Introduction
Inflammatory bowel disease (IBD), which primarily
manifests as ulcerative colitis (UC) or Crohn's disease, is
considered a chronic inflammatory disease of the rectum and colon
with an unknown etiology (1,2). IBD
is mainly characterized by abdominal pain, diarrhea, mucus,
purulent stool, and pathological lesions limited to the mucosa and
sub-mucosa (1,2). The prevalence of IBD is higher in
Europe and USA; however, the morbidity of IBD in Asian countries is
increasing yearly, particularly in China, which has the highest
rate amongst Asian countries. At present, the morbidity of UC in
China is ~3.44 per 100,000 individuals (3). The pathogenic process of UC is
associated with dysregulation of the intestinal flora and
intestinal mucosal immune disarrangements (4), and is characterized by bacterial
diversity loss and transfer of microflora (5,6). It
has been demonstrated that the dextran sulfate sodium (DSS)-induced
colitis model is the best UC model for simulating the pathological
processes of UC, including abnormal cytokine production, and
infiltration of neutrophils and macrophages into colonic epithelial
cells (7,8). In a previous study on DSS-induced
mouse models, the number of lactic acid bacteria decreased notably,
whereas that of Vibrio desulfuricans significantly
increased, suggesting that such changes in the microflora maybe
closely associated with intestinal inflammation (9). Numerous studies on the intestinal
microflora have been performed to prevent or treat UC (10), including transplantation of fecal
bacteria, which achieves its therapeutic effect by returning the
normal functionality of the intestinal flora (11). Clinically, numerous immunomodulators
and non-steroidal anti-inflammatory drugs (such as sulfadiazine and
glucocorticoids) have been widely applied for treating UC (12,13).
However, the above therapeutic strategies usually cause various
adverse effects, such as vomiting, hepatorenal toxicity, systemic
edema and anemia (10,11). Traditional Chinese Medicines (TCMs)
possess strong anti-inflammatory effects with fewer side-effects
(14). As a result, TCMs have
attracted increasing attention due to their anti-inflammatory
properties, particularly as a treatment for IBD (15-17).
Leonurine (YMJ), as an active ingredient, was first
extracted as a TCM from Leonurus heterophyllus (18). YMJ promotes uterine contraction, and
prevents osteoporosis, mastitis and myocardial fibrosis (19). The above functions of YMJ maybe
associated with its anti-inflammatory effects. Previous studies
reported that YMJ could inhibit neuro-inflammation, suppress the
PI3K/AKT/NF-κB signaling pathway and prevent endometritis (20-22).
Therefore, it was speculated that YMJ may also possess beneficial
effects as a treatment for UC. TCMs prevent and treat IBD by
regulating the intestinal microflora, such as Chlorogenic
acid, Escherichia coli, F. prausnitzii and
Clostridium pratense (23).
The effects of YMJ on UC and the associated mechanisms have not
been explored, to the best of our knowledge. Therefore, the present
study evaluated the preventative effects of YMJ on IBD, and whether
they involved changes in the intestinal flora.
The aim of the present study was to investigate the
therapeutic effects of YMJ on DSS-induced UC in C57BL/6J mice, and
to explore the functional regulation of YMJ on intestinal flora in
mice. Improvement of colitis in mice was evaluated by estimating
changes in weight, disease activity score and colon histopathology.
In order to examine the effects of YMJ on intestinal inflammation
in IBD, the cytokine levels in serum were determined by ELISA,
whereas the infiltration of immune cells in the colonic tissues of
mice were evaluated using immunohistochemical analysis. The
mechanism of YMJ-mediated regulation of intestinal flora in mice
was evaluated using 16S ribosomal RNA (rDNA) sequencing, and by
estimating the changes in the abundance and composition of fecal
microflora.
Materials and methods
Animals
C57BL/6J wild-type mice (weighting 18-20 g; 8 weeks
old) were purchased form Shanghai SLAC Laboratory Animal Co., Ltd.
The mice were maintained in standard cages in a specific pathogen
free animal room at a temperature of 22±2˚C and a humidity of
55±5%, with a light/dark cycle of 12 h. All mice were provided
ad libitum access to sterilized water and standard chow.
This study was performed in accordance with Guide of the Care and
Use of Laboratory Animals published by the US National Institutes
of Health (NIH) (24). The present
study was approved by the Ethical Committee of Nanjing Medical
University (Nanjing, China) (approval no. IACUC-1904036).
Trial grouping, UC model establishment
and sample collection
A total of 15 mice were randomly divided into three
groups: A control group (n=5), a DSS group (n=5) and a DSS+YMJ
group (n=5). During the 15 days of experiments, the mice were
administered different treatments. The mice in the control group
were provided double-distilled water for 15 days ad libitum,
without any other treatments. In the DSS group, the mice were
provided double-distilled water for 7 days ad libitum,
followed by double-distilled water containing 2.5% DSS (cat. no.
42867; Sigma-Aldrich; Merck KGaA)for the subsequent 8 days. In the
DSS+YMJ group, the mice were provided double-distilled water
containing 1 mM YMJ (cat. no. PA12123; Weng Jiang Reagent Co.,
Ltd.) for 7 days, followed by administration of double-distilled
water containing 2.5% DSS for the 8 subsequent days.
Following the above treatments for 15 days, the skin
surrounding the anus was disinfected with 75% ethanol to stimulate
defecation. Then, the feces of mice were collected and stored in a
sterile Eppendorf tube at -80˚C for extracting DNA and bacterial
microflora. The mice were monitored closely daily for signs of
pain, distress and behavior, through evaluating the appetite,
activity levels and hydration status. During the above processes,
no mice died in any of the groups. The mice were euthanized using
150 mg/kg pentobarbital (Sigma-Aldrich; Merck KGaA), and a 0.1 ml
retro-orbital blood sample was collected(centrifuged at room
temperature for 2 h at 2,000 x g)for detection of inflammatory
factors in the serum, and the whole colonic tissues of mice were
obtained and fixed in neutral formalin solution for 2 h at room
temperature for subsequent experiments. The death of the mice was
confirmed by cardiac arrest, respiratory arrest, pupillary dilation
and the absence of a nerve reflex.
Evaluation of weight and disease
activity
During the experiment, the weight of the mice was
recorded at the same time every day, and the weight-change curve
was drawn accordingly. Mice exhibiting blood in their stool and
moribund mice were also observed. The severity of DSS-induced
colitis was evaluated using the disease activity index (DAI), which
involves three assessing indices, as illustrated in Table I (25). The DAI value in this study was
defined as the average score of the above three assessing indices.
Mice were anesthetized by intraperitoneal injection of
pentobarbital (50 mg/kg; Sigma-Aldrich; Merck KGaA) and then were
euthanized using 150 mg/kg pentobarbital. The entire large
intestine was separated from the anal to the ileocecal junction,
and the length of the colon between the ileocecal junction and the
anus was measured.
 | Table IScoring system for disease activity
index. |
Table I
Scoring system for disease activity
index.
| Score | Weight loss | Fecal
consistency | Bleeding |
|---|
| 0 | 0 | Normal | No |
| 1 | 0-5% | Slight changed | Trace |
| 2 | 5-10% | Mild diarrhea | Mild occult
blood |
| 3 | 10-20% | Diarrhea | Significant
bleeding |
| 4 | >20% | Severe
diarrhea | Massive
bleeding |
Hematoxylin and eosin (H&E)
staining
Colon histopathology was evaluated by H&E
staining. Briefly, ~24 h after fixing with neutral formalin, the
mice colonic tissues were dehydrated using a graded series of
alcohol solutions and embedded in paraffin. The embedded colonic
tissues were sliced into sections of a thickness of 5 µm. The
sections were placed in an oven and heated at 65˚C for 30 min.
Next, the sections were stained with hematoxylin (cat. no. C0107;
Beyotime Institute of Biotechnology) and eosin (cat. no. C0109;
Beyotime Institute of Biotechnology), as reported elsewhere
(26). Finally, the sections were
sealed with neutral resin and scanned using a light microscope
(Ax70; Olympus Corporation) (magnification, x400) with CellSens
Standard v.1.6 software (Olympus Corporation).
ELISA
The expression of inflammatory factors in serum was
determined using ELISA. Briefly, retro-orbital blood (0.1 ml) was
collected from mice and incubated for 2 h at room temperature, and
then centrifuged at 2,000 x g at 4˚C for 10 min. Next, the serum
levels of interleukin (IL)-6, tumor necrosis factor-α (TNF-α) and
IL-1β were detected using a Mouse IL-6 ELISA kit (cat. no. PI326),
Mouse TNF-α ELISA kit (cat. no. PT512) and Mouse IL-1β ELISA kit
(cat. no. PI301), respectively (all from Beyotime Institute of
Biotechnology).
