Introduction
Endometrial cancer (EC) is the second most common
gynecological type of cancer, with 417,367 new cases and 97,370 new
deaths worldwide in 2021. The incidence of EC is increasing every
year (1). Currently, adjuvant
chemotherapy, including cisplatin (DDP), is used in clinical
practice to improve the therapeutic effect and prognosis of EC.
However, patients who undergo long-term chemotherapy can often
develop drug resistance, thus limiting its effect (2,3).
Oncological nursing has also played a significant role in the
prognosis of human malignancies, including EC (4,5).
Therefore, the identification of novel mechanisms and tumor active
markers to reverse the resistance of tumor cells to
chemotherapeutic drugs and improve the sensitivity of chemotherapy
have become the main means to improve the prognosis of patients
with cancer.
F-box only protein 31 (FBXO31), which belongs to the
FBXO family, serves a significant role in DNA damage response and
cell cycle regulation, and is involved in the development and
occurrence of several types of tumors (6). A previous study showed that FBXO31
could act as a tumor suppressor gene in cholangiocarcinoma, while
it could sensitize cancer stem cell-like cells to DDP via promoting
ferroptosis and proteasome-mediated degradation of glutathione
peroxidase 4(7). In addition,
FBXO31 inhibited lipogenesis and tumor progression in glioma by
promoting the ubiquitination and degradation of CD147(8). Another study also suggested that the
c-Myc oncogene could impair the inhibitory effect of FBXO31 on
tumor development, thus promoting the progression of ovarian cancer
(9). The aforementioned findings
indicated that FBXO31 was downregulated in different types of
cancer and it was, therefore, considered as a candidate tumor
suppressor gene. The UALCAN database (https://ualcan.path.uab.edu/analysis.html) was applied
to predict the expression levels of FBXO31 in patients with EC and
the high expression of FBXO31in EC and the association between
FBXO31 expression and EC prognosis were found. However, its effects
on EC and DDP resistance remain to be elucidated.
The JASPAR database (https://jaspar.genereg.net/) was used to predict the
binding capacity of Krüppel-like factor 9 (KLF9) transcription
factors on FBXO31 promoter. The KLF family is a significant group
of transcription factors in eukaryotes (10). Their carboxyl terminus contains
three highly conserved zinc finger domains of Cys2/His2, which bind
to promoters or enhancers to regulate the expression of their
corresponding target genes, thus exerting several biological
functions (11). KLF9 can be used
as a prognostic marker of EC (12,13).
A previous study has shown that EC cell proliferation and invasion
can be inhibited by upregulating KLF9 in EC (14). However, the regulatory relationship
between FBXO31 and KLF9 in EC has not so far been reported.
Therefore, the current study aimed to investigate
the role and regulatory mechanism of FBXO31 and its effect on DDP
resistance in EC.
Materials and methods
Databases
The UALCAN database was applied to predict the
expression levels of FBXO31 in patients with EC and the association
between FBXO31 expression and EC prognosis (15). Additionally, the JASPAR database
was used to predict the binding capacity of KLF9 transcription
factors on FBXO31 promoter (16).
Cell culture
The immortalized human endometrial epithelial cells
hEEC (cat. no. MZ-3223; Ningbo Mingzhou Biotechnology Co., Ltd.)
and the EC cell lines HEC-1A (cat. no. BNCC338711; BeNa Culture
Collection), RL95-2 (cat. no. BNCC356242; BeNa Culture Collection)
and Ishikawa (cat. no. BNCC342468; BeNa Culture Collection) were
cultured in DMEM supplemented with 10% fetal bovine serum (FBS;
Thermo Fisher Scientific, Inc.) at 37˚C in an incubator with 5%
CO2.
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was used to detect mRNA expression in cells.
Total RNA was extracted from 1x104 cells using a TRIzol
reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. The first-strand cDNA was synthesized
using random primers according to the manufacturer's protocols. The
mRNA expression levels were determined by RT-qPCR using the Bio-Rad
CFX96TM Real-Time PCR System (Bio-Rad Laboratories, Inc.) with the
SYBR Green Kit (Takara Bio, Inc.), according to the manufacturer's
instructions. The thermocycling conditions used were: 5 min at
95˚C, followed by 40 cycles at 95˚C for 15 sec and at 60˚C for 34
sec. GAPDH served as an internal control gene for normalization and
the expression levels were calculated using the 2-ΔΔCq
method (17). The PCR primers were
as follows: FBXO31 forward: 5'-ACAGCGTTCAGAAGATGGCT-3', reverse:
5'-GGTGGATCCTGAACAGAGGC-3'; KLF9 forward:
5'-TGGGAGCAGTCCATGGGATA-3', reverse: 5'-AAAGGCAGCGTGCAGAGTAT-3';
GAPDH forward: 5'-ACAGCGTTCAGAAGATGGCT-3', reverse:
5'-GGTGGATCCTGAACAGAGGC-3'. Each experiment was repeated three
times.
Western blot analysis
Ishikawa cells were harvested and lysed with RIPA
buffer (Thermo Fisher Scientific, Inc.). Protein concentration was
measured using a bicinchoninic assay (Beyotime Institute of
Biotechnology). The 30 µg protein/lane was separated on 12% gels
using SDS-PAGE and were then transferred onto a PVDF membrane
(MilliporeSigma). Following blocking with 5% non-fat milk for 1 h
at room temperature, the membrane was incubated with primary
antibodies FBXO31 (1:1,000; cat. no. ab86137; Abcam), Bcl-2
(1:1,000; cat. no. ab182858; Abcam), Bax (1:1,000; cat. no.
ab32503; Abcam), cleaved caspase3 (1:1,000; cat. no. ab32042;
Abcam), caspase3 (1:1,000; cat. no. ab32351; Abcam), KLF9 (1:1,000;
cat. no. ab227920; Abcam), GAPDH (1:1,000; cat. no. ab9485; Abcam)
at 4˚C overnight. The next day, the membranes were incubated with
the corresponding anti-rabbit IgG conjugated to HRP (1:5,000; cat.
no. sc-2357; Santa Cruz Biotechnology, Inc.) for 2 h at room
temperature. Finally, the protein bands were visualized using the
Enhanced ECL Chemiluminescent Substrate Kit (Shanghai Yeasen
Biotechnology Co., Ltd.) and the signal intensity was measured with
ImageJ software (version 1.48; National Institutes of Health).
Cell transfection
For transfection, cells were cultured into plates
for 24 h and were then transfected with FBXO31 overexpression
(Oe-FBXO31) plasmid or the corresponding negative control vector
(Oe-NC) and small interfering (si)-RNA clones targeting KLF9
(si-KLF9#1 and si-KLF9#2) or the corresponding si-NC (Shanghai
GenePharma Co., Ltd.) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37˚C for an
additional 48 h, according to the manufacturer's instructions.
