Introduction
Primary liver cancer is the sixth most commonly
diagnosed cancer and the third leading cause of cancer death
worldwide (1). Primary liver
cancer mainly includes hepatocellular carcinoma (HCC) and
intrahepatic cholangiocarcinoma (2-5).
HCC accounts for 90% of primary liver cancer cases (6). The occurrence and development of
liver cancer involves a variety of processes and pathways,
including ERK, PI3K/ AKT and Wnt/β-catenin signaling pathways.
These oncogenic signaling pathways can be activated by a variety of
oncogenic drivers during liver carcinogenesis (7). Among them, the MAPK pathway acts on
multiple links of tumor development by regulating the survival,
senescence, proliferation, apoptosis, epithelial-mesenchymal
transition (EMT), migration and invasion of cancer cells (8-11).
A common hallmark of tumor growth signaling mediated by the
Ras/Raf/MEK/ERK signaling axis in the MAPK pathway is the
persistent activation of ERK1/2. Ras and Raf are well-known
oncogenes and the Ras/Raf/MEK/ERK signaling pathway is activated in
~50% of patients with early liver cancer and almost all patients
with advanced liver cancer (12).
By specifically inhibiting MEK1 and then blocking ERK1/2
phosphorylation, the proliferation of liver cancer cells can be
inhibited in a dose-dependent manner and its cytological effects
involve cell cycle arrest, apoptosis and tumorigenicity (13).
It has been recognized that cancer cells maintain
cell survival and reproduction by reorganizing lipid metabolism
(14). The liver is the main organ
for lipid metabolism and homeostasis maintenance and the role of
lipid metabolism reprogramming in the occurrence of liver cancer
has attracted much attention. During the transformation of
non-alcoholic steatohepatitis into liver cancer, a variety of
oncogenic signals and fatty acid metabolism signals are
synergistically activated and targeting the lipoprotein
lipase/fatty acid-binding protein 4/carnitine palmitoyltransferase
I fatty acid metabolism signaling axis can prevent hepatitis from
turning into liver cancer, it is suggested that the activation of
fatty acid metabolism signaling axis is the initiation and
maintenance factor of hepatocellular carcinogenesis (15). Mitochondria are a key place for
lipolysis and anabolism. Mitochondrial fission can promote the
shift of glucose metabolism from glycolysis to oxidative
phosphorylation, thereby alleviating the energy pressure of tumor
survival. Mitochondrial fission can promote fatty acid synthesis
and inhibit fatty acid oxidation in liver cancer cells, regulate
lipid metabolism reprogramming and promote proliferation,
metastasis and tumor growth of liver cancer cells in vivo
(16). Upregulation of fatty acid,
cholesterol synthesis and fatty acid oxidation changes are the main
characteristics of fatty acid metabolic re-programming (17,18).
Signaling molecules in fatty acids and cholesterol metabolism
regulation are becoming new therapeutic targets for liver cancer
(19).
Fatty acid hydroxylase domain containing 2 (FAXDC2),
also called C5orf4, is a member of the fatty acid hydroxylase
superfamily. This family regulates the hydroxylation modification
of fatty acids and generates 2-hydroxylated fatty acids by
catalyzing the hydroxylation at the C2 position of fatty acids
(20). One part of hydroxylated
fatty acids enter the β-oxidation pathway for degradation, another
part participates in the biosynthesis of cholesterol and
sphingomyelin (21). In addition
to energy storage, sphingolipids and steroids in lipids constitute
the main components of biological membranes that are necessary for
cell signal transduction (18).
The region of cell membrane rich in cholesterol and sphingomyelin
forms ordered functional microdomains, termed lipid rafts (LRs)
(18). In response to external
stimuli, the receptors and adapter proteins in the membrane are
concentrated to the LRs, forming an orderly signal sorting center.
Controlling the content of cholesterol and sphingomyelin can
artificially achieve the dynamic remodeling of lipid rafts
(22,23), thereby changing the transduction
properties of intracellular signals. It has been reported that the
expression of FAXDC2 is downregulated in prostate cancer and
neuroblastoma and low expression of FAXDC2 is an unfavorable factor
for disease prognosis (24,25).
Another study has shown that FAXDC2 can regulate macrophage
differentiation by regulating ERK signaling-related mechanisms
(26). However, the roles of
FAXDC2 in liver cancer remain unknown.
The present study attempted to combine
bioinformatics and experimental biology strategies to explore the
function and possible underling mechanism of FAXDC2 in the
occurrence and development of hepatocellular carcinoma, in order to
provide clues in finding fatty acid metabolic therapeutic targets
for the disease diagnosis and treatment.
Materials and methods
Tumor Immune Estimation Resource
(TIMER) 2.0
TIMER (http://timer.cistrome.org/) is an extensive tool
designed for the systematic examination of immune infiltrates in
different types of cancer using data from The Cancer Genome Atlas
(TCGA) database. It consists of a collection of 10,897 samples from
32 types of cancer. In the present study, the Gene Differential
Expression (Gene_DE) module was employed to evaluate the variations
in expression of the FAXDC2 gene between tumor tissues and adjacent
normal tissues across all TCGA tumors. The statistical significance
of these differences was determined using the Wilcoxon rank sum
test.
cBioPortal
The cBioPortal for Cancer Genomics (http://cbioportal.org) is a robust online platform
that serves as a comprehensive resource for cancer genomics data
derived from various platforms. The present study utilized the
platform's summary information, plots and co-expression analyses
for different types of cancer to investigate the expression of the
FAXDC2 gene in cancer.
LinkedOmics analysis
LinkedOmics (linkedomics.org) is an open-access portal that
integrates multi-omics and clinical data sourced from the TCGA
project, comprising data from 11,158 patients across 32 types of
cancer. For the present study, the LinkFinder tab within
LinkedOmics was used to identify genes that were differentially
expressed (DEGs) in association with FAXDC2. The search and target
datasets used in the analysis were derived from RNA sequencing
(RNA-seq). To determine the significance of the findings,
statistical analyses using Pearson's and Spearman's correlation
tests were conducted. Additionally, the LinkInterpreter tab was
employed to perform over-representation enrichment analysis and
gene set enrichment analysis (GSEA).
Kaplan-Meier plotter
The Kaplan-Meier plotter (http://kmplot.com/analysis) is a powerful tool that
enables the assessment of the effects of 54K genes (mRNA, miRNA and
protein) on survival outcomes across 21 different types of cancer.
The data sed in this tool are sourced from various databases,
including Gene Expression Omnibus, European Genome-phenome Archive
and TCGA. The primary objective of this tool is to identify and
validate survival biomarkers through a meta-analysis approach. To
generate the Kaplan-Meier plots, the ‘survplot’ R package
(http://www.cbs.dtu.dk/~eklund/survplot/) was employed.
Statistical significance was evaluated using log-rank testing.
UALCAN analysis
UALCAN (ualcan.path.uab.edu/index.html) is a user-friendly web
portal that facilitates comprehensive analyses of TCGA gene
expression data, incorporating TCGA level 3 RNA-seq data and
clinical information from 31 different types of cancer. the present
study, UALCAN was employed to examine the relative expression of
the FAXDC2 gene within normal samples and across specific tumor
subgroups, including cervical squamous cell carcinoma and
endocervical adenocarcinoma (LIHC).
Western blotting
Cellular proteins were extracted using RIPA lysis
buffer (Elabscience Biotechnology, Inc.). The protein
concentrations were determined using the BCA assay. For gel
electrophoresis, protein samples (10-25 µl/sample) were
loaded onto a 10% acrylamide gel. Electrophoresis was conducted at
a constant voltage of 80 V until the target protein bands
were adequately separated. Following electrophoresis, the proteins
were transferred onto nitrocellulose (NC) membranes using a
constant current of 300 mA. The NC membranes were then immersed in
Tris-buffered saline (TBST) containing 0.05% Tween 20 and 5%
skimmed milk for 1.5 h at room temperature. After removing excess
milk, the NC membrane was incubated with primary antibody (1:1,000)
at room temperature for 90 min. Subsequently, the membrane
was washed three times with TBST for 5 min each. Next, the NC
membrane was incubated at room temperature for 90 min with the
secondary antibody (diluted at a volume ratio of 1:5,000), followed
by three washes with TBST for 5 min each. Super-sensitive ECL
chemiluminescent substrate (Biosharp, cat. no. BL520A) was applied
to the film for imaging. Densitometry was performed using ImageJ
1.52a software (http://imagej.org, National Institutes
of Health).
The anti-Phospho-p44/42 MAPK (Erk1/2) (cat. no.
4370), anti-(BCL-2) (cat. no. 4223), anti-p44/42 MAPK (Erk1/2)
(cat. no. 4695), anti-CDK4 (cat. no. 12790), anti-CDK2 (cat. no.
2546), anti-E-Cadherin (cat. no. 3195), anti-p-AKT (cat. no. 9271)
and anti-AKT (cat. no. 9272) antibodies were purchased from Cell
Signaling Technology, Inc. The anti-p53 antibody was purchased from
ProteinTech Group, Inc. (1:1,000; cat. no. 10442-1-AP), the
anti-Vimentin (cat. no. T55134), anti-EGFR (cat. no. T55112)
antibodies were purchased from Abmart Biomedicine (Shanghai) Co.,
Ltd. and the anti-BAX antibody was purchased from Boster Biological
Technology (cat. no. BA0315-2). ACTIN was used as internal
references, and antibody was purchased from Abmart Biomedicine
(Shanghai) Co., LTD. (1:1,000; cat. no. M20011). Goat anti-mouse
IgG HRP-conjugated and anti-rabbit IgG HRP-conjugated secondary
antibodies were purchased from CWBio (1:5,000; cat. nos. CW0102 and
CW0103).
RNA extraction and reverse
transcription-quantitative (RT-q) PCR
Total RNA was isolated using the Vazyme RNA
extraction kit (Vazyme Biotech Co., Ltd.) according to the
manufacturer's protocol. cDNA was synthesized using the Vazyme cDNA
synthesis kit (Vazyme Biotech Co., Ltd.) according to the
manufacturer's protocol. Real-time PCR was conducted using SYBR
Premix Ex Taq II (Takara Bio, Inc.) according to the manufacturer's
protocol. The TaqMan qPCR cycling conditions were as follows: 50̊C
for 2 min, 95̊C for 5 min, followed by 40 cycles at 95̊C for 15
sec-40 cycles, 60̊C for 40 sec-40 cycles. The primers utilized in
the PCR analysis were obtained from TsingKe Biological Technology.
The expression level of GAPDH is used as an internal reference for
quantification, and the relative expression of related genes was
calculated using the 2-ΔΔCq method (27). The specific primer sequences are
provided in Table SI. This
experiment was replicated three times.
Cell culture and transfection
Hepg2 cells were maintained in Dulbecco's Modified
Eagle's Medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum [FBS; Serana (WA) Pty.
Ltd.]. The cells were cultured at a temperature of 37˚C in a
humidified incubator with 5% CO2. Transfection of Hepg2
cells was performed using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol, cell density, approximately 80%, with 5
µg of plasmid per dish at room temperature for 10 min.