Western blot analysis
The expression levels of inflammatory proteins,
including NF-κB (also known as p65) and phosphorylated (p)-p65, in
mouse colonic tissues were evaluated by western blotting. The
colonic tissues were cut into small pieces and treated with
nucleoprotein lysate (200 µl) (cat. no. P0013; Beyotime Institute
of Biotechnology) to obtain protein lysates. The concentration of
protein lysates was determined using a BCA Protein assay kit (cat.
no. P0010S, Beyotime Institute of Biotechnology). A total of 0.5 µg
protein (per lane) lysates were then subjected to SDS-PAGE, and
then electro-transferred to PVDF membranes (Amersham; Cytiva). The
PVDF membranes were first blocked with 5% bovine serum albumin
(cat. no. A8806; Sigma-Aldrich; Merck KGaA) at room temperature for
1 h, and then incubated with a rabbit anti-mouse p65 polyclonal
antibody (cat. no. ab16502; 1:2,000), rabbit anti-mouse p65
(phospho S316, phosphorylated p65, p-p65) polyclonal antibody (cat.
no. ab254097; 1:2,000) and rabbit anti-mouse GAPDH monoclonal
antibody (cat. no. ab181602; 1:2,000) at 4˚C overnight.
Subsequently, the PVDF membranes were incubated with horseradish
peroxidase-labeled goat anti-rabbit IgG antibody (cat. no.
ab205718; 1:1,000) at room temperature for 2 h. All of the above
antibodies were purchased from Abcam. Finally, signals were
visualized using BeyoECL Moon Imaging kit (cat. no. P0018FS;
Beyotime Institute of Biotechnology). The western blotting images
were captured using Quantity One version 4.6.0 (Bio-Rad
Laboratories, Inc.).
Extraction of 16S rDNA from mouse
feces, and biological analysis of composition and abundance changes
in intestinal microflora
DNA was extracted from mouse feces using a DNA
extraction kit (cat. no. D0065S; Beyotime Institute of
Biotechnology), according to the manufacturer's instructions.
Briefly, a 200-mg feces sample was added to a 2-ml centrifugal tube
and subjected to a high centrifugation speed of 10,000 x g at 4˚C
for 45 min. Next, the supernatants were discarded, and the
precipitates were re-suspended using 100 ml pre-cooled PBS and
treated with phenol, followed by centrifugation of the solutions at
a speed of 10,000 x g at 4˚C for 10 min. Finally, the purified
genomic DNA was extracted by adding pre-cooled absolute ethanol.
The concentration of total DNA was detected using an ultraviolet
spectrophotometer (NanoDrop 2000 UV; NanoDrop Technologies; Thermo
Fisher Scientific, Inc.). The 16S rDNA extraction, biological
analysis and abundance changes in intestinal microflora were
performed using the Meiji cloud platform as described in previous
studies (27,28). The above genomic DNA was used to
generate the 16S rDNA. The V3, V4 and V5 hypervariable regions of
the prokaryotic 16S rDNA were used for synthesizing the amplicons.
The DNA libraries were validated using the Agilent Bioanalyzer
(Agilent Technologies, Inc.) and quantified with a Qubit 2.0
fluorometer. Then, the DNA libraries were analyzed using the
Illumina MiSeq platform (Illumina, Inc.) and MiSeq Control Software
v.2.2.0 (Illumina, Inc.). The raw reads were filtered and matched
to the sequences spanning the V3-V4 amplicon with PANDAseq, as
described previously (29). The
merged sequences with 97% identity of nucleotide sequence were
clustered into operational taxonomic units (OTUs)using UPARSE
(30). The abundance, diversity,
similarity and composition of the bacteria were generated as
described previously (28).
Moreover, the principal co-ordinates analysis was also conducted
according to the previously studies reported (28-30).
Statistical analysis
Continuous variables were represented as the mean ±
standard deviation of at least 6 repeats, and analyzed using SPSS
(version 20.0; IBM Corp.). ANOVA followed by a Tukey's post-hoc
test was used to compare differences between multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
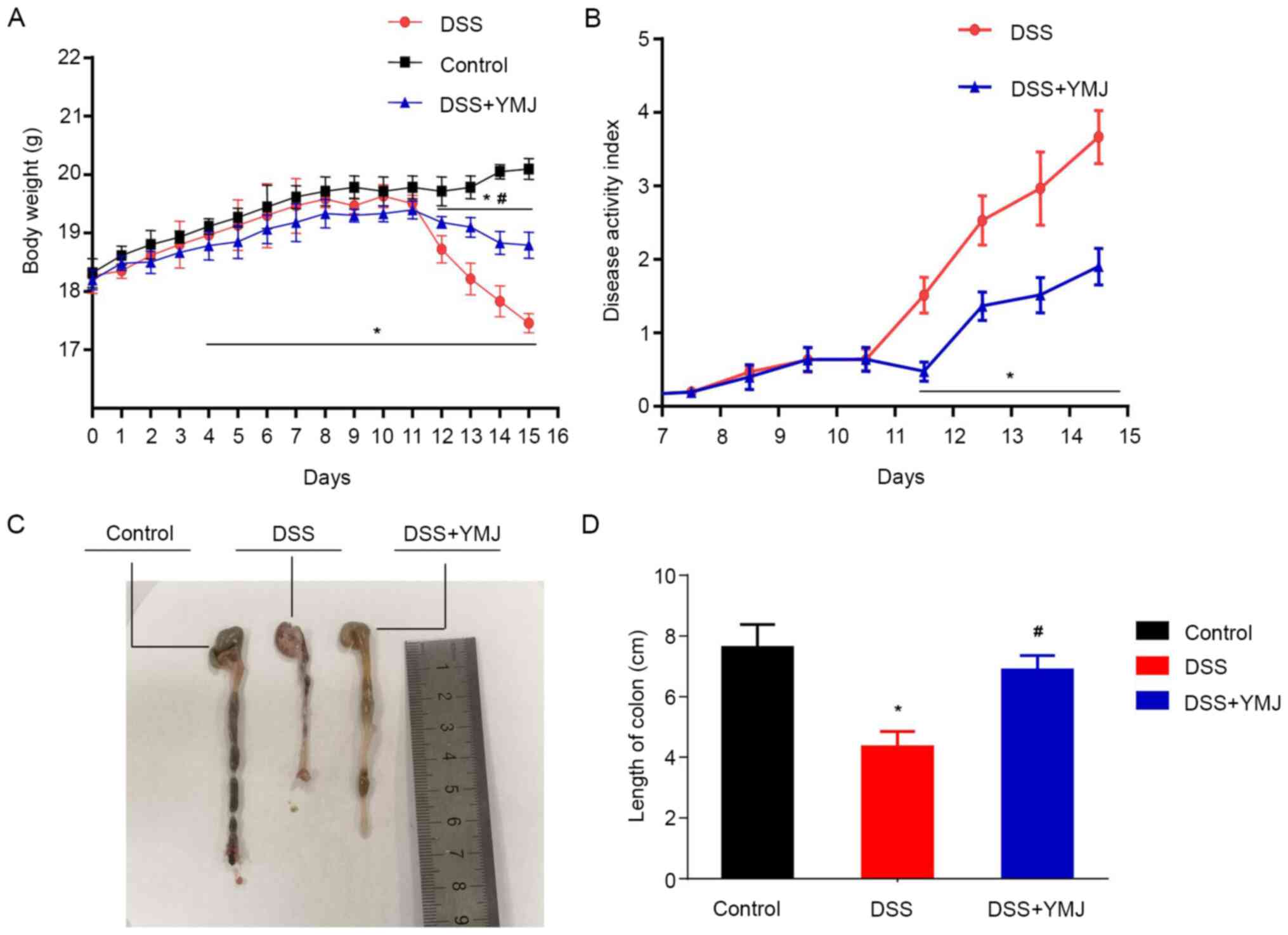
YMJ enhances body weight and reduces
the DAI score in the UC mice
The weight of the mice in the control group
increased steadily, and the mice did not exhibit diarrhea or
hematochezia. At ~4 days post-DSS induction (DSS group), diarrhea
and blood in the stool were recorded, and the weight of the mice
significantly decreased compared with that of the control mice
(Fig. 1A; P<0.05). However, the
diarrhea and blood in the stool of mice in the TMJ-treated group
(DSS+YMJ group) was notably reduced, and the weight of mice in
DSS+YMJ group was significantly higher compared with that of mice
in the DSS group from days 12-15 (Fig.
