Introduction
Alzheimer's disease (AD) is a progressive
neurodegenerative disease that impacts ~50 million people per year,
worldwide. In addition, AD it is the most common form (60-80%) of
dementia (1). Two major risk
factors for AD are traumatic brain injury and cerebrovascular
diseases (2). Decreased function of
the central cholinergic system leads to impaired cognitive ability
that is positively correlated with acetylcholinesterase (AchE)
activity, which reflects the state of cellular metabolism and the
activity of cholinergic neurons (3). Currently, there is no cure for
Alzheimer's disease (AD). Acetylcholinesterase inhibitors and
N-methyl-D-aspartate receptor antagonists are the main drugs used
for the clinical treatment of AD (4). The mean age of AD patients was over 70
years old, and they would die without effective treatment within 10
years after diagnosis (5,6).
Eukaryotes have an evolutionary defense system
against the destructive effect of reactive oxygen species (ROS)
overproduction, which is considered crucial for brain health
(7,8). Malondialdehyde (MDA), superoxide
dismutase (SOD) and glutathione peroxidase (GSH-Px) are common
indicators of oxidative Stress (9).
Nitric oxide synthase (iNOS) is a gas intercellular signaling
carrier. Jiang et al found that inhibiting iNOS could reduce
the risk of Alzheimer's disease in rat (10). In the last few decades, great
progresses have been made in understanding early onset familial and
delayed sporadic AD at molecular levels (11-14).
Numerous biological processes and >60% human
genes are regulated by microRNAs (miR) than can be found in most
body tissues, including brain tissues, cerebrospinal fluid and
serum (15). Dysregulation of miRNA
is therefore crucial in neurodegenerative diseases (16). Previous in vitro and in
vivo studies have explored the role of miRNAs in the
pathogenesis of AD, and demonstrated that miR-92a-3p, miR-181c-5p
and miR-210-3p, miRNA-132, miRNA-107 are in abnormal high or low
expression level in the brains of patients with AD (17-19).
miRNA-22 over-expression may be neuroprotective for
neurodegenerative diseases as well as neurodevelopmental disorders
by inhibiting apoptosis (20). For
instance, miR-132 contributes to dendritic growth of newborn
neurons in the hippocampus of adult mice (21). Furthermore, deletion of miR-132 and
miR-212 results in induction of tau aggregation and impairment of
cognitive skills in mice (22). In
addition, expressions of miR-212 and miR-23a are upregulated in
post-mortem frontal cortex tissues of patients with AD or with mild
cognitive impairment (23).
Neuron apoptosis during AD is closely associated
with MAPK pathway (24). MAPKs are
serine-threonine kinases that are naturally highly expressed in the
central nervous system. Cellular activity includes proliferation,
differentiation, survival, death, and transformation (25). The mammalian MAPK family consists of
p38 MAPK, ERK and c-Jun NH2-terminal kinases. The p38 signaling
pathway is involved in the increase of inflammation and apoptosis
subsequent to the overproduction of ROS during oxidative stress,
and MAPK1 is one of the important members of the family (26).
Therefore, we hypothesized that miR-132 may be able
to downregulate the expression of MAPK1, thus inhibiting the p38
signaling pathway and protecting the brain tissues of rats with AD
from inflammatory injury and apoptosis.
Materials and methods
Animals
A total of 70 SPF Sprague-Dawley rats (weight, 235±5
g) were purchased from the Experimental Animal Center of Capital
Medical University. A rat model of AD was established by
intracerebroventricular administration of 20 µg Aβ25-35 (4 µg/µl, 5
µl; purity ≥97%, Sigma) as previously described (27). Briefly, rats were anesthetized with
intraperitoneal injection of 3% pentobarbital sodium (30 mg/kg) and
their head was fixed in the table by stereotaxic device (Ruiwode
Life Technology Co., Ltd, China) for later injection. Subsequently,
a hole was drilled on the right parietal bone (anteroposteriorly,
1mm; laterally right, 1.5 mm; dorsoventrally, 4 mm). Model rats
were received intracerebroventricular injection of 5 µl of Aβ25-35
solution (4 µg/µl) at the speed of 1 µl/min and the normal rats
were injected with normal saline by same method. The Aβ25-35
solution was incubated at 37˚C for 96 h to induce aggregation
before usage. The rats were free to move and eat after surgery.
Rats were separated into seven groups of 10 rats as
follows: Normal group (untreated rats), model group (rat model of
AD), Ad-miR-132 negative control (NC) group (rats injected with
negative control of miR-132 adenovirus vector in the hippocampal
CA1 region), Ad-miR-132 group (model rats injected with miR-132
adenovirus vector in the hippocampal CA1 region), Ad-small
interfering (si)MAPK1 NC (model rats injected with negative control
of siRNA adenovirus vector of MAPK1 gene in the hippocampal CA1
region), Ad-siMAPK1 (model rats injected with siRNA adenovirus
vector of MAPK1 in the hippocampal CA1 region), and Ad-miR-132 +
Ad-MAPK1 group (model rats injected with miR-132 adenovirus vector
and overexpressing adenovirus vector of MAPK1 in the hippocampal
CA1 region). The adenoviral vectors used in this experiment were
purchased from Tianjin Saierbio Biotechnology Co., Ltd.. After one
week, rats were anesthetized with intraperitoneal injection of 3%
pentobarbital sodium (30 mg/kg) and, after removal of eyeballs, 0.5
ml of blood samples were collected from the ophthalmic vein, and
brains were harvested. Brain tissues and venous blood of five rats
per group were used for detection of AChE, ROS, MDA, SOD and GSH-Px
in serum. A part of hippocampus tissue was fixed with 10% neutral
formalin solution at room temperature for 24 h, dehydrated by
gradient alcohol (30-100%), and then embedded in paraffin. The
remaining part of brain tissue was stored in liquid nitrogen for
further experiments.