FAXDC2 overexpression was achieved by inserting the coding sequence
(CDS) of human FAXDC2 into the pCMV-HA vector (Agilent
Technologies, Inc.) to generate pCMV-HA-FAXDC2, pCMV-HA was used as
a negative control for overexpression. For FAXDC2 knockdown,
specific short interfering (si)RNA targeting FAXDC2 was synthesized
by Sangon Biotech Co., Ltd. Transfection of siRNA was performed
using Lipofectamine® RNAiMAX (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructionsat
room temperature for 10 min and then place it in a 37̊C incubator
for 48 h. The sequences of the siRNAs used are provided in Table SII.
CCK-8 assay for cell viability
Cell viability of HepG2 cells was assessed using
Cell Counting Kit-8 (CCK-8; Beyotime Institute of Biotechnology).
Following transfection with the plasmid vector, the cells were
incubated for 24, 48 and 72 h. After the respective time points, 20
µl of CCK-8 reagent was added to each well and the cells were then
incubated for 1 h at 37˚C in a CO2 incubator. The
absorbance of the medium was quantified at a wavelength of 450 nm
using a microplate reader (Bio-Rad Laboratories, Inc.) This
measurement was used to evaluate cell viability.
Flow cytometry
Flow cytometry was employed to evaluate cell cycle
progression and apoptosis 24 h after cell transfection. For cell
cycle analysis, a total of 5,000 cells were fixed, stained with
propidium iodide at 37̊C water bath for 30 min, and analyzed using
Becton Dickinson (BD) FACS Calibur flow cytometry (BD Biosciences).
To assess apoptosis, the Annexin V Apoptosis Detection Kit (BD
Biosciences) was used following the manufacturer's instructions, by
treating with Annexin V on ice for 10 min, then adding propidium
iodide, mixing, and ice Let stand for 5 min before performing flow
cytometry analysis. For this assay, 5x104 cells were
labeled with annexin V and propidium iodide and then subjected to
flow cytometry analysis. Flow cytometry analysis of apoptosis was
performed using FLOWJO version 10.8.1 (flowjo.com/),
and flow cytometry analysis cycle was performed using MODFIT LT
version 4.0.5 (https://www.solvusoft.com/zh-cn/file-extensions/software/verity-software-house/).
The apoptosis rate is a comparative analysis by calculating the
percentage of early + late apoptotic cells.
Cell migration and invasion
assays
To conduct cell migration and invasion assays,
24-well plates with 8 µm chambers inserted into each well
(Corning, Inc.) were used. For migration assays, 5x104
cells were directly added to the upper chamber. In invasion assays,
an additional layer of Matrigel was applied to the insert to
establish a matrix barrier at 37̊C for 3 h, followed by seeding
1x105 cells into the upper chamber. Further, 800
µl of DMEM supplemented with 10% FBS was added to each lower
chamber. The cells were then incubated at 37˚C for a specific
duration to allow migration or invasion. The cells that migrated or
invaded through the chambers were fixed and stained using 0.1%
crystal violet and 20% methanol for 30 min at room temperature in
the dark. Subsequently, the cells were observed and counted under
an inverted microscope (Nikon Eclipse; Nikon Corporation).
Vector construction
The CDS region of the FAXDC2 gene was amplified
using the polymerase chain reaction (PCR) method and homologous
recombination was performed simultaneously. The homologous
recombination reagent from Vazyme Biological Company was used for
this purpose. The amplified CDS region was then inserted into the
pCMV-HA vector (Promega Corporation). To validate the sequence
integrity and accuracy of the constructed vector, Sanger sequencing
analysis was performed by Tsingke Biotechnology Co, Beijing,
China.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 8.0 software (GraphPad Software; Dotmatics). The data are
expressed as mean ± standard deviation. Differences between
two groups were evaluated using unpaired t-tests. In TIMER 2.0, the
Wilcoxon rank sum test was employed. Each experiment was repeated a
minimum of three times to ensure reproducibility. The significance
of survival curves was assessed using the log-rank test. For
LinkedOmics analysis, Pearson and Spearman's correlation tests were
used. Spearman's correlation test was used for cBioPortal analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
The expression of FAXDC2 is
downregulated in tumor tissues
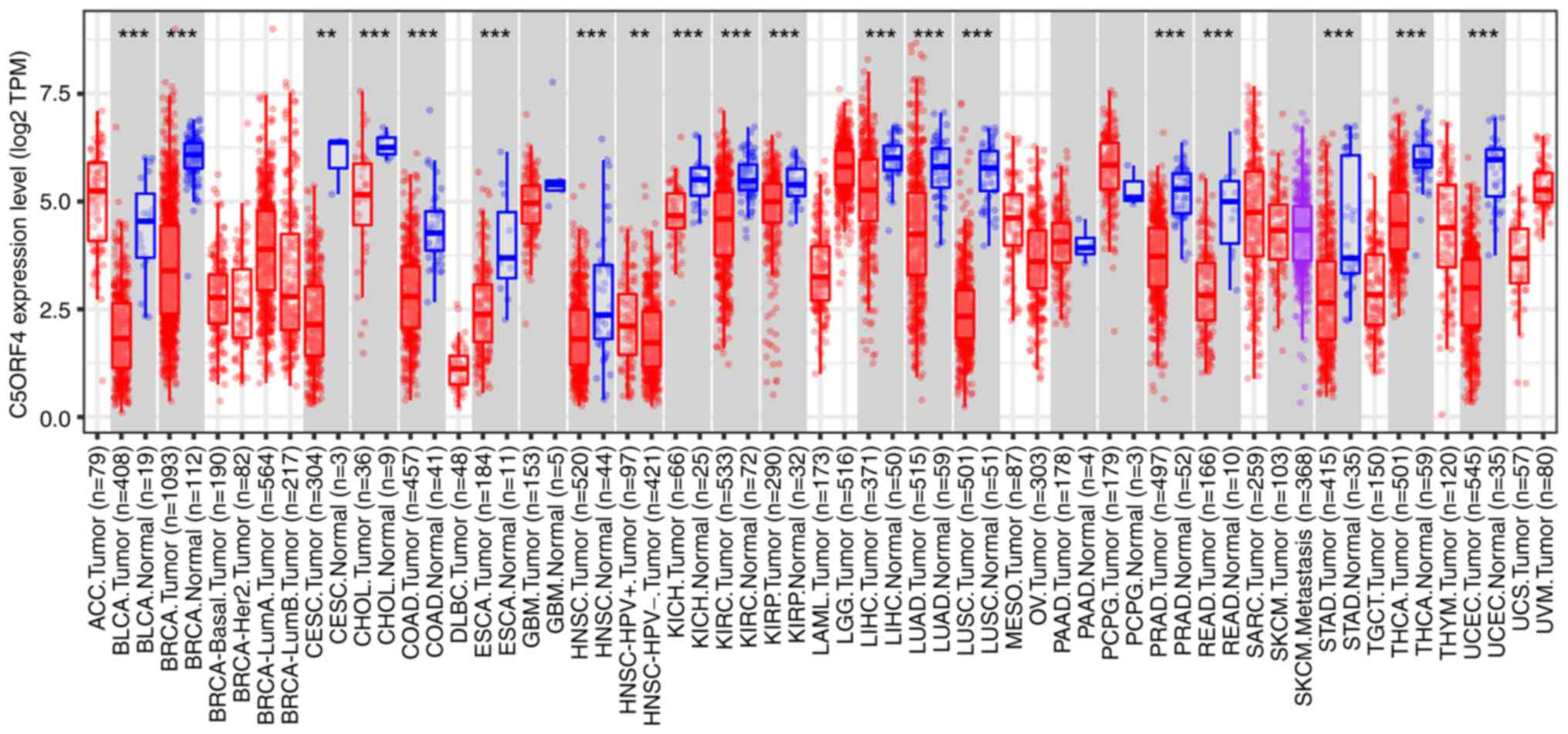
TIMER2.0 was employed to conduct an analysis
comparing the expression of FAXDC2 in various human tumor tissues
with normal tissues. Compared with normal tissues, FAXDC2 exhibited
significant downregulation in several types of cancer, including
lung squamous cell carcinoma, lung adenocarcinoma, hepatocellular
carcinoma (LIHC), prostate cancer, colorectal cancer, cervical
squamous cell carcinoma and endocervical adenocarcinoma, as well as
18 other types of tumor tissue where it consistently demonstrated
significant downregulation (Fig.
1). This suggested that FAXDC2 may have a relatively conserved
role in carcinogenesis.