1A; P<0.05). Meanwhile, the weight of mice in DSS+YMJ group
was also significantly lower compared with that of mice in the
control group from days 12-15 (Fig.
1A; P<0.05). Furthermore, the DAI values were significantly
lower in the DSS+YMJ group compared with the DSS group from days
12-15 (Fig. 1B; P<0.05). These
results suggested that the YMJ could improve the body weight via
reducing the DAI scores in UC mice, hence, the reasons for how YMJ
triggered body weight enhancement was investigated in the following
experiments.
YMJ treatments increases the length of
the colon in the UC mice
To determine the effect of YMJ on the colon length
of the UC mice, the length of the colons of the treated mice was
measured. The results showed that, compared with the control group,
the colon length of mice in the DSS group was significantly
shortened (Fig. 1C and D; P<0.05). However, the length of the
colon in the YMJ-treated mice (DSS+YMJ group) was significantly
lengthened compared with that in the DSS mice (Fig. 1C and D; P<0.05). These results suggest that
YMJ significantly alleviates the UC induced by DSS.
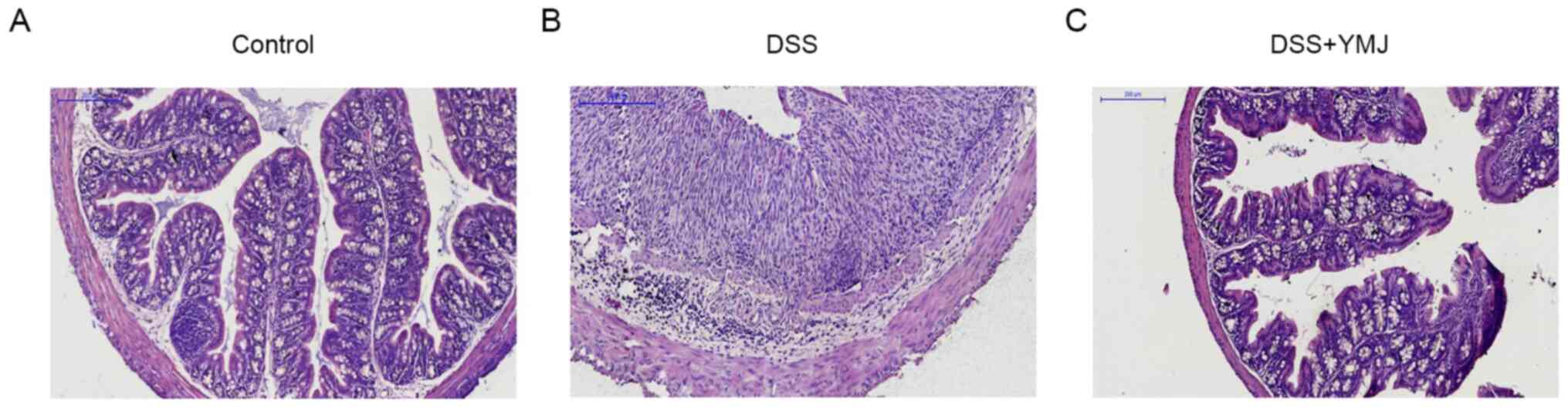
YMJ alleviates inflammatory
infiltration
The H&E staining findings indicated that the
mucosal epithelium of mice in the blank control group was intact
and the glands were well arranged, without congestion, edema,
ulcers or inflammatory cell infiltration (Fig. 2A). The cell atypia of the DSS group
was obvious, and it was accompanied by extensive inflammatory cell
infiltration (Fig. 2B). The
intestinal mucosa of mice in the DSS+YMJ group was clear at all
levels, and inflammatory cell infiltration could be observed
locally, but cell atypia was not obvious (Fig. 2C). Furthermore, in the DSS group,
the histological structure of the colon was lost, including
disintegration of the epithelium, damage of the barrier and a
decrease of the crypt, which was accompanied by infiltration of
granulocytes and single nuclear cells into the mucosa and
sub-mucosa (Fig. 2B). By contrast,
in the YMJ-treated mice, the extent and severity of colon injury
were significantly reduced (Fig.
2C).
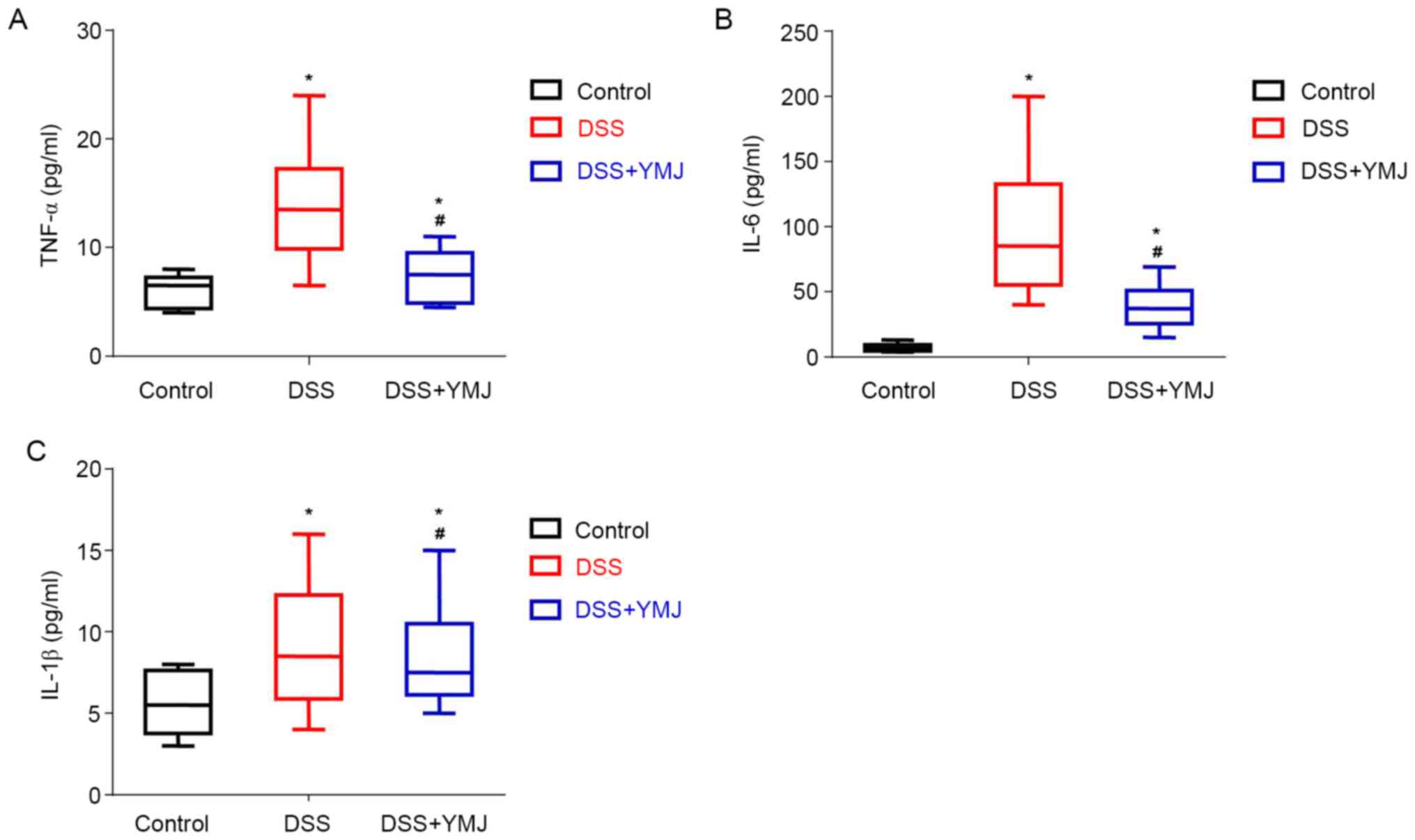
YMJ decreases the serum levels of
inflammatory factors in the UC mice
The serum levels of inflammatory factors in the UC
mice were examined by ELISA. The results showed that the serum
levels of TNF-α (Fig. 3A), IL-6
(Fig. 3B) and IL-1β (Fig. 3C) were significantly higher in the
mice of the DSS group compared with that of the mice in the control
group (P<0.05). The YMJ treatment significantly decreased the
serum levels of inflammatory factors compared with that observed in
the mice of the DSS group (Fig. 3A;
P<0.05). However, the serum levels of TNF-α (Fig. 3A), IL-6 (Fig. 3B) and IL-1β (Fig. 3C) in the DSS+YMJ group were also
significantly lower than that in the control group (P<0.05).