Rats were euthanized in the following situations: i)
During the study, a rat showed weight loss (rapid loss of 20% of
the original weight), loss of appetite (complete loss of appetite
for 24 h or 50% loss of appetite for 3 days), would not voluntary
eat or drink, or failed to or were reluctant in standing; ii)
euthanasia was performed for tissue collection and subsequent
experiments. Rats were sacrificed by rapid cervical dislocation
following anesthesia with intraperitoneal injection of 3%
pentobarbital sodium (30 mg/kg). The death of rats was confirmed by
the absence of breath and heartbeat and when rats showed pupil
dilation. The experiment was conducted in accordance with the 3R
principles and approved by the Animal Ethics Committee of Beijing
Tiantan Hospital, Capital Medical University.
Dual-luciferase reporter assay
The binding site of miR-132 to the 3'-UTR of the
MAPK1 gene was analyzed via the biological prediction website
microRNA.org (http://www.microrna.org/microrna/home.do). The
screening of 3'-UTR on this website was mainly analyzed from three
aspects: The sequence matching, the thermal stability of the double
strand of miRNA and the mRNA and the conservation of target sites.
Subsequently, the targeting relationship between miR-132 and MAPK1
was verified by dual-luciferase reporter assay. The 3'-UTR of the
MAPK1 gene and the 3'-UTR of the mutated MAPK1 gene were inserted
into the luciferase reporter gene vector pGL3-Basic and named
PGL3-MAPK1 wild-type (WT) and PGL3-MAPK1 mutant (MUT) respectively
(Shanghai GenePharma Co., Ltd.). The Renila Luciferase internal
reference plasmid and the two reporter vectors were co-transfected
into HEK 293T cells (American Type Culture Collection) with
Ad-miR-132 and Ad-miR-132 NC by Lipofectamine 3000 (Thermo Fisher
Scientific, USA). After 24 h transfection, dual luciferase assay
was performed. Protein of cells in each group was extracted using
RIPA (cat. no. R0010; Beijing Solarbio Science & Technology
Co., Ltd.) containing PMSF (0.1 mM). The detection of luciferase
activity was performed using a kit from Promega Corporation
according to the manufacturers' instructions. The relative
luciferase activity=firefly luciferase activity/Renilla
luciferase activity (15).
Reverse transcription quantitative
(RT-q) PCR
Total RNA was extracted from brain tissues using
TRIzol (Thermo Fisher Scientific, Inc.). RNA was reverse
transcribed into cDNA using TaqMan MicroRNA Assays Reverse
Transcription Primer (Thermo Fisher Scientific, Inc.). Quantitative
PCR was performed using SYBR® Premix Ex Taq™ II Kit
(Takara). The following components were added in the mixture: 25 µl
of SYBR® Premix Ex Taq™ II (2x), 2 µl of PCR upstream
and downstream primers, 1 µl of ROX Reference Dye (50x), 4 µl of
DNA template and 16 µl of ddH2O. Fluorescence
quantitative PCR was performed with ABI PRISM® 7300
(Kunke Equipment Co., Ltd., Shanghai, China). The reaction
conditions were as follows: 10 min pre-denaturation at 95˚C,
followed by 32 cycles at 95˚C for 15 sec and 60˚C for 30 sec, and
72˚C for 1 min. The relative expression levels were normalized to
endogenous control U6 and were expressed as 2-ΔΔCq and
calculated as follows (28): ΔCt=Ct
(target gene)-Ct (U6) and ΔΔCt=ΔCt
(experimental group)-ΔCt (control group). The
sequences of the primers used are present in Table I.
 | Table ISequences of the primers used for
reverse transcription quantitative PCR. |
Table I
Sequences of the primers used for
reverse transcription quantitative PCR.
| Name | Sequences |
|---|
| miR-132 | |
|
Forward |
5'-TGGATCCCCCCCAGTCCCCGTC CCTCAG-3' |
|
Reverse |
5'-TGAATTCGGATACCTTGGCCGG GAGGAC-3' |
| U6 | |
|
Forward |
5'-CTCGCTTCGGCAGCACA-3' |
|
Reverse |
5'-AACGCTTCACGAATTTGCGT-3' |
Western blotting
Total protein was extracted from brain tissues using
RIPA (cat. no. R0010; Beijing Solarbio Science & Technology
Co., Ltd.) containing PMSF (0.1 mM). The protein concentration was
determined using BCA kit (Thermo Fisher Scientific, Inc.). The
sample was mixed with the loading buffer and heated at 100˚C in a
water bath for 10 min. Proteins (50 µg) were separated by 10%
SDS-PAGE and transferred onto a PVDF membrane (cat. no. ISEQ00010;
EMD Millipore). Membranes were blocked using 5% skim milk at 4˚C
for 2 h and washed with 0.1% TBST. Membranes were incubated with
primary antibodies against phosphorylated (p) p38MAPK (ab31828;
1:1,000; Abcam), MAPK1 (ab31828; 1:5,000; Abcam), iNOS (ab213987;
1:5,000; Abcam) and GAPDH (ab22555; 1:2,000; Abcam) overnight at
4˚C. After three washes with TBST for 6 min, membranes were
incubated with the secondary HRP-labeled goat anti-rabbit IgG
antibody (TA140003; 1:5,000; OriGene Technologies, Inc.) at room
temperature for 2 h. Membranes were washed three times with TBST
for 6 min and placed into TBS. Enhanced chemiluminescence reagent
(BB-3501; Cytiva) was used to detect the signal on the membrane.
Images were acquired on a Bio-Rad image analysis system (Bio-Rad
Laboratories, Inc.). The data were analyzed via densitometry using
ImageJ software V2.1.4.7 (National Institutes of Health) and
normalized to expression of the internal control GAPDH.
