspondingly, the percentage of cells in the
G2/M phase decreased compared with untreated cells. These results
suggested that matrine could lead to a shorter DNA synthesis phase
in SO-Rb50/VCR cells. This was partially confirmed by the result
that matrine reduces the cyclin D1 content in treated cells
compared to untreated cells. As a key regulator of cell cycle G1/S
transition, cyclin D1 expression has previously been reported to be
disturbed in a number of malignancies, due to gene amplification,
chromosomal translocation, or mutations (38). It has been reported that tumor cells
frequently display increased expression of cyclin D1(39).
The effects of matrine on proliferation, apoptosis
and cell cycle arrest of RB cells have been verified by several
research groups (40-42).
The present study demonstrated a novel function of matrine in
treatment of SO-Rb50/VCR cells. Although specific mechanisms were
not distinguished, the present study further supports the
hypothesis that matrine has broad-spectrum anti-cancer activity. An
additional finding of the present study was that matrine did not
increase the sensitivity of cells to VCR. This finding differed
from previous studies, in which matrine was shown to reverse
resistance to paclitaxel (43),
cisplatin and afatinib (44).
In summary, the results of the present study
demonstrated that matrine promotes apoptosis of SO-Rb50/VCR cells
and arrests the cell cycle. The present study revealed that matrine
possesses this function not only in general RB cells but also in
vincristine resistant RB cells.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials.
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
BZ, LC, BL, QL, FG, ZZ and HB performed the
experiments and analyzed the data. BZ and YW designed the present
study and wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rao R and Honavar SG: Retinoblastoma.
Indian J Pediatr. 84:937–944. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Steinmetz B, Hackl H, Slabáková E,
Schwarzinger I, Smějová M, Spittler A, Arbesu I, Shehata M, Souček
K and Wieser R: The oncogene EVI1 enhances transcriptional and
biological responses of human myeloid cells to all-trans retinoic
acid. Cell Cycle. 13:2931–2943. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhan XX, Liu Y, Yang JF, Wang GY, Mu L,
Zhang TS, Xie XL, Wang JH, Liu YM, Kong QF, et al:
All-trans-retinoic acid ameliorates experimental allergic
encephalomyelitis by affecting dendritic cell and monocyte
development. Immunology. 138:333–345. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Philippeit C, Busch M and Dünker N:
Epigenetic control of trefoil factor family (TFF) peptide
expression in human retinoblastoma cell lines. Cell Physiol
Biochem. 34:1001–1014. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chawla B, Jain A and Azad R: Conservative
treatment modalities in retinoblastoma. Indian J Ophthalmol.
61:479–485. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Abramson DH, Shields CL, Munier FL and
Chantada GL: Treatment of retinoblastoma in 2015: Agreement and
disagreement. JAMA Ophthalmol. 133:1341–1347. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Varan A, Kiratli H, Aydın B, Tarlan B,
Poyraz CB, Akyüz C and Büyükpamukçu M: The treatment of
retinoblastoma with four-drug regimen including cisplatin,
etoposide, vincristine, and cyclophosphamide. Pediatr Hematol
Oncol. 29:529–537. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chantada G and Schaiquevich P:
Intra-arterial Chemotherapy for Retinoblastoma. JAMA Ophthalmol.
134:1202–1203. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lou Y, Qian W, Meng H, Mai W, Tong H, Tong
Y, Huang J and Jin J: High efficacy of arsenic trioxide plus
all-trans retinoic acid based induction and maintenance therapy in
newly diagnosed acute promyelocytic leukemia. Leuk Res. 37:37–42.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Masetti R, Vendemini F, Zama D, Biagi C,
Gasperini P and Pession A: All-trans retinoic acid in the treatment
of pediatric acute promyelocytic leukemia. Expert Rev Anticancer
Ther. 12:1191–1204. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Silverman JA and Deitcher SR:
Marqibo® (vincristine sulfate liposome injection)
improves the pharmacokinetics and pharmacodynamics of vincristine.
Cancer Chemother Pharmacol. 71:555–564. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang H, Li B, Bai SW and Wang HJ:
Constitutively active Akt contributes to vincristine resistance in
human retinoblastoma cells. Cancer Invest. 28:156–165.