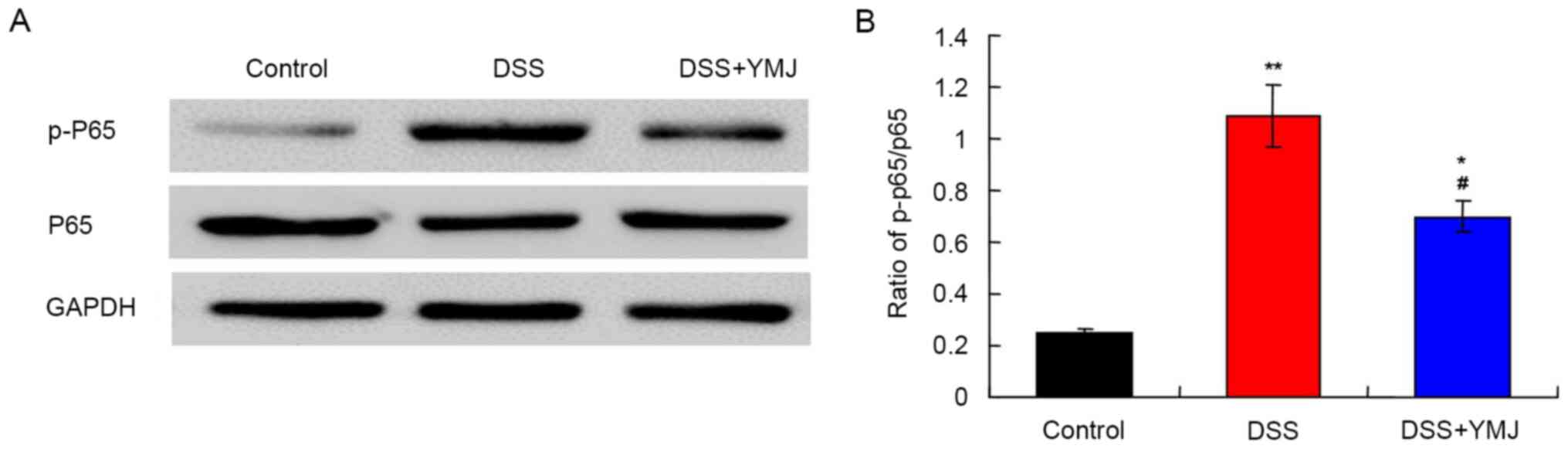
YMJ downregulates the NF-κB signaling
pathway in the UC mice
The western blotting results (Fig. 4A) demonstrated that compared with
the control group, the p-p65/p65 ratio was significantly higher in
the DSS group (Fig. 4B; P<0.01).
Notably, the p-p65/p65 ratio was significantly downregulated in the
DSS+YMJ group compared with that in the DSS group (Fig. 4B; P<0.05). Therefore, DSS
activated the NF-κB signaling pathway, whereas YMJ inhibited this
pathway.
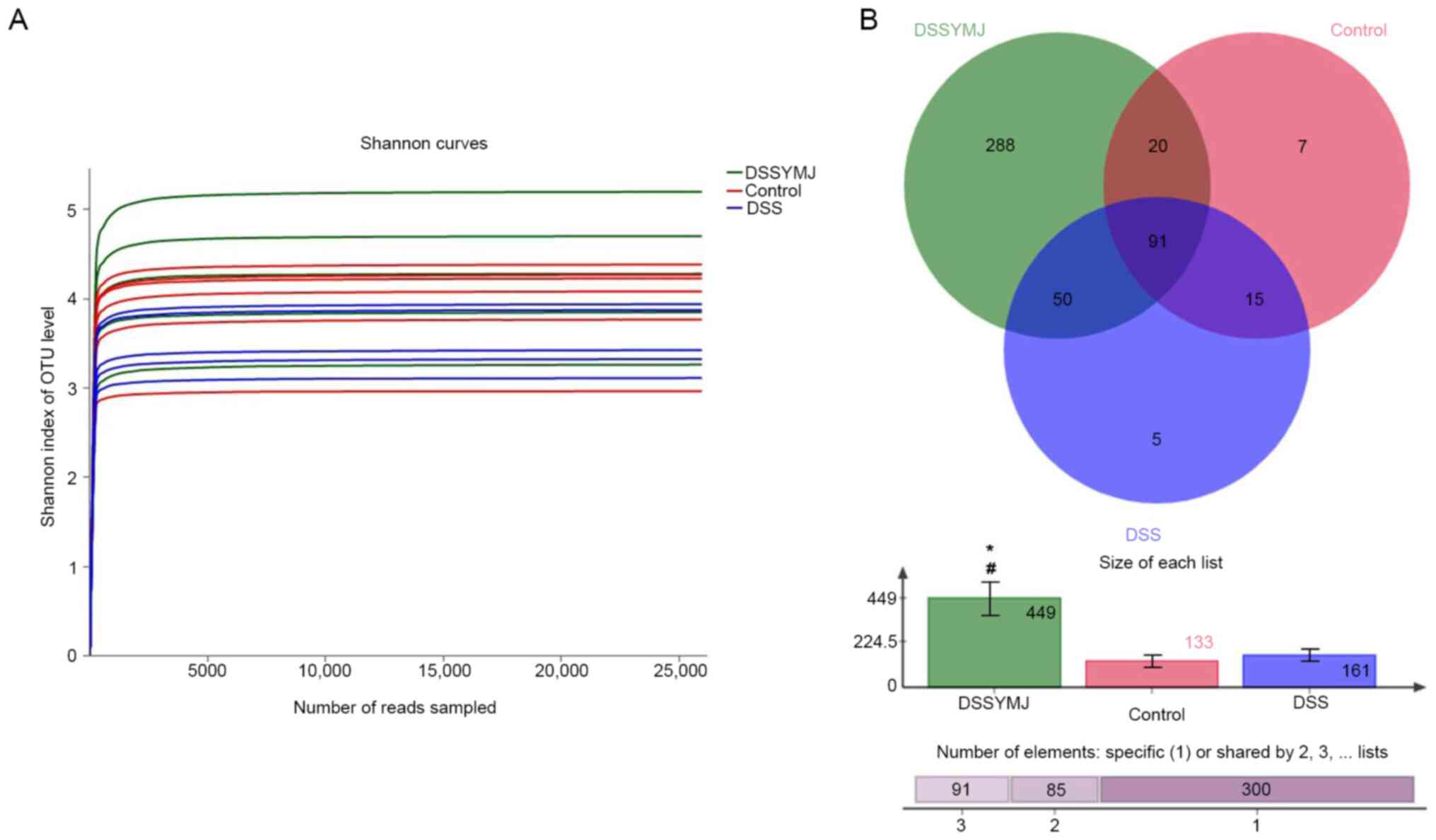
YMJ increases the quantity of
intestinal flora in mice
The fecal DNA content of each sample was >100 ng,
and the optical density 260/280 ratio was 1.8-2.0, which met the
amplification requirements (Fig.
5A). After adding >25,000 sequences, only a small number of
OTUs were produced, suggesting that the sequencing quantity (number
of base reads) was appropriate. The analysis of the similarity of
intestinal microflora using Venn diagrams showed that there were
more intestinal microflora in the DSS+YMJ group than in the control
and DSS groups (Fig. 5B;
P<0.05). However, there were no significant differences in the
quantity of intestinal microflora between the control and DSS
groups (Fig. 5B; P>0.05).