Morris water maze
The water maze is a circular pool (150 cm in
diameter and 60 cm in height) filled with water at the temperature
of 20-25˚C. The pool was divided into four quadrants as follows:
Lower right, upper right, lower left and upper left, with a
platform installed in the lower right quadrant. After 5 days of
navigation training, the rats were placed in water and the time to
find the platform within 2 min (escape latency) was recorded. If
the rat failed to locate the platform within 2 min, they would be
guided to the platform and allowed to stay on it for 10 sec
(recorded as 2 min). On the 5th day, the platform in the water was
removed to conduct the space exploration experiment. Each rat was
placed in the same place of the pool and the time spent in the
lower right quadrant, along with the number of times the rat swum
by the original platform position were recorded, which corresponds
to the number of times passing through the platform.
Hematoxylin and eosin (H&E)
staining
The brain tissues were fixed in 10% neutral formalin
solution at room temperature for 24 h, dehydrated with gradient
alcohol (30-100%), dewaxed with xylene, embedded in a wax bath and
sliced (4-6 µm). Sections were dewaxed with xylene, hydrated with
gradient alcohol (100-70%), washed with distilled water for 1 min,
stained with hematoxylin for 3 min and then rinsed with tap water.
Sections were immersed in 0.5% hydrochloric acid for 10 sec,
immersed in water for 10 min and stained in eosin solution for 5
min. Slices were conventionally dehydrated and dewaxed again, and
mounted by neutral gum. Sections were were observed under an
optical microscope (XP-330; Shanghai Bingyu Industry Co., Ltd.).
Five fields per section were randomly selected and the number of
pyknotic nerve cells was determined.
TUNEL assay
Paraffin embedded sections were dewaxed, rehydrated
by 100-70% gradient alcohol, immersed in 3%
H2O2 for 12 min and incubated with proteinase
K (20 µg/ml in Tris/HCl) for 30 min at room temperature. Sections
were washed three times with PBS for 6 min and sections were
incubated with 20 µg/ml proteinase K without DNase (ST533; Beyotime
Institute of Biotechnology) at 37˚C for 15 min. Then, TUNEL
reaction mixture was added and incubated at 37˚C for 1 h in a wet
box (C1088; Beyotime Institute of Biotechnology). Samples were
washed three times with PBS for 6 min and observed under a
fluorescence microscope (ECLIPSE Ti; Nikon Corporation) and cells
exhibiting green fluorescent were TUNEL-positive cells. The
apoptotic index was calculated as follows: Apoptotic rate=number of
TUNEL-positive cells/total number of cells x100.
Detection of AChE and iNOS in brain
tissues and ROS, MDA, SOD and GSH-Px in serum
The levels of iNOS and AChE in brain tissues were
determined using ELISA kits (cat. nos. 69-98762 and 69-30132,
respectively; Merck KGaA). The blood sample were stand at room
temperature for 2 h and centrifuged at 10,000 x g for 10 min to
obtain serum. The levels of MDA and SOD in serum were measured with
the use of ROS (cat. no. E004-1-1), MDA (cat. no. A003-1-2), GSH-Px
(cat. no. A006-2-1), and SOD (cat. no. A001-3-3) kits from Nanjing
