Introduction
Short bowel (SB) syndrome occurs as a consequence of
malabsorption from an intestinal surface area insufficient to
maintain growth and a normal nutritional status (1,2).
Intestinal adaptation is a well-known phenomenon that increases the
absorptive capacity following surgical resection of the small
intestine. The most prominent feature of intestinal adaptation is
the proliferation of intestinal cells that increases crypt depth
and enlarges the length and width of the villi (3). It is known that micronutrients
affect several aspects of intestinal adaptation. Wang et al
(4) reported that intestinal
adaptation was facilitated by retinoic acid, an active form of
vitamin A. The authors demonstrated that the intravenous
administration of retinoic acid exerted trophic effects in rats
with SB by inhibiting apoptosis and stimulating crypt cell
proliferation (4).
Vitamin A is a fat-soluble vitamin required for a
number of physiological activities. Retinyl esters (REs),
animal-derived forms of vitamin A, are hydrolyzed into retinol
before entering the absorptive epithelial cells. By contrast,
β-carotene, a vegetable-derived form of vitamin A, is converted
into retinal after entering the absorptive epithelial cells and is
then reduced to retinol within the cells (5). The retinol thus produced binds to
cellular retinol-binding protein II (CRBP II, gene symbol
Rbp2) in absorptive epithelial cells, forming a retinol-CRBP
II complex. This complex is esterified to REs by lecithin retinol
acyltransferase (LRAT, gene symbol Lrat). REs are then
discharged basolaterally into the lymphatic vessels as a component
of chylomicrons and are delivered to the liver through the general
circulation (6). In hepatocytes,
REs are hydrolyzed into retinol and complexed with retinol-binding
protein (RBP, gene symbol Rbp4). The retinol-RBP complex is
transferred to hepatic stellate cells (HSCs) where the retinol
binds to CRBP I (gene symbol Rbp1) and is again esterified
to REs by LRAT for storage (7,8).
CRBP I and II are members of the retinoid-binding
protein family (9) which
constitutes the calycin protein superfamily together with the fatty
acid-binding proteins (FABPs) (10). In mammals, CRBP I is expressed
ubiquitously (11,12), whereas CRBP II is expressed
specifically in the intestine and the fetal liver (13). CRBP II expression increases
perinatally, a change that is associated with the growth and
differentiation of the intestine (13). CRBP II knockout mice have been
shown to be more susceptible to vitamin A deficiency, suggesting
that CRBP II plays an important role in vitamin A homeostasis
(14). Takase et al
(15) reported that jejunal
bypass surgery led to a marked increase in the levels of CRBP II in
the residual jejunal segment of rats. The upregulation of the
Rbp2 gene in rats with SB was also reported by Dodson et
al (16). It has been
demonstrated that the upregulation of the mRNA expression of
Rbp2 results in the growth of absorptive epithelial cells,
presumably through increased vitamin A absorption by those cells
(17).
As noted above, vitamin A is thought to be trophic
to the bowel, and may therefore be important during adaptation in
SB syndrome, and the deficiency of vitamin A may have negative
consequences for adaptation. In the present study, we examined the
effect of significant bowel resection on vitamin A absorption,
transport and metabolism. For this purpose, we used a rat model of
SB and analyzed vitamin A-related factors by reverse
transcription-quantitative PCR (RT-qPCR), western blot analysis and
immunohistochemistry, as well as measurements of vitamin A in
tissues and cultured cells.