Considering the involvement of FAXDC2 in the pathway
of fatty acid synthesis and the significance of the liver in fat
synthesis and metabolism, LIHC was selected to explore the role of
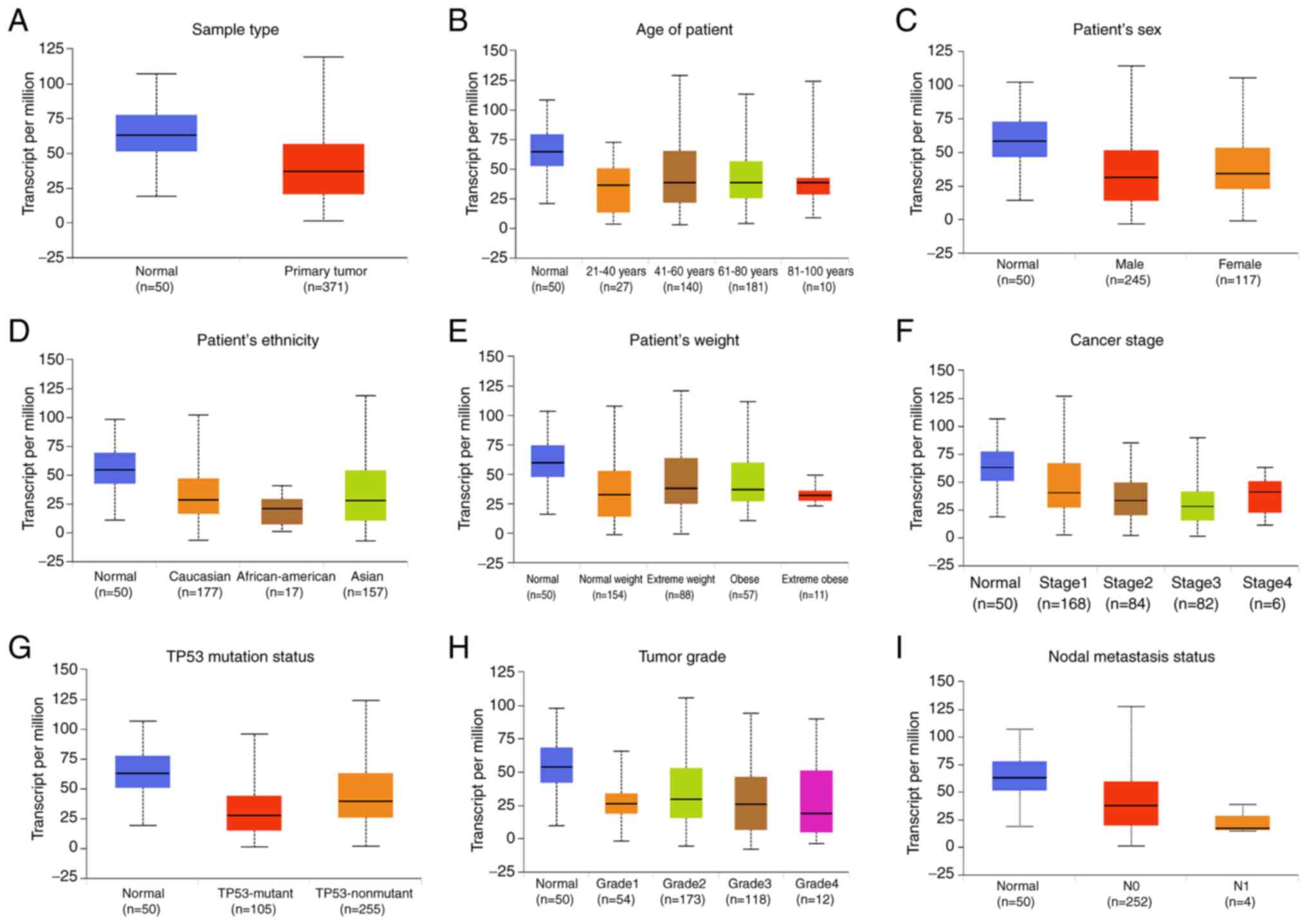
FAXDC2 in cancer. UALCAN was used to classify LIHC samples from the
TCGA database based on sample type, cancer stage, body weight, age,
tumor grade and lymph node metastasis status. Expression of FAXDC2
was lower in various LIHC subgroups Compared with normal tissues
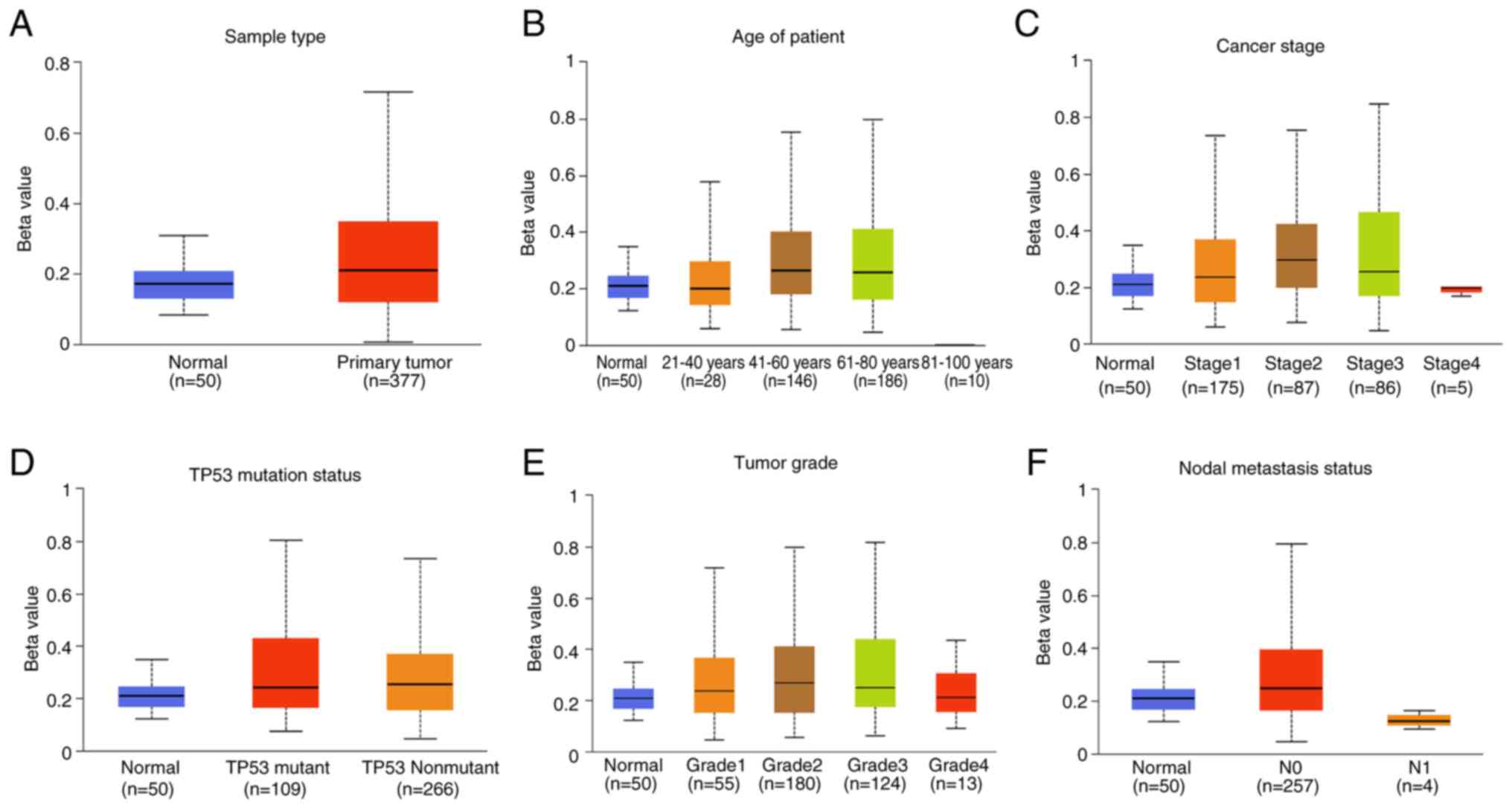
(Fig. 2). Moreover, the
methylation level of the FAXDC2 gene promoter was observed to be
higher in various types of cancer tissue compared with normal
tissues (Fig. 3).
High expression of FAXDC2 in LIHC
patients has good prognosis
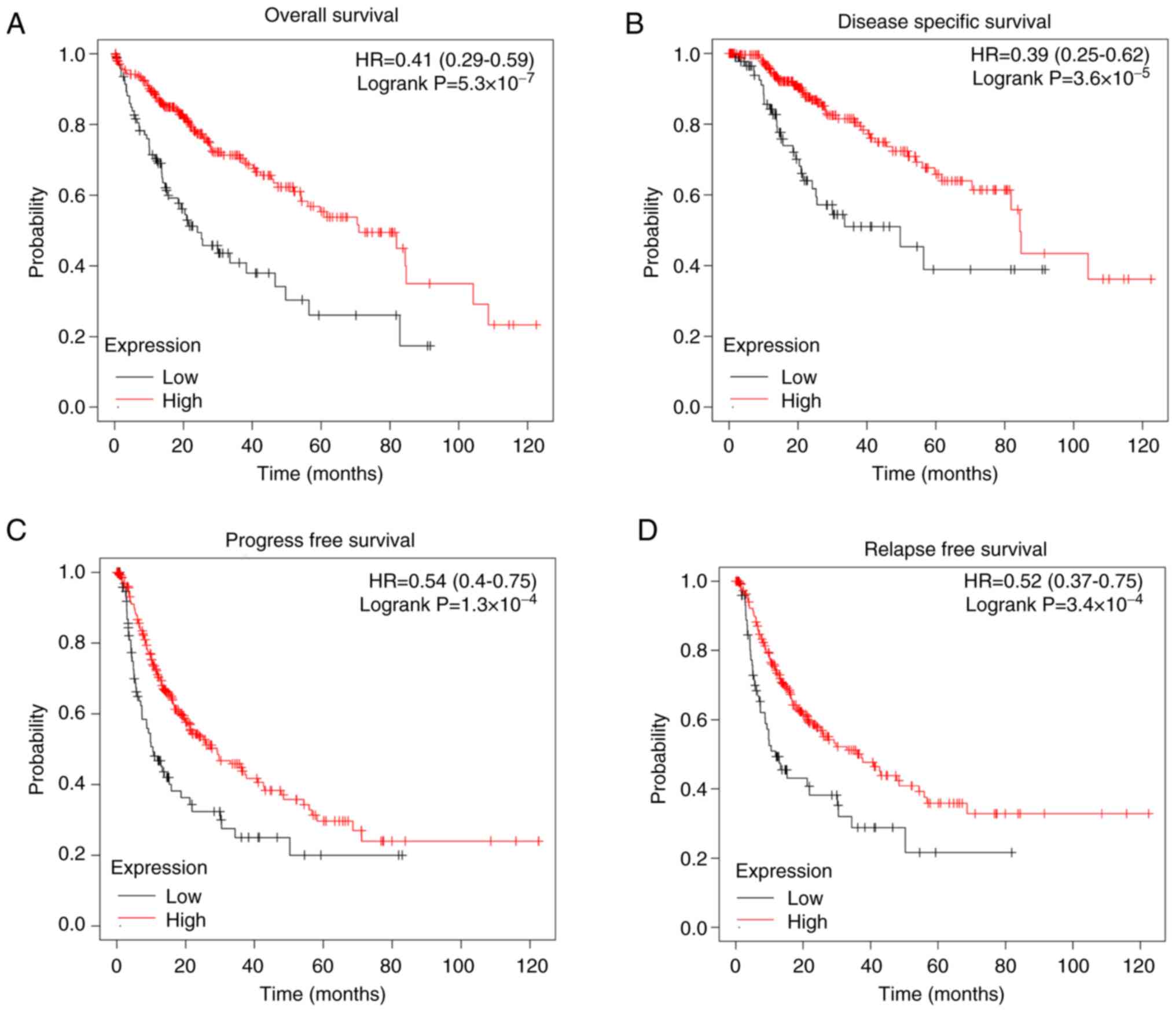
To explore the prognostic significance of FAXDC2 in
LIHC, the present study conducted Kaplan-Meier survival analysis.
The LIHC cohort was divided into subgroups based on the expression
level of FAXDC2. The results revealed that patients with low
expression of FAXDC2 had a poorer prognosis compared with other
subgroups. Kaplan-Meier survival curves (Fig. 4) demonstrated that LIHC patients
with high FAXDC2 expression had longer overall survival [OS; hazard
ratio (HR)=0.41 (0.29-0.59); P=5.3 x10-7], disease-free
survival [DSS; HR=0.39 (0.25-0.62), P=3.6 x10-5],
progression-free survival [PFS; HR=0.54 (0.4-0.75), P=1.3
x10-4] and recurrence-free survival [RFS; HR=0.52
(0.37-0.75), P=3.4 x10-4] compared with those with low
FAXDC2 expression.
Collectively, these results indicated that high
expression of FAXDC2 is closely associated with favorable clinical
outcomes in LIHC patients, suggesting its potential as a valuable
prognostic biomarker.