Jiancheng Bioengineering Institute.
Statistical analyses
All data were analyzed using SPSS v21.0 statistical
software (IBM Corp.). Data were expressed as the means ± standard
deviation. The comparison among multiple groups was performed using
one-way analysis of variance followed by Tukey post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-132 inhibits MAPK1 gene
expression
The biological prediction site microrna.org (http://www.microrna.org/microrna/home.do) predicted
that miR-132 and MAPK1 had specific binding sites (Fig. 1A). The results from the
dual-luciferase reporter assay demonstrated that the luciferase
activity in the subgroup PGL3-MAPK1 WT of Ad-miR-132 group was
significantly lower compared with that in the Ad-miR-132 NC group
(P<0.05). However, the luciferase activity in the subgroup
PGL3-MAPK1 MUT did not change significantly (P>0.05; Fig. 1B). miR-132 could inhibit the
expression of MAPK1.
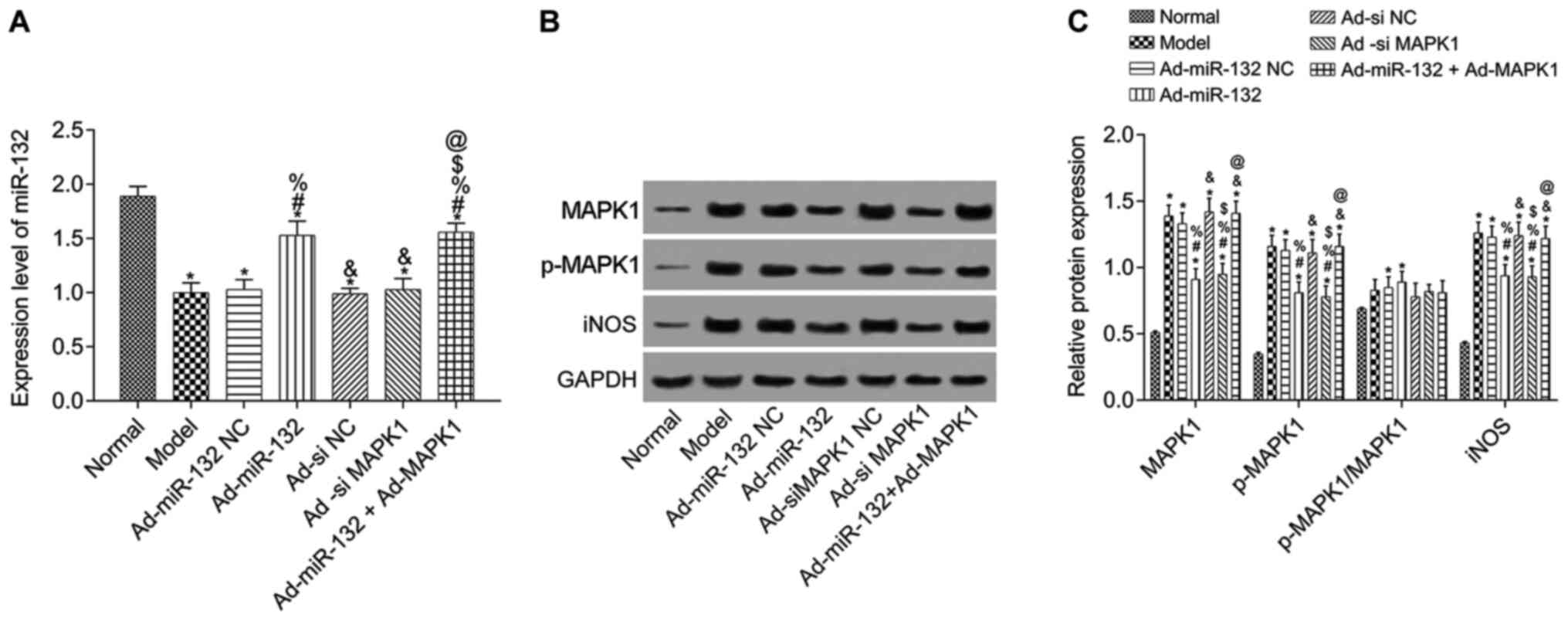
Expression of miR-132, MAPK1, p-MAPK1
and iNOS
To further verify the results of dual-luciferase
reporter assay, miR-132 expression level was detected by RT-qPCR
and the expression of MAPK1, p-MAPK1 and iNOS was evaluated by
western blotting in hippocampus tissue of rats with AD (Fig. 2). Compared with the Normal group,
miR-132 expression level was significantly downregulated in model
group, and expression of MAPK1, p-MAPK1 and iNOS was significantly
upregulated (P<0.05). Compared with the Model group, miR-132
expression was significantly increased in the Ad-miR-132 group and
the Ad-miR-132 + Ad-MAPK1 group (P<0.05). The other groups had
similar levels of miR-132 expression (Fig. 2A). Furthermore, the expression of
MAPK1, p-MAPK1 and iNOS in Ad-miR-132 NC group, Ad-siMAPK1 NC group
and Ad-miR-132 Ad-MAPK1 group was similar to that of the Model
group (P>0.05). However, the expression of MAPK1, p-MAPK1 and
iNOS in Ad-miR-132 group and Ad-siMAPK1 group were significantly
decreased compared with the Model group (all P<0.05). In
addition, compared with Ad-miR-132 group, the expression of MAPK1,
p-MAPK1 and iNOS in Ad-miR-132 + Ad-MAPK1 group was significantly
increased (P<0.05, Fig. 2B and
C).
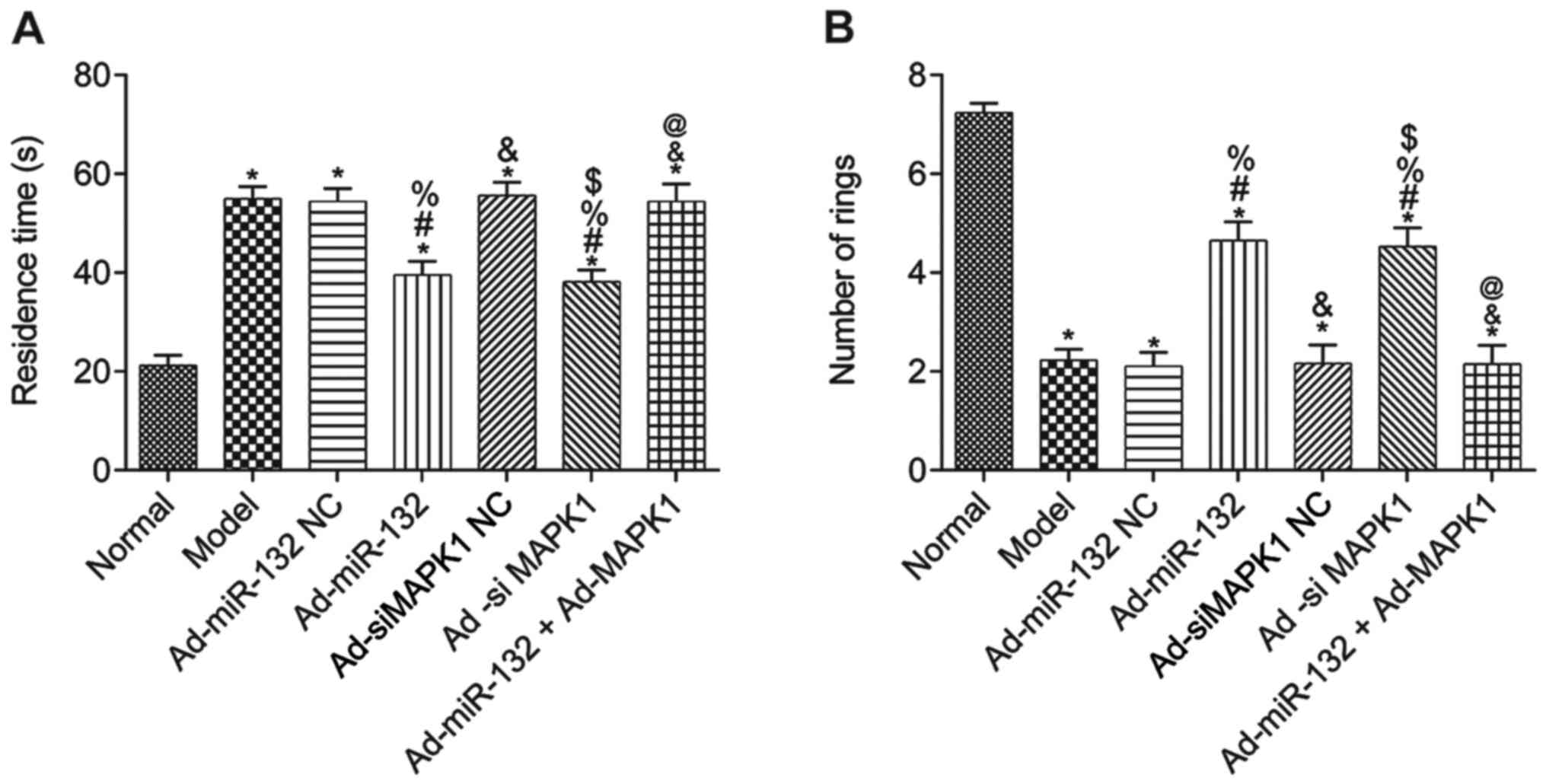
Learning and memory ability
The results from the water maze test demonstrated
that compared with the Normal group, the escape latency of rats in
all other groups was significantly elevated, and the number of
times passing through the rings was significantly decreased
(P<0.05; Fig. 3A and B). Furthermore, compared with the Model
group, there was no significant difference in Ad-miR-132 NC group,
Ad-siMAPK1 NC group and Ad-miR-132 + Ad-MAPK1 group in the two
experiments (P>0.05; Fig. 3A and
B). However, the Ad-miR-132 group
and Ad-siMAPK1 group had significantly decreased escape latency and
significantly elevated number of times passing through the rings
compared with the Model group (P<0.05; Fig. 3A and B). In addition, compared with the
Ad-miR-132 group, the Ad-miR-132 + Ad-MAPK1 group had significantly
elevated escape latency and significantly decreased number of times
passing through the rings (P<0.05; Fig. 3A and B).