After transfection for 48 h, RT-qPCR and western blotting were used
to detect the transfection effect as aforementioned. The
transfected cells were treated with DDP or control PBS medium,
incubated for 24 h and harvested for further analysis. The
sequences were as follows: si-KLF9#1 sense,
5'-UGUAAUGGGCUUUGAGAUGGG-3' and antisense,
5'-CAUCUCAAAGCCCAUUACAGA-3'; si-KLF9#2 sense,
5'-UUUUCACGCGUCUGUUUCCUG-3' and antisense,
5'-GGAAACAGACGCGUGAAAACU-3' and si-NC sense,
5'-UUCUCCGAACGUGUCACGUTT-3' and antisense,
5'-ACGUGACACGUUCGGAGAATT-3'.
Cell Counting Kit 8 (CCK-8) and EdU
assays
Cell viability was assessed using a commercial CCK-8
assay kit (Nanjing Jiancheng Bioengineering Institute). The
absorbance at wavelength of 450 nm was measured using a microplate
reader. In addition, EdU assay was carried out to evaluate the
proliferation ability of EC cells using the EdU Cell Proliferation
Kit (YuhengBio). EdU-positive cells were counted and images were
captured under a fluorescent microscope (Leica Microsystems
GmbH).
Wound healing assay
Wound healing assay was used to detect the cell
migration. Ishikawa cells were plated into a 6-well plate and cell
transfection techniques were used to overexpress FBXO31 or
interfere with KLF9 expression. Subsequently, a linear scratch was
gently created using a sterile 10-µl plastic tip. The medium was
removed from the plate and the plate was then washed using PBS to
remove non-attached cells. The attached cells were cultured in
serum-free DMEM. The migrated cells were observed under a light
microscope at magnification of x100 and the wound width was
measured at 0 and 24 h. The recovery rate of the wound was
calculated using the following equation: [(width at 0 h-width at 24
h)/width at 0 h] x100%.
Transwell assay
Transwell assay was used to detect the cell
invasion. For Transwell assays, Ishikawa cells were seeded into
24-well Transwell cell culture chambers with filters of 8-µm pores.
Transwell apical chambers precoated with 1% Matrigel basement
membrane gel (Corning, Inc.) at 37˚C for 30 min were used for cell
invasion. The upper chambers pre-coated or not with Matrigel (40
µl/well) were supplemented with Ishikawa cells in DMEM. The lower
chamber was filled-up with DMEM containing 10% FBS. The cells were
routinely cultured for 24 h. Subsequently, cells on the surface of
the membranes were collected and stained with 0.5% crystal violet
for 15 min at room temperature. Finally, images of the migrated and
invaded cells in five randomly selected fields were captured under
a light microscope at magnification of x200 and the cell migration
and invasion rates were determined using ImageJ software (version
1.48; National Institutes of Health).
Flow cytometric analysis
Flow cytometric analysis was used to detect
apoptosis. After treatment with the indicated compounds, Ishikawa
cells were seeded into 6-well plates at a density of 106
cells/well and incubated for an additional 24 h. Subsequently,
cells were stained with annexin V-FITC/PI (5 µl/5 µl) at room
temperature in the dark for 15 min using an apoptosis detection
kit, according to the manufacturer's instructions. Cell suspensions
were examined by flow cytometry (Gallios; Beckman Coulter, Inc.).
The flow cytometry data were analyzed with CellQuest Pro software
(version 3.3; BD Biosciences). Apoptosis rate was calculated as the
sum of the early apoptosis rate (the lower right quadrant) and the
late apoptosis rate (the upper right quadrant).
Luciferase reporter assay
Luciferase reporter assay was used to detect
promoter activity of FBXO31. Wild-type (WT) or mutant (MUT) FBXO31
promoter were cloned into the pGL3 Basic vector (Promega
Corporation). Following overexpressed FBXO31 or interfere with KLF9
expression, Ishikawa cells were seeded into 12-well plates and
co-transfected with plasmids encompassing the promoter region of
FBXO31, the pRL-SV40 Renilla luciferase reporter vector
(Promega Corporation) and KLF9 overexpression plasmid or control
vector using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). The luciferase activity was measured at
48 h after transfection using the Dual Luciferase Assay System
(Promega Corporation) and was normalized to Renilla
luciferase activity.
Chromatin immunoprecipitation (ChIP)
assay
ChIP assay was used to verify the relationship
between FBXO31 and KLF9. Following overexpressed FBXO31 or
interfere with KLF9 expression, a total of 4x106
Ishikawa cells were treated with 3.7% formaldehyde for 10 min at
25˚C to crosslink DNA with proteins followed by centrifugation at
300 x g for 3 min at 25˚C, and washed in pre-cooled PBS for 10 min
at 25˚C. Subsequently, the cells were lysed in 300 µl SDS lysis
buffer [1% SDS, 10 mM EDTA and 50 mM Tris-HCl (pH 8.0)] and the
cell lysates were sonicated to shear DNA to fragments of 200 to 500
bp in length. The DNA fragments from the 100 µl cell lysates were
then immunoprecipitated overnight at 4˚C with an antibody against
KLF9 (1:300). IgG (1:100) served as control antibody. Then, all
samples were supplemented with protein A/G beads followed by
incubation at 4˚C for 2 h. Beads were then washed with 1 ml each of
low salt immune complex wash buffer, high salt immune complex wash
buffer, LiCl immune complex wash buffer and TE buffer (all Merck
KGaA) at 4˚C. The bound immunocomplex was eluted by adding 300 µl
of fresh elution buffer [10 mM Tris; 1 mM EDTA (pH 8.0)]. To
reverse cross-linking, the samples were treated with proteinase K.
Subsequently, DNA was eluted and purified using the
phenol/chloroform/isoamyl alcohol extraction method. Finally, the
recruitment of KLF9 on FBXO31 promoter was assessed by RT-qPCR
using the SYBR Green Kit (Takara Bio, Inc.) according to the
manufacturer's instructions. Input groups served as a positive
control and IgG groups served as a negative control. The
thermocycling conditions used were: 5 min at 95˚C, followed by 40
cycles at 95˚C for 15 sec and at 60˚C for 34 sec. The PCR products
were resolved by agarose gel electrophoresis on 2% gel with
ethidium bromide and semi-quantitatively analyzed by ImageJ
software (version 1.38).