Differentially expressed genes related
to FAXDC2
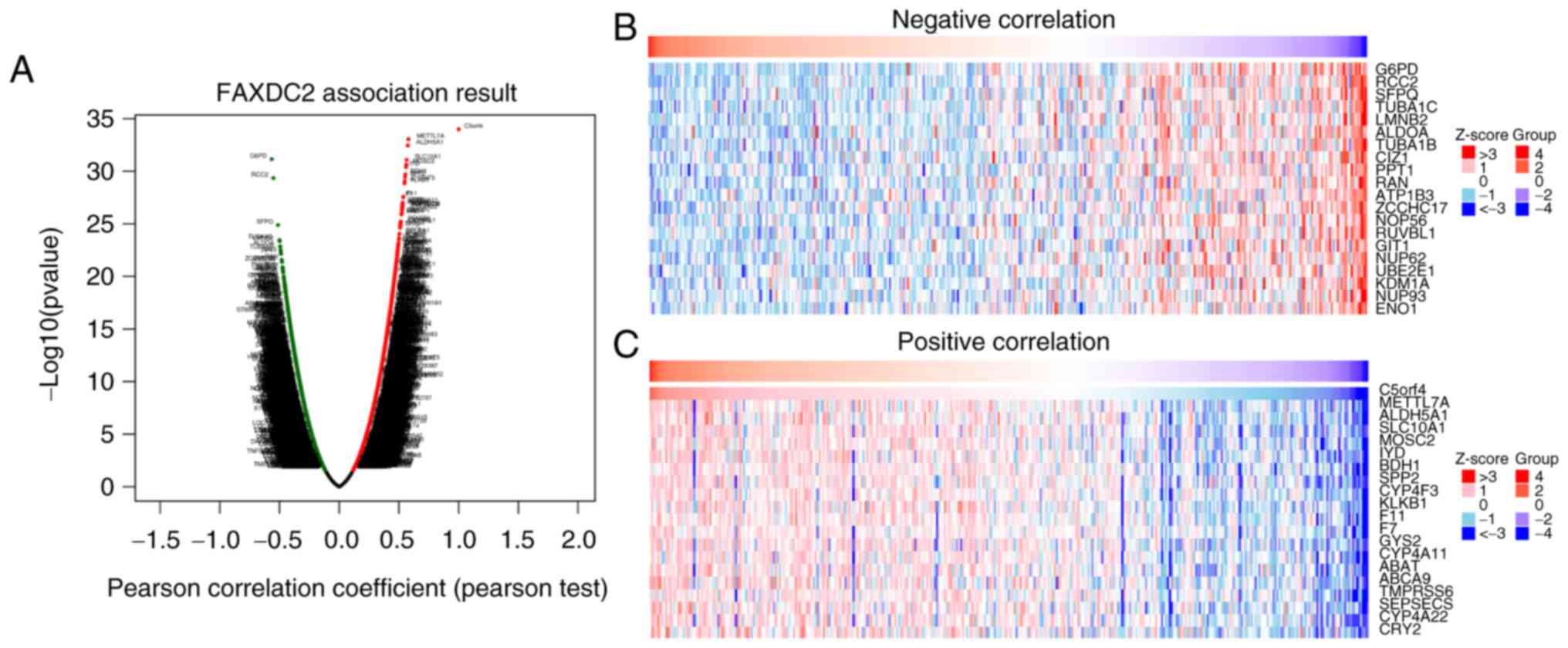
In LIHC, the present study identified genes
associated with FAXDC2 using LinkedOmics and displayed them in a
heatmap (28). As shown in the
volcano plot (Fig. 5A), the top 50
differentially expressed genes or negatively or positively
correlated genes were significantly expressed and the top 20
correlated differentially expressed genes were shown in the heatmap
(Fig. 5B and C).
Functional enrichment analysis of
FAXDC2
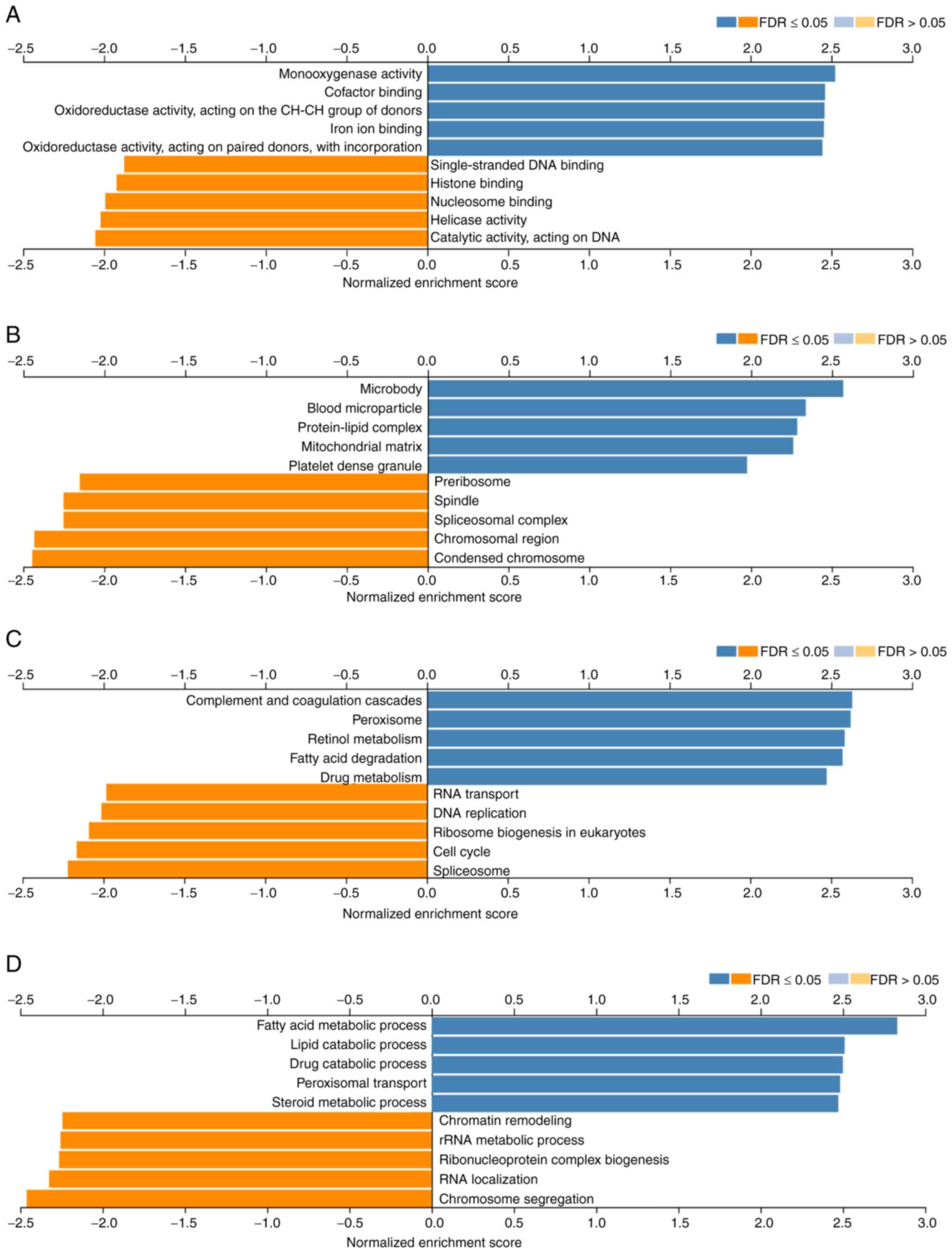
Next, gene ontology analysis was performed using the
GSEA LinkInterpreter module to classify FAXDC2-related genes into
three categories based on molecular functions, involved biological
processes and cellular locations where they occur. The five up- or
downregulated pathways for each pathway were as follows. Those
mainly involved in molecular functions, showed upregulation in
molecular functions such as oxidoreductase activity, iron ion
binding and cofactor binding and in monooxygenase activity,
single-strand DNA binding, histone binding, nucleosome binding and
helicase activity. Molecular functions such as catalytic activity
acting on DNA were downregulated (Fig.
6A). In the category of cellular components, the FAXDC2-related
genes were mainly enriched and upregulated in cellular components
such as microsomes, blood particles, protein-lipid complexes,
mitochondrial matrix and platelet dense granules, while
downregulated in chromatin, spindle, spliceosome complexes and
pre-ribosomes (Fig. 6B). Further
analysis revealed functional KEGG pathways that may be involved in
the development and progression of LIHC, including complement and
coagulation cascades, peroxisomes, retinol metabolism, fatty acid
degradation; upregulated in pathways such as drug metabolism and
downregulated in pathways such as RNA transport, DNA replication,
ribosome biogenesis in eukaryotes, cell cycle and spliceosome
(Fig. 6C). In the biological
process category, FAXDC2 related differentially expressed genes
were mainly enriched in peroxisome transport, amino acid
metabolism, lipid catabolism, drug catabolism, steroid metabolism,
regulation of chromosome organization, chromatin reorganization
plasticity, rRNA metabolic process and RNA localization (Fig. 6D).
In summary, the differential expression of FAXDC2 in
LIHC tissues involved ab-normal changes in multiple signaling
pathways and biological processes.
FAXDC2 is associated with multiple
signaling pathways involved in cancer regulation
To identify FAXDC2 as a putative cancer regulator,
common signaling pathways or key regulators that are aberrantly
expressed in tumors were investigated. It is known that PI3K, ERK
and other signaling pathways or signal transduction pathways are
often abnormally expressed in LIHC tissues (13,29,30).
Therefore, we conducted co-expression analysis of key regulatory
molecules of these signaling pathways and FAXDC2 was performed.
In LIHC, the cBioPortal platform was used to
correlate the expression level of FAXDC2 with key molecules
aberrantly expressed in tumor tissue (such as CDC25B, CDK2, CDK4,
AKT1, TNFα, MAPK3 and PARP1). Finally, it was found that key
regulators in tumorigenesis, such as AKT1 and MAPK3, were
significantly associated with the expression of FAXDC2 in LIHC
(Fig. 7). Pearson correlation
coefficient analysis showed that the expression level of FAXDC2 was
negatively correlated with the expression levels of CDC25B, CDK2,
CDK4, MAPK3 and PARP1, positively correlated with the expression
levels of EGFR, AKT1 and CDH1, but had no significant correlation
with MTOR. These results suggested that FAXDC2 may affect cancer
progression through multiple signaling pathways and is closely
related to life activities such as cell proliferation, migration
and apoptosis.