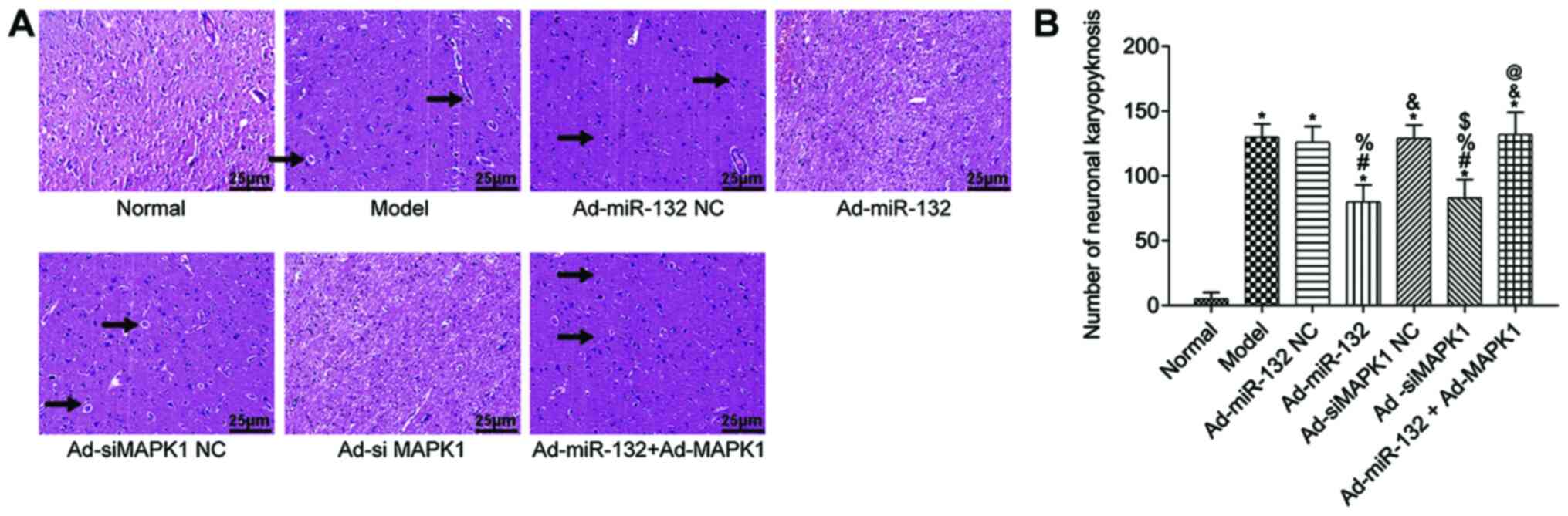
Pathological changes of brain
tissues
The pathological changes of rat hippocampus tissues
were detected by HE staining (Fig.
4). The hippocampus tissue of Normal group showed regular
structure and no obvious pathological damage (irregular structure,
neuronal shrinkage and deep staining color). However, other groups
presented with different degrees of pathological damage, which were
less severe in the Ad-miR-132 group and the Ad-siMAPK1 group. The
quantification results were consist with above description
(Fig. 4B).
Apoptosis of brain neurons in
rats
The apoptosis of hippocampal neurons was detected by
TUNEL staining (Fig. 5). The
results demonstrated that the apoptosis rate of hippocampus neurons
was significantly in all groups compared with the Normal group
(P<0.05). Compared with the Model group, there were no
statistical difference in the apoptotic rate in the Ad-miR-132 NC
group, Ad-siMAPK1 NC group and Ad-miR-132 + Ad-MAPK1 group
(P>0.05). However, the Ad-miR-132 group and Ad-siMAPK1 group had
significantly decreased apoptosis rate compared with the Model
group (P<0.05). In addition, compared with Ad-miR-132 group, the
apoptosis rate of hippocampus neurons in the Ad-miR-132 + Ad-MAPK1
group was significantly elevated (P<0.05).