2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ma X, Hu M, Wang H and Li J: Discovery of
traditional Chinese medicine monomers and their synthetic
intermediates, analogs or derivatives for battling P-gp-mediated
multi-drug resistance. Eur J Med Chem. 159:381–392. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu Y, Xu Y, Ji W, Li X, Sun B, Gao Q and
Su C: Anti-tumor activities of matrine and oxymatrine: Literature
review. Tumour Biol. 35:5111–5119. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yang N, Han F, Cui H, Huang J, Wang T,
Zhou Y and Zhou J: Matrine suppresses proliferation and induces
apoptosis in human cholangiocarcinoma cells through suppression of
JAK2/STAT3 signaling. Pharmacol Rep. 67:388–393. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen J, Mei Q, Xu Y-C, Du J, Wei Y and Xu
Z-M: Effects of matrine injection on T-lymphocyte subsets of
patients with malignant tumor after gamma knife radiosurgery. J
Chin Integr Med. 4:78–79. 2006.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
17
|
Huang S, Fan W, Liu P and Tian J: Meta
analysis of compound matrine injection combined with cisplatin
chemotherapy for advanced gastric cancer. Zhongguo Zhong Yao Za
Zhi. 36(22):3198–202. 2011.PubMed/NCBI(In Chinese).
|
|
18
|
Zhang LP, Jiang JK, Tam JW, Zhang Y, Liu
XS, Xu XR, Liu BZ and He YJ: Effects of Matrine on proliferation
and differentiation in K-562 cells. Leuk Res. 25:793–800.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jiang H, Hou C, Zhang S, Xie H, Zhou W,
Jin Q, Cheng X, Qian R and Zhang X: Matrine upregulates the cell
cycle protein E2F-1 and triggers apoptosis via the mitochondrial
pathway in K562 cells. Eur J Pharmacol. 559:98–108. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li H, Tan G, Jiang X, Qiao H, Pan S, Jiang
H, Kanwar JR and Sun X: Therapeutic effects of matrine on primary
and metastatic breast cancer. Am J Chin Med. 38:1115–1130.
2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu T, Song Y, Chen H, Pan S and Sun X:
Matrine inhibits proliferation and induces apoptosis of pancreatic
cancer cells in vitro and in vivo. Biol Pharm Bull. 33:1740–1745.
2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhao B, Li B, Bai S, Shen L, Ren R, Jonas
JB, Xu X, Lu Q and Liu Q: Effects of matrine on proliferation and
apoptosis of cultured retinoblastoma cells. Graefes Arch Clin Exp
Ophthalmol. 250:897–905. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang XQ, Wang YC, Guo YT and Tang X:
Effect of piperlongumine on drug resistance reversal in human
retinoblastoma HXO-RB44/VCR and SO-Rb50/CBP cell lines. Int J Clin
Exp Pathol. 8:2525–2534. 2015.PubMed/NCBI
|
|
24
|
Shields CL, Ramasubramanian A, Thangappan
A, et al: Chemoreduction for group E retinoblastoma: comparison of
chemoreduction alone versus chemoreduction plus low-dose external
radiotherapy in 76 eyes. Ophthalmology. 116:544–551 e541.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Schueler AO, Anastassiou G, Jurklies C,
Havers W, Wieland R and Bornfeld N: De novo intraocular
retinoblastoma development after chemotherapy in patients with
hereditary retinoblastoma. Retina. 26:425–431. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Filho JP, Correa ZM, Odashiro AN, Coutinho
AB, Martins MC, Erwenne CM and Burnier MN Jr: Histopathological
features and P-glycoprotein expression in retinoblastoma. Invest
Ophthalmol Vis Sci. 46:3478–3483. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
O'Driscoll L, Walsh N, Larkin A, Ballot J,
Ooi WS, Gullo G, O'Connor R, Clynes M, Crown J and Kennedy S:
MDR1/P-glycoprotein and MRP-1 drug efflux pumps in pancreatic
carcinoma. Anticancer Res. 27 (4B):2115–2120. 2007.PubMed/NCBI
|
|
28
|
Xu X, Ling Q, Gao F, He ZL, Xie HY and
Zheng SS: Hepatoprotective effects of marine and kuhuang in liver
transplant recipients. Am J Chin Med. 37:27–34. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang S, Qi J, Sun L, Cheng B, Pan S, Zhou
M and Sun X: Matrine induces programmed cell death and regulates
expression of relevant genes based on PCR array analysis in C6
glioma cells. Mol Biol Rep. 36:791–799. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang Y, Zhang H, Yu P, Liu Q, Liu K, Duan
H, Luan G, Yagasaki K and Zhang G: Effects of matrine against the
growth of human lung cancer and hepatoma cells as well as lung
cancer cell migration. Cytotechnology. 59:191–200. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Qin X-G, Hua Z, Shuang W, Wang YH and Cui
YD: Effects of matrine on HepG2 cell proliferation and expression
of tumor relevant proteins in vitro. Pharm Biol. 48:275–281.