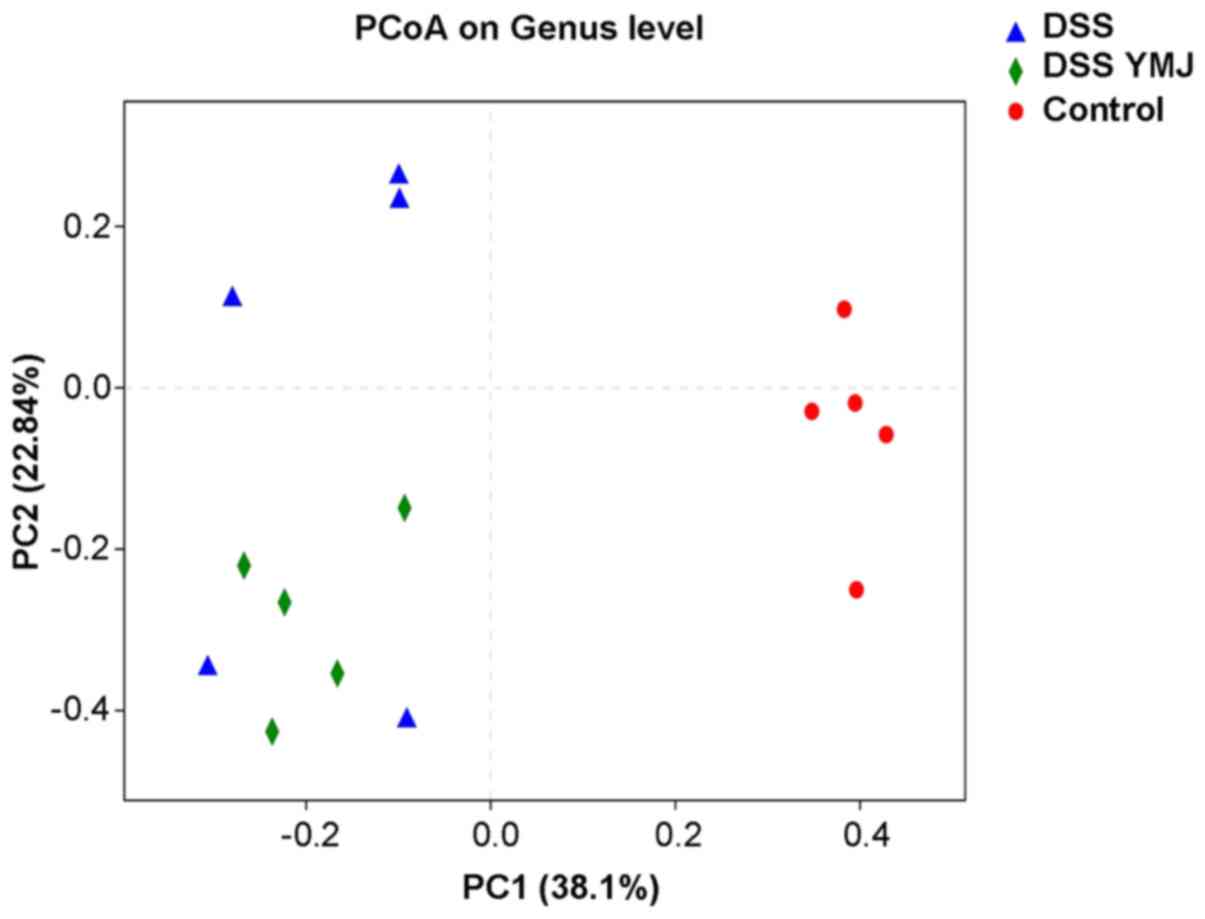
YMJ improves intestinal microflora
diversity in DSS mice
To further visualize similarities or differences
amongst the DSS+YMJ group and mice in the other two groups,
principal co-ordinates analysis was performed based on weighted
UniFrac distance (Fig. 6). In the
intra-group analysis, the sample distances in each group were
close, and the differences were small; thus, they could be
clustered into groups. The poor degree of aggregation of samples in
the DSS group indicated that the changes to the bacterial flora
caused by DSS were irregular. Regarding the distance between
groups, the DSS+YMJ group was closer to the control group than it
was to the DSS group. However, the difference between mice in the
YMJ-treated group and the DSS group was not significant, suggesting
that YMJ treatment could improve the intestinal flora diversity of
DSS mice slightly.
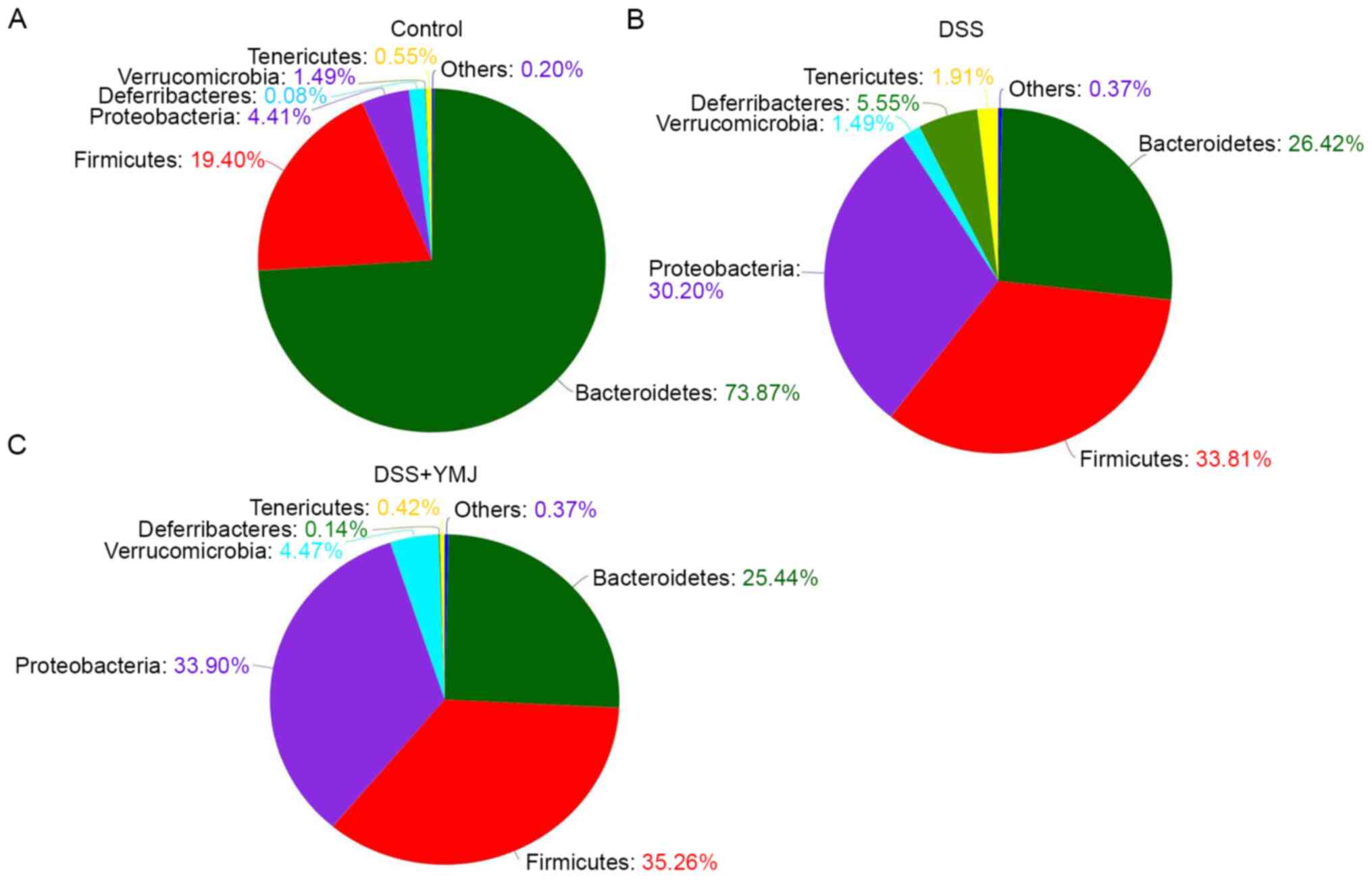
Top intestinal microflora at the
phylum level in the feces of mice
The results of intestinal flora structure analysis
showed that the top six species in each group were
Bacteroidetes, Firmicutes, Proteobacteria,
Verrucomicrobia, Tenericutes and
Deferribacteres. The dominant bacteria in each group were
the same, namely Bacteroidetes, Firmicutes,
Proteobacteria and Verrucomicrobia (Fig. 7) Therefore, compared with those of
the control group, the order and composition ratio of dominant
bacteria at the phylum level in the DSS group exhibited a
substantial change, and Bacteroidetes decreased
significantly, whereas Firmicutes, Proteobacteria,
Verrucomicrobia, Tenericutes and
Deferribacteres increased significantly.
Compared with the DSS group, the order of microflora
level in the DSS+YMJ group was consistent, although the composition
ratio was slightly different (Fig.
7). The differences were primarily observed in
Tenericutes (0.42% in the DSS+YMJ group,1.91% in the DSS
group and 0.55% in the control group) and Deferribacteres
(0.14% in the DSS+YMJ group, 5.55% in the DSS group and 0.08% in
the control group). The order of microflora level and the
composition ratio in the DSS+YMJ group were close to those of the
control group.