Levels of AChE, ROS, MDA, SOD and
GSH-Px in serum of rats
The levels of AChE, ROS, MDA, SOD and GSH-Px in the
serum of rats from each group are presented in Fig. 6. Compared with the Normal group, all
groups had presented significantly decreased serum levels of SOD
and GSH-Px, as well as significantly elevated AChE, ROS and MDA
levels (P<0.05). There were no difference in the serum levels of
AChE, ROS, MDA, SOD and GSH-Px between the Model group, Ad-miR-132
NC group, Ad-siMAPK1 NC group and Ad-miR-132 + Ad-MAPK1 group
(P>0.05). Furthermore, compared with the Model group, the
Ad-miR-132 group and Ad-siMAPK1 group presented significantly
elevated levels of serum SOD and GSH-Px, and decreased levels of
AChE, ROS and MDA (P<0.05). In addition, compared with the
Ad-miR-132 group, the Ad-miR-132 + Ad-MAPK1 group had significantly
decreased serum levels of SOD and GSH-Px, and elevated serum levels
of AChE, ROS and MDA (P<0.05).
 | Figure 6Serums levels of AChE, ROS, MDA, SOD
and GSH-PX in rats from different groups (n=5). Serum levels of (A)
AChE, (B) ROS, (C) MDA, (D) SOD and (E) GSH-PX.
*P<0.05 vs. Normal group; #P<0.05 vs.
Model group; %P<0.05 vs. Ad-miR-132 NC group;
&P<0.05 vs. Ad-miR-132 group;
$P<0.05 vs. Ad-siMAPK1 NC group;
@P<0.05 vs. Ad-siMAPK1 group. NC, negative control;
miR, microRNA; si, small interfering; GSH-Px, glutathione
peroxidase; MDA, malondialdehyde; ROS, reactive oxygen species;
SOD, superoxide dismutase; AChE, acetylcholinesterase. |
Discussion
AD is a progressive neurodegenerative disease
characterized by loss of memory and cognitive function and is
considered as a major cause of dementia (27). Recently, numerous potential
biomarkers have been used in combination with therapeutic targets
for the treatment of AD (29). For
example, Lin et al (30)
used Osthole to upregualte miRNA-101a-3p. Several miRNAs have been
reported to be involved in a variety of diseases (16-19).
Because of their stability and endogenous nature, miRNAs may be
used as treatment options in AD (31).
Certain miRNAs can control the formation, maturation
and function of synapse, and their abnormal expressions might be
the basis of synaptic dysfunction (32). Furthermore, a number of specific
miRNAs are dysregulated in patients with AD, including the miRNAs
of key genes of AD, such as amyloid precursor protein or
beta-secretase 1, or of neuronal functions, such as glutamate
receptors (33). Che et al
(34) demonstrated that miR-132 can
regulate the angiogenesis of patients with cerebral ischemia
through NF-κB and vascular endothelial growth factor pathways, and
reported that overexpression of miR-132 in vitro could
decrease iNOS expression. In the present study, specific binding
sites of miR-132 and MAPK1 were found through bioinformatics
analysis, and dual-luciferase reporter assay confirmed that miR-132
can negatively target MAPK1 gene. This study therefore hypothesized
that miR-132 may be involved in the occurrence and development of
AD by regulating MAPK signaling pathway, which may provide a
promising new pathway for the treatment of AD.
In the present study, rats were treated with miR-132
analogs and siMAPK1, and the results demonstrated that after p38
signaling pathway was disturbed, the learning ability, memory and
brain tissue disorder of AD rats were significantly improved, the
apoptosis of nerve cells was decreased, and the serum levels of
AChE was significantly decreased. Previous studies on neural and
non-neuronal cells have demonstrated that p38 regulates apoptosis
through a variety of mechanisms, including activation of p53, as
well as phosphorylation of c-JUN and c-fos, induction of Bax
transposition, and involvement in Fas-FasL-mediated apoptosis,
enhancement of c-myc expression and activation of caspase-3
(35,36). In addition, p38 MAPK can enhance the
expression of TNF-α, thereby activating p38-induced apoptosis
(37). ERK is involved in cell
activation and migration and plays an important role in synaptic
plasticity and memory in vivo (38). Amyloid beta has been reported to
induce JNK activation and cell death (39). Therefore, miR-132 may improve
learning ability and memory, improving brain disorder in rats with
AD and protecting nerve cells from apoptosis by inhibiting the p38
signaling pathway.
Numerous studies have reported that miRNAs can
regulate oxidative stress (40-42).
Increased oxidative stress can lead to cell apoptosis and serves a
key role in neurodegenerative diseases, such as AD (42). In the present study, rats treated
with miR-132 analogs and siMAPK1 presented with decreased serum
levels of ROS and MDA, elevated serum levels of SOD and GSH-Px and
downregulated iNOS. Overall, these results suggested that oxidative
stress was decreased and that the antioxidant defense systems were
stimulated. Che et al (34)
reported that miR-132 overexpression in vitro inhibited
iNOS, which is consistent with the present results. Subsequently,
inhibition of p38 signaling pathway may improve brain damage,
decrease oxidative stress and improve cognitive dysfunction.
In conclusion, the present study demonstrated that
miR-132 inhibit iNOS expression in brain tissue, decreased
oxidative stress and ameliorated the cognitive function in AD rats
through p38 signaling pathway These findings may help understanding
the pathogenesis of AD and may serve the development of novel
clinical treatment. However, the association between miR-132
signaling pathway and AD are not fully understood, and the
downstream molecules of the p38 signaling pathway need to be
further investigated. In addition, since miR-132 and miR-212 are
both related to AD, the role of miR-212 in AD requires further
investigation.
Acknowledgements
Not applicable.
Funding
This study was supported by the Beijing Municipal
Administration of Hospitals' Youth Program (grant no. QML20180506)
and the Natural Science Foundation of Beijing (grant no.
17G10258).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LS contributed to manuscript concept. YD, JZ and XS
carried out the experiments and analyzed data. GM, GL and ZM
prepared the experimental data and performed statistical analysis.