Statistical analysis
All data are expressed as the mean ± SD. All
statistical analyses and graph generation were performed using
GraphPad 8.0 statistical software (Dotmatics). The results were
compared using one-way ANOVA followed by Tukey's post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
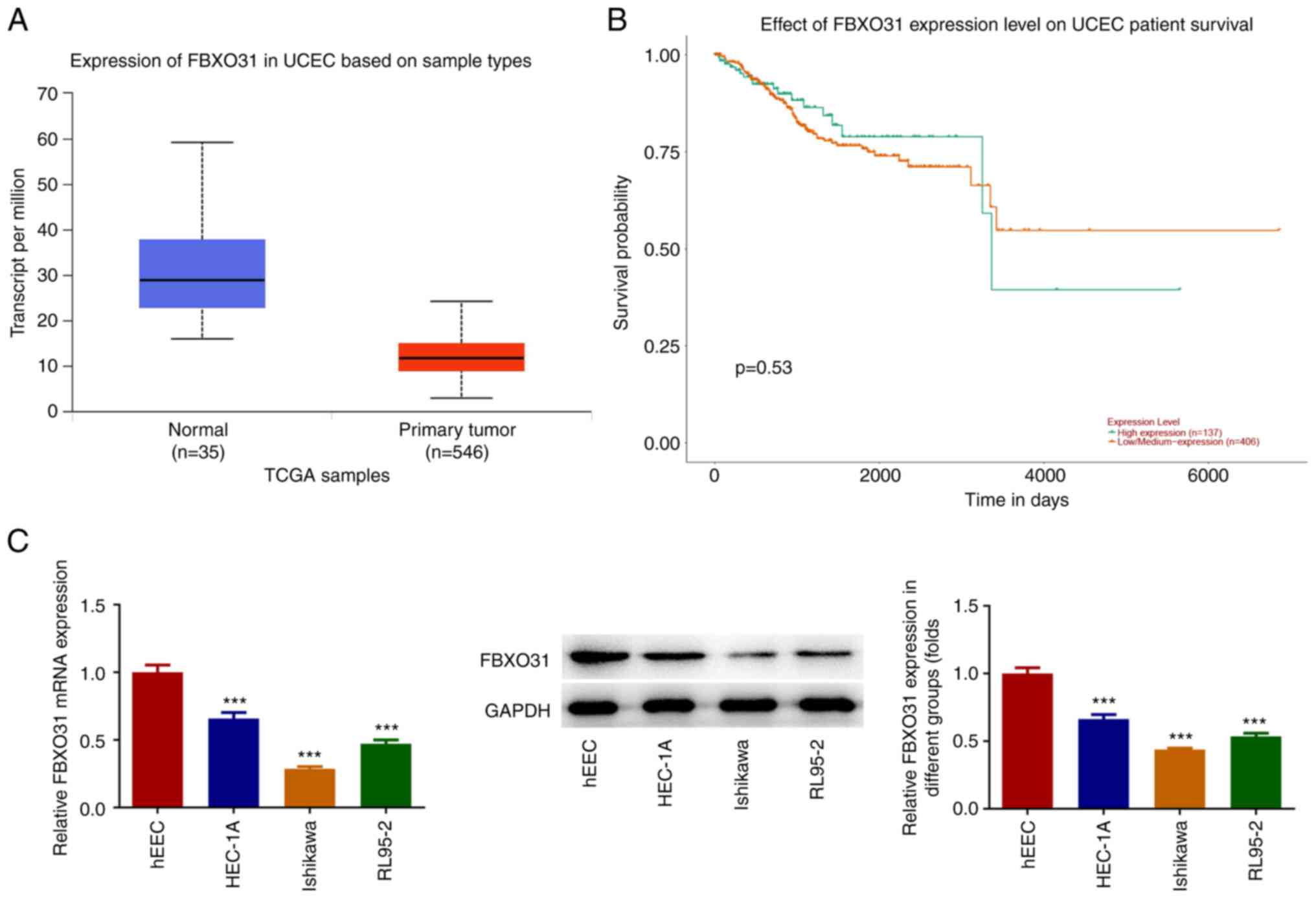
FBXO31 is downregulated in EC
Bioinformatics analysis using the UALCAN database
showed that FBXO31 was downregulated in EC tissues (Fig. 1A). In addition, FBXO31
downregulation in EC was associated with poor prognosis (Fig. 1B). Furthermore, the expression
levels of FBXO31 were detected in EC cell lines by RT-qPCR and
western blot analysis. The results demonstrated that compared with
control cells, the expression levels of FBXO31 were significantly
decreased in the EC cell lines (Fig.
1C). Among them, Ishikawa cells displayed the most
significantly reduced FBXO31 levels and, therefore, these cells
were selected for the follow-up experiments.
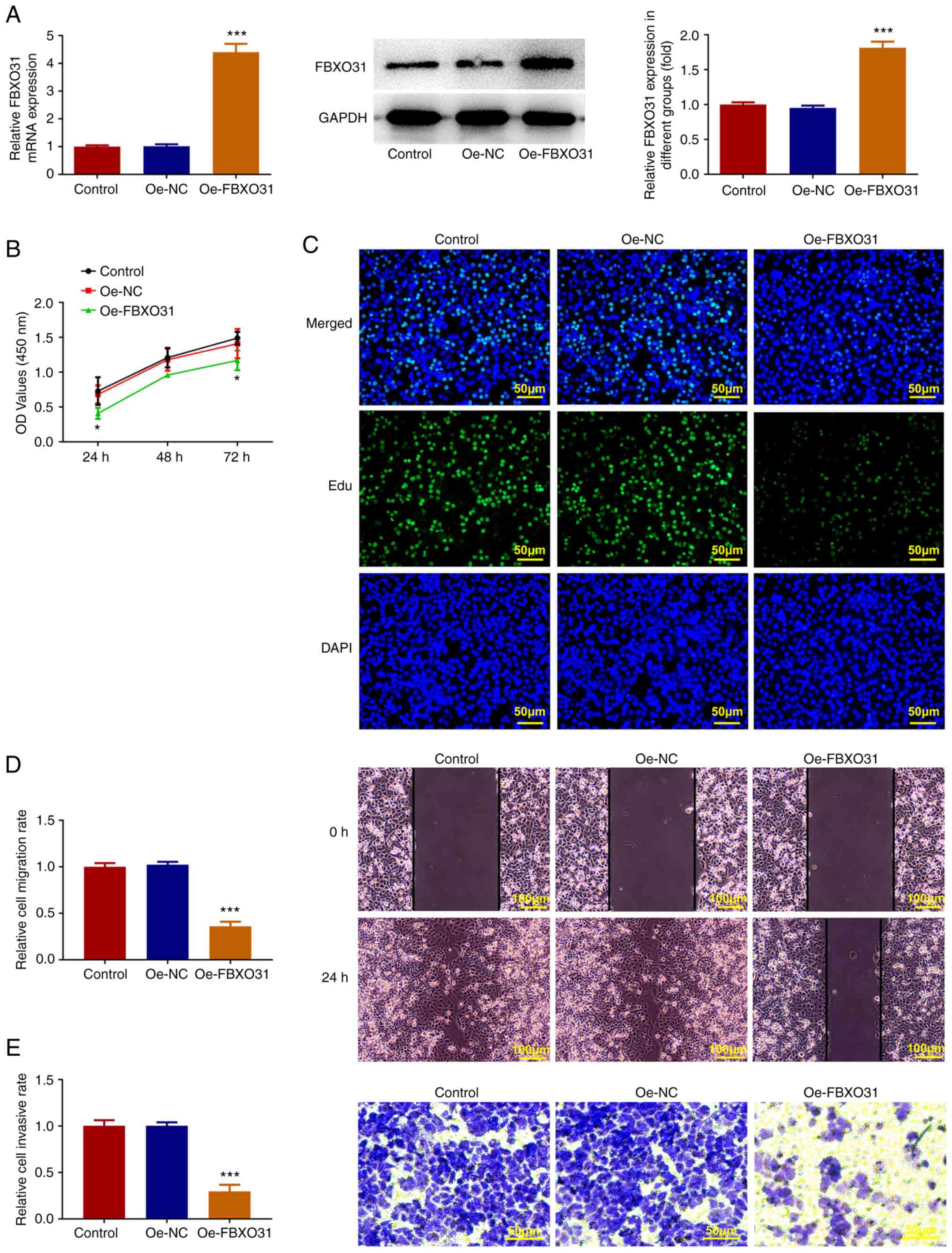
FBXO31 overexpression inhibits the
malignant progression of EC cells
An Oe-FBXO31 plasmid was constructed and cells were
then divided into the control, Oe-NC group and Oe-FBXO31 groups.