Top 10 intestinal microflora at the
genus level in the feces of mice
The present study further specified annotations at
the genus level and listed the top 10 intestinal microflora, as
shown in Table II. The results
revealed that the highest proportion of Escherichia
coli-Shigella was found in the DSS group (15.25%),
followed by Helicobacter (14.65%), Bifidobacterium
(13.72%), Turicibacter (12.58%), Polymorphic bacillus
(11.88%) and Mucispirillum (5.60%). After treatment with
YMJ, the dominant microflora in the intestinal tract of mice were
probiotics, including Parasutterella (30.80%),
Ackermania (10.17%), Polymorphic bacillus (12.05%),
Escherichia coli-Shigella (11.76%),
Turicibacter (4.84%) and Parabacterioides (4.42%).
The intestinal microflora in the mice of the control group
primarily included Bifidobacterium (67.52%),
Ruminococcus (5.11%) and Polymorphic bacillus
(4.75%).
 | Table IITop 10 species at the genus levels in
feces of mice. |
Table II
Top 10 species at the genus levels in
feces of mice.
| | DSS | DSS+YMJ |
|---|
| Control Genus | Constituent
ratio | Genus | Constituent
ratio | Genus | Constituent
ratio |
|---|
|
Bifidobacterium | 67.52% | Escherichia
coli-Shigella | 15.25% |
Parasutterella | 30.80% |
|
Ruminococcus | 5.11% |
Helicobacter | 14.65% |
Ackermania | 10.17% |
| Polymorphic
bacillus | 4.75% |
Bifidobacterium | 13.72% | Polymorphic
bacillus | 12.05% |
|
Lachnospira | 3.90% |
Turicibacter | 12.58% | Escherichia
coli-Shigella | 11.76% |
|
Lactobacillus | 1.83% | Polymorphic
bacillus | 11.88% |
Turicibacter | 4.84% |
|
Ackermania | 1.73% | Mucispirillum | 5.60% |
Parabacterioides | 4.42% |
| Streptococcus
fragmentosus | 1.33% |
Enterococcus | 3.00% |
Helicobacter | 4.00% |
|
Rikenellaceae | 1.30% | Non-classified
streptococcus | 2.69% |
Bifidobacterium | 3.73% |
|
Paraprentium | 1.02% |
Parasutterella | 2.10% |
Lactobacillus | 2.25% |
|
Parabacterioides | 0.92% |
Ackermania | 2.03% |
Lachnospira | 1.72% |
Discussion
The present study explored the effects of YMJ on
DSS-induced mouse models of UC. The DSS-induced UC mouse model is a
common experimental method used to investigate intestinal
inflammation (31). Previous
studies have shown that YMJ can improve mucosal injury, and can
reduce the extent and severity of injury (22,32).
In addition, YMJ could also alleviate the inflammatory damage
induced by DSS through inhibition of the NF-κB signaling pathway
and decreasing the secretion of inflammatory factors (33). Furthermore, YMJ can inhibit
pathogenic bacteria, such as Escherichia
coli-Shigella, increase the proportion of
Parasutterella and Ackermania, and alleviate
intestinal inflammation (33).
Intestinal mucosa, as a mucosal layer of the
intestinal wall, prevents the entrance of toxins and pathogens into
the intestinal cavity (34). Once
the mucosal barrier is penetrated, the sub-mucosal layer will be
exposed to a large number of intraluminal antigens, including food,
bacteria and cytokines that are produced in the process of the
innate immune response. Histopathological evaluation showed that
YMJ could alleviate mucosal injury, improve weight loss and the DAI
score of mice, and alleviate diarrhea and hematochezia (35).
YMJ inhibited the NF-κB signaling pathway, which
resulted in reduced infiltration of immune cells and a decrease in
the secretion of inflammatory cytokines (33). When NF-κB is not activated, it forms
a complex with IκBα. When inflammatory factors, growth factors or
chemokines (such as TNF-α and IL-1β) in the colon of mice activate
NF-κB, IκBα is phosphorylated and then degraded by the
ubiquitin-proteasome signaling pathway (36). TNF-α, IL-6 and IL-1β have also been
demonstrated to act as proinflammatory factors, which are important
for modulating inflammation. The results of the present study
showed that YMJ decreased the serum levels of inflammatory factors,
including TNF-α, IL-6 and IL-1β, in the UC mouse model.
Furthermore, YMJ modulated the NF-κB signaling pathway by
downregulating the p-p65/p65 ratio. These results suggest that DSS
activates the NF-κB signaling pathway, and YMJ notably inhibited
the activation of the NF-κB signaling pathway toa certain extent,
and reduced the inflammatory damage in the UC mouse model.
Bacteroidetes and Firmicutes are the
two most abundant phyla in the human intestinal tract, followed by
Proteobacteria, Verrucomicrobia and
Verrucomicrobia. The composition of intestinal microflora in
the C57BL/6J wild-type mice is similar to that of the human
intestinal flora (37); thus, mice
were selected as animal models in the present study. At the UC
stage, changes in intestinal microflora always trigger associated
inflammatory pathways to further expand the inflammatory response,
which leads to the deterioration of the disease (38,39).
The present findings indicated that YMJ could improve the abundance
and distribution of bacteria; therefore, the anti-inflammatory
effects of YMJ may be closely associated with the regulation of
intestinal microflora. YMJ could reverse the dominant position of
two types of inflammatory bacteria in the intestinal tract of model
mice, such as Parasutterella and Ackermania. In
addition, YMJ could increase the abundance and proportion of
probiotics in the intestinal tract of experimental mice, including
Bifidobacterium, Bacillus, Lactococcus and
Lactobacillus. Following YMJ treatment, the levels of
Parasutterella, which belongs to the core symbiotic
bacteria, increased significantly. Parasutterella can stably
colonize the intestinal tract of mice without initiating an immune
response or inducing fluctuations of the intestinal flora (40). A previous study reported that
bacteria maybe involved in the maintenance of bile acid homeostasis
and cholesterol metabolism through alterations in bile acid
transporter genes in the ileum, and bile acid synthesis genes in
the liver (41). YMJ also induced
the increase in Ackermania, a bacterium that degrades
mucoprotein in the intestines. Notably, Ackermania is
negatively correlated with obesity, diabetes, inflammation and
metabolic disorders (42,43), and positively correlated with
behavioral indicators of anxiety and depression (44-46).
Ackermania can regulate intestinal mucus thickness and
maintain intestinal integrity (46,47).
The present study demonstrated that YMJ could reduce inflammatory
factors to prevent the initiation of inflammation, inhibit
pathogenic bacteria, such as Escherichia coli and
Shigella, and increase the proportion of
Parasutterella and Ackermania. Therefore, YMJ could
further reduce intestinal inflammation, improve intestinal
microflora and alleviate the severity of UC. Furthermore, the
species and abundance of intestinal microflora in mice undergoing
YMJ treatment were improved.
The present study has various limitations. First, a
positive drug group was not included, since this is a preliminary
study on the effect of YMJ on DSS-induced UC. In subsequent
studies, the effect of YMJ should be investigated and compared with
positive drugs. Second, the correlation between YMJ and intestinal
microflora also requires further study, which would be beneficial
for clinical application. Third, the targeting bacteria screened in
this study have not been studied in depth. Further research on YMJ
treatment for UC is required. Finally, due to the preliminary
experimental findings on the safety of YMJ, the effects of
leonurine treatment on the microflora of normal mice have not been
investigated. In future studies, the effects of YMJ should be
determined.