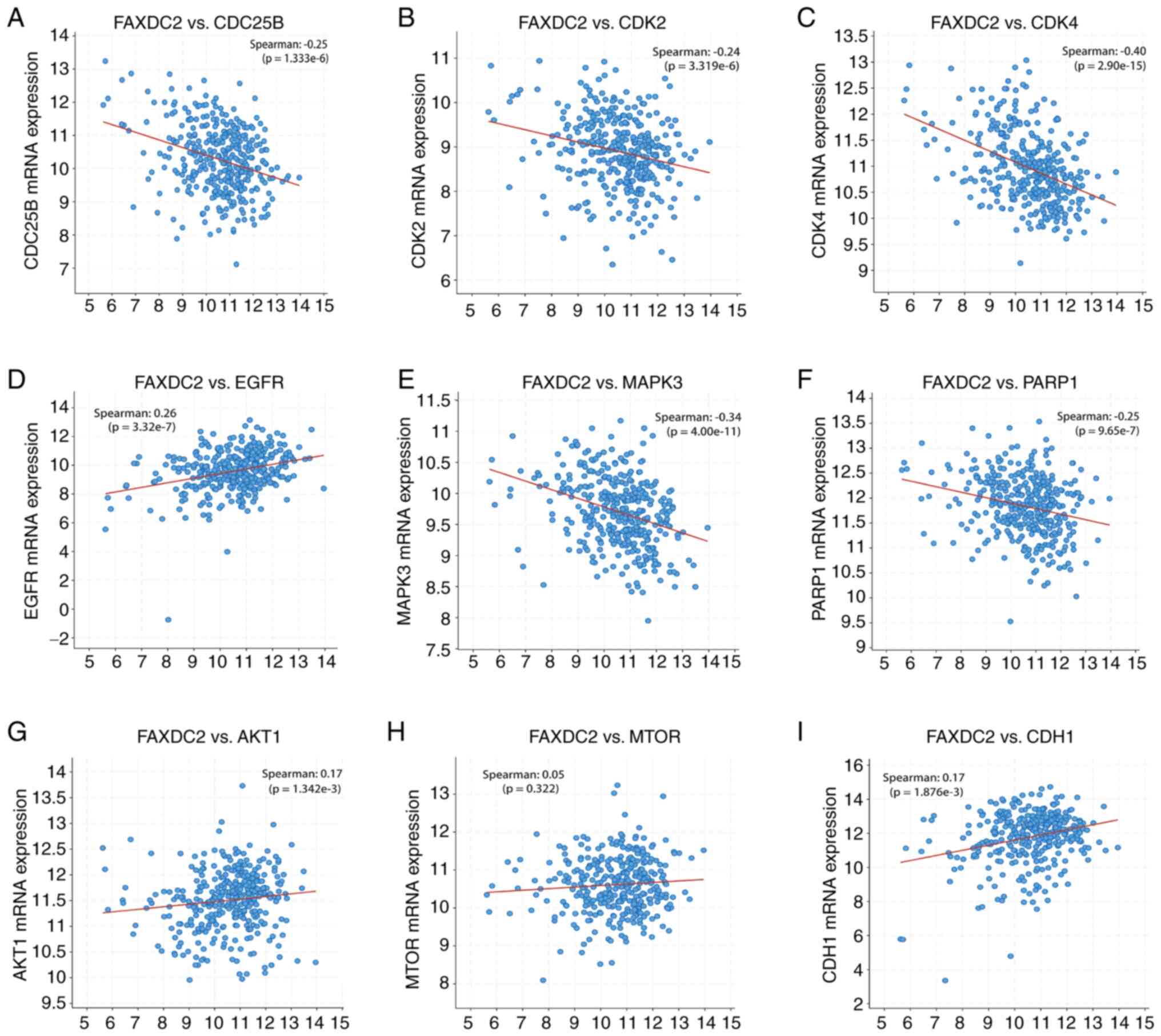 | Figure 7Correlation of FAXDC2 mRNA expression
levels with other gene expression levels in LIHC samples
(cBioPortal). (A) FAXDC2 and CDC25B, (B) FAXDC2 and CDK2, (C)
FAXDC2 and CDK4, (D) FAXDC2 and EGFR, (E) FAXDC2 and MAPK3, (F)
FAXDC2 and PARP1, (G) FAXDC2 and AKT1, (H) FAXDC2 and MTOR, (I)
FAXDC2 and CDH1. Statistical significance was calculated by t-test.
FAXDC2, fatty acid hydroxylase domain containing 2; CDC25B, cell
division cycle 25B; CDK2, cyclin-dependent kinase 2; EGFR,
epidermal growth factor receptor; MAPK3, mitogen-activated protein
kinase 3; PARP1, Poly (ADP-Ribose) Polymerase 1; AKT1, AKT
serine/threonine kinase 1, also known as protein kinase B (PKB);
MTOR, mechanism target of rapamycin kinase; CDH1, cadherin 1. |
Overexpression of FAXDC2 regulates
cycle distribution of HepG2 cells
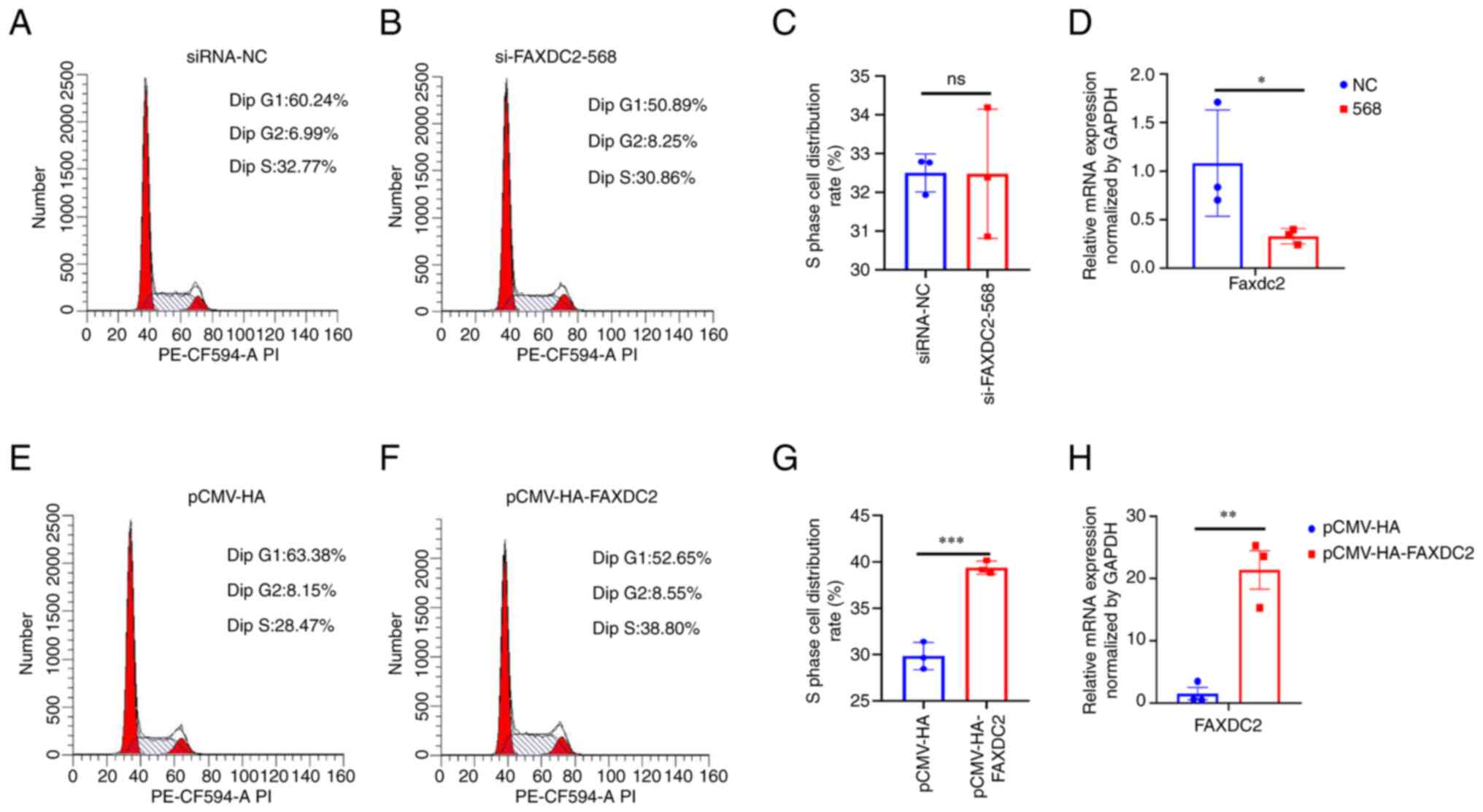
In order to verify the association between FAXDC2
and liver cancer from an experimental point of view, the present
study overexpressed and silenced HepG2 cells and per-formed flow
cytometry to analyze the cycle changes of HepG2 cells, as shown in
the Fig. 8. The cycle distribution
of HepG2 cells treated with si-FAXDC2-568 and FAXDC2 overexpression
was detected by flow cytometry and it was found that the cell cycle
distribution of si-FAXDC2 had no significant change, while the
number of cells in S phase of cells overexpressing FAXDC2 increased
significantly and the number of cells in
G1/G0 phase significantly decreased. This
indicated that the overexpression of FAXDC2 can regulate the cell
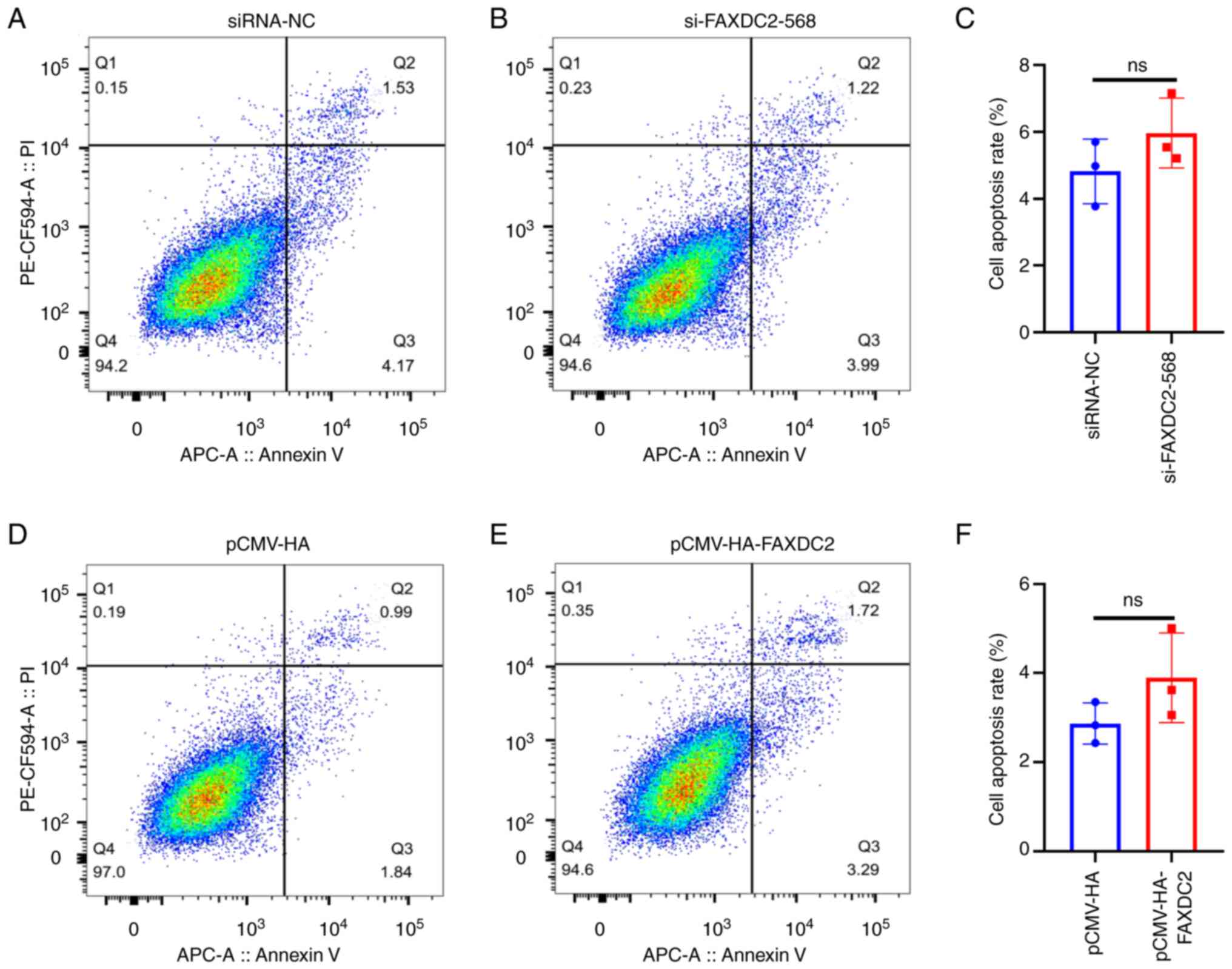
cycle distribution. In addition, through the detection of cell
apoptosis, it was found that the apoptosis rate of HepG2 cells
treated with overexpression of FAXDC2 has no significant change
compared with the control group and the apoptosis rate of HepG2
cells treated with si-FAXDC2 had no significant change (Fig. 9).