The transfection efficacy was verified by RT-qPCR and western blot
analysis (Fig. 2A). Furthermore,
cell viability and proliferation were assessed by CCK-8 and EdU
staining assays. Cell viability was significantly decreased in the
Oe-FBXO31 group compared with the Oe-NC group (Fig. 2B and C). Additionally, the invasion and
migration abilities of Ishikawa cells were evaluated by wound
healing and Transwell assays. The results revealed that the
invasion and migration abilities of Ishikawa cells were notably
attenuated following FBXO31 overexpression (Fig. 2D and E).
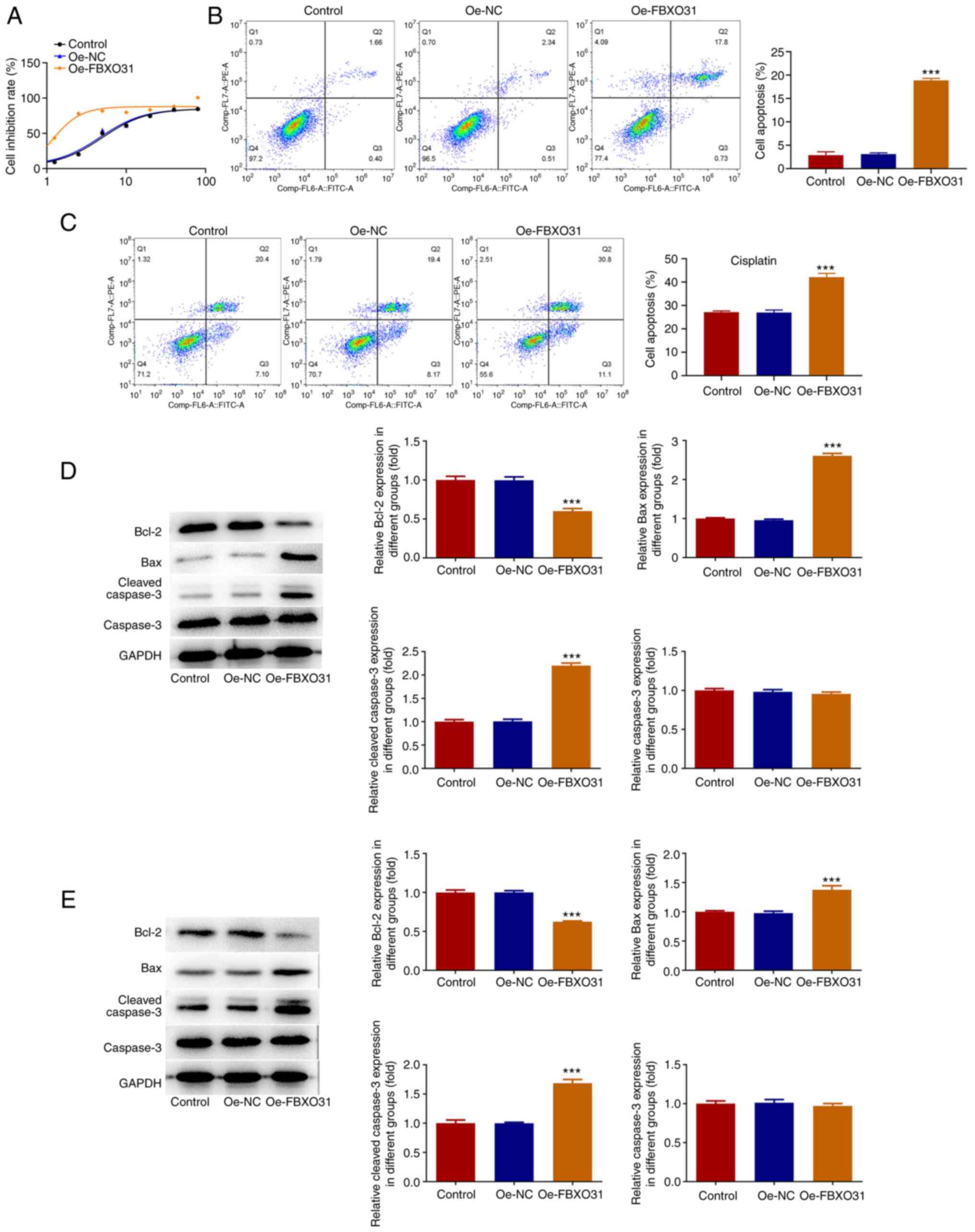
FBXO31 overexpression enhances the
sensitivity of EC cells to DDP
CCK-8 assay was carried out to measure the half
inhibitory concentration (IC50) of DDP in EC cells
following FBXO31 overexpression, compared with the other groups.
The IC50 of the control and Oe-FBXO31 group was 4.991
and 1.142 µg/ml, respectively, thus indicating that the
IC50 of DDP was significantly decreased in the Oe-FBXO31
group compared with the Oe-NC group (Fig. 3A). This finding suggested that
FBXO31 overexpression could enhance the sensitivity of EC cells to
DDP. Furthermore, cell apoptosis was assessed by flow cytometric
analysis after cell treatment with PBS or DDP (IC50).
The results showed that following cell treatment with PBS, the
apoptosis rate of cells in the Oe-FBXO31 group was significantly
increased (apoptosis rate, 22%) compared with the Oe-NC group
(Fig. 3B). Following treatment of
cells with 1.142 µg/ml DDP, the cell apoptosis rate was notably
elevated in the Oe-FBXO31 group (apoptosis rate, ~44%) compared
with the Oe-NC group (Fig. 3C).
Western blot analysis demonstrated that Bax and cleaved caspase 3
were markedly upregulated and Bcl2 was downregulated in PBS-treated
cells in the Oe-FBXO31 group compared with the Oe-NC group
(Fig. 3D). Consistently, the
expression levels of Bax and cleaved caspase 3 were significantly
enhanced and those of Bcl2 were markedly reduced in FBXO31
overexpressing cells treated with 1.142 µg/ml DDP compared with the
Oe-NC group. However, compared with PBS the expression of
apoptosis-related proteins changed more significantly following DDP
intervention (Fig. 3E).
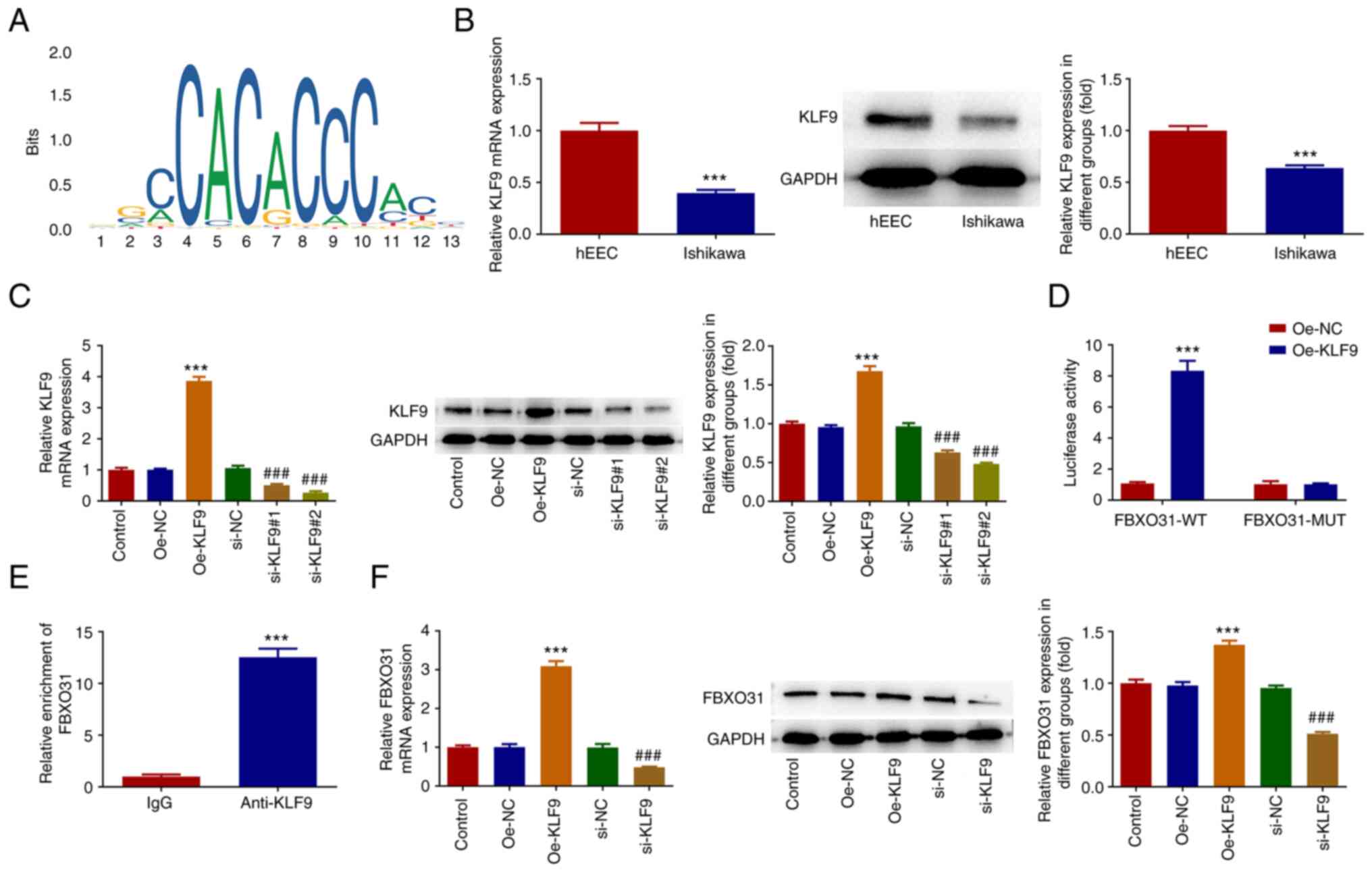
KLF9 promotes the transcription of
FBXO31
The JASPAR database predicted the binding sites of
the KLF9 transcription factor on FBXO31 promoter (Fig. 4A). RT-qPCR and western blot
analysis showed that the expression of KLF9 was abnormally
decreased in EC cells (Fig. 4B).
Subsequently, EC cells were transfected with KLF9 overexpression
and interference plasmids, and the transfection efficacy was
detected by qPCR and western blot analysis (Fig. 4C). The si-KLF9#2 clone was selected
for the follow-up experiments. The promoter activity of FBXO31 was
detected by luciferase assay. Therefore, the results showed that
the promoter activity in the FBXO31-wild type (WT) + Oe-KLF9 group
was significantly enhanced compared with the FBXO31-WT + Oe-NC
group (Fig. 4D). ChIP results also
verified the binding capacity of KLF9 on FBXO31 promoter (Fig. 4E). In addition, FBXO31 was
significantly upregulated in KLF9-overexpressing cells and markedly
downregulated in KLF9-depleted Ishikawa cells (Fig. 4F).
KLF9-regulated FBXO31 inhibits the
progression of EC and enhances the sensitivity of EC cells to
DDP
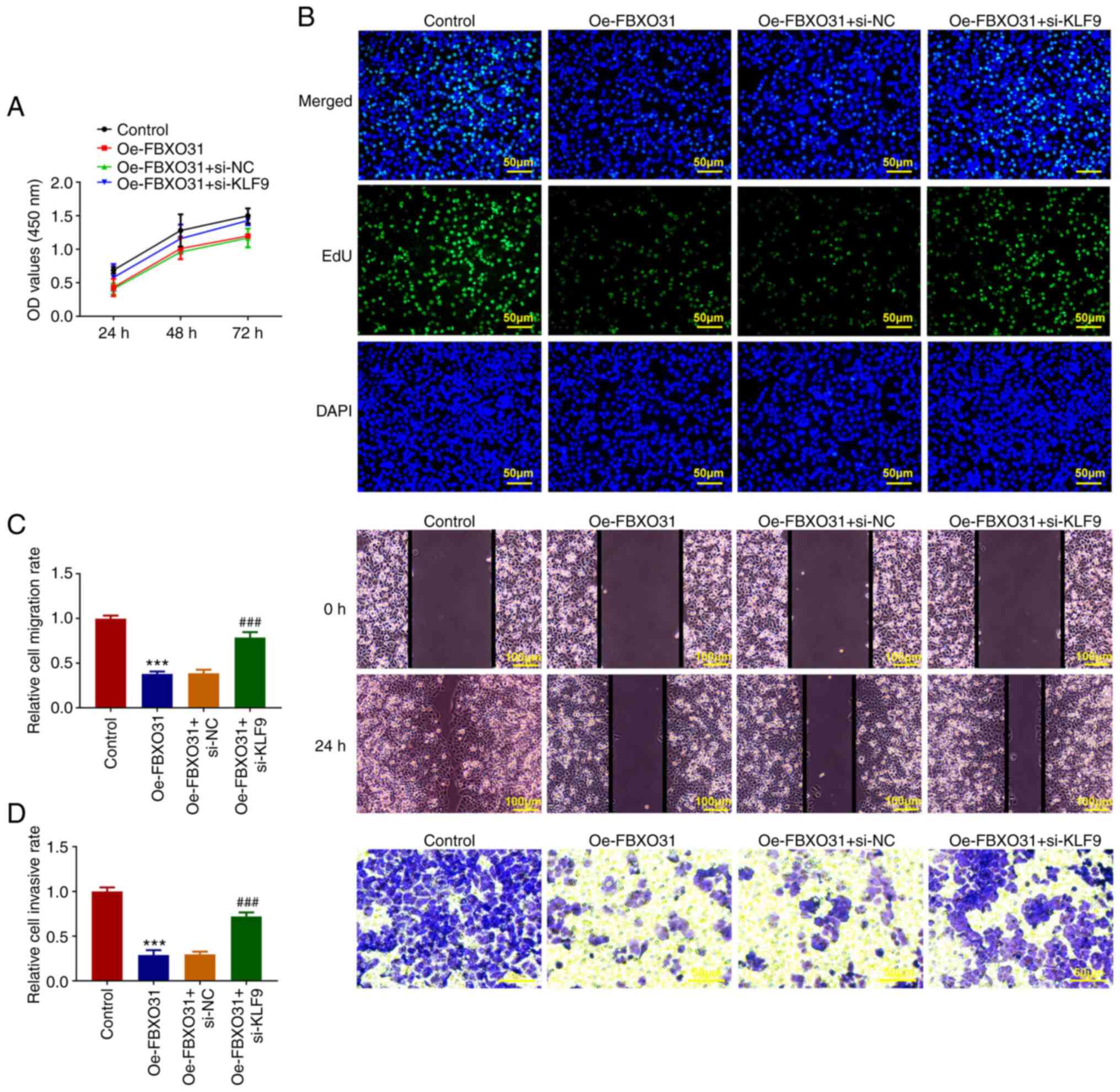
Cells were divided into the control, Oe-FBXO31,
Oe-FBXO31 + si-NC and Oe-FBXO31 + si-KLF9 groups. The CCK-8 and EdU
staining assay results showed that compared with the Oe-FBXO31 +
si-NC group, the viability and proliferation capacity of cells in
the Oe-FBXO31 + si-KLF9 group were significantly increased
(Fig. 5A and B). Furthermore, wound healing and
Transwell assays demonstrated that cell invasion and migration were
notably enhanced in the Oe-FBXO31 + si-NC group compared with the
Oe-FBXO31 + si-KLF9 group (Fig. 5C
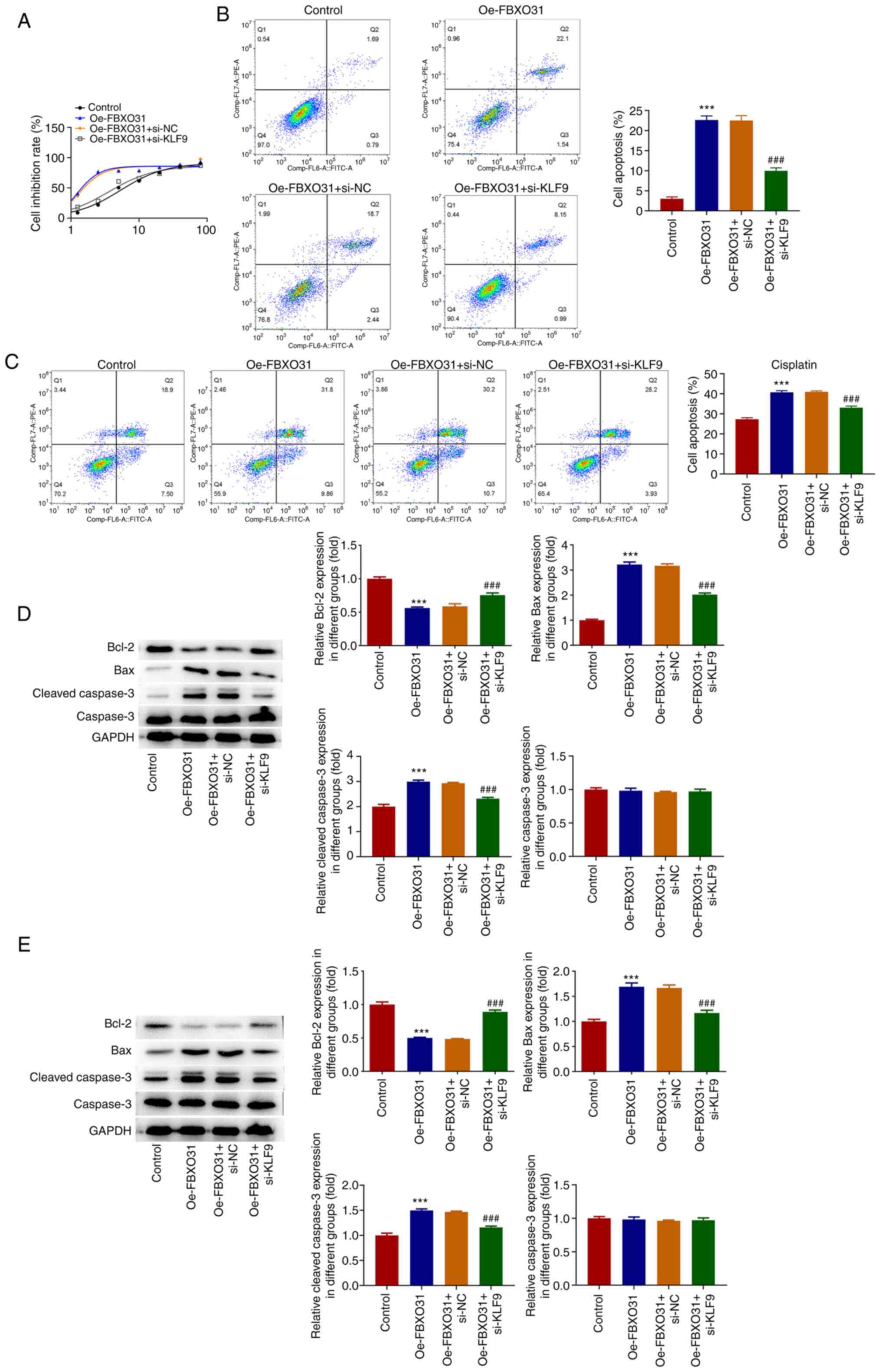
and D). Subsequently, CCK-8 assays
were performed to detect the IC50 of DDP in transfected
EC cells. The IC50 values were 5.339 µg/ml in the
control group, 1.196 µg/ml in the Oe-FBXO31 group, 1.260 µg/ml in
the Oe-FBXO31 + si-NC group and 3.565 µg/ml in the Oe-FBXO31 +
si-KLF9 group, thus indicating that the IC50 value of
DDP in the Oe-FBXO31 + si-KLF9 group was significantly increased
compared with that in the Oe-FBXO31 + si-NC group (Fig. 6A). Furthermore, flow cytometry was
used to assess cell apoptosis in PBS- and DDP-treated cells. The
results revealed that the cell apoptosis rate in PBS-treated cells
in the Oe-FBXO31 + si-KLF9 group was significantly reduced compared
with the Oe-FBXO31 + si-NC group (apoptosis rate, 10%; Fig. 6B). After DDP intervention, cell
apoptosis was significantly reduced in the Oe-FBXO31 + si-KLF9
group, compared with the Oe-FBXO31 + si-NC group (apoptosis rate,
35%; Fig. 6C). Additionally,
western blotting results showed that after PBS intervention, Bax
and cleaved caspase 3 were markedly downregulated, while Bcl2 was
upregulated in the Oe-FBXO31 + si-NC group compared with the
Oe-FBXO31 + si-KLF9 group. Consistently, treatment with DDP further
downregulated Bax and cleaved caspase 3, and upregulated Bcl2 in
the Oe-FBXO31 + si-NC group compared with the Oe-FBXO31 + si-KLF9
group. However, compared with cells treated with PBS, the
expression of apoptosis-related proteins more significantly changed
in DDP-treated EC cells (Fig. 6D
and E).
Discussion
EC, one of the most common types of cancer in women,
with an increasing annual incidence and tendency towards younger
ages, has gradually evolved into a public social and health issue
(18). Currently, there are no
effective strategies to inhibit the progression of EC. Therefore,
elucidating the pathogenesis of EC is of far-reaching significance
for the development of novel and efficient approaches of diagnosis
and treatment in clinic. The present study demonstrated that FBXO31
expression is decreased in EC cell lines. Overexpression of FBXO31
in EC cells can significantly inhibit the proliferation, invasion
and migration of EC cells, promote apoptosis, and enhance the
sensitivity of EC cells to DDP. In addition, it was found that KLF9
can transcriptionally activate the expression of FBXO31 in EC
cells, thereby influencing the malignant progression of EC. The
present study discussed for the first time, to the best of the
authors' knowledge, the expression of FBXO31 in EC cells and its
regulatory effect on the malignant progression of EC cells and the
sensitivity of EC cells to DDP. Furthermore, it discussed for the
first time the regulatory mechanism of FBXO31 in EC and found that
KLF9 can transcriptionally regulate FBXO31 and thus regulate the
malignant process of EC.
The bioinformatics analysis results using the UALCAN
database predicted that the expression of FBXO31 in patients with
EC was significantly reduced and that FBXO31 downregulation was
associated with poor prognosis in patients with EC. The experiments
of the present study also showed that FBXO31 was abnormally
downregulated in EC cell lines. Previous studies demonstrated that
the expression levels of FBXO31 were significantly associated with
poor prognosis in patients with breast cancer, thus suggesting that
FBXO31 could be a potential clinical target and prognostic
biomarker for patients with breast cancer (19,20).
In addition, the loss of FBXO31-mediated degradation of
dual-specificity phosphatase 6 could dysregulate ERK and PI3K/AKT
signaling and promote prostate tumorigenesis (21). However, the regulatory effect of
FBXO31 in EC remains elusive. In the present study, FBXO31
overexpression significantly inhibited EC cell viability,
proliferation, invasion and migration. At present, DDP-based
chemotherapy regimen is mostly used in clinical treatment of EC,
and has achieved good results. However, some EC patients have
long-term use, resulting in drug resistance, resulting in
chemotherapy failure. Therefore, it is urgent to find a program
that can reverse tumor drug resistance, so as to improve the
prognosis of these patients (22,23).
A previous study showed that FBXO31 exerted a significant
regulatory role in drug resistance in esophageal squamous cell
carcinoma and could be a potential indicator or target for it
(24). The results of the present
study suggested that FBXO31 overexpression could improve the
sensitivity of Ishikawa cells to DDP chemotherapy.
Bioinformatics analysis using the JASPAR website
predicted that the transcription factor KLF9 could bind to FBXO31
promoter, thus indicating that KLF9 could transcriptionally
regulate FBXO31 expression. In addition, the expression levels of
KLF9 were also significantly decreased in EC cells. KLF9 has been
widely studied in cancer. The expression of KLF9 in EC tissues was
reduced and its decreased expression was associated with the highly
metastatic capacity of EC cells. Another study demonstrated that
KLF9 could inhibit the proliferation and invasion of EC cells via
inhibiting the Wnt/β-catenin signaling pathway (14). However, the regulatory association
between KLF9 and FBXO31 in EC has not been previously reported. In
the current study, KLF9 expression was silenced in FBXO31
overexpressing EC cells and the results showed that KLF9 knockdown
could reverse the effects of FBXO31 overexpression on EC cell
proliferation, invasion, migration and apoptosis. In addition,
FBXO31 regulated by KLF9 could enhance the sensitivity of EC cells
to DDP chemotherapy.
The present study had certain limitations. It only
performed experiments in cells, and did not perform verification
and further discussion in animals and clinically. Future studies
will be in animals and clinically. These future studies should
select more EC cell lines for experiments and further verification
in other EC cell lines should be conducted.
In conclusion, the results of the current study
suggested that the KLF9-regulated FBXO31 expression could inhibit
the progression of EC and enhance the sensitivity of Ishikawa cells
to DDP, thus providing strong evidence for KLF9 and FBXO31 as
clinical therapeutic targets of EC and its resistance to DDP. In
addition, the FBXO31 and KLF9 genes in the present study can be
used as important factors in disease diagnosis, providing important
theoretical basis and technical support for disease prevention and
screening.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data sets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
MY conceived the present study. MY and CN performed
the experiments and wrote the manuscript. MY processed the
experimental data and ensured the authenticity and accuracy of the
experimental data. Both authors read and approved the final
manuscript. MY and CN confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhong Y, Lin H, Li Q, Liu C and Shen J:
CircRNA_100565 contributes to cisplatin resistance of NSCLC cells
by regulating proliferation, apoptosis and autophagy via
miR-337-3p/ADAM28 axis. Cancer Biomark. 30:261–273. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang B and Zhang Y, Li R, Li J, Lu X and
Zhang Y: Oncolytic adenovirus Ad11 enhances the chemotherapy effect
of cisplatin on osteosarcoma cells by inhibiting autophagy. Am J
Transl Res. 12:105–117. 2020.PubMed/NCBI
|
|
4
|
Challinor JM, Alqudimat MR, Teixeira TOA
and Oldenmenger WH: Oncology nursing workforce: Challenges,
solutions, and future strategies. Lancet Oncol. 21:e564–e574.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Passarello K, Kurian S and Villanueva V:
Endometrial cancer: An overview of pathophysiology, management, and
care. Semin Oncol Nurs. 35:157–165. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tan Y, Liu D, Gong J, Liu J and Huo J: The
role of F-box only protein 31 in cancer. Oncol Lett. 15:4047–4052.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhu Z, Zheng Y, He H, Yang L, Yang J, Li
M, Dai W and Huang H: FBXO31 sensitizes cancer stem cells-like
cells to cisplatin by promoting ferroptosis and facilitating
proteasomal degradation of GPX4 in cholangiocarcinoma. Liver Int.
42:2871–2888. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Feng Y, Liu M, Xie P, Dong R and Hao Z:
FBXO31 suppresses lipogenesis and tumor progression in glioma by
promoting ubiquitination and degradation of CD147. Prostaglandins
Other Lipid Mediat. 163(106667)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Islam S, Dutta P, Sahay O, Gopalakrishnan
K, Muhury SR, Parameshwar P, Shetty P and Santra MK:
Feedback-regulated transcriptional repression of FBXO31 by c-Myc
triggers ovarian cancer tumorigenesis. Int J Cancer. 150:1512–1524.
2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yang HW, Xia T, Chen ZL, Feng SQ, Peng Y,
Zhou L, Gan L and Yang ZQ: Cloning, chromosomal localization and
expression patterns of porcine Kruppel-like factors 4, -5, -7 and
the early growth response factor 2. Biotechnol Lett. 29:157–163.
2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pei J and Grishin NV: A new family of
predicted Kruppel-like factor genes and pseudogenes in placental
mammals. PLoS One. 8(e81109)2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Viola L, Londero AP, Bertozzi S, Orsaria
M, Marzinotto S, Antoniazzi F, Renda V, Cinel J, Fruscalzo A, Lellé
RJ and Mariuzzi L: Prognostic role of kruppel-like factors 5, 9,
and 11 in endometrial endometrioid cancer. Pathol Oncol Res.
26:2265–2272. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Korani M, Fallah S, Tehranian A,
Nourbakhsh M, Samadikuchaksaraei A, Pour MS and Maleki J: The
evaluation of the FOXO1, KLF9 and YT521 genes expression in human
endometrial cancer. Clin Lab. 59:483–489. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yan X, Zhang H, Ke J, Zhang Y, Dai C, Zhu
M, Jiang F, Zhu H, Zhang L, Zuo X, et al: Progesterone receptor
inhibits the proliferation and invasion of endometrial cancer cells
by up regulating Kruppel-like factor 9. Transl Cancer Res.
9:2220–2230. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi B and Varambally
S: UALCAN: A portal for facilitating tumor subgroup gene expression
and survival analyses. Neoplasia. 19:649–658. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fornes O, Castro-Mondragon JA, Khan A, van
der Lee R, Zhang X, Richmond PA, Modi BP, Correard S, Gheorghe M,
Baranasic D, et al: JASPAR 2020: Update of the open-access database
of transcription factor binding profiles. Nucleic Acids Res.
48:D87–D92. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Makker V, MacKay H, Ray-Coquard I, Levine
DA, Westin SN, Aoki D and Oaknin A: Endometrial cancer. Nat Rev Dis
Primers. 7(88)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang X, Zhang T, Zhang S and Shan J:
Prognostic values of F-box members in breast cancer: An online
database analysis and literature review. Biosci Rep.
39(BSR20180949)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu Y, Pan B, Qu W, Cao Y, Li J and Zhao
H: Systematic analysis of the expression and prognosis relevance of
FBXO family reveals the significance of FBXO1 in human breast
cancer. Cancer Cell Int. 21(130)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Duan S, Moro L, Qu R, Simoneschi D, Cho H,
Jiang S, Zhao H, Chang Q, de Stanchina E, Arbini AA and Pagano M:
Loss of FBXO31-mediated degradation of DUSP6 dysregulates ERK and
PI3K-AKT signaling and promotes prostate tumorigenesis. Cell Rep.
37(109870)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ding N, Zhang T, Yu X and Zhuang S: T-Box
transcription factor 2 enhances chemoresistance of endometrial
cancer by mediating NRF2 expression. Curr Protein Pept Sci.
23:563–570. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lin TC, Wang KH, Chuang KH, Kao AP and Kuo
TC: Oct-4 induces cisplatin resistance and tumor stem cell-like
properties in endometrial carcinoma cells. Taiwan J Obstet Gynecol.
62:16–21. 2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lv L, Wang SC, Mo JY, Huang KL, Xu ML and
Liu J: Effects and mechanisms of FBXO31 on Taxol chemoresistance in
esophageal squamous cell carcinoma. Biochem Biophys Res Commun.
586:129–136. 2022.PubMed/NCBI View Article : Google Scholar
|