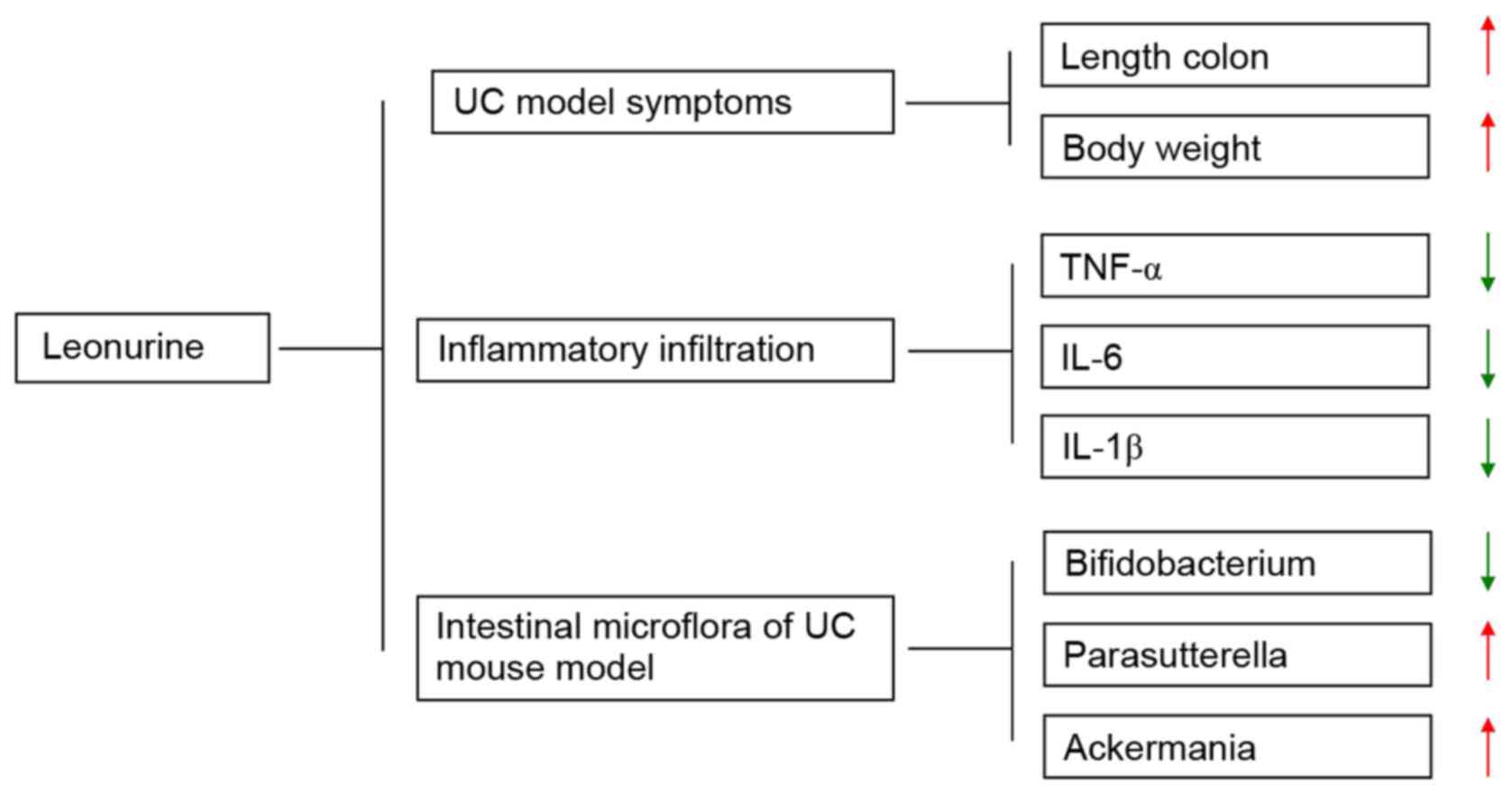
In conclusion, YMJ improved the disease outcomes of
UC to a certain extent, reduced the serum levels of inflammatory
factors and increased the ratio of beneficial bacteria in the
intestinal tract (Fig. 8).
Therefore, YMJ has clinical potential and value for the treatment
of UC through modulating the intestinal microflora and reducing the
inflammatory response in the intestinal tract. Future studies
should focus on the selected targeting of intestinal microflora,
and clarify the correlation between YMJ and intestinal microflora
in UC animal models.
Acknowledgements
Not applicable.
Funding
Funding: This work was funded by the Natural Science Foundation
of Jiangsu province (grant no. BK20180678), the Natural Science
Foundation of Jiangsu Universities (grant no. 17KJB330004), Project
of ‘Nursing Science’ Funded by the Priority Academic Program
Development of Jiangsu Higher Education Institutions (General
Office, the People's Government of Jiangsu Province; grant no.
[2018]-No. 87), the key discipline project of ‘Nursing Science’ of
Jiangsu province during the 13th five-year plan (Jiangsu Provincial
Primary and Secondary School Teaching and Research Office; grant
no. [2016]-No. 9), ‘Nursing Science’ of Jiangsu University Brand
Professional Construction Project (Jiangsu Colleges and
Universities; grant no. [2015]-No. 11) and ‘Centre for Health
Promotion and Nursing Cooperative Innovation’ Constructive Project
of Nanjing Medical University (grant no. JX21831803/004).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request. The sequencing data generated during this study using
samples obtained from experimental animals have been upload to
China National GeneBank database (db.cngb.org/;
accession no. CNP0001816).
Authors' contributions
YW, ZL, MJ conceived and designed the experiments.
SZ, TZ, YT, RW and TX performed experiments and analyzed the data.
SZ and TZ contributed to analysis. YW wrote the manuscript and
performed the statistical analysis. TL performed the experiments
and literature review. SZ, YW, ZL, MJ confirmed the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Guide of Care and Use of Laboratory Animals of National
Institutes of Health (revised in 1996). The present study was
approved by the Ethical Committee of Nanjing Medical University
(Nanjing, China) (approval no. IACUC-1904036).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yu YR and Rodriguez JR: Clinical
presentation of Crohn's, ulcerative colitis, and indeterminate
colitis: Symptoms, extraintestinal manifestations, and disease
phenotypes. Semin Pediatr Surg. 26:349–355. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cheng C, Hua J, Tan J, Qian W, Zhang L and
Hou X: Identification of differentially expressed genes, associated
functional terms pathways, and candidate diagnostic biomarkers in
inflammatory bowel diseases by bioinformatics analysis. Exp Ther
Med. 18:278–288. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ng SC, Tang W, Ching JY, Wong M, Chow CM,
Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, et al: Asia-Pacific
Crohn's and Colitis Epidemiologic Study (ACCESS) Study Group:
Incidence and phenotype of inflammatory bowel disease based on
results from the Asia-pacific Crohn's and colitis epidemiology
study. Gastroenterology. 145:158–165.e2. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sartor RB and Wu GD: Roles for intestinal
bactetria, viruses, and fungi in pathogenesis of inflammatory bowel
diseases and therapeutic approaches. Gastroenterology.
152:327–339.e4. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kostic AD, Xavier RJ and Gevers D: The
microbiome in inflammatory bowel disease: Current status and the
future ahead. Gastroenterology. 146:1489–1499. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Michail S, Durbin M, Turner D, Griffiths
AM, Mack DR, Hyams J, Leleiko N, Kenche H, Stolfi A and Wine E:
Alterations in the gut microbiome of children with severe
ulcerative colitis. Inflamm Bowel Dis. 18:1799–1808.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen ZM, Peto R, Iona A, Guo Y, Chen YP,
Bian Z, Yang L, Zhang YY, Lu F, Chen JS, Collins R and Li LM: China
Kadoorie Biobank Collaborative Group. Emerging tobacco-related
cancer risks in China: A nationwide, prospective study of 0.5
million adults. Cancer. 121 (Suppl 17):3097–106. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lin Y, Yang X, Yue W, Xu X, Li B, Zou L
and He R: Chemerin aggravates DSS-induced colitis by suppressing M2
macrophage polarization. Cell Mol Immunol. 11:355–366.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Håkansson Å, Tormo-Badia N, Baridi A, Xu
J, Molin G, Hagslätt ML, Karlsson C, Jeppsson B, Cilio CM and Ahrné
S: Immunological alteration and changes of gut microbiota after
dextran sulfate sodium (DSS) administration in mice. Clin Exp Med.
15:107–120. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Paramsothy S, Kamm MA, Kaakoush NO, Walsh
AJ, van den Bogaerde J, Samuel D, Leong RW, Connor S, Ng W,
Paramsothy R, Xuan W, Lin E, Mitchell HM and Borody TJ: Multidonor
intensive faecal microbiota transplantation for active ulcerative
colitis: a randomised placebo-controlled trial. Lancet.
389:1218–28. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Weingarden AR and Vaughn BP: Intestinal
microbiota, fecal microbiota transplantation, and inflammatory
bowel disease. Gut Microbes. 8:238–252. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ishiguro Y, Ohkawara T, Sakuraba H,
Yamagata K, Hiraga H, Yamaguchi S, Fukuda S, Munakata A, Nakane A
and Nishihira J: Macrophage migration inhibitory factor has a
proinflammatory activity via the p38 pathway in
glucocorticoid-resistant ulcerative colitis. Clin Immunol.
120:335–341. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhou H, Wong YF, Wang J, Cai X and Liu L:
Sinomenine ameliorates arthritis via MMPs, TIMPs, and cytokines in
rats. Biochem Biophys Res Commun. 376:352–357. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yu XH, Wang YF, Dai FY, Zhao JH and Li P:
The protective effects of Berberine and Hesperidin on inflammatory
factor-stimulating cardiac fibroblasts. Eur Rev Med Pharmacol Sci.