2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li J, Yang S and Zhu G: Postnatal calpain
inhibition elicits cerebellar cell death and motor dysfunction.
Oncotarget. 8:87997–88007. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li Q, Cheng H, Zhu G, Yang L, Zhou A, Wang
X, Fang N, Xia L, Su J, Wang M, et al: Gambogenic acid inhibits
proliferation of A549 cells through apoptosis-inducing and cell
cycle arresting. Biol Pharm Bull. 33:415–420. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Savill J and Fadok V: Corpse clearance
defines the meaning of cell death. Nature. 407:784–788.
2000.PubMed/NCBI View
Article : Google Scholar
|
|
35
|
Birkinshaw RW and Czabotar PE: The BCL-2
family of proteins and mitochondrial outer membrane
permeabilisation. Semin Cell Dev Biol. 72:152–162. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Opferman JT and Kothari A: Anti-apoptotic
BCL-2 family members in development. Cell Death Differ. 25:37–45.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kim EM, Jung CH, Song JY, Park JK and Um
HD: Pro-apoptotic Bax promotes mesenchymal-epithelial transition by
binding to respiratory complex-I and antagonizing the malignant
actions of pro-survival Bcl-2 proteins. Cancer Lett. 424:127–135.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Diehl JA and Knudsen KE: Splice variants
and phosphorylated isoforms of cyclin D1 in tumorigenesis. In:
D-type Cyclins and Cancer. Hinds PW, Brown NE (eds). Springer,
Berlin, pp 91-109, 2018.
|
|
39
|
VanArsdale T, Boshoff C, Arndt KT and
Abraham RT: Molecular pathways: Targeting the cyclin D-CDK4/6 axis
for cancer treatment. Clin Cancer Res. 21:2905–2910.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Shao Q, Zhao X and Yao L: Matrine inhibits
the growth of retinoblastoma cells (SO-Rb50) by decreasing
proliferation and inducing apoptosis in a mitochondrial pathway.
Mol Biol Rep. 41:3475–3480. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhao B, Li B, Bai S, Shen L, Ren R, Jonas
JB, Xu X, Lu Q and Liu Q: Effects of matrine on proliferation and
apoptosis of cultured retinoblastoma cells. Graefes Arch Clin Exp
Ophthalmol. 250:897–905. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yu W, Li B, Ren RJ, Gao F, Li LQ, Liu XC
and Wang Y: The effects of matrine on cell proliferation and
telomerase activity in retinoblastoma cells in vitro. Zhonghua Yan
Ke Za Zhi. 42:594–599. 2006.PubMed/NCBI(In Chinese).
|
|
43
|
Luo SX, Deng WY, Wang XF, Lü HF, Han LL,
Chen BB, Chen XB and Li N: Molecular mechanism of
indirubin-3'-monoxime and Matrine in the reversal of paclitaxel
resistance in NCI-H520/TAX25 cell line. Chin Med J (Engl).
126:925–929. 2013.PubMed/NCBI
|
|
44
|
Liao XZ, Tao LT, Liu JH, Gu YY, Xie J,
Chen Y, Lin MG, Liu TL, Wang DM, Guo HY, et al: Matrine combined
with cisplatin synergistically inhibited urothelial bladder cancer
cells via down-regulating VEGF/PI3K/Akt signaling pathway. Cancer
Cell Int. 17(124)2017.PubMed/NCBI View Article : Google Scholar
|