Knee osteoarthritis (KOA) is a multi-etiological,
chronic disabling disease that affects the entire knee joint, which
is the most common site of involvement in OA (1). KOA is classified as primary or
secondary depending on etiology. The pathogenesis of primary KOA is
complex and involves numerous factors, such as mechanical stress,
inflammation, metabolism, immunity and genetics, with age,
genetics, body weight, sex and race being risk factors (2,3). By
contrast, secondary KOA is primarily caused by either trauma,
congenital articular dysplasia or iatrogenic injury. The
pathological changes of KOA are not passive degenerative or
wear-and-tear lesions but active changes caused by an imbalance
between articular tissue damage and repair (4). They are typically accompanied by
lesions in articular or subchondral bones, ligaments, synovium,
joint capsule and periarticular muscular structures (5). The primary clinical manifestations
are pain and limited mobility, which reduce the quality of life of
patients (6). Surveys have
indicated a higher incidence of KOA symptoms in the Chinese
population aged ≥60 (19.4%) (7),
with the incidence rate in women (10.3%) being higher than that in
men (5.7%) (8). The ageing
population has led to an increase in the proportion of older people
with OA. Therefore, OA has become a major healthcare and economic
burden (9). Currently available
pharmacological therapies such as NSAIDs are palliative as they are
mainly aimed at symptomatic relief of inflammation and pain and the
delay of disease progression (10). There are presently no drugs that
halt disease progression or reverse pathological changes in the
entire joint and most patients with end-stage KOA require surgical
treatment (11,12). The definition of KOA, as well as
identification of risk factors and pathophysiological mechanisms,
are being improved and developed as researchers investigate
pathogenesis of the disease (13).
However, the scientific, rational and effective treatment of KOA
remains a challenge in clinical practice (14). The present study reviews the
epidemiological characteristics, risk factors, histopathological
manifestation, pathogenesis, diagnosis, treatment modalities and
the latest KOA research.
In the United States, Osteoarthritis is the second
leading cause of incapacity in men >50 years of age (24). The disease is also one of the main
causes of disability (24) and
affects the quality of life and economic status of patients.
Therefore, greater emphasis on the prevention and treatment of KOA
in older people is important to decrease disability rates and
improve quality of life in the elderly population.
Family history is a risk factor for KOA. In a
previous study, the heritability frequencies of femoral, tibial,
patellar and total cartilage volumes were estimated to be 61, 76,
66 and 73%, respectively (25).
Given the relatively high heritability of tibial cartilage volume
and greater susceptibility of the elderly to tibial fractures,
attention should be paid to the protection of the tibia during
middle and older age. KOA is also associated with abnormalities in
the COL2A1 gene, which is associated with type II collagen
synthesis. One-third of the mutations in the COL2A1 gene are
dominant negative mutations, affecting glycine residues in the α1
chain G-X-Y repeat sequence. These mutations disrupt the triple
helix structure of collagen and are common in type II
achondroplasia and hypochondroplasia (26). Which suggests that KOA is
influenced by genetics (27).
High body mass index is a risk factor for KOA. A
previous study reported a 35% increase in KOA risk with every
5-unit increase in body mass index (28). This is primarily attributed to the
fact that greater body weight increases the weight-bearing pressure
of the knee joint, thereby increasing the probability of KOA.
Sex differences exist in the incidence rates of KOA.
In rural Tianjin, the prevalence of KOA was higher in women than in
men (14.1% vs. 6.5%) (29-32).
The causes of sex differences in KOA incidence remain unclear and
may be associated with estrogen levels (33).
Race affects the incidence of KOA. Through the
investigation of the prevalence of KOA among the elderly population
in an urban area in China and the white population in the United
States, it was shown that the prevalence rates of radiological and
clinical KOA in Chinese women were 46.6 and 15.4%, respectively,
which were considerably higher than the rates in white American
women of the same age group, 34.8 and 11.6% respectively. The
radiological and clinical KOA prevalence rates in elderly Chinese
men were 27.6 and 7.1% (34),
respectively, similar to those in white American men, 30.8 and 6.9%
respectively (35).
KOA is associated with articular injuries, including
articular surface fractures, joint dislocation and ligament and
meniscus injury (36). These
traumatic injuries increase the incidence of KOA to different
extents. For example, there a near 7-fold and 8-fold increase in
the odds for the development of KOA post ACL injury and ACL
reconstruction have been reported (37).
KOA occurs as a result of combined effects of
multiple factors, but there is currently no consensus on the exact
risk factors. Specifically, age, genetics, body weight, sex, race
and trauma have been recognized as risk factors by a number of
studies, but opinion is divided on the roles of diet, smoking and
exercise, which require further investigation (43-46).
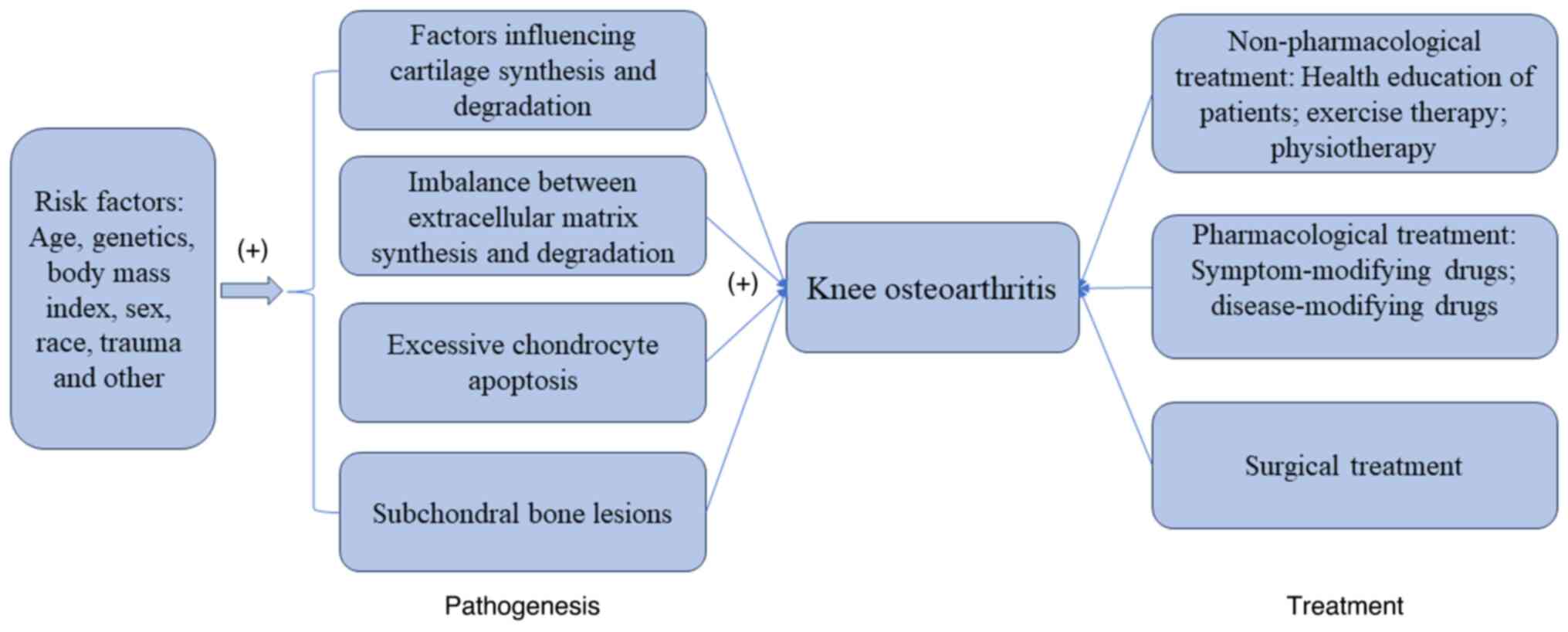
This article summarizes the risk factors, pathogenesis and
treatment of KOA in the form of graphs (Fig. 1).
The primary histopathological characteristics of KOA
include degradation and degenerative changes of the articular
cartilage matrix. However, these are not limited to articular
cartilage and may even involve the entire joint. Other
manifestations include articular cartilage softening, fibrosis,
ulceration, and the loss of articular cartilage, synovial
hyperemia, swelling and hyperplasia, subchondral bone sclerosis and
eburnation and osteophyte and subchondral cyst formation (47,48).