23:5468–5476. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li W, Zhao X, Lv X, Han W and Wang H:
Silibinin retards colitis-associated carcinogenesis by repression
of Cdc25C in mouse model. Inflamm Bowel Dis. 25:1187–1195.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
DeVore NM and Scott EE: Nicotine and
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone binding and access
channel in human cytochrome P450 2A6 and 2A13 enzymes. J Biol Chem.
287:26576–26585. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhu L, Gu P and Shen H: Protective effects
of berberine hydrochloride on DSS-induced ulcerative colitis in
rats. Int Immunopharmacol. 68:242–251. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liang H, Liu P, Wang Y, Song S and Ji A:
Protective effects of alkaloid extract from Leonurus
heterophyllus on cerebral ischemia reperfusion injury by middle
cerebral ischemic injury (MCAO) in rats. Phytomedicine. 18:811–818.
2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yang D, Jia W and Zhu YZ: Leonurine, a
potential agent of traditional Chinese medicine: Recent updates and
future perspectives. Nat Prod Commun. 11:1757–1761. 2016.PubMed/NCBI
|
|
20
|
Jin M, Li Q, Gu Y, Wan B, Huang J, Xu X,
Huang R and Zhang Y: Leonurine suppresses neuroinflammation through
promoting oligodendrocyte maturation. J Cell Mol Med. 23:1470–1485.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hu ZC, Gong LF, Li XB, Fu X, Xuan JW, Feng
ZH and Ni WF: Inhibition of PI3K/Akt/NF-κB signaling with leonurine
for ameliorating the progression of osteoarthritis: In vitro and in
vivo studies. J Cell Physiol. 234:6940–6950. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wu H, Dai A, Chen X, Yang X, Li X, Huang
C, Jiang K and Deng G: Leonurine ameliorates the inflammatory
responses in lipopolysaccharide-induced endometritis. Int
Immunopharmacol. 61:156–161. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang Z, Wu X, Cao S, Cromie M, Shen Y,
Feng Y, Yang H and Li L: Chlorogenic acid ameliorates experimental
colitis by promoting growth of Akkermansia in mice.
Nutrients. 9(E677)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
US National Institutes of Health (NIH):
Guide of the Care and Use of Laboratory Animals. NIH Publication,
pp852-853, 1996.
|
|
25
|
Han F, Zhang H, Xia X, Xiong H, Song D,
Zong X and Wang Y: Porcine β-defensin 2 attenuates inflammation and
mucosal lesions in dextran sodium sulfate-induced colitis. J
Immunol. 194:1882–1893. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liang YN, Yu JG, Zhang DB, Zhang Z, Ren
LL, Li LH, Wang Z and Tang ZS: Indigo Naturalis ameliorates dextran
sulfate sodium-induced colitis in mice by modulating the intestinal
microbiota community. Molecules. 24(4086)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zeng SL, Li SZ, Xiao PT, Cai YY, Chu C,
Chen BZ, Li P, Li J and Liu EH: Citrus polymethoxyflavones
attenuate metabolic syndrome by regulating gut microbiome and amino
acid metabolism. Sci Adv. 6(eaax6208)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ye Z, Zhang N, Wu C, Zhang X, Wang Q,
Huang X, Du L, Cao Q, Tang J, Zhou C, et al: A metagenomic study of
the gut microbiome in Behcet's disease. Microbiome.
6(135)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Masella AP, Bartram AK, Truszkowski JM,
Brown DG and Neufeld JD: PADNAseq: Paired-end assembler for
illumina sequences. BMC Bioinformatics. 13:1–7. 2013.
|
|
30
|
Edgar RC: UPARSE: Highly accurate OTU
sequences from microbial amplicon reads. Nat Methods. 10:996–998.
2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang WF, Yang Y, Su X, Xu DY, Yan YL, Gao
Q and Duan MH: Deoxyschizandrin suppresses dss-induced ulcerative
colitis in mice. Saudi J Gastroenterol. 22:448–455. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nie J and Liu X: Leonurine attenuates
hyperalgesia in mice with induced adenomyosis. Med Sci Monit.
23:1701–1706. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li N, Xu Q, Liu Q, Pan D, Jiang Y, Liu M,
Liu M, Xu H and Lin C: Leonurine attenuates fibroblast-like
synoviocyte-mediated synovial inflammation and joint destruction in
rheumatoid arthritis. Rheumatology (Oxford). 56:1417–1427.
2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Olson A, Diebel LN and Liberati DM:
Exogenous phosphatidylcholine supplementation improves intestinal
barrier defense against Clostridium difficile toxin. J Trauma Acute
Care Surg. 77:570–575; discussion 576. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shang X, Pan H, Wang X, He H and Li M:
Leonurus japonicus Houtt.: Ethnopharmacology, phytochemistry
and pharmacology of an important traditional Chinese medicine. J
Ethnopharmacol. 152:14–32. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Shen J, Cheng J, Zhu S, Zhao J, Ye Q, Xu
Y, Dong H and Zheng X: Regulating effect of baicalin on
IKK/IKB/NF-κB signaling pathway and apoptosis-related proteins in
rats with ulcerative colitis. Int Immunopharmacol. 73:193–200.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Haange SB, Jehmlich N, Hoffmann M, Weber
K, Lehmann J, von Bergen M and Slanina U: Disease development is
accompanied by changes in bacterial protein abundance and functions
in a refined model of dextran sulfate sodium (DSS)-induced colitis.
J Proteome Res. 18:1774–1786. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Rodríguez-Nogales A, Algieri F,
Garrido-Mesa J, Vezza T, Utrilla MP, Chueca N, Fernández-Caballero
JA, García F, Rodríguez-Cabezas ME and Gálvez J: The administration
of Escherichia coli Nissle 1917 ameliorates development of
DSS-induced colitis in mice. Front Pharmacol. 9(468)2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Li M, Wu Y, Hu Y, Zhao L and Zhang C:
Initial gut microbiota structure affects sensitivity to DSS-induced
colitis in a mouse model. Sci China Life Sci. 61:762–769.
2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chen YJ, Wu H, Wu SD, Lu N, Wang YT, Liu
HN, Dong L, Liu TT and Shen XZ: Parasutterella, in
association with irritable bowel syndrome and intestinal chronic
inflammation. J Gastroenterol Hepatol. 33:1844–1852.
2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ju T, Kong JY, Stothard P and Willing BP:
Defining the role of Parasutterella, a previously
uncharacterized member of the core gut microbiota. ISME J.
13:1520–1534. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sheng L, Jena PK, Liu HX, Hu Y, Nagar N,
Bronner DN, Settles ML, Bäumler AJ and Wan YY: Obesity treatment by
epigallocatechin-3-gallate-regulated bile acid signaling and its
enriched Akkermansia muciniphila. FASEB J: Jun 8, 2018 (Epub
ahead of print). doi: 10.1096/fj.201800370R.
|
|
43
|
Moreira GV, Azevedo FF, Ribeiro LM, Santos
A, Guadagnini D, Gama P, Liberti EA, Saad M and Carvalho C:
Liraglutide modulates gut microbiota and reduces NAFLD in obese
mice. J Nutr Biochem. 62:143–154. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Demirci M, Tokman HB, Uysal HK, Demiryas
S, Karakullukcu A, Saribas S, Cokugras H and Kocazeybek BS: Reduced
Akkermansia muciniphila and Faecalibacterium
prausnitzii levels in the gut microbiota of children with
allergic asthma. Allergol Immunopathol (Madr). 47:365–371.
2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kosciow K and Deppenmeier U:
Characterization of a phospholipid-regulated beta-galactosidase
from Akkermansia muciniphila involved in mucin degradation.
Microbiologyope: Feb 6, 2019.
|
|
46
|
McGaughey KD, Yilmaz-Swenson T, Elsayed
NM, Cruz DA, Rodriguiz RM, Kritzer MD, Peterchev AV, Roach J,
Wetsel WC and Williamson DE: Relative abundance of
Akkermansia spp. and other bacterial phylotypes correlates
with anxiety- and depressive-like behavior following social defeat
in mice. Sci Rep. 9(3281)2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Cruz-Aguliar RM, Wantia N, Clavel T,
Vehreschild MJ, Buch T, Bajbouj M, Haller D, Busch D, Shmid RM and
Stein-Thoeringer CK: An open-labeled study on fecal microbiota
transfer in irritable bowel syndrome patients reveals improvement
in abdominal pain associated with the relative abundance of
Akkermansia muciniphila. Digestion. 100:127–138.
2019.PubMed/NCBI View Article : Google Scholar
|