Materials and methods
Animals and surgical procedures
Protocols for animal experimentation were approved
by the Animal Research Committee, Akita University Graduate School
of Medicine, Akita, Japan. All animal experiments adhered to the
‘Guidelines for Animal Experimentation’ of the University. Male
Sprague-Dawley rats (ages ranged from 8 to 11 weeks, 280–480 g body
weight) were used in this study. Twelve hours after fasting, each
animal was anesthetized by an intraperitoneal injection of
pentobarbital. In the sham-operated rats, the gut was simply
divided 10 cm proximal to the ileocecal junction and anastomosed
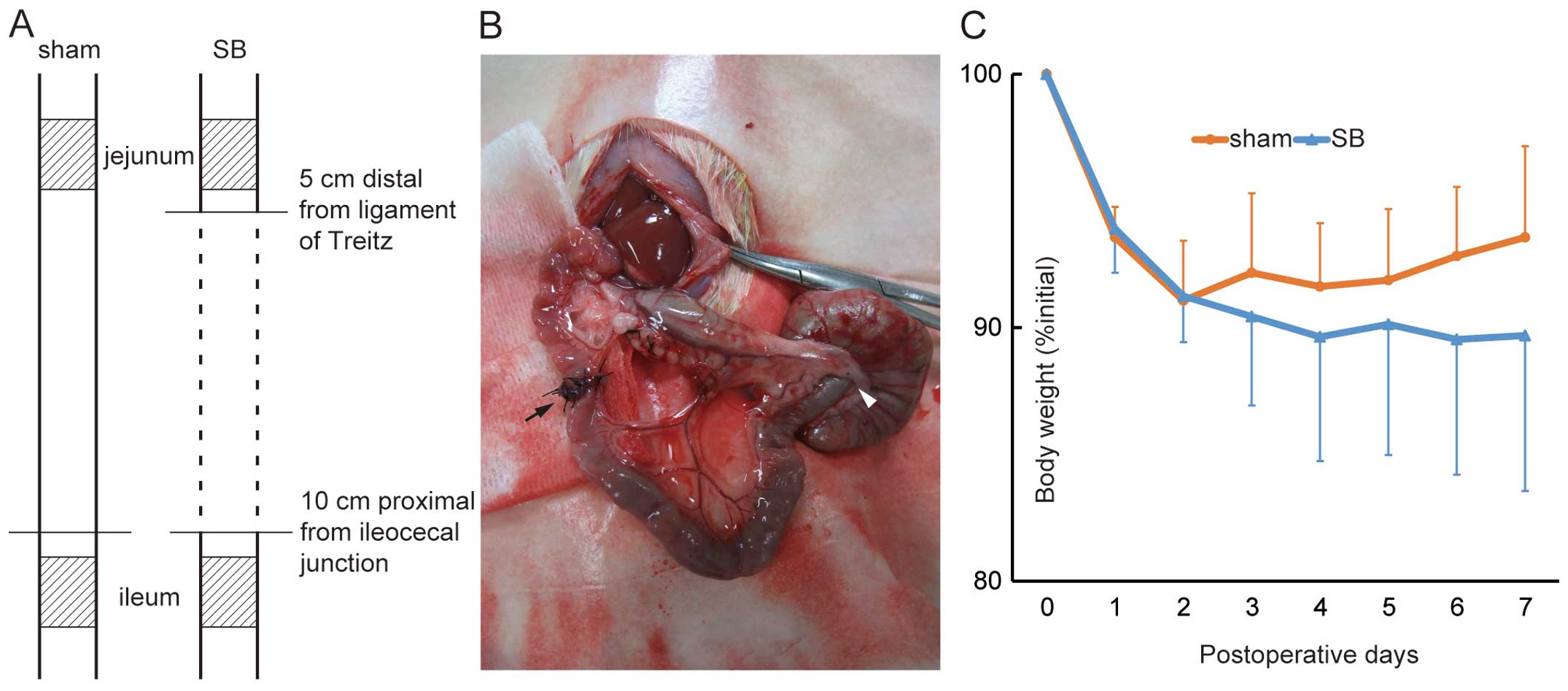
without resecting any part (Fig.
1A). For the induction of SB in the animals, the small bowel
was resected from a point 5 cm distal to the ligament of Treitz to
a point 10 cm proximal to the ileocecal junction, resulting in a
75% resection of the small intestine. Mesenteric vessels were
ligated with sutures, and bowel continuity was restored by
end-to-end anastomosis (Fig. 1B,
arrow). The rats were fasted for 24 h, but were allowed free access
to water. The rats with SB and sham-operated rats were then fed
with Elental (Ajinomoto Pharmaceuticals, Tokyo, Japan) according to
the manufacturer’s instructions. On day 7, the animals were
sacrificed with an intraperitoneal injection of pentobarbital, and
the livers and small intestines were excised and immersed in 10%
formalin. For RNA preparation, small sections of the livers and
small intestines were quick-frozen in liquid nitrogen and kept
frozen at −85°C.