During development of KOA, the balance between
cartilage synthesis and degradation is primarily affected by
pro-degenerative cytokines, such as interleukins (ILs) and tumour
necrosis factor-α (TNF-α), and pro-anabolic factors such as
insulin-like growth factor 1 (IGF-1), transforming growth factor-β
(TGF-β) and bone morphogenetic proteins (BMPs) (49). Pro-degenerative factors primarily
include IL-1β, -6, -15, -17 and -18 and TNF-α, with IL-1β and TNF-α
being strong activators of cartilage extracellular matrix (ECM)
degradation. Pro-anabolic factors include IGF-1, members of the
TGF-β family, BMP-2 and -7, IL-4 and -10 and anti-inflammatory or
regulatory cytokines secreted by the synovium, cartilage or other
tissues, including fibroblast growth factor 2 and IL-4, -6, -10 and
-14 (50-52).
Free radicals are also associated with cartilage destruction and
matrix degradation in KOA. For example, reactive oxygen species can
inhibit the proliferation of articular chondrocytes, promote their
apoptosis and cause an imbalance in synthesis and degradation of
collagen and proteoglycans (PGs) in the cartilage matrix, thereby
inducing cartilage injury (53).
ECM, synthesized and secreted by chondrocytes, is
primarily composed of water, collagen and PGs. These components
form a complex network structure that provides nutrients and
support to chondrocytes and serves as a site for physiological
activity of chondrocytes (54). A
previous study showed that cartilage injury in KOA occurs primarily
in the form of ECM degeneration (55), which manifests as an imbalance
between degradation and synthesis. Signaling pathways associated
with degradation comprise MAPKs, NF-κB, the Wnt/β-catenin and Notch
signaling pathways and toll-like receptors (56).
A small amount of apoptosis occurs in normal
cartilage and is generally limited to the surface layer. It is an
essential physiological process for growth, development, functional
regulation and maintenance of internal environment stability in
cartilage (57,58). However, excessive apoptosis of
cartilage is a pathological process and one of the pathogenic
mechanisms of KOA (59). In a
previous study, flow cytometry data showed that ~5% of normal
chondrocytes and 22% of OA chondrocytes undergo apoptosis. Staining
of cartilage sections revealed that apoptotic cells existed on the
surface and middle cartilage layers, with PGs notably decreased in
apoptotic regions, and the number of apoptotic cells was positively
associated with KOA severity (60). Chondrocyte apoptosis in KOA is
primarily regulated by the mitochondrial, death receptor and
endoplasmic reticulum stress response pathways (61,62).
A previous study investigated the widely recognized Fas pathway
among death receptor pathways, and the NO pathway among the various
mitochondrial pathways (62-66).
Genes involved in regulation of apoptosis mainly include the BCL-2
and interlukin-1β-converting enzyme (ICE) gene families and the p53
and c-myc genes.
Subchondral bone hardening and osteophyte formation,
known as bone remodeling, are characteristic manifestations of KOA
(67). Studies have shown that
subchondral bone lesions appear during the early stage of KOA and
may occur earlier than cartilage degeneration (68). Although there is a lack of
consensus in this aspect, it has been widely recognized that
subchondral bone lesions promote cartilage degeneration (68-70).
KOA is primarily diagnosed based on clinical
symptoms, signs, imaging examinations and laboratory
examinations.
KOA primarily manifests as knee joint pain that is
aggravated by fatigue or weather changes and cold and wet weather,
and relieved after rest or the application of heat. Patients
typically experience either dull and needle-like pain or joint
soreness (71). The pain
originates from tissue around the knee joint, such as subchondral
bone microfractures, synovitis, joint capsular strain caused by
osteohypertrophy and increased intraosseous venous pressure
(72). In the early stage of
disease, pain is only felt during continuous loading or overuse of
the joint. When joint weakness or movement disorders develop during
middle-to-late stages of the disease, pain occurs with mild
exertion or during rest or night-time (73). Patients also experience limited
joint mobility upon waking in the morning, known as morning
stiffness, which usually lasts <30 min (74) and is limited to the affected
joints.
A physical examination should be performed to
determine whether symptoms are associated with joints. It usually
consists of joint palpation, testing of the active and passive
range of motion of joints and assessment of pain using the pain
index. Common signs include the following: i) Tenderness and
tactile pain in the knee joint and joint edges, with tender points
mostly located near the joint line, joint capsule and insertion
points of the collateral ligament. This may be associated with
muscle spasms, bursitis and tendonitis (71); ii) hypertrophy and swelling of the
knee joint resulting from osteophyte formation leading to
osteohypertrophy with or without aseptic inflammatory exudation
from the synovial membrane within the joint, which may be
accompanied by an increase in local skin temperature of the knee
joint (75); iii) obviously
palpable articular crepitus sounds during active or passive joint
flexion and extension, primarily related to articular cartilage
detachment and degeneration of joints such as the patella and
femur, which result in uneven articular surfaces that rub against
each other and the presence of cartilage fragments in the joint
(5); iv) limited joint range of
motion and movement abnormalities, which may be related to pain,
exudate adhesion, flexion contracture, muscle spasms, cartilage
loss, degeneration, mechanical obstruction caused by cartilage or
the menisci or intra-articular loose bodies (76) and v) joint varus deformity or
valgus deformity (77).
X-ray is the first-choice imaging modality in the
diagnosis of KOA. In general, anteroposterior and lateral
radiographs of the knee joint, particularly the weight-bearing
views, are required for the comparison of bilateral knee joints
(78). In the early stage of
disease, X-ray findings are usually negative, with small
osteophytes occasionally seen on the superior and inferior patellar
edges. Advanced disease manifests as narrowing of the joint space,
bone sclerosis, cystic changes, osteophyte formation along joint
edges, subchondral ossification or cystic changes, or joint
deformation (79). Further disease
progression leads to the occurrence of manifestations such as
subchondral bone osteosclerosis, subarticular cysts, bone
resorption and intra-articular loose bodies (80). The Chinese Medical Association
Guidelines for the Diagnosis and Treatment of OA (2018 edition)
summarized the three typical X-ray manifestations of OA, namely
asymmetric joint space narrowing in the affected joints,
subchondral bone sclerosis or cystic changes and osteophyte
formation along joint edges (39).
Computed tomography (CT) and magnetic resonance
imaging (MRI) are primarily used for differential diagnosis. CT
possesses limitations, such as for certain minor soft tissue
injuries, CT images may not be sensitive enough and is less
commonly used in clinical practice (81). It is primarily used to locate loose
bodies or debris in the joint, and to determine patellofemoral
joint structure (82). MRI can
show lesions in the cartilage, bone marrow and soft tissue of the
knee joint, provide indications of early cartilage and synovial
lesions and reveal structural abnormalities in periarticular
tissues such as ligaments and menisci (83). It also enables the evaluation of
bone resorption around the knee joint. With semi-quantitative and
quantitative analysis of cartilage, the degree of lesions can be
evaluated in a more accurate manner, which provides early
diagnostic value (84).
Quantitative T2-weighted MRI may serve as an indicator for early
KOA diagnosis and evaluation of disease condition (85-89),
but it is of limited use in the guidance of clinical treatment of
KOA (90) as it is difficult to
distinguish inflammatory arthritis OA with MRI.
Ultrasound examinations are of use in detecting
joint exudation, cartilage lesions and popliteal cysts and in
guiding punctures and injections in joints and the surrounding soft
tissue (91). Radionuclide bone
imaging assists in determining the metabolic status of osseous
tissue, which contributes to early diagnosis (92).
The Guidelines for the Diagnosis and Treatment of OA
(2018 Edition) published in China first proposed the concept and
strategy of stepwise treatment. Basic treatment, including patient
education, exercise therapy, physical therapy and action support
therapy, is applicable to all patients with OA. Appropriate basic
treatment regimens can be selected for patients with early-stage
KOA. For those with aggravated disease, secondary pharmacological
treatment can be administered with the appropriate drug
administration routes and drug types selected based on affected
sites and risk factors of the patient. If basic and pharmacological
treatment prove ineffective, surgical treatment can be performed.
The surgical plan should be developed by taking the lesion site and
degree, general condition and willingness of the patient into
consideration (39). At present,
the treatment principle for KOA is combined use of
non-pharmacological and pharmacological therapies, with surgery
only performed when necessary. Treatment of symptomatic KOA should
be aimed at the following: i) Control 0of symptoms; ii) improvement
of limited joint function; iii) enhancement of quality of life; iv)
reduction of disability rate and v) avoidance of excessive
medication. In 2000, the American College of Rheumatology (ACR) and
the European League Against Rheumatism (EULAR) recommended the
following treatment options for patients with KOA:
Non-pharmacological, pharmacological and surgical treatment options
(96).
EULAR has classified pharmacological agents for KOA
treatment into two primary categories, namely symptom- and
disease-modifying drugs (103-105).