Overexpression of FAXDC2 inhibits
HepG2 cell invasion
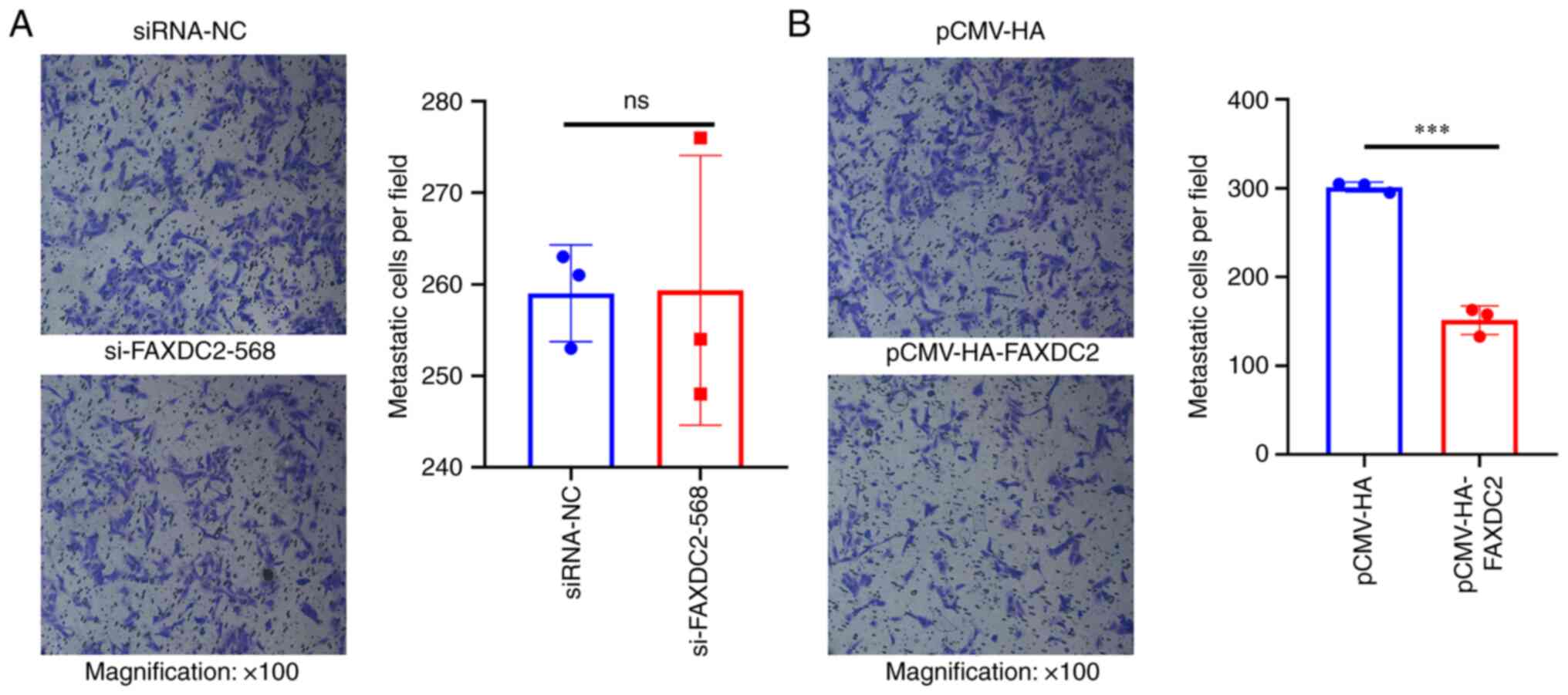
In order to detect the effect of endogenous
differential expression of FAXDC2 in HepG2 cells on the invasion
function of HepG2 cell lines, Transwell was used to detect the
effect of FAXDC2 on the invasion ability of HepG2 cells. Compared
with the control group, the invasive ability of HepG2 cells knocked
down by FAXDC2 had no significant change (Fig. 10A) and HepG2 cells treated with
overexpression of FAXDC2 had weaker invasive ability (Fig. 10B).
Overexpression of FAXDC2 inhibits
proliferation of HepG2 cells
The above results suggested that FAXDC2 plays an
inhibitory role in the occurrence and development of liver cancer.
In order to verify whether the overexpression of FAXDC2 leads to
the weakening of cell proliferation through arresting the cell
cycle, CCK-8 assay was then performed on HepG2 cells (Fig. 11A and B). The results showed that after
overexpression of FAXDC2, the number of living cells was
significantly reduced compared with the control, suggesting that
FAXDC2 reduced the proliferation activity of HepG2 cells. To reveal
how the overexpression of FAXDC2 regulated the proliferation and
invasion ability of HepG2 cells at the molecular level, the changes
in the expression levels of genes related to proliferation,
invasion and apoptosis at the protein and mRNA level were detected
by western blotting and qPCR (Fig.
11C-E), as shown in the figure, after overexpressing FAXDC2 to
treat HepG2 cells, the expression of Vimentin and TGFβ1 related to
invasion and EMT were significantly downregulated, the protein
expression levels of E-cadherin were significantly upregulated and
the expression levels of cycle-related proteins CDK2 and CDC25B
were significantly downregulated, while the genes related to
apoptosis such as P53, Bax, Bcl2 and CASP9 demonstrated no
significant changes.
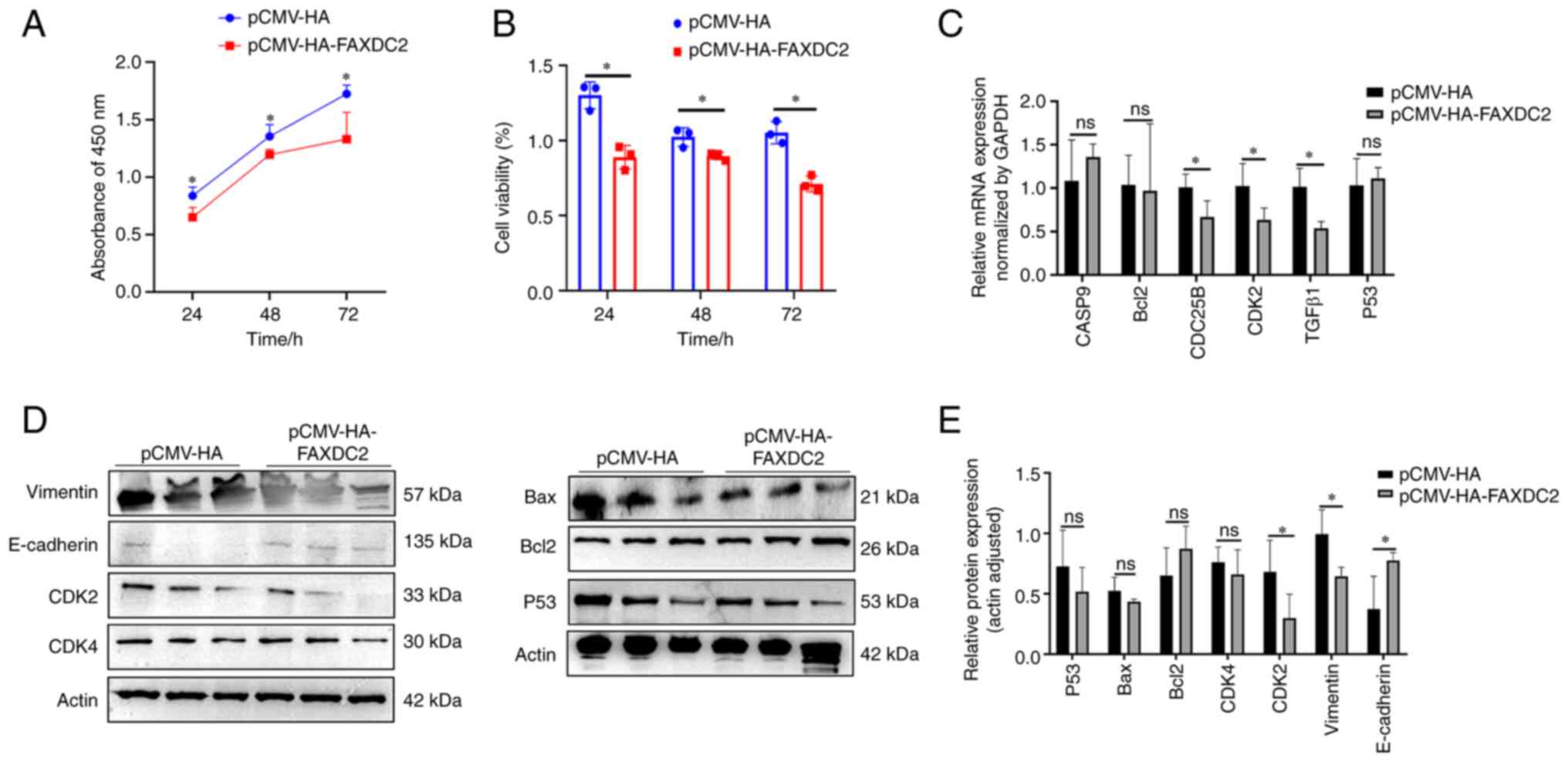 | Figure 11Evaluation of the effect of FAXDC2
overexpression on the proliferation of HepG2 cells. (A) 450nm
absorbance of FAXDC2 overexpressed HepG2 cells. (B) Cell viability
of FAXDC2-overexpressed HepG2 cells. (C) qPCR detection of FAXDC2
overexpression, changes in mRNA levels of genes related to
apoptosis and proliferation, (D) Effects of overexpression of
FAXDC2 on proliferation, apoptosis, migration and invasion-related
proteins were (E) Quantitative statistics of panel D. The
experimental results were calculated using the ImageJ tool and
GraphPad Prism 8.0 was used for statistical quantification. The
comparison between the experimental group and the control group is
represented by a histogram (n=3). Statistical significance was
calculated by t-test. *P<0.05, ns, no significant.