A pair of rats underwent surgery for each trial: 1
animal for SB and 1 for the sham operation. From the 19 pairs of
rats, 13 rats with SB and 14 sham-operated completed the trial. The
jejuna of the sham-operated rats were collected from 8 animals out
of the 14 animals that underwent surgery. Over the first 2 days
following surgery, both the rats with SB and the sham-operated rats
lost weight (Fig. 1C).
Afterwards, the sham-operated rats gradually regained weight,
whereas the rats with SB continued to lose weight.
RNA extraction and RT-qPCR
The frozen rat livers and intestines were dissolved
in TRIzol reagent (Life Technologies, Carlsbad, CA, USA) and total
RNA was isolated. The cDNAs were synthesized from 5 μg of
total RNA using SuperScript III Reverse Transcriptase (Life
Technologies) using an oligo(dT)18 primer. The
quantification of cDNA was carried out using the LightCycler 480
SYBR-Green I Master (Roche Diagnostics, Meylan, France). Transcript
amounts were normalized against the glyceraldehyde 3-phosphate
dehydrogenase (Gapdh) transcript. The nucleotide sequences
used are summarized in Table
I.
 | Table IPrimers used for RT-qPCR. |
Table I
Primers used for RT-qPCR.
| Gene | Forward | Reverse | Length (bp) |
|---|
| Gapdh |
ACAGCAACTCCCATTCTTCC |
TCCACCACCCTGTTGCTGTA | 118 |
| Rbp1 |
AGGCATAGATGACCGCAAGT |
TCATCACCCTCAATCCACTG | 117 |
| Rbp2 |
GAAACACCCTGGTGTGCGTG |
GAACACTTGTCGACACACCT | 122 |
| Rbp4 |
GTTTTCTCGTGACCCCAATG |
ACTGTTTCTTGAGGGTCTGC | 139 |
| Lrat |
GCGAACACTTTGTGACCTAC |
GACAGCTGAAGCAAGACAAC | 119 |
| Apoa4 |
TGAAGGCTGTGGTGCTGA |
CCTCCTTGGCATTGTTGC | 126 |
| Apob |
CAGCCATAGGCACTGTGAGTC |
TGTCCCTCCACTCCATTTTG | 126 |
| Fabp1 |
TTCTCCGGCAAGTACCAAGT |
TTCATGCACGATTTCTGACAC | 126 |
| Fabp2 |
TGATGGCACTTGGAAAGTAGAC |
CCTTCCTGTGTGATCGTCAG | 126 |
Organelle fractionation
Small sections of tissue (50–150 mg) were
homogenized in 0.5 ml of cell disruption buffer (0.25 M sucrose, 20
mM HEPES, pH 7.5) using a Polytron CT 2100 homogenizer (Kinematica
AG, Littau-Luzern, Switzerland). The samples were further
homogenized by 20 strokes using a Dounce tissue grinder (Wheaton,
Millville, NJ, USA). The homogenates were centrifuged for 7 min at
1,100 × g. The supernatants were further centrifuged at 1,900 × g
for 30 min. The pellets were set aside as mitochondrial fractions
and the supernatants were again centrifuged at 105,000 × g for 1 h.
The pellets were set aside as microsomal fractions and final
supernatants were cytosol fractions. The pelleted organelle
fractions were dissolved in 100 μl of cell disruption
buffer. Protein concentrations in all the organelle fractions were
determined using the BCA protein assay reagent (Thermo Scientific,
Rockford, IL, USA).
Western blot analysis
A total of 50 μg of each organelle fraction
was separated by SDS-PAGE and transferred onto PVDF membranes
(Atto, Tokyo, Japan). The membranes were incubated with primary
antibodies against either CRBP I (1:200 dilution, sc-30106; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA), CRBP II (1:10
dilution, HPA035866; Sigma-Aldrich, St. Louis, MO, USA) or LRAT
(1:200 dilution; Immuno-Biological Laboratories, Gunma, Japan). A
peroxidase-conjugated secondary antibody (1:5,000 dilution,
111-035-003; Jackson ImmunoResearch, West Grove, PA, USA) was
applied. Bound antibodies were detected by ECL chemiluminescence
(GE Healthcare Life Sciences, Piscataway, NJ, USA) and recorded on
X-ray film (Fujifilm, Tokyo, Japan). As the positive controls,
whole cell lysates from HEK293T cells transfected with Rbp1
or Rbp2 plasmids were used.