LS and YD involved in drafting the manuscript or revising it
critically. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of Beijing Tiantan Hospital, Capital Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Herrera-Espejo S, Santos-Zorrozua B,
Álvarez-González P, Lopez-Lopez E and Garcia-Orad Á: A systematic
review of microRNA expression as biomarker of late-onset
Alzheimer's disease. Mol Neurobiol. 56:8376–8391. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pan Y, Liu R, Terpstra E, Wang Y, Qiao F,
Wang J, Tong Y and Pan B: Dysregulation and diagnostic potential of
microRNA in Alzheimer's disease. J Alzheimers Dis. 49:1–12.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ossenkoppele R, Mattsson N, Teunissen CE,
Barkhof F, Pijnenburg Y, Scheltens P, van der Flier WM and
Rabinovici GD: Cerebrospinal fluid biomarkers and cerebral atrophy
in distinct clinical variants of probable Alzheimer's disease.
Neurobiol Aging. 36:2340–2347. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wu X, Cai H, Pan L, Cui G, Qin F, Li Y and
Cai Z: Small molecule natural products and Alzheimer's disease.
Curr Top Med Chem. 19:187–204. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Panza GA, Taylor BA, MacDonald HV, Johnson
BT, Zaleski AL, Livingston J, Thompson PD and Pescatello LS: Can
exercise improve cognitive symptoms of Alzheimer's disease? J Am
Geriatr Soc. 66:487–495. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Torvinen-Kiiskinen S, Tolppanen AM,
Koponen M, Tanskanen A, Tiihonen J, Hartikainen S and Taipale H:
Proton pump inhibitor use and risk of hip fractures among
community-dwelling persons with Alzheimer's disease-a nested
case-control study. Aliment Pharmacol Ther. 47:1135–1142.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nikolova G and Mancheva V: Analysis of the
parameters of oxidative stress in patients with Parkinson's
disease. Com Clin Pathol. 22:151–155. 2012.
|
|
8
|
Bouchez C and Devin A: mitochondrial
biogenesis and mitochondrial reactive oxygen species (ROS): A
complex relationship regulated by the cAMP/PKA signaling pathway.
Cells. 8(287)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gökçe Çokal B, Yurtdaş M, Keskin Güler S,
Güneş HN, Ataç Uçar C, Aytaç B, Durak ZE, Yoldaş TK, Durak İ and
Çubukçu HC: Serum glutathione peroxidase, xanthine oxidase, and
superoxide dismutase activities and malondialdehyde levels in
patients with Parkinson's disease. Neurol Sci. 38:425–431.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jiang P, Li C, Xiang Z and Jiao B:
Tanshinone IIA reduces the risk of Alzheimer's disease by
inhibiting iNOS, MMP-2 and NF-κBp65 transcription and translation
in the temporal lobes of rat models of Alzheimer's disease. Mol Med
Rep. 10:689–694. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kumar S, Reddy AP, Yin X and Reddy PH:
Novel MicroRNA-455-3p and its protective effects against abnormal
APP processing and amyloid beta toxicity in Alzheimer's disease.
Biochim Biophys Acta Mol Basis Dis. 1865:2428–2440. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Davidson YS, Raby S, Foulds PG, Robinson
A, Thompson JC, Sikkink S, Yusuf I, Amin H, DuPlessis D, Troakes C,
et al: TDP-43 pathological changes in early onset familial and
sporadic Alzheimer's disease, late onset Alzheimer's disease and
Down's syndrome: Association with age, hippocampal sclerosis and
clinical phenotype. Acta Neuropathol. 122:703–713. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cruchaga C, Del-Aguila JL, Saef B, Black
K, Fernandez MV, Budde J, Ibanez L, Deming Y, Kapoor M, Tosto G, et
al: Polygenic risk score of sporadic late-onset Alzheimer's disease
reveals a shared architecture with the familial and early-onset
forms. Alzheimers Dement. 14:205–214. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jin SC, Pastor P, Cooper B, Cervantes S,
Benitez BA, Razquin C and Goate A: Ibero-American Alzheimer Disease
Genetics Group Researchers and Cruchaga C. Pooled-DNA sequencing
identifies novel causative variants in PSEN1, GRN and MAPT in a
clinical early-onset and familial Alzheimer's disease
Ibero-American cohort. Alzheimers Res Ther. 4(34)2012.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Duan Q and Si E: MicroRNA-25 aggravates
Aβ1-42-induced hippocampal neuron injury in Alzheimer's disease by
downregulating KLF2 via the Nrf2 signaling pathway in a mouse
model. J Cell Biochem. 120:15891–15905. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yang K, Feng S, Ren J and Zhou W:
Upregulation of microRNA-196a improves cognitive impairment and
alleviates neuronal damage in hippocampus tissues of Alzheimer's
disease through downregulating LRIG3 expression. J Cell Biochem.
120:17811–17821. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Siedlecki-Wullich D, Català-Solsona J,
Fábregas C, Hernández I, Clarimon J, Lleó A, Boada M, Saura CA,
Rodríguez-Álvarez J and Miñano-Molina AJ: Altered microRNAs related
to synaptic function as potential plasma biomarkers for Alzheimer's
disease. Alzheimers Res Ther. 11(46)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Luikart BW, Bensen AL, Washburn EK,
Perederiy JV, Su KG, Li Y, Kernie SG, Parada LF and Westbrook GL:
miR-132 mediates the integration of newborn neurons into the adult
dentate gyrus. PLoS One. 6(e19077)2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang WX, Rajeev BW, Stromberg AJ, Ren N,
Tang G, Huang Q, Rigoutsos I and Nelson PT: The expression of
microRNA miR-107 decreases early in Alzheimer's disease and may
accelerate disease progression through regulation of beta-site
amyloid precursor protein-cleaving enzyme 1. J Neurosci.