FAXDC2, fatty acid hydroxylase domain containing 2; qPCR,
quantitative PCR; Bax, apoptosis regulator; P53, human tumor
suppressor gene; E-cadherin, cadherin E; CDK2, cyclin-dependent
kinase 2; CDK4, cyclin-dependent kinase 4; Vimentin, vimentin;
Bcl2, apoptosis regulator; TGFβ1, transforming growth factor β1;
Actin, actin. |
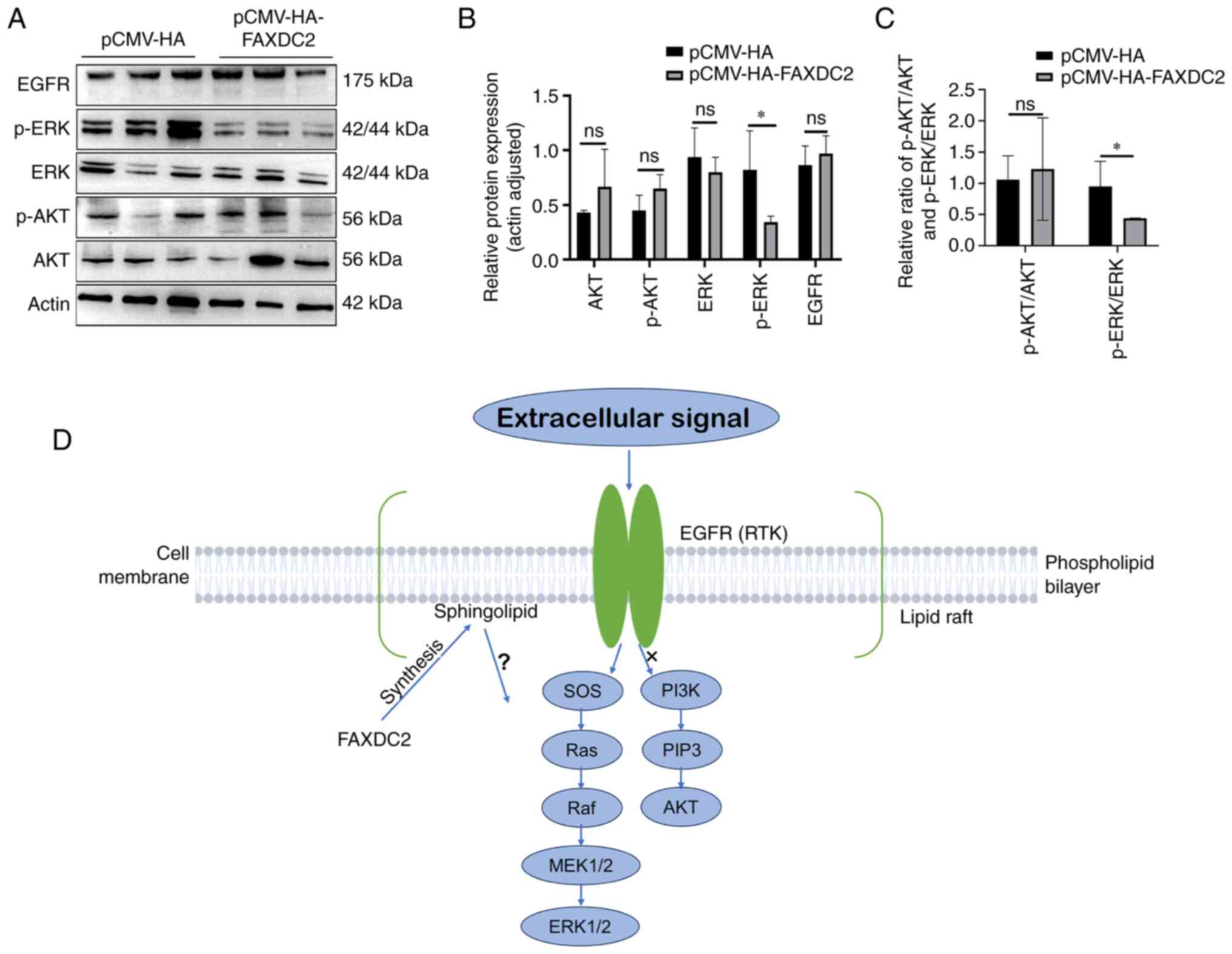
Overexpression of FAXDC2 inhibits ERK
signaling in liver cancer cells
Based on the previous analysis results of the
cBioPortal platform it was hypothesized that FAXDC2 may exert cell
biological functions through AKT or ERK signaling in the process of
liver cancer. The effect of FAXDC2 overexpression on ERK signaling
and AKT signaling was then evaluated by western blotting (Fig. 12). The results of western blotting
(Fig. 12A and B) showed that following the
overexpression of FAXDC2, the expression level of p-ERK was
significantly downregulated and the expression levels of AKT, p-AKT
and EGFR showed no significant change, which indicated that FAXDC2
may be activated in the HepG2 cell line through Inhibit ERK
phosphorylation to play an inhibitory effect on liver cancer
(Fig. 12C).
Discussion
The reprogramming of lipid metabolism plays an
important role in the occurrence and development of liver cancer.
As a member of the fatty acid hydroxylase family, FAXDC2 has a
potential role in the synthesis of cholesterol and sphingomyelin.
Although more attention has been paid to FAXDC2 in the past decade,
its function in liver cancer has not been investigated. The present
study explored the role of FAXDC2 in the development of liver
cancer based on bioinformatics and experimental biology. It
produced the following findings: i) In the majority of cancer
tissues, including liver cancer, FAXDC2 demonstrated downregulation
and its low expression was found to be associated with a
detrimental prognosis in cancer patients. ii) The presence of
FAXDC2 exhibited inhibitory effects on the proliferation, migration
and invasion of liver cancer cells. iii) It is possible that FAXDC2
exerted its influence on the growth of liver cancer cells by
suppressing the ERK signaling pathway.
The expression of FAXDC2 is inversely
correlated with the development of liver cancer
Bioinformatics results showed that FAXDC2 is
expressed in 18 cancer tissues including lung squamous cell
carcinoma, lung adenocarcinoma, liver cancer, prostate cancer and
colorectal cancer. This suggested that FAXDC2 might have a
universal function in cancer and the liver, as the main site of
fatty acid synthesis and metabolism, and this has become a key
research target of the authors. Meanwhile, abnormally high levels
of FAXDC2 gene promoter methylation were detected in a number of
LIHC subgroups by UALCAN analysis, suggesting that epigenetic
downregulation of FAXDC2 might contribute to the development of
LIHC. Through Kaplan-Meier plotter analysis, significant
differences were observed in the survival curves between patients
with high and low expression of FAXDC2. This analysis confirmed
that FAXDC2 expression is downregulated in prostate cancer and
neuroblastoma and low expression of FAXDC2 is indicative of an
unfavorable prognosis for these diseases (24,25),
in accordance with the experimental findings of the present
study.
In summary, mutations in FAXDC2 can affect the
initial stages of liver cancer development and low expression of
FAXDC2 is associated with a poor prognosis in patients with liver
cancer.
FAXDC2 inhibits the proliferation and
invasion of liver cancer cells
The disruption of the cell cycle plays a significant
role in cancer development (31).
The flow cytometry results of the present study indicated that the
overexpression of FAXDC2 led to an increase in the S phase of the
cell cycle in HepG2 cells. This increase in the S phase was
accompanied by a decrease in proliferation activity and
downregulation of CDK2, a marker involved in transitioning from the
S phase to the G2 phase. It has been demonstrated in a
previous study that arresting cells in the S phase can inhibit the
progression of liver cancer (32).
Therefore, it was hypothesized that FAXDC2 induces cell cycle
arrest in the S phase, thereby inhibiting cell proliferation and
impeding the development of liver cancer.
The results of Transwell experiments showed that the
overexpression of FAXDC2 reduced the invasion ability of HepG2.
Combined with the results of western blotting, the expression of
Vimentin was downregulated and the expression of E-Cadherin was
upregulated. Upregulation of Vimentin expression and downregulation
of E-Cadherin expression are the hallmarks of EMT in metastatic
cancer and the upregulation of Vimentin is associated with tumor
growth, invasion and poor prognosis (33-35).
Although the above roles of FAXDC2 in cancer cell had not been
reported before to the best of the authors' knowledge, a similar
cytology function is well demonstrated on its superfamily gene,
FAXDC1(36). It has been reported
that FAXDC1 can inhibit the proliferation of colorectal cancer
cells, migration, EMT progression and tumor growth (37). FAXDC2 might play a similar function
to FAXDC1 and inhibits the occurrence and development of liver
cancer.
Notably, the opposite was not evident in the
FAXDC2-knockdown HepG2 cell line, which was consistent with the
results detected by flow cytometry and it was hypothesized that the
reason for this result might be a compensatory mechanism that
rescues the effect of low expression of FAXDC2.
FAXDC2 may inhibit the proliferation
and invasion of liver cancer cells by downregulating ERK
signaling
It is known that ERK signal plays a role in
promoting cell proliferation and invasion in cancer (38-41).
The analysis results of the cBioPortal platform showed that the
co-expression of FAXDC2 and MAPK3 in liver cancer was negatively
correlated, but not significantly correlated, with AKT. These
results suggested that FAXDC2 might also regulate liver
carcinogenesis by inhibiting ERK signaling in liver cancer cells.
Although the mechanism of FAXDC2 inhibiting ERK has not been
elucidated in the present study, some clues gives reason to
hypothesize that sphingomyelin in lipid rafts may play a key role
in this process. It has been reported that overactivation of EGFR
and downstream ERK1/2 by regulating lipid raft dynamics leads to
accelerated cell proliferation and thus promotes the development of
liver cancer (42). Considering
that FAXDC2 is involved in synthesis of sphingomyelin, it is
probable that FAXDC2 regulates the dynamics of lipid rafts by
affecting the content of sphingomyelin and then affects ERK signal
transduction. Indeed, in megakaryocytes, FAXDC2 promotes
megakaryocyte differentiation by regulating ERK phosphorylation and
upregulating RUNX1, and when FAXDC2 is expressed the mRNA level of
ganglioside (GM3) synthase, a sialic acid-containing
Glycosphingolipids are expressed on the cell surface. Jin et
al (26) speculate that FAXDC2
may regulate ERK signaling by regulating the synthesis of
sphingolipids such as GM3. It can be seen that in the liver cancer,
FAXDC2 might also perform dynamic regulation mechanism of lipid
rafts, but cell differentiation is different from tumorigenesis,
the mechanism of action of FAXDC2 in the development of liver
cancer needs to be further elucidated.