Traditional Chinese medicine and Chinese herbal medications can
also be used for the treatment of KOA (106). Modern pharmacological studies
have shown that Yanghe decoction has the effect of preventing
chondrocyte apoptosis and exerts an anti-inflammatory action during
KOA treatment (107,108).
Non-steroidal anti-inflammatory drugs (NSAIDs) exert
anti-inflammatory effects by decreasing the synthesis of
prostaglandins through the inhibition of cyclooxygenase (COX) and
are used as first-line therapy in KOA treatment (110). NSAIDs include salicylic and
phenylpropionic acid, acetic acid derivatives, fenamates, enolic
acids, naphthalenones and COX inhibitors, with acetylsalicylic
acid, ibuprofen, naproxen, indomethacin and sulindac commonly used
in clinical practice (111).
Traditional NSAIDs produce toxic side effects due to the lack of
selectivity towards COX-1 or COX-2(112). To address this, second-generation
NSAIDs, a class of COX-2 selective inhibitors, have been designed
to avoid adverse reactions associated with traditional NSAIDs,
including gastrointestinal complications, cardiovascular events,
renal toxicity, the exacerbation of hypertension and fluid
retention (113). Representative
drugs include celecoxib and rofecoxib. Studies have demonstrated
that celecoxib enables an increase in PG content of the cartilage
matrix, cartilage repair and delay of cartilage degeneration
(114,115). However, COX-2 selective
inhibitors increase risk of cardiovascular disease (116). Therefore, consideration of
various factors and rational drug use is necessary for the clinical
application of NSAIDs.
Inhibition of inflammation and alleviation of
symptoms can be achieved through aspiration of joint effusion and
subsequent injection of corresponding medications (117). Steroid hormones (118) and hyaluronic acid (119) are commonly used as intracavitary
injection drugs. The intra-articular injection of glucocorticoids
enables inhibition of abnormal connective tissue proliferation,
reduction of synovitis, significant alleviation of joint pain and
improvement of joint function (120). However, it is important to note
that repeated injections of intra-articular glucocorticoids over a
long period of time can lead to cartilage loss (121). Hyaluronic acid attenuates joint
pain, increases joint mobility, decreases synovitis and promotes
anabolism of chondrocytes in patients with OA, but the exact
mechanisms of action require further investigation (122). Both glucocorticoids and
hyaluronic acid effectively relieve joint pain and swelling but
should not be used repeatedly due to the damaging effects of
injections on cartilage, such as scratching of the cartilage by the
syringe needle (123,124). Additionally, intra-articular
injections of autologous platelet-rich plasma for treatment of KOA
have demonstrated good therapeutic effects (125,126) and possess good prospects for
application in clinical practice.
Chondroitin sulphate is glycosaminoglycan that
promotes the anabolism of chondrocytes, stimulates PG and
hyaluronic acid synthesis and inhibits ECM degradation by enzymes,
which contribute to relief of joint pain and improvement of joint
function (137).
Diacerein is an anthraquinone is extracted from Da
Huang (Radix et Rhizoma Rhei). It inhibits inflammatory factors and
MMPs, promotes the synthesis of growth factors and stabilizes
lysosomal membranes, thereby achieving anti-inflammatory, analgesic
and cartilage-protecting effects (138). A previous study demonstrated that
the combined use of glucosamine and diacerein improves meniscal
damage (139). Diarrhea may occur
as a rare adverse reaction.
MMPs may degrade type II collagen and PGs in the
ECM, which results in cartilage destruction (140). Tetracyclines (141) inhibit MMP-1 release from type B
synoviocytes, as well as nitrous oxide production. This leads to
the reduction of damage to knee articular cartilage by inflammatory
mediators, which contributes to the improvement of disease in
patients with KOA (141). The
development of MMP inhibitors has gained increasing research
interest in the field of pharmacological therapies for OA (142).
Surgical treatment is recommended for patients with
advanced KOA who respond poorly after 6 months of conservative
treatment, and whose condition severely affects daily living
(103,151). However, the effects of surgical
treatment may be influenced by factors such as pain, joint
function, anatomical factors and patient's physical function
(152). Current surgical
treatment methods for KOA include knee arthroscopy (153), bone marrow stimulation (154) and osteotomy (155-157)
and joint replacement (158),
fusion (159) and arthroplasty
(160). For patients with
early-to-mid-stage KOA, joint arthroscopy is a better option as it
provides the advantages of a smaller wound and rapid recovery
(153). Artificial joint
replacement, which is the terminal treatment for KOA, may be
considered for patients with advanced KOA who experience joint
ankylosis, severe damage to joint structures and intense pain
(158). However, there is need
for further exploration into the prevention and treatment of
postoperative complications following artificial joint replacement
(159). Osteotomy techniques such
as high tibial osteotomy are primarily used for the treatment of
KOA with varus and valgus deformities (161).
KOA is a common chronic disease in orthopedic
practice and one of the primary causes of disability and pain in
older people (162). Although
etiology and pathogenesis of KOA have not yet been fully
elucidated, research on its pathogenic mechanisms has shifted from
the macroscopic level, including looking at biomechanics, articular
cartilage and subchondral bone lesions to the microscopic level,
including investigation of inflammatory cytokines and neural
mechanisms (140). However,
current research remains limited (9). For example, certain progress has been
made in the investigation of KOA-associated inflammatory cytokines
(163) from the single-factor
perspective, but the complex mechanisms of association among
multiple factors have not yet been determined (164). Given the lack of early symptoms
and signs and specific diagnostic indicators in imaging and
laboratory examinations, differential diagnosis of KOA in clinical
practice is of importance. Clinicians are required to make
judgments based on analysis of risk factors, symptoms, signs and
results of imaging and laboratory examinations. Identifying
specific diagnostic markers via measurement of markers in the
peripheral blood, joint fluid and other body fluids of patients may
facilitate early detection, diagnosis and treatment of KOA.
However, the combination of various factors, such as risk factors,
symptoms, signs, imaging and laboratory findings is required for
confirmed diagnosis and staging to provide a basis for clinical
treatment.
Numerous factors, including risk factors,
psychosocial factors, symptoms, signs, imaging and laboratory
findings, should be taken into consideration for the determination
of KOA treatment regimens as disease characteristics and general
conditions differ between individuals. Prevention should take
priority, followed by individualized and standardized treatment via
formulation of treatment regimens suited to every patient to
maximize clinical effects. KOA treatment is currently limited to
symptomatic therapy such as anti-inflammatory treatment, analgesia
and limited functional improvement. In previous studies,
researchers have developed pharmacological agents to promote
cartilage repair and regeneration (165,166), inhibit cartilage degradation
(167), promote cartilage matrix
secretion (168), specifically
inhibit inflammatory factors and pathways (169) and maintain the joint cavity
environment (170,171). However, the majority of these
drugs are still undergoing clinical trials. In addition,
sociopsychological support should also be provided to patients
during treatment process to assist in the recovery of social
function. With the continuous improvements in understanding of the
pathogenesis of KOA, it is anticipated that drugs that directly
target pathogenic factors of the disease will emerge in the future
and create new breakthroughs in disease treatment. It is
anticipated that KOA may be prevented, controlled or cured through
active intervention rather than passive treatment. Future research
efforts should be directed towards the prevention of KOA,
etiological treatment, maximum recovery of joint structure and
function and the limitation or even reversal of KOA
progression.
Not applicable.
Funding: The present study was supported by the Yunnan
Provincial Clinical Orthopedic Trauma Medical Center (grant no.
ZX20191001) and the Science and Technology Plan Project of The
Science and Technology Department of Yunnan Province (grant no.
202101AY070001-296).
Not applicable.
YX and TJ conceived the study. RG and JL designed
the study and drafted, reviewed and edited the manuscript. CY, CZ,
FC and JW wrote the manuscript. JC, HN, KK and ZW analysed the
literature. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Prieto-Alhambra D, Judge A, Javaid MK,
Cooper C, Diez-Perez A and Arden NK: Incidence and risk factors for
clinically diagnosed knee, hip and hand osteoarthritis: Influences
of age, gender and osteoarthritis affecting other joints. Ann Rheum
Dis. 73:1659–1664. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Busija L, Bridgett L, Williams SR, Osborne
RH, Buchbinder R, March L and Fransen M: Osteoarthritis. Best Pract
Res Clin Rheumatol. 24:757–768. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Felson DT, Lawrence RC, Dieppe PA, Hirsch
R, Helmick CG, Jordan JM, Kington RS, Lane NE, Nevitt MC, Zhang Y,
et al: Osteoarthritis: New insights. Part 1: The disease and its
risk factors. Ann Intern Med. 133:635–646. 2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Michael JW, Schlüter-Brust KU and Eysel P:
The epidemiology, etiology, diagnosis, and treatment of
osteoarthritis of the knee. Dtsch Arztebl Int. 107:152–162.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Andriacchi TP, Mündermann A, Smith RL,
Alexander EJ, Dyrby CO and Koo S: A framework for the in vivo
pathomechanics of osteoarthritis at the knee. Ann Biomed Eng.
32:447–457. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dawson J, Linsell L, Zondervan K, Rose P,
Carr A, Randall T and Fitzpatrick R: Impact of persistent hip or
knee pain on overall health status in elderly people: A
longitudinal population study. Arthritis Rheum. 53:368–374.
2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xiang YJ and Dai SM: Prevalence of
rheumatic diseases and disability in China. Rheumatol Int.
29:481–490. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tang X, Wang S, Zhan S, Niu J, Tao K,
Zhang Y and Lin J: The prevalence of symptomatic knee
osteoarthritis in China: Results from the China Health and
retirement longitudinal study. Arthritis Rheumatol. 68:648–653.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sarzi-Puttini P, Cimmino MA, Scarpa R,
Caporali R, Parazzini F, Zaninelli A, Atzeni F and Canesi B:
Osteoarthritis: An overview of the disease and its treatment
strategies. Semin Arthritis Rheum. 35:1–10. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bannuru RR, Osani MC, Vaysbrot EE, Arden
NK, Bennell K, Bierma-Zeinstra SMA, Kraus VB, Lohmander LS, Abbott
JH, Bhandari M, et al: OARSI guidelines for the non-surgical
management of knee, hip, and polyarticular osteoarthritis.
Osteoarthritis Cartilage. 27:1578–1589. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Brophy RH and Fillingham YA: AAOS Clinical
practice guideline summary: Management of osteoarthritis of the
Knee (Nonarthroplasty), Third edition. J Am Acad Orthop Surg.
30:e721–e729. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Peng H, Ou A, Huang X, Wang C, Wang L, Yu
T and Zhang Y and Zhang Y: Osteotomy around the knee: The surgical
treatment of osteoarthritis. Orthop Surg. 13:1465–1473.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jang S, Lee K and Ju JH: Recent updates of
diagnosis, pathophysiology, and treatment on osteoarthritis of the
knee. Int J Mol Sci. 22(2619)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bichsel D, Liechti FD, Schlapbach JM and
Wertli MM: Cross-sectional analysis of recommendations for the
treatment of hip and knee osteoarthritis in clinical guidelines.
Arch Phys Med Rehabil. 103:559–569.e5. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bahar E, Shamarina D, Sergerie Y and
Mukherjee P: Descriptive overview of pertussis epidemiology among
older adults in Europe during 2010-2020. Infect Dis Ther.
11:1821–1838. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Madry H, Kon E, Condello V, Peretti GM,
Steinwachs M, Seil R, Berruto M, Engebretsen L, Filardo G and
Angele P: Early osteoarthritis of the knee. Knee Surg Sports
Traumatol Arthrosc. 24:1753–1762. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cross M, Smith E, Hoy D, Nolte S, Ackerman
I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, et al:
The global burden of hip and knee osteoarthritis: estimates from
the global burden of disease 2010 study. Ann Rheum Dis.
73:1323–1330. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Woolf AD and Pfleger B: Burden of major
musculoskeletal conditions. Bull World Health Organ. 81:646–656.
2003.PubMed/NCBI
|
|
19
|
Abbassy AA, Trebinjac S and Kotb N: The
use of cellular matrix in symptomatic knee osteoarthritis. Bosn J
Basic Med Sci. 20:271–274. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang Y, Xu L, Nevitt MC, Aliabadi P, Yu
W, Qin M, Lui LY and Felson DT: Comparison of the prevalence of
knee osteoarthritis between the elderly Chinese population in
Beijing and whites in the United States: The Beijing Osteoarthritis
Study. Arthritis Rheum. 44:2065–2071. 2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
(AUS) AOA: Lay summary 2015 annual report
hip and knee replacement: Suppliment report, 2015.
|
|
22
|
Zhou Z, Cui J, Wu S, Geng Z and Su J: Silk
fibroin-based biomaterials for cartilage/osteochondral repair.
Theranostics. 12:5103–5124. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hiligsmann M, Cooper C, Arden N, Boers M,
Branco JC, Luisa Brandi M, Bruyère O, Guillemin F, Hochberg MC,
Hunter DJ, et al: Health economics in the field of osteoarthritis:
An Expert's consensus paper from the European Society for Clinical
and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO).
Semin Arthritis Rheum. 43:303–313. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Arden N and Nevitt MC: Osteoarthritis:
Epidemiology. Best Pract Res Clin Rheumatol. 20:3–25.
2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hunter DJ, Snieder H, March L and Sambrook
PN: Genetic contribution to cartilage volume in women: A classical
twin study. Rheumatology (Oxford). 42:1495–1500. 2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Barat-Houari M, Sarrabay G, Gatinois V,
Fabre A, Dumont B, Genevieve D and Touitou I: Mutation update for
COL2A1 gene variants associated with type II collagenopathies. Hum
Mutat. 37:7–15. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chapman K and Valdes AM: Genetic factors
in OA pathogenesis. Bone. 51:258–264. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jiang L, Tian W, Wang Y, Rong J, Bao C,
Liu Y, Zhao Y and Wang C: Body mass index and susceptibility to
knee osteoarthritis: A systematic review and meta-analysis. Joint
Bone Spine. 79:291–297. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ji S, Liu L, Li J, Zhao G, Cai Y, Dong Y,
Wang J and Wu S: Prevalence and factors associated with knee
osteoarthritis among middle-aged and elderly individuals in rural
Tianjin: A population-based cross-sectional study. J Orthop Surg
Res. 18(266)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hunter DJ and Felson DT: Osteoarthritis.
BMJ. 332:639–642. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jordan JM, Luta G, Renner JB, Dragomir A,
Hochberg MC and Fryer JG: Ethnic differences in self-reported
functional status in the rural south: The Johnston County
Osteoarthritis Project. Arthritis Care Res. 9:483–491.
1996.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Srikanth VK, Fryer JL, Zhai G, Winzenberg
TM, Hosmer D and Jones G: A meta-analysis of sex differences
prevalence, incidence and severity of osteoarthritis.
Osteoarthritis Cartilage. 13:769–781. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shu Z, Miao X, Tang T, Zhan P, Zeng L and
Jiang Y: The GSK-3β/β-catenin signaling pathway is involved in
HMGB1-induced chondrocyte apoptosis and cartilage matrix
degradation. Int J Mol Med. 45:769–778. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xu L, Nevitt MC, Zhang Y, Yu W, Alibadi P
and Felson DT: High prevalence of knee, but not hip or hand
osteoarthritis in Beijing elders: Comparison with data of Caucasian
in United States. Zhonghua Yi Xue Za Zhi. 83:1206–1209.
2003.PubMed/NCBI(In Chinese).
|
|
35
|
Guccione AA, Felson DT, Anderson JJ,
Anthony JM, Zhang Y, Wilson PW, Kelly-Hayes M, Wolf PA, Kreger BE
and Kannel WB: The effects of specific medical conditions on the
functional limitations of elders in the Framingham Study. Am J
Public Health. 84:351–358. 1994.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Øiestad BE, Engebretsen L, Storheim K and
Risberg MA: Knee osteoarthritis after anterior cruciate ligament
injury: A systematic review. Am J Sports Med. 37:1434–1443.
2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Webster KE and Hewett TE: Anterior
cruciate ligament injury and knee osteoarthritis: An umbrella
systematic review and meta-analysis. Clin J Sport Med. 32:145–152.
2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xie F, Li SC, Fong KY, Lo NN, Yeo SJ, Yang
KY and Thumboo J: What health domains and items are important to
patients with knee osteoarthritis? A focus group study in a
multiethnic urban Asian population. Osteoarthritis Cartilage.
14:224–230. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Stitik TP and Foye PM: The prevalence of
knee pain and symptomatic knee osteoarthritis among veteran
traumatic amputees and nonamputees. Arch Phys Med Rehabil.
86(1273)2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wigley RD, Zhang NZ, Zeng QY, Shi CS, Hu
DW, Couchman K, Duff IF and Bennett PH: Rheumatic diseases in
China: ILAR-China study comparing the prevalence of rheumatic
symptoms in northern and southern rural populations. J Rheumatol.
21:1484–1490. 1994.PubMed/NCBI
|
|
41
|
Palmer KT: Occupational activities and
osteoarthritis of the knee. Br Med Bull. 102:147–170.
2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lau EC, Cooper C, Lam D, Chan VN, Tsang KK
and Sham A: Factors associated with osteoarthritis of the hip and
knee in Hong Kong Chinese: Obesity, joint injury, and occupational
activities. Am J Epidemiol. 152:855–862. 2000.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Fuller-Thomson E, Stefanyk M and
Brennenstuhl S: The robust association between childhood physical
abuse and osteoarthritis in adulthood: Findings from a
representative community sample. Arthritis Rheum. 61:1554–1562.
2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Rossignol M, Leclerc A, Allaert FA,
Rozenberg S, Valat JP, Avouac B, Coste P, Litvak E and Hilliquin P:
Primary osteoarthritis of hip, knee, and hand in relation to
occupational exposure. Occup Environ Med. 62:772–777.
2005.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ni J, Wang P, Yin KJ, Huang JX, Tian T,
Cen H, Sui C, Xu Z and Pan HF: Does smoking protect against
developing osteoarthritis? Evidence from a genetically informed
perspective. Semin Arthritis Rheum. 55(152013)2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Morales-Ivorra I, Romera-Baures M,
Roman-Viñas B and Serra-Majem L: Osteoarthritis and the
Mediterranean Diet: A systematic review. Nutrients.
10(1030)2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Uryu N, Okada K and Kawakita K: Analgesic
effects of indirect moxibustion on an experimental rat model of
osteoarthritis in the knee. Acupunct Med. 25:175–183.
2007.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Torelli SR, Rahal SC, Volpi RS, Sequeira
JL and Grassioto IQ: Histopathological evaluation of treatment with
chondroitin sulphate for osteoarthritis induced by continuous
immobilization in rabbits. J Vet Med A Physiol Pathol Clin Med.
52:45–51. 2005.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kim JR, Yoo JJ and Kim HA: Therapeutics in
osteoarthritis based on an understanding of its molecular
pathogenesis. Int J Mol Sci. 19(674)2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wojdasiewicz P, Poniatowski ŁA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014(561459)2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wang Q, Pan X, Wong HH, Wagner CA, Lahey
LJ, Robinson WH and Sokolove J: Oral and topical boswellic acid
attenuates mouse osteoarthritis. Osteoarthritis Cartilage.
22:128–132. 2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Yoshida A, Morihara T, Matsuda K, Sakamoto
H, Arai Y, Kida Y, Kawata M and Kubo T: Immunohistochemical
analysis of the effects of estrogen on intraarticular neurogenic
inflammation in a rat anterior cruciate ligament transection model
of osteoarthritis. Connect Tissue Res. 53:197–206. 2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Sutipornpalangkul W, Morales NP and
Harnroongroj T: Free radicals in primary knee osteoarthritis. J Med
Assoc Thai. 92 (Suppl 6):S268–S274. 2009.PubMed/NCBI
|
|
54
|
Guilak F, Nims RJ, Dicks A, Wu CL and
Meulenbelt I: Osteoarthritis as a disease of the cartilage
pericellular matrix. Matrix Biol. 71-72:40–50. 2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Aurich M, Squires GR, Reiner A,
Mollenhauer JA, Kuettner KE, Poole AR and Cole AA: Differential
matrix degradation and turnover in early cartilage lesions of human
knee and ankle joints. Arthritis Rheum. 52:112–119. 2005.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Yao Q, Wu X, Tao C, Gong W, Chen M, Qu M,
Zhong Y, He T, Chen S and Xiao G: Osteoarthritis: Pathogenic
signaling pathways and therapeutic targets. Signal Transduct Target
Ther. 8(56)2023.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Taniguchi N, Caramés B, Ronfani L, Ulmer
U, Komiya S, Bianchi ME and Lotz M: Aging-related loss of the
chromatin protein HMGB2 in articular cartilage is linked to reduced
cellularity and osteoarthritis. Proc Natl Acad Sci USA.
106:1181–1186. 2009.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Johnson EO, Charchandi A, Babis GC and
Soucacos PN: Apoptosis in osteoarthritis: Morphology, mechanisms,
and potential means for therapeutic intervention. J Surg Orthop
Adv. 17:147–152. 2008.PubMed/NCBI
|
|
59
|
Kawaguchi H: Endochondral ossification
signals in cartilage degradation during osteoarthritis progression
in experimental mouse models. Mol Cells. 25:1–6. 2008.PubMed/NCBI
|
|
60
|
Hashimoto S, Ochs RL, Rosen F, Quach J,
McCabe G, Solan J, Seegmiller JE, Terkeltaub R and Lotz M:
Chondrocyte-derived apoptotic bodies and calcification of articular
cartilage. Proc Natl Acad Sci USA. 95:3094–3099. 1998.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Liu L, Luo P, Yang M, Wang J, Hou W and Xu
P: The role of oxidative stress in the development of knee
osteoarthritis: A comprehensive research review. Front Mol Biosci.
9(1001212)2022.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Ryu JH, Shin Y, Huh YH, Yang S, Chun CH
and Chun JS: Hypoxia-inducible factor-2α regulates Fas-mediated
chondrocyte apoptosis during osteoarthritic cartilage destruction.
Cell Death Differ. 19:440–450. 2012.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Chen H, Wang J, Hu B, Wu X, Chen Y, Li R
and Yuan W: MiR-34a promotes Fas-mediated cartilage endplate
chondrocyte apoptosis by targeting Bcl-2. Mol Cell Biochem.
406:21–30. 2015.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Chou CT, Yang JS and Lee MR: Apoptosis in
rheumatoid arthritis-expression of Fas, Fas-L, p53, and Bcl-2 in
rheumatoid synovial tissues. J Pathol. 193:110–116. 2001.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Lee MS, Trindade MC, Ikenoue T, Goodman
SB, Schurman DJ and Smith RL: Regulation of nitric oxide and bcl-2
expression by shear stress in human osteoarthritic chondrocytes in
vitro. J Cell Biochem. 90:80–86. 2003.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Chen WP, Jin GJ, Xiong Y, Hu PF, Bao JP
and Wu LD: Rosmarinic acid down-regulates NO and PGE2
expression via MAPK pathway in rat chondrocytes. J Cell Mol Med.
22:346–353. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Bijlsma JW, Berenbaum F and Lafeber FP:
Osteoarthritis: An update with relevance for clinical practice.
Lancet. 377:2115–2126. 2011.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Wu X, Crawford R, Xiao Y, Mao X and
Prasadam I: Osteoarthritic subchondral bone release exosomes that
promote cartilage degeneration. Cells. 10(251)2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Mansell JP, Collins C and Bailey AJ: Bone,
not cartilage, should be the major focus in osteoarthritis. Nat
Clin Pract Rheumatol. 3:306–307. 2007.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Ajami S, Javaheri B, Chang YM, Maruthainar
N, Khan T, Donaldson J, Pitsillides AA and Liu C: Spatial links
between subchondral bone architectural features and cartilage
degeneration in osteoarthritic joints. Sci Rep.
12(6694)2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
de Rooij M, van der Leeden M, Heymans MW,
Holla JF, Häkkinen A, Lems WF, Roorda LD, Veenhof C,
Sanchez-Ramirez DC, de Vet HC and Dekker J: Prognosis of pain and
physical functioning in patients with knee osteoarthritis: A
systematic review and meta-analysis. Arthritis Care Res (Hoboken).
68:481–492. 2016.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Emmert D, Rasche T, Stieber C, Conrad R
and Mücke M: Knee pain-symptoms, diagnosis and therapy of
osteoarthritis. MMW Fortschr Med. 160:58–64. 2018.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
73
|
Sinusas K: Osteoarthritis: Diagnosis and
treatment. Am Fam Physician. 85:49–56. 2012.PubMed/NCBI
|
|
74
|
Kolasinski SL, Neogi T, Hochberg MC, Oatis
C, Guyatt G, Block J, Callahan L, Copenhaver C, Dodge C, Felson D,
et al: 2019 American college of Rheumatology/Arthritis foundation
guideline for the management of osteoarthritis of the hand, hip,
and knee. Arthritis Care Res (Hoboken). 72:149–162. 2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Pessler F, Dai L, Diaz-Torne C,
Gomez-Vaquero C, Paessler ME, Zheng DH, Einhorn E, Range U,
Scanzello C and Schumacher HR: The synovitis of ‘Non-Inflammatory’
orthopaedic arthropathies: A quantitative histological and
immunohistochemical analysis. Ann Rheum Dis. 67:1184–1187.
2008.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Katsuragawa Y, Saitoh K, Tanaka N, Wake M,
Ikeda Y, Furukawa H, Tohma S, Sawabe M, Ishiyama M, Yagishita S, et
al: Changes of human menisci in osteoarthritic knee joints.
Osteoarthritis Cartilage. 18:1133–1143. 2010.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Krackow KA, Mandeville DS, Rachala SR,
Bayers-Thering M and Osternig LR: Torsion deformity and joint
loading for medial knee osteoarthritis. Gait Posture. 33:625–629.
2011.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Incze MA: I have arthritis of the knees:
What Should I Do? JAMA Intern Med. 179(736)2019.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Huang J, Chen X, Xia M, Lv S and Tong P:
West Lake staging: A new staging system orchestrated by X-ray and
MRI on knee osteoarthritis. J Orthop Surg (Hong Kong).
29(23094990211049587)2021.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Sukerkar PA and Doyle Z: Imaging of
osteoarthritis of the knee. Radiol Clin North Am. 60:605–616.
2022.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Zarringam D, Saris DBF and Bekkers JEJ:
The value of SPECT/CT for knee osteoarthritis: A systematic review.
Cartilage. 12:431–437. 2021.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Bousson V, Lowitz T, Laouisset L, Engelke
K and Laredo JD: CT imaging for the investigation of subchondral
bone in knee osteoarthritis. Osteoporos Int. 23 (Suppl
8):S861–S865. 2012.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Watson PJ, Carpenter TA, Hall LD and Tyler
JA: Cartilage swelling and loss in a spontaneous model of
osteoarthritis visualized by magnetic resonance imaging.
Osteoarthritis Cartilage. 4:197–207. 1996.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Hayashi D, Guermazi A, Kwoh CK, Hannon MJ,
Moore C, Jakicic JM, Green SM and Roemer FW: Semiquantitative
assessment of subchondral bone marrow edema-like lesions and
subchondral cysts of the knee at 3T MRI: A comparison between
intermediate-weighted fat-suppressed spin echo and Dual Echo Steady
State sequences. BMC Musculoskelet Disord. 12(198)2011.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Blumenkrantz G and Majumdar S:
Quantitative magnetic resonance imaging of articular cartilage in
osteoarthritis. Eur Cell Mater. 13:76–86. 2007.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Mosher TJ and Dardzinski BJ: Cartilage MRI
T2 relaxation time mapping: Overview and applications. Semin
Musculoskelet Radiol. 8:355–368. 2004.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Nissi MJ, Rieppo J, Töyräs J, Laasanen MS,
Kiviranta I, Jurvelin JS and Nieminen MT: T(2) relaxation time
mapping reveals age- and species-related diversity of collagen
network architecture in articular cartilage. Osteoarthritis
Cartilage. 14:1265–1271. 2006.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Dardzinski BJ, Mosher TJ, Li S, Van Slyke
MA and Smith MB: Spatial variation of T2 in human articular
cartilage. Radiology. 205:546–550. 1997.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Liebl H, Joseph G, Nevitt MC, Singh N,
Heilmeier U, Subburaj K, Jungmann PM, McCulloch CE, Lynch JA, Lane
NE and Link TM: Early T2 changes predict onset of radiographic knee
osteoarthritis: Data from the osteoarthritis initiative. Ann Rheum
Dis. 74:1353–1359. 2015.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Conaghan P: Is MRI useful in
osteoarthritis? Best Pract Res Clin Rheumatol. 20:57–68.
2006.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Zhao X, Gong W, Li X, Yang W, Yang D and
Liu Z: Back propagation neural network-based ultrasound image for
diagnosis of cartilage lesions in knee osteoarthritis. J Healthc
Eng. 2021(2584291)2021.PubMed/NCBI View Article : Google Scholar
|
|
92
|
McCrae F, Shouls J, Dieppe P and Watt I:
Scintigraphic assessment of osteoarthritis of the knee joint. Ann
Rheum Dis. 51:938–942. 1992.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Garnero P, Mazières B, Guéguen A, Abbal M,
Berdah L, Lequesne M, Nguyen M, Salles JP, Vignon E and Dougados M:
Cross-sectional association of 10 molecular markers of bone,
cartilage, and synovium with disease activity and radiological
joint damage in patients with hip osteoarthritis: The ECHODIAH
cohort. J Rheumatol. 32:697–703. 2005.PubMed/NCBI
|
|
94
|
Schouten JS, Van den Ouweland FA,
Valkenburg HA and Lamberts SW: Insulin-like growth factor-1: A
prognostic factor of knee osteoarthritis. Br J Rheumatol.
32:274–280. 1993.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Manicourt DH, Fujimoto N, Obata K and
Thonar EJ: Serum levels of collagenase, stromelysin-1, and TIMP-1.
Age- and sex-related differences in normal subjects and
relationship to the extent of joint involvement and serum levels of
antigenic keratan sulfate in patients with osteoarthritis.
Arthritis Rheum. 37:1774–1783. 1994.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Zhang W, Doherty M, Arden N, Bannwarth B,
Bijlsma J, Gunther KP, Hauselmann HJ, Herrero-Beaumont G, Jordan K,
Kaklamanis P, et al: EULAR evidence based recommendations for the
management of hip osteoarthritis: Report of a task force of the
EULAR Standing Committee for International Clinical Studies
Including Therapeutics (ESCISIT). Ann Rheum Dis. 64:669–681.
2005.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Zeng CY, Zhang ZR, Tang ZM and Hua FZ:
Benefits and mechanisms of exercise training for knee
osteoarthritis. Front Physiol. 12(794062)2021.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Wellsandt E and Golightly Y: Exercise in
the management of knee and hip osteoarthritis. Curr Opin Rheumatol.
30:151–159. 2018.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Li Y, Su Y, Chen S, Zhang Y, Zhang Z, Liu
C, Lu M, Liu F, Li S, He Z, et al: The effects of resistance
exercise in patients with knee osteoarthritis: A systematic review
and meta-analysis. Clin Rehabil. 30:947–959. 2016.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Page CJ, Hinman RS and Bennell KL:
Physiotherapy management of knee osteoarthritis. Int J Rheum Dis.
14:145–151. 2011.PubMed/NCBI View Article : Google Scholar
|
|
101
|
van Doormaal MCM, Meerhoff GA, Vliet
Vlieland TPM and Peter WF: A clinical practice guideline for
physical therapy in patients with hip or knee osteoarthritis.
Musculoskeletal Care. 18:575–595. 2020.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Peter WF, Jansen MJ, Hurkmans EJ, Bloo H,
Dekker J, Dilling RG, Hilberdink W, Kersten-Smit C, de Rooij M,
Veenhof C, et al: Physiotherapy in hip and knee osteoarthritis:
Development of a practice guideline concerning initial assessment,
treatment and evaluation. Acta Reumatol Port. 36:268–281.
2011.PubMed/NCBI
|
|
103
|
Kloppenburg M, Kroon FP, Blanco FJ,
Doherty M, Dziedzic KS, Greibrokk E, Haugen IK, Herrero-Beaumont G,
Jonsson H, Kjeken I, et al: 2018 update of the EULAR
recommendations for the management of hand osteoarthritis. Ann
Rheum Dis. 78:16–24. 2019.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Rausch Osthoff AK, Niedermann K, Braun J,
Adams J, Brodin N, Dagfinrud H, Duruoz T, Esbensen BA, Günther KP,
Hurkmans E, et al: 2018 EULAR recommendations for physical activity
in people with inflammatory arthritis and osteoarthritis. Ann Rheum
Dis. 77:1251–1260. 2018.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Kroon FPB, Carmona L, Schoones JW and
Kloppenburg M: Efficacy and safety of non-pharmacological,
pharmacological and surgical treatment for hand osteoarthritis: A
systematic literature review informing the 2018 update of the EULAR
recommendations for the management of hand osteoarthritis. RMD
Open. 4(e000734)2018.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Wang HM, Liu JN and Zhao Y: Progress on
integrated Chinese and Western medicine in the treatment of
osteoarthritis. Chin J Integr Med. 16:378–384. 2010.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Xu X, Wan Y, Gong L, Ma Z and Xu T:
Chinese herbal medicine Yanghe decoction for knee osteoarthritis: A
protocol for systematic review and meta-analysis. Medicine
(Baltimore). 99(e21877)2020.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Xia H, Cao D and Yang F, Yang W, Li W, Liu
P, Wang S and Yang F: Jiawei Yanghe decoction ameliorates cartilage
degradation in vitro and vivo via Wnt/β-catenin signaling pathway.
Biomed Pharmacother. 122(109708)2020.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Hochberg MC and Dougados M:
Pharmacological therapy of osteoarthritis. Best Pract Res Clin
Rheumatol. 15:583–593. 2001.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Nelson AE, Allen KD, Golightly YM, Goode
AP and Jordan JM: A systematic review of recommendations and
guidelines for the management of osteoarthritis: The chronic
osteoarthritis management initiative of the U.S. bone and joint
initiative. Semin Arthritis Rheum. 43:701–712. 2014.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Bindu S, Mazumder S and Bandyopadhyay U:
Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A
current perspective. Biochem Pharmacol. 180(114147)2020.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Bacchi S, Palumbo P, Sponta A and
Coppolino MF: Clinical pharmacology of non-steroidal
anti-inflammatory drugs: A review. Antiinflamm Antiallergy Agents
Med Chem. 11:52–64. 2012.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Jones R: Nonsteroidal anti-inflammatory
drug prescribing: Past, present, and future. Am J Med. 110
(Suppl):4S–7S. 2001.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Puljak L, Marin A, Vrdoljak D, Markotic F,
Utrobicic A and Tugwell P: Celecoxib for osteoarthritis. Cochrane
Database Syst Rev. 5(CD009865)2017.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Mastbergen SC, Bijlsma JW and Lafeber FP:
Selective COX-2 inhibition is favorable to human early and
late-stage osteoarthritic cartilage: A human in vitro study.
Osteoarthritis Cartilage. 13:519–526. 2005.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Solomon SD, McMurray JJ, Pfeffer MA,
Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E and
Bertagnolli M: Adenoma Prevention with Celecoxib (APC) Study
Investigators. Cardiovascular risk associated with celecoxib in a
clinical trial for colorectal adenoma prevention. N Engl J Med.
352:1071–1080. 2005.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Elsawy NA, Ibrahiem AH, Younis GA,
Meheissen MA and Abdel-Fattah YH: Clinical examination, ultrasound
assessment and aspiration of knee effusion in primary knee
osteoarthritis patients. J Orthop Surg Res. 18(422)2023.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Henricsdotter C, Ellegaard K, Klokker L,
Bartholdy C, Bandak E, Bartels EM, Bliddal H and Henriksen M:
Changes in ultrasound assessed markers of inflammation following
intra-articular steroid injection combined with exercise in knee
osteoarthritis: Exploratory outcome from a randomized trial.
Osteoarthritis Cartilage. 24:814–821. 2016.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Wang SZ, Guo YD, Zhang XJ and Wang C:
Intra-articular injection of compound betamethasone and hyaluronic
acid for the treatment of moderate-severe knee osteoarthritis: A
randomized controlled trial. Zhongguo Gu Shang. 34:424–428.
2021.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
120
|
Deyle GD, Allen CS, Allison SC, Gill NW,
Hando BR, Petersen EJ, Dusenberry DI and Rhon DI: Physical therapy
versus glucocorticoid injection for osteoarthritis of the knee. N
Engl J Med. 382:1420–1429. 2020.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Zhang Y, Ruan G, Zheng P, Huang S, Zhou X,
Liu X, Hu W, Feng H, Lin Y, He J, et al: Efficacy and safety of
GLucocorticoid injections into InfrapaTellar faT pad in patients
with knee ostEoarthRitiS: Protocol for the GLITTERS randomized
controlled trial. Trials. 24(6)2023.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Jin L, Xu K, Liang Y, Du P, Wan S and
Jiang C: Effect of hyaluronic acid on cytokines and immune cells
change in patients of knee osteoarthritis. BMC Musculoskelet
Disord. 23(812)2022.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Georgiev T and Angelov AK: Modifiable risk
factors in knee osteoarthritis: Treatment implications. Rheumatol
Int. 39:1145–1157. 2019.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Liao CD, Chen HC, Huang MH, Liou TH, Lin
CL and Huang SW: Comparative efficacy of intra-articular injection,
physical therapy, and combined treatments on pain, function, and
sarcopenia indices in knee osteoarthritis: A network meta-analysis
of randomized controlled trials. Int J Mol Sci.
24(6078)2023.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Xie X, Zhang C and Tuan RS: Biology of
platelet-rich plasma and its clinical application in cartilage
repair. Arthritis Res Ther. 16(204)2014.PubMed/NCBI View
Article : Google Scholar
|
|
126
|
Duymus TM, Mutlu S, Dernek B, Komur B,
Aydogmus S and Kesiktas FN: Choice of intra-articular injection in
treatment of knee osteoarthritis: Platelet-rich plasma, hyaluronic
acid or ozone options. Knee Surg Sports Traumatol Arthrosc.
25:485–492. 2017.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Taqi A, Gran S and Knaggs RD: Current use
of analgesics and the risk of falls in people with knee
osteoarthritis: A population-based cohort study using primary care
and hospital records. Osteoarthr Cartil Open.
3(100165)2021.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Sharma SK, Vij AS and Sharma M: Mechanisms
and clinical uses of capsaicin. Eur J Pharmacol. 720:55–62.
2013.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Liu J and Zang YJ: Comparative study
between three analgesic agents for the pain management during
extracorporeal shock wave lithotripsy. Urol J. 10:942–945.
2013.PubMed/NCBI
|
|
130
|
Guo J, Hu X, Wang J, Yu B, Li J, Chen J,
Nie X, Zheng Z, Wang S and Qin Q: Safety and efficacy of compound
methyl salicylate liniment for topical pain: A multicenter
real-world study in China. Front Pharmacol.
13(1015941)2022.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Riddle DL, Moxley G and Dumenci L:
Response to comments in: Statin use is associated with reduced
incidence and progression of knee osteoarthritis in the Rotterdam
study by Clockaerts et al. Ann Rheum Dis.
72(e12)2013.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Sondergaard BC, Madsen SH,
Segovia-Silvestre T, Paulsen SJ, Christiansen T, Pedersen C,
Bay-Jensen AC and Karsdal MA: Investigation of the direct effects
of salmon calcitonin on human osteoarthritic chondrocytes. BMC
Musculoskelet Disord. 11(62)2010.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Armagan O, Serin DK, Calisir C,
Dokumacioglu A, Ozgen M, Oner S and Alatas O: Inhalation therapy of
calcitonin relieves osteoarthritis of the knee. J Korean Med Sci.
27:1405–1410. 2012.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Tanaka H, Ueta Y, Yamashita U, Kannan H
and Yamashita H: Biphasic changes in behavioral, endocrine, and
sympathetic systems in adjuvant arthritis in Lewis rats. Brain Res
Bull. 39:33–37. 1996.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Sawitzke AD, Shi H, Finco MF, Dunlop DD,
Harris CL, Singer NG, Bradley JD, Silver D, Jackson CG, Lane NE, et
al: Clinical efficacy and safety of glucosamine, chondroitin
sulphate, their combination, celecoxib or placebo taken to treat
osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum
Dis. 69:1459–1464. 2010.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Jordan KM, Arden NK, Doherty M, Bannwarth
B, Bijlsma JW, Dieppe P, Gunther K, Hauselmann H, Herrero-Beaumont
G, Kaklamanis P, et al: EULAR Recommendations 2003: An evidence
based approach to the management of knee osteoarthritis: Report of
a Task Force of the Standing Committee for International Clinical
Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis.
62:1145–1155. 2003.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Bishnoi M, Jain A, Hurkat P and Jain SK:
Chondroitin sulphate: A focus on osteoarthritis. Glycoconj J.
33:693–705. 2016.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Fidelix TS, Macedo CR, Maxwell LJ and
Fernandes Moça Trevisani V: Diacerein for osteoarthritis. Cochrane
Database Syst Rev: CD005117, 2014.
|
|
139
|
Reginster JY, Deroisy R, Rovati LC, Lee
RL, Lejeune E, Bruyere O, Giacovelli G, Henrotin Y, Dacre JE and
Gossett C: Long-term effects of glucosamine sulphate on
osteoarthritis progression: A randomised, placebo-controlled
clinical trial. Lancet. 357:251–256. 2001.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Loeser RF, Goldring SR, Scanzello CR and
Goldring MB: Osteoarthritis: A disease of the joint as an organ.
Arthritis Rheum. 64:1697–1707. 2012.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Sadowski T and Steinmeyer J: Effects of
tetracyclines on the production of matrix metalloproteinases and
plasminogen activators as well as of their natural inhibitors,
tissue inhibitor of metalloproteinases-1 and plasminogen activator
inhibitor-1. Inflamm Res. 50:175–182. 2001.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Malemud CJ: Inhibition of MMPs and
ADAM/ADAMTS. Biochem Pharmacol. 165:33–40. 2019.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Rosenbaum CC, O'Mathúna DP, Chavez M and
Shields K: Antioxidants and antiinflammatory dietary supplements
for osteoarthritis and rheumatoid arthritis. Altern Ther Health
Med. 16:32–40. 2010.PubMed/NCBI
|
|
144
|
Gui T, Luo L, Chhay B, Zhong L, Wei Y, Yao
L, Yu W, Li J, Nelson CL, Tsourkas A, et al: Superoxide
dismutase-loaded porous polymersomes as highly efficient
antioxidant nanoparticles targeting synovium for osteoarthritis
therapy. Biomaterials. 283(121437)2022.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Mathieu S, Soubrier M, Peirs C, Monfoulet
LE, Boirie Y and Tournadre A: A Meta-analysis of the impact of
nutritional supplementation on osteoarthritis symptoms. Nutrients.
14(1607)2022.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Yao H, Xu J, Wang J, Zhang Y, Zheng N, Yue
J, Mi J, Zheng L, Dai B, Huang W, et al: Combination of magnesium
ions and vitamin C alleviates synovitis and osteophyte formation in
osteoarthritis of mice. Bioact Mater. 6:1341–1352. 2021.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Suantawee T, Tantavisut S, Adisakwattana
S, Tanavalee A, Yuktanandana P, Anomasiri W, Deepaisarnsakul B and
Honsawek S: Oxidative stress, vitamin e, and antioxidant capacity
in knee osteoarthritis. J Clin Diagn Res. 7:1855–1859.
2013.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Bhattacharya I, Saxena R and Gupta V:
Efficacy of vitamin E in knee osteoarthritis management of North
Indian geriatric population. Ther Adv Musculoskelet Dis. 4:11–19.
2012.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Karsdal MA, Tanko LB, Riis BJ, Sondergard
BC, Henriksen K, Altman RD, Qvist P and Christiansen C: Calcitonin
is involved in cartilage homeostasis: Is calcitonin a treatment for
OA? Osteoarthritis Cartilage. 14:617–624. 2006.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Saberianpour S, Abolbashari S, Modaghegh
MHS, Karimian MS, Eid AH, Sathyapalan T and Sahebkar A: Therapeutic
effects of statins on osteoarthritis: A review. J Cell Biochem.
123:1285–1297. 2022.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Cook JL and Payne JT: Surgical treatment
of osteoarthritis. Vet Clin North Am Small Anim Pract. 27:931–944.
1997.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Gomoll AH, Filardo G, de Girolamo L,
Espregueira-Mendes J, Marcacci M, Rodkey WG, Steadman JR,
Zaffagnini S and Kon E: Surgical treatment for early
osteoarthritis. Part I: Cartilage repair procedures. Knee Surg
Sports Traumatol Arthrosc. 20:450–466. 2012.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Krych AJ, Bert JM and Levy BA: Treatment
of OA of the knee in the middle-aged athlete: The role of
arthroscopy. Sports Med Arthrosc Rev. 21:23–30. 2013.PubMed/NCBI View Article : Google Scholar
|
|
154
|
Gobbi A, Lane JG and Dallo I: Editorial
commentary: Cartilage restoration-what is currently available?
Arthroscopy. 36:1625–1628. 2020.PubMed/NCBI View Article : Google Scholar
|
|
155
|
Yasunaga Y, Fujii J, Tanaka R, Yasuhara S,
Yamasaki T, Adachi N and Ochi M: Rotational acetabular osteotomy.
Clin Orthop Surg. 9:129–135. 2017.PubMed/NCBI View Article : Google Scholar
|
|
156
|
Li OL, Pritchett S, Giffin JR and Spouge
ARI: High Tibial osteotomy: An update for radiologists. AJR Am J
Roentgenol. 218:701–712. 2022.PubMed/NCBI View Article : Google Scholar
|
|
157
|
Han J, Zeng Z, Pei F and Zheng T: An
implementation study of periarticular knee osteotomy in the
treatment of knee osteoarthritis. Am J Transl Res. 13:4771–4779.
2021.PubMed/NCBI
|
|
158
|
Liao CD, Huang SW, Huang YY and Lin CL:
Effects of Sarcopenic obesity and its confounders on knee range of
motion outcome after total knee replacement in older adults with
knee osteoarthritis: A retrospective study. Nutrients.
13(3717)2021.PubMed/NCBI View Article : Google Scholar
|
|
159
|
Liu CY, Li CD, Wang L, Ren S, Yu FB, Li
JG, Ma JX and Ma XL: Function scores of different surgeries in the
treatment of knee osteoarthritis: A PRISMA-compliant systematic
review and network-meta analysis. Medicine (Baltimore).
97(e10828)2018.PubMed/NCBI View Article : Google Scholar
|
|
160
|
Xue YY, Shi JN, Zhang K, Zhang HH and Yan
SH: The effects of total knee arthroplasty on knee proprioception
of patients with knee osteoarthritis: A meta-analysis. J Orthop
Surg Res. 17(258)2022.PubMed/NCBI View Article : Google Scholar
|
|
161
|
He M, Zhong X, Li Z, Shen K and Zeng W:
Progress in the treatment of knee osteoarthritis with high Tibial
osteotomy: A systematic review. Syst Rev. 10(56)2021.PubMed/NCBI View Article : Google Scholar
|
|
162
|
Dell'Isola A, Allan R, Smith SL, Marreiros
SS and Steultjens M: Identification of clinical phenotypes in knee
osteoarthritis: A systematic review of the literature. BMC
Musculoskelet Disord. 17(425)2016.PubMed/NCBI View Article : Google Scholar
|
|
163
|
Lin M, Li X, Liang W, Liu J, Guo J, Zheng
J and Liu X: Needle-knife therapy improves the clinical symptoms of
knee osteoarthritis by inhibiting the expression of inflammatory
cytokines. Exp Ther Med. 7:835–842. 2014.PubMed/NCBI View Article : Google Scholar
|
|
164
|
Du X, Liu ZY, Tao XX, Mei YL, Zhou DQ,
Cheng K, Gao SL, Shi HY, Song C and Zhang XM: Research progress on
the pathogenesis of knee osteoarthritis. Orthop Surg: Jul 12, 2023
doi: 10.1111/os.13809 (Epub ahead of print).
|
|
165
|
Dai W, Cheng J, Yan W, Cao C, Zhao F, Li
Q, Hu X, Wang J and Ao Y: Enhanced osteochondral repair with
hyaline cartilage formation using an extracellular matrix-inspired
natural scaffold. Sci Bull (Beijing): Aug 1, 2023 doi:
10.1016/j.scib.2023.07.050 (Epub ahead of print).
|
|
166
|
Li C, Liu Y, Weng T, Yang M, Wang X and
Chai W: Fabrication of injectable Kartogenin-conjugated composite
hydrogel with a sustained drug release for cartilage repair.
Pharmaceutics. 15(1949)2023.PubMed/NCBI View Article : Google Scholar
|
|
167
|
Deng C, Chen Y, Zhao X, Yu L, Xiao Y, Li
H, Zhang Y, Ai K, Zhou D, Bai X, et al: Apoptotic neutrophil
membrane-camouflaged liposomes for dually targeting synovial
macrophages and fibroblasts to attenuate osteoarthritis. ACS Appl
Mater Interfaces: Jul 31, 2023 doi: 10.1021/acsami.3c05861 (Epub
ahead of print).
|
|
168
|
Liu R, Liu Z, Chen H, He S, Wang S, Dai J
and Li X: Ginkgolide K delays the progression of osteoarthritis by
regulating YAP to promote the formation of cartilage extracellular
matrix. Phytother Res: Aug 1, 2023 doi: 10.1002/ptr.7953 (Epub
ahead of print).
|
|
169
|
Kuppa SS, Kim HK, Kang JY, Lee SC, Yang
HY, Sankaranarayanan J and Seon JK: Polynucleotides suppress
inflammation and stimulate matrix synthesis in an in vitro
Cell-based osteoarthritis model. Int J Mol Sci.
24(12282)2023.PubMed/NCBI View Article : Google Scholar
|
|
170
|
Chen S, Meng C, He Y, Xu H, Qu Y, Wang Y,
Fan Y, Huang X and You H: An in vitro and in vivo study: Valencene
protects cartilage and alleviates the progression of osteoarthritis
by anti-oxidative stress and anti-inflammatory effects. Int
Immunopharmacol. 123(110726)2023.PubMed/NCBI View Article : Google Scholar
|
|
171
|
Wang X, Cai Y, Wu C, Liang J, Tang K, Lin
Z, Chen L, Lu Y and Wang Q: Conversion of senescent cartilage into
a pro-chondrogenic microenvironment with antibody-functionalized
copper sulfate nanoparticles for efficient osteoarthritis therapy.
J Nanobiotechnology. 21(258)2023.PubMed/NCBI View Article : Google Scholar
|