28:1213–1223. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jovicic A, Zaldivar Jolissaint JF, Moser
R, Silva Santos Mde F and Luthi-Carter R: MicroRNA-22 (miR-22)
overexpression is neuroprotective via general anti-apoptotic
effects and may also target specific Huntington's disease-related
mechanisms. PLoS One. 8(e54222)2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Smith PY, Hernandez-Rapp J, Jolivette F,
Lecours C, Bisht K, Goupil C, Dorval V, Parsi S, Morin F, Planel E,
et al: miR-132/212 deficiency impairs tau metabolism and promotes
pathological aggregation in vivo. Hum Mol Genet. 24:6721–6735.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hansen KF, Sakamoto K, Aten S, Snider KH,
Loeser J, Hesse AM, Page CE, Pelz C, Arthur JS, Impey S and
Obrietan K: Targeted deletion of miR-132/-212 impairs memory and
alters the hippocampal transcriptome. Learn Mem. 23:61–71.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Weinberg RB, Mufson EJ and Counts SE:
Evidence for a neuroprotective microRNA pathway in amnestic mild
cognitive impairment. Front Neurosci. 9(430)2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li Y, Ba M, Du Y, Xia C, Tan S, Ng KP and
Ma G: Aβ1-42 increases the expression of neural KATP subunits
Kir6.2/SUR1 via the NF-κB, p38 MAPK and PKC signal pathways in rat
primary cholinergic neurons. Hum Exp Toxicol. 38:665–674.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jiang S, Zhao Y, Zhang T, Lan J, Yang J,
Yuan L, Zhang Q, Pan K and Zhang K: Galantamine inhibits
β-amyloid-induced cytostatic autophagy in PC12 cells through
decreasing ROS production. Cell Prolif. 51(e12427)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Meng M, Ai D, Sun L, Xu X and Cao X: EGb
761 inhibits Aβ1-42-induced neuroinflammatory response by
suppressing P38 MAPK signaling pathway in BV-2 microglial cells.
Neuroreport. 30:434–440. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Galoyan AA, Sarkissian JS, Chavushyan VA,
Meliksetyan IB, Avagyan ZE, Poghosyan MV, Vahradyan HG, Mkrtchian
HH and Abrahamyan DO: Neuroprotection by hypothalamic peptide
proline-rich peptide-1 in Abeta25-35 model of Alzheimer's disease.
Alzheimers Dement. 4:332–344. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Brennan S, Keon M, Liu B, Su Z and Saksena
NK: Panoramic visualization of circulating microRNAs across
neurodegenerative diseases in humans. Mol Neurobiol. 56:7380–7407.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lin Y, Liang X, Yao Y, Xiao H, Shi Y and
Yang J: Osthole attenuates APP-induced Alzheimer's disease through
up-regulating miRNA-101a-3p. Life Sci. 225:117–131. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gao S, Lin J, Wang T, Shen Y, Li Y, Yang
W, Zhou K and Hu H: Qingxin kaiqiao fang ameliorates memory
impairment and inhibits apoptosis in APP/PS1 double transgenic mice
through the MAPK pathway. Drug Des Devel Ther. 13:459–475.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sadlon A, Takousis P, Alexopoulos P,
Evangelou E, Prokopenko I and Perneczky R: miRNAs identify shared
pathways in Alzheimer's and Parkinson's diseases. Trends Mol Med.
25:662–672. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Prakash D and Sudhandiran G: Dietary
flavonoid fisetin regulates aluminium chloride-induced neuronal
apoptosis in cortex and hippocampus of mice brain. J Nutr Biochem.
26:1527–1539. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Che F, Du H, Zhang W, Cheng Z and Tong Y:
MicroRNA-132 modifies angiogenesis in patients with ischemic
cerebrovascular disease by suppressing the NF-κB and VEGF pathway.
Mol Med Rep. 17:2724–2730. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hwang S, Jeong H, Hong EH, Joo HM, Cho KS
and Nam SY: Low-dose ionizing radiation alleviates Aβ42-induced
cell death via regulating AKT and p38 pathways in Drosophila
Alzheimer's disease models. Biol Open. 8(bio036657)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kim SJ, Hwang SG, Shin DY, Kang SS and
Chun JS: p38 kinase regulates nitric oxide-induced apoptosis of
articular chondrocytes by accumulating p53 via NFkappa B-dependent
transcription and stabilization by serine 15 phosphorylation. J
Biol Chem. 277:33501–33508. 2002.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Muraleva NA, Kolosova NG and Stefanova NA:
p38 MAPK-dependent alphaB-crystallin phosphorylation in Alzheimer's
disease-like pathology in OXYS rats. Exp Gerontol. 119:45–52.
2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chen Y, Liang Z, Blanchard J, Dai CL, Sun
S, Lee MH, Grundke-Iqbal I, Iqbal K, Liu F and Gong CX: A
non-transgenic mouse model (icv-STZ mouse) of Alzheimer's disease:
Similarities to and differences from the transgenic model (3xTg-AD
mouse). Mol Neurobiol. 47:711–725. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hu Z, Jiao R, Wang J, Wang P, Zhu Y, Zhao
J, De Jager P, Bennett DA, Jin L and Xiong M: Shared causal paths
underlying Alzheimer's dementia and type 2 diabetes. Sci Rep.
10(4107)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ishimoto T, Sugihara H, Watanabe M,
Sawayama H, Iwatsuki M, Baba Y, Okabe H, Hidaka K, Yokoyama N,
Miyake K, et al: Macrophage-derived reactive oxygen species
suppress miR-328 targeting CD44 in cancer cells and promote redox
adaptation. Carcinogenesis. 35:1003–1011. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ebi H, Sato T, Sugito N, Hosono Y, Yatabe
Y, Matsuyama Y, Yamaguchi T, Osada H, Suzuki M and Takahashi T:
Counterbalance between RB inactivation and miR-17-92 overexpression
in reactive oxygen species and DNA damage induction in lung
cancers. Oncogene. 28:3371–3379. 2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Taupin P: A dual activity of ROS and
oxidative stress on adult neurogenesis and Alzheimer's disease.
Cent Nerv Syst Agents Med Chem. 10:16–21. 2010.PubMed/NCBI View Article : Google Scholar
|