The present study had limitations. The molecular
mechanism of FAXDC2 function by inhibiting ERK and content of
sphingomyelin were not determined. These issues should be
investigated via metabolomics.
The present study demonstrated, for the first time
to the best of the authors' knowledge, that FAXDC2 may be a novel
inhibitor of liver cancer proliferation, migration and invasion
through ERK signal related mechanism. The present study provided
experimental evidence for the development of liver cancer and the
search for new diagnostic and interventional methods.
Supplementary Material
Quantitative PCR primer
sequences.
siRNA sequence.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported in part by grants from
the National Natural Science Foundation of China (grant nos.
81970324 and 81974019), Shanghai Key Laboratory of Regulatory
Biology, Institute of Biomedical Sciences, East China Normal
University, National Key Research and Development Program of China
(grant no. 2018YFA0108700), Guangdong Provincial Special Support
Program for Prominent Talents (grant no. 2021JC06Y656), Science and
Technology Planning Project of Guangdong Province (grant nos.
2020B1111170011 and 2022B1212010010), Guangdong special funds for
science and technology innovation strategy, China (Stability
support for scientific research institutions affiliated to
Guangdong Province- grant no. GDCI 2021), the Marine Economy
Development Project of Department of Natural Resources of Guangdong
Province [grant no. GDNRC(2022)039], Guangzhou Science and
Technology Plan Project (grant no. 202201000006) and the Special
Project of Dengfeng Program of Guangdong Provincial People's
Hospital (grant no. KJ012019119).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XF conceived the study and wrote and edited the
manuscript. Methodology was performed by XF, XW and WY. ZP, SX, QZ
and XY performed experiments. Data analysis was performed by YL,
PZ, FL, ZJ, XY, WY and YW. Data validation was performed by ZP, PZ,
XW and FL. ZP constructed figures and wrote the manuscript and XW
and WY provided guidance. XF and ZP confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48.
2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vogel A, Meyer T, Sapisochin G, Salem R
and Saborowski A: Hepatocellular carcinoma. Lancet. 400:1345–1362.
2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chidambaranathan-Reghupaty S, Fisher PB
and Sarkar D: Hepatocellular carcinoma (HCC): Epidemiology,
etiology and molecular classification. Adv Cancer Res. 149:1–61.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33.
2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Podlasek A, Abdulla M, Broering D and
Bzeizi K: Recent advances in locoregional therapy of hepatocellular
carcinoma. Cancers (Basel). 15(3347)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Whittaker S, Marais R and Zhu AX: The role
of signaling pathways in the development and treatment of
hepatocellular carcinoma. Oncogene. 29:4989–5005. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xiang T, Fei R, Wang Z, Shen Z, Qian J and
Chen W: Nicotine enhances invasion and metastasis of human
colorectal cancer cells through the nicotinic acetylcholine
receptor downstream p38 MAPK signaling pathway. Oncol Rep.
35:205–210. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xu CY, Qin MB, Tan L, Liu SQ and Huang JA:
NIBP impacts on the expression of E-cadherin, CD44 and vimentin in
colon cancer via the NF-κB pathway. Mol Med Rep. 13:5379–5385.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Reddy KB, Nabha SM and Atanaskova N: Role
of MAP kinase in tumor progression and invasion. Cancer Metastasis
Rev. 22:395–403. 2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Llovet JM, Villanueva A, Lachenmayer A and
Finn RS: Advances in targeted therapies for hepatocellular
carcinoma in the genomic era. Nat Rev Clin Oncol. 12:408–424.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wiesenauer CA, Yip-Schneider MT, Wang Y
and Schmidt CM: Multiple anticancer effects of blocking MEK-ERK
signaling in hepatocellular carcinoma. J Am Coll Surg. 198:410–421.
2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cheng C, Geng F, Cheng X and Guo D: Lipid
metabolism reprogramming and its potential targets in cancer.
Cancer Commun (Lond). 38(27)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yang H, Deng Q, Ni T, Liu Y, Lu L, Dai H,
Wang H and Yang W: Targeted inhibition of LPL/FABP4/CPT1 fatty acid
metabolic axis can effectively prevent the progression of
nonalcoholic steatohepatitis to liver cancer. Int J Biol Sci.
17:4207–4222. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wu D, Yang Y, Hou Y, Zhao Z, Liang N, Yuan
P, Yang T, Xing J and Li J: Increased mitochondrial fission drives
the reprogramming of fatty acid metabolism in hepatocellular
carcinoma cells through suppression of Sirtuin 1. Cancer Commun
(Lond). 42:37–55. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang W, Bai L, Li W and Cui J: The lipid
metabolic landscape of cancers and new therapeutic perspectives.
Front Oncol. 10(605154)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Beloribi-Djefaflia S, Vasseur S and
Guillaumond F: Lipid metabolic reprogramming in cancer cells.
Oncogenesis. 5(e189)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Du D, Liu C, Qin M, Zhang X, Xi T, Yuan S,
Hao H and Xiong J: Metabolic dysregulation and emerging
therapeutical targets for hepatocellular carcinoma. Acta Pharm Sin
B. 12:558–580. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Alderson NL, Rembiesa BM, Walla MD,
Bielawska A, Bielawski J and Hama H: The human FA2H gene encodes a
fatty acid 2-hydroxylase. J Biol Chem. 279:48562–48568.
2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Guo L, Zhang X, Zhou D, Okunade AL and Su
X: Stereospecificity of fatty acid 2-hydroxylase and differential
functions of 2-hydroxy fatty acid enantiomers. J Lipid Res.
53:1327–1335. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xue J, Yu Y, Zhang X, Zhang C, Zhao Y, Liu
B, Zhang L, Wang L, Chen R, Gao X, et al: Sphingomyelin synthase 2
inhibition ameliorates cerebral ischemic reperfusion injury through
reducing the recruitment of toll-like receptor 4 to lipid rafts. J
Am Heart Assoc. 8(e012885)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen S, He H, Yang H, Tan B, Liu E, Zhao X
and Zhao Y: The role of lipid rafts in cell entry of human
metapneumovirus. J Med Virol. 91:949–957. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Peng Y, Song Y and Wang H: Systematic
elucidation of the aneuploidy landscape and identification of
aneuploidy driver genes in prostate cancer. Front Cell Dev Biol.
9(723466)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang P, Ma K, Ke X, Liu L, Li Y, Liu Y
and Wang Y: Development and validation of a five-RNA-based
signature and identification of candidate drugs for neuroblastoma.
Front Genet. 12(685646)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jin Q, Ren Y, Wang M, Suraneni PK, Li D,
Crispino JD, Fan J and Huang Z: Novel function of FAXDC2 in
megakaryopoiesis. Blood Cancer J. 6(e478)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu H, Wilson KR, Firth AM, Macri C,
Schriek P, Blum AB, Villar J, Wormald S, Shambrook M, Xu B, et al:
Ubiquitin-like protein 3 (UBL3) is required for MARCH
ubiquitination of major histocompatibility complex class II and
CD86. Nat Commun. 13(1934)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gu Z, Eils R and Schlesner M: Complex
heatmaps reveal patterns and correlations in multidimensional
genomic data. Bioinformatics (Oxford, England). 32:2847–2849.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Huynh H, Nguyen TT, Chow KH, Tan PH, Soo
KC and Tran E: Over-expression of the mitogen-activated protein
kinase (MAPK) kinase (MEK)-MAPK in hepatocellular carcinoma: Its
role in tumor progression and apoptosis. BMC Gastroenterol.
3(19)2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wu Y, Zhang Y, Qin X, Geng H, Zuo D and
Zhao Q: PI3K/AKT/mTOR pathway-related long non-coding RNAs: Roles
and mechanisms in hepatocellular carcinoma. Pharmacol Res.
160(105195)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shen L, Tian SJ, Song HL, Chen X, Guo H,
Wan D, Wang YR, Wang FW and Liu LJ: Cytotoxic tricycloalternarene
compounds from endophyte alternaria sp. W-1 associated with
laminaria japonica. Mar Drugs. 16(402)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chow AK, Ng L, Sing Li H, Cheng CW, Lam
CS, Yau TC, Cheng PN, Fan ST, Poon RT and Pang RW: Anti-tumor
efficacy of a recombinant human arginase in human hepatocellular
carcinoma. Curr Cancer Drug Targets. 12:1233–1243. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Takkunen M, Grenman R, Hukkanen M,
Korhonen M, García de Herreros A and Virtanen I: Snail-dependent
and -independent epithelial-mesenchymal transition in oral squamous
carcinoma cells. J Histochem Cytochem. 54:1263–1275.
2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
de Araujo VC, Pinto Júnior DS, de Sousa
SO, Nunes FD and de Araujo NS: Vimentin in oral squamous cell
carcinoma. Eur Arch Otorhinolaryngol. 250:105–109. 1993.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hama H: Fatty acid 2-Hydroxylation in
mammalian sphingolipid biology. Biochim Biophys Acta. 1801:405–414.
2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sun L, Yang X, Huang X, Yao Y, Wei X, Yang
S, Zhou D, Zhang W, Long Z, Xu X, et al: 2-Hydroxylation of fatty
acids represses colorectal tumorigenesis and metastasis via the YAP
transcriptional axis. Cancer Res. 81:289–302. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Webb DJ, Nguyen DH and Gonias SL:
Extracellular signal-regulated kinase functions in the urokinase
receptor-dependent pathway by which neutralization of low density
lipoprotein receptor-related protein promotes fibrosarcoma cell
migration and matrigel invasion. J Cell Sci. 113:123–134.
2000.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen L, Guo P, He Y, Chen Z, Chen L, Luo
Y, Qi L, Liu Y, Wu Q, Cui Y, et al: HCC-derived exosomes elicit HCC
progression and recurrence by epithelial-mesenchymal transition
through MAPK/ERK signalling pathway. Cell Death Dis.
9(513)2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yang M, Yu X, Li X, Luo B, Yang W, Lin Y,
Li D, Gan Z, Xu J and He T: TNFAIP3 is required for FGFR1
activation-promoted proliferation and tumorigenesis of premalignant
DCIS.COM human mammary epithelial cells. Breast Cancer Res.
20(97)2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang K, Ji W, Yu Y, Li Z, Niu X, Xia W and
Lu S: FGFR1-ERK1/2-SOX2 axis promotes cell proliferation,
epithelial-mesenchymal transition, and metastasis in
FGFR1-amplified lung cancer. Oncogene. 37:5340–5354.
2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhang G, Li X, Chen Q, Li J, Ruan Q, Chen
YH, Yang X and Wan X: CD317 activates EGFR by regulating its
association with lipid rafts. Cancer Res. 79:2220–2231.
2019.PubMed/NCBI View Article : Google Scholar
|