Immunohistochemistry and
histochemistry
Paraffin-embedded ilea were sectioned at 3-μm
intervals. To unmask the antigens, the specimens were boiled in
citrate buffer (0.01 M, pH 6.0) for 25 min. For the inactivation of
endogenous peroxidase activity, the slides were immersed in 1%
H2O2 for 20 min. The specimens were incubated
with either CRBP II antibody (1:10 dilution) or LRAT antibody
(1:100 dilution) overnight at 4°C. The slides were treated with a
1:500 dilution of peroxidase-conjugated secondary antibody and
incubated with DAB substrate (Roche Diagnostics). The nuclei were
counterstained with Mayer’s hematoxylin solution (Wako Pure
Chemical, Osaka, Japan). Hematoxylin and eosin (H&E) staining
was also performed for histological comparisons. The specimens were
recorded digitally using the NanoZoomer Digital Pathology system
(Hamamatsu Photonics, Hamamatsu, Japan).
Quantitative analysis of retinol and REs
by high performance liquid chromatography (HPLC)
Small sections of tissue (30–100 mg) were
homogenized in 0.5 ml of cell disruption buffer (20 mM Tris-HCl, pH
8.0) using a Polytron CT 2100 homogenizer. Follwoing the addition
of 0.5 ml methanol and 1.75 ml dichloromethane, the homogenates
were vigorously agitated with a Vortex mixer for 1 min.
Subsequently, 0.5 ml of distilled water and 2.75 ml of hexane were
added and agitated with a Vortex mixer for 5 sec, and the samples
were then centrifuged for 5 min at 3,000 × g. The supernatants were
set aside in a pear-shaped flask. The same procedures were carried
out using the lower layer of the centrifuged samples without the
addition of water. The dried supernatant was dissolved in 200
μl of N1 (mixture of 125 ml benzene, 25 ml tert-butyl methyl
ether, 0.2 ml ethanol and 350 ml hexane) and assessed by HPLC
(Elite LaChrom; Hitachi, Tokyo, Japan) with a silica gel column
(SL12S03-1506WT; YMC, Kyoto, Japan) for the quantification of
retinol. The flow-through fraction containing REs was collected,
dried and saponized in KOH (0.33 M in ethanol) for 30 min at 40°C.
Subsequently, 1.75 ml of dichloromethane and 2.75 ml of hexane were
added to the saponized samples, and mixed for 30 sec. Distilled
water (1 ml) was added and mixed for 30 sec and centrifuged for 5
min at 3,000 × g. The supernatant was set aside in a pear-shaped
flask. The same procedures were repeated. The retinol contained in
the extracts was analyzed by HPLC.
Plasmids
The cDNA for CRBP II (Rbp2) and LRAT
(Lrat) was amplified from the isolated rat HSCs by reverse
transcription-PCR using primers designed from the reported cDNA
sequences (GenBank accession no. NM_012640 for Rbp2 and
GenBank accession no. NM_022280 for Lrat) and inserted into
the KpnI and EcoRI sites of pcDNA3.1 (Life
Technologies). The primer pairs used for the cloning of Rbp2
and Lrat were 5′-GACTGGTACCATGACGAAGGACCAGAATGG-3′ and
5′-GGAATTCTCACTTCTTTTTGAACACTTGTC-3′; 5′-GACGGTACCATGAAGAACTCAATGC
TGGAG-3′ and ′-GACTGGAATTCCTAGCCAGACATCATCCAC-3′, respectively.
Forty cycles of PCR were required to amplify the cDNA for
Rbp2 from the HSCs. The cloned cDNAs were verified by
sequencing.
Transfection
Transfection of the plasmids into HEK293T cells was
carried out using Lipofectamine 2000 reagent (Life Technologies). A
total of 2 μg each of Rbp2 and Lrat cDNA was
added to a 6-cm dish (n=8 for each condition). Eighteen hours after
transfection, the cells were treated with 10 μM retinol
(Sigma-Aldrich) in Dulbecco’s modified Eagle’s medium (DMEM; Life
Technologies) supplemented with fetal bovine serum (SAFC
Bioscience, Lenexa, KS, USA) for 10 min. The cells were collected,
quick-frozen in liquid nitrogen and stored at −85°C until vitamin A
quantification by HPLC.
Statistical analysis
Data are expressed as the means ± standard deviation
(SD). The statistical significance of the differences was evaluated
by an unpaired Student’s t-test for data shown in Figs. 2 and 5, and by ANOVA combined with Tukey’s
test for data shown in Fig. 6.
P-values <0.05 were considered to indicate a statistically
significant difference.
Results
Upregulation of Rbp2 and apolipoprotein
A-IV (Apoa4) mRNA epxression in the intestines of rats with SB
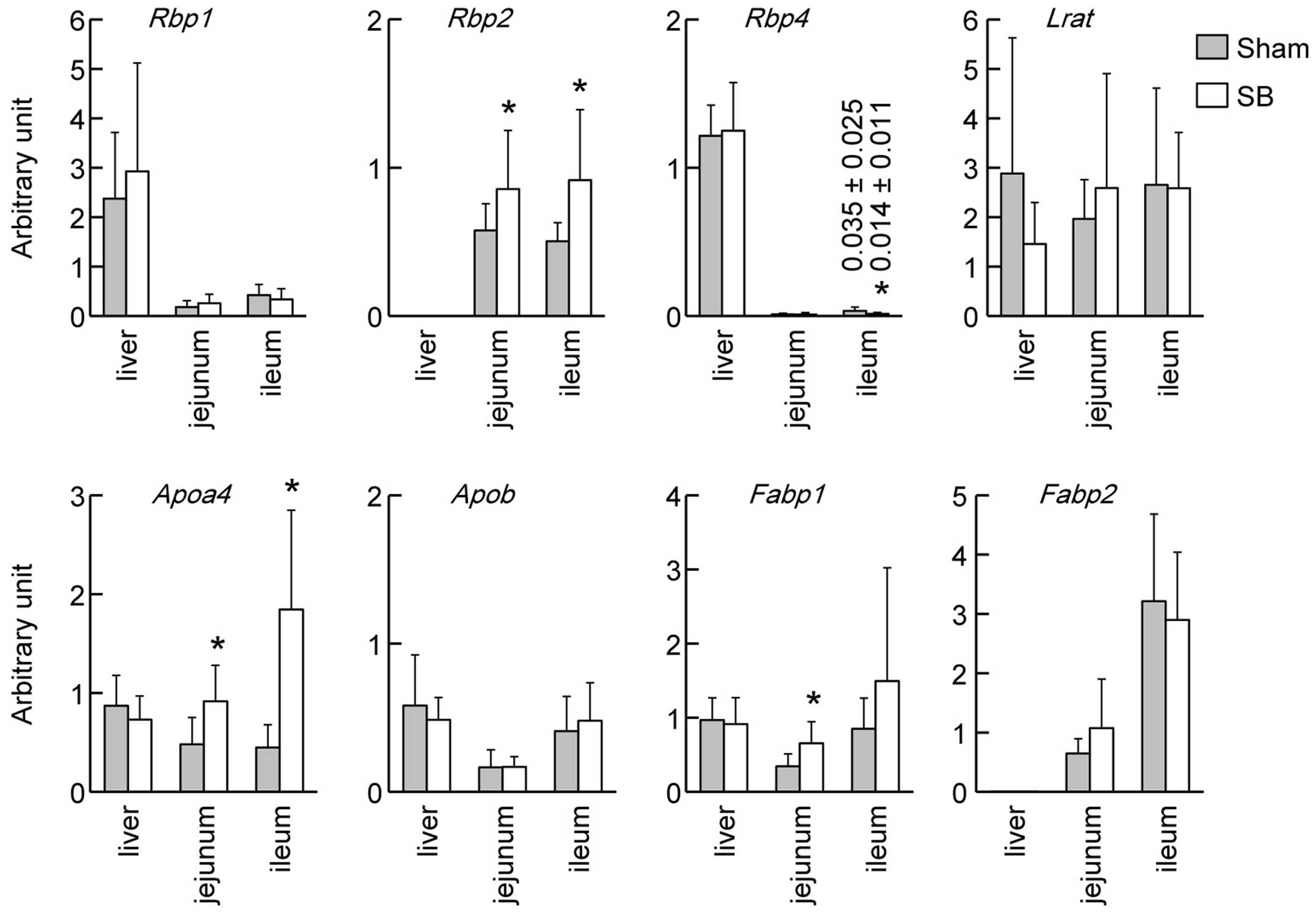
We examined the mRNA expression levels of genes
related to retinoid absorption, transport and metabolism (Fig. 2). Rbp1 mRNA was
predominantly expressed in the liver and no significant changes
were observed in its expression levels between the rats with SB and
the sham-operated rats in either the liver or the intestine. The
mRNA expression levels of Rbp4 in the ilea of rats with SB
and sham-operated rats differed significantly (P<0.05), although
the mRNA expression of Rbp4 in the intestines was
considerably lower than that in the livers. Rbp2 mRNA was
specifically expressed in the intestines, and the mRNA expression
levels of Rbp2 in the rats with SB were higher than those in
the sham-operated rats in both the jejuna and the ilea (P<0.05).
Lrat mRNA coding for retinol-esterifying enzyme was
expressed in both the liver and the intestine and did not show any
significant differences between the rats with SB and the
sham-operated animals. The intestinal mRNA expression levels of
Apoa4 in the rats with SB were higher than those in the
sham-opearted rats in both the jejuna and the ilea (P<0.05). The
mRNA expression of Fabp1 in the jejuna of rats with SB was
higher than that in the sham-operated rats (P<0.05).
Co-localization of CRBP II and LRAT in
the intestinal absorptive epithelial cells of rats with SB
We analyzed the cellular locations of CRBP II and
LRAT proteins in the small intestines of rats with SB and
sham-operated rats. CRBP II was observed mainly in the absorptive
epithelial cells in the ilea of rats with SB (Fig. 3A and C, arrows), while weak
staining was observed in the ilea of the sham-operated rats
(Fig. 3F and H). Immunohistologic
staining of LRAT revealed its presence in the absorptive epithelial
cells and lamina propria mucosae (Fig. 3B, D, G and I); the staining
intensities seemed to be at the same level in the rats with SB and
the sham-operated rats. In the rats with SB, the absorptive
epithelial cells were positive for both CRBP II and LRAT staining
(Fig. 3C and D, arrows). Some
fibroblast-like cells in the lamina propria mucosae seemed to be
double-positive (Fig. 3C and D,
closed arrowheads) and others to be positive only for LRAT
(Fig. 3C and D, open arrowheads).
No histological differences were observed between the intestines of
the sham-operated rats and the rats with SB, as shown by H&E
staining (Fig. 3E and J).
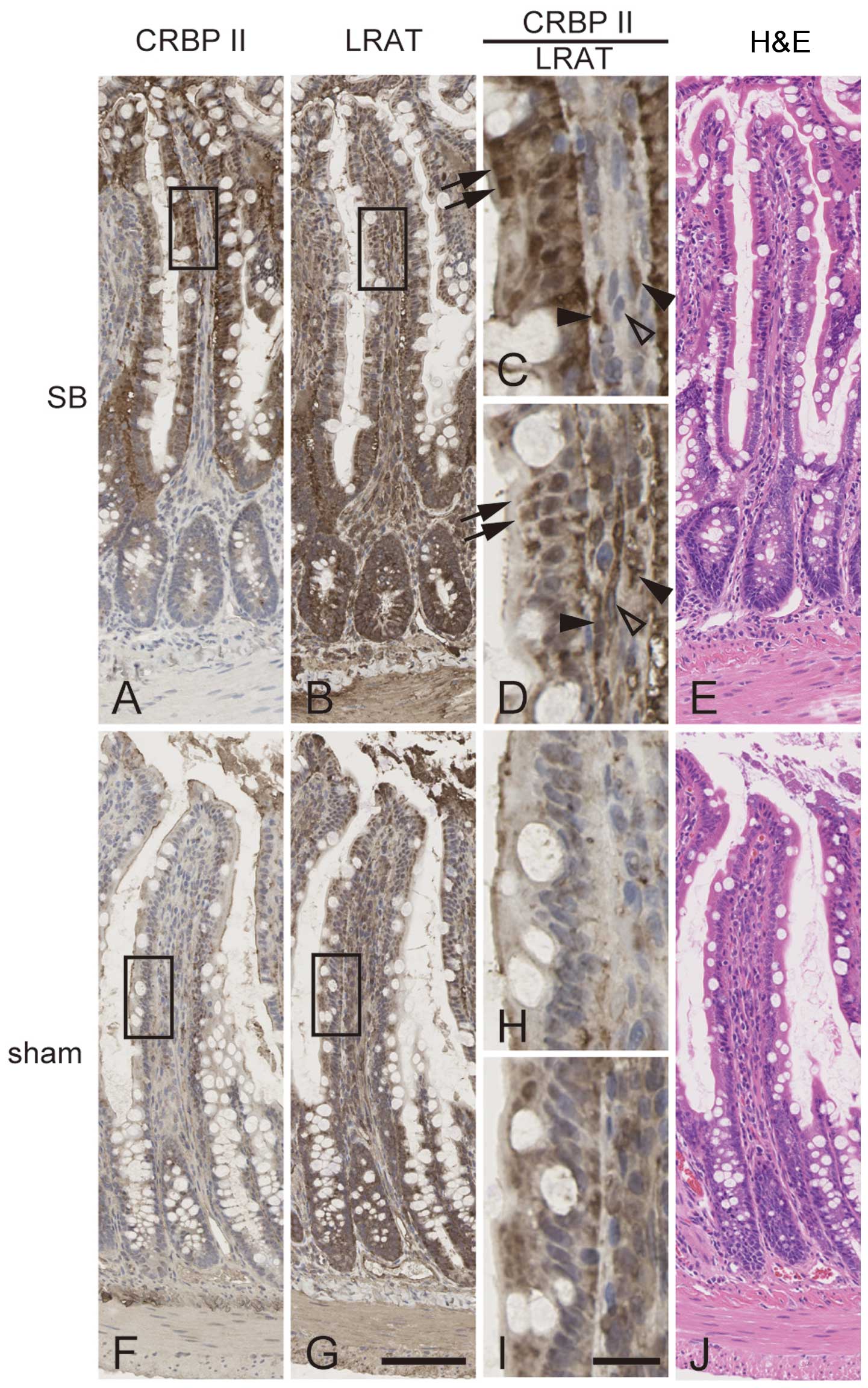 | Figure 3Cellular localizations of cellular
retinol-binding protein II (CRBP II) and lecithin retinol
acyltransferase (LRAT). Immunohistological staining of serial
sections of ileum of rats with short bowel (SB) syndrome (A–D) and
that of sham-operated rats (F–I) was performed using antibodies
against CRBP II (A, C, F and H) or LRAT (B, D, G and I).
High-magnification images of rectangles in (A, B, F and G) are
shown in (C, D, H and I), respectively. H&E-stained sections
are shown (E and J). Arrows, absorptive epithelial cells positive
for both CRBP II and LRAT. Closed arrowheads, fibroblast-like cells
in lamina propria mucosae that were positive for both CRBP II and
LRAT. Open arrowheads, fibroblast-like cells positive for LRAT and
negative for CRBP II. Scale bar in G is 100 μm and applies
to (A, B, E, F and J). Scale bar in (I) is 20 μm and applies
to (C, D and H). |
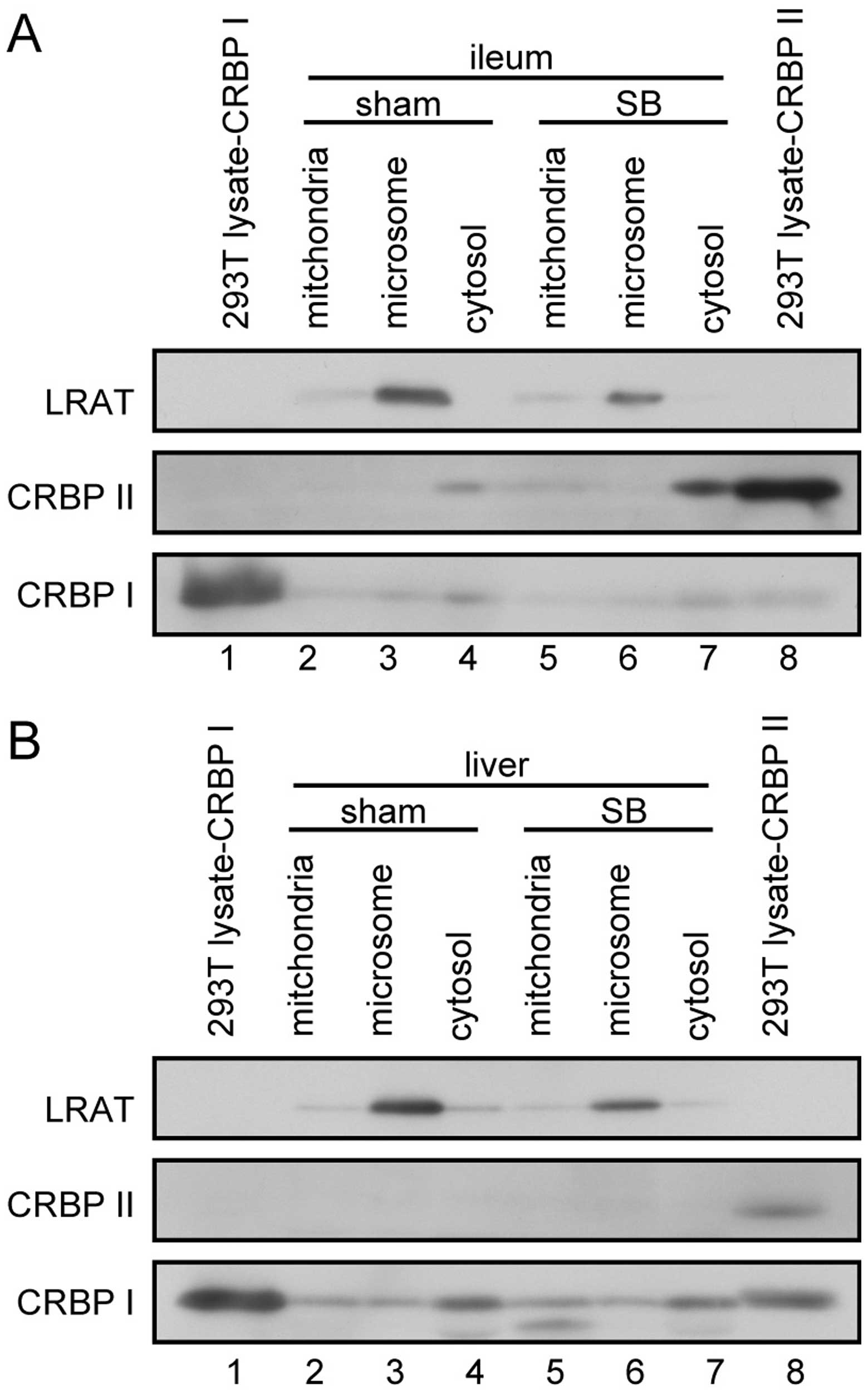
Subcellular localization of LRAT, CRBP I
and CRBP II in the livers and intestines of rats with SB and
sham-operated rats
We then analyzed the subcellular distributions of
retinoid metabolism-related gene products in the liver and ileum.
CRBP II was expressed in the cytosolic fraction of the ileum
(Fig. 4A), but not in the liver
(Fig. 4B). LRAT was expressed in
both the liver and the ileum (Fig.
4), localized in the microsomal fractions. CRBP I was mainly
expressed in the liver (Fig. 4B);
its expression levels were higher in the cytosolic than in the
mitochondrial and microsomal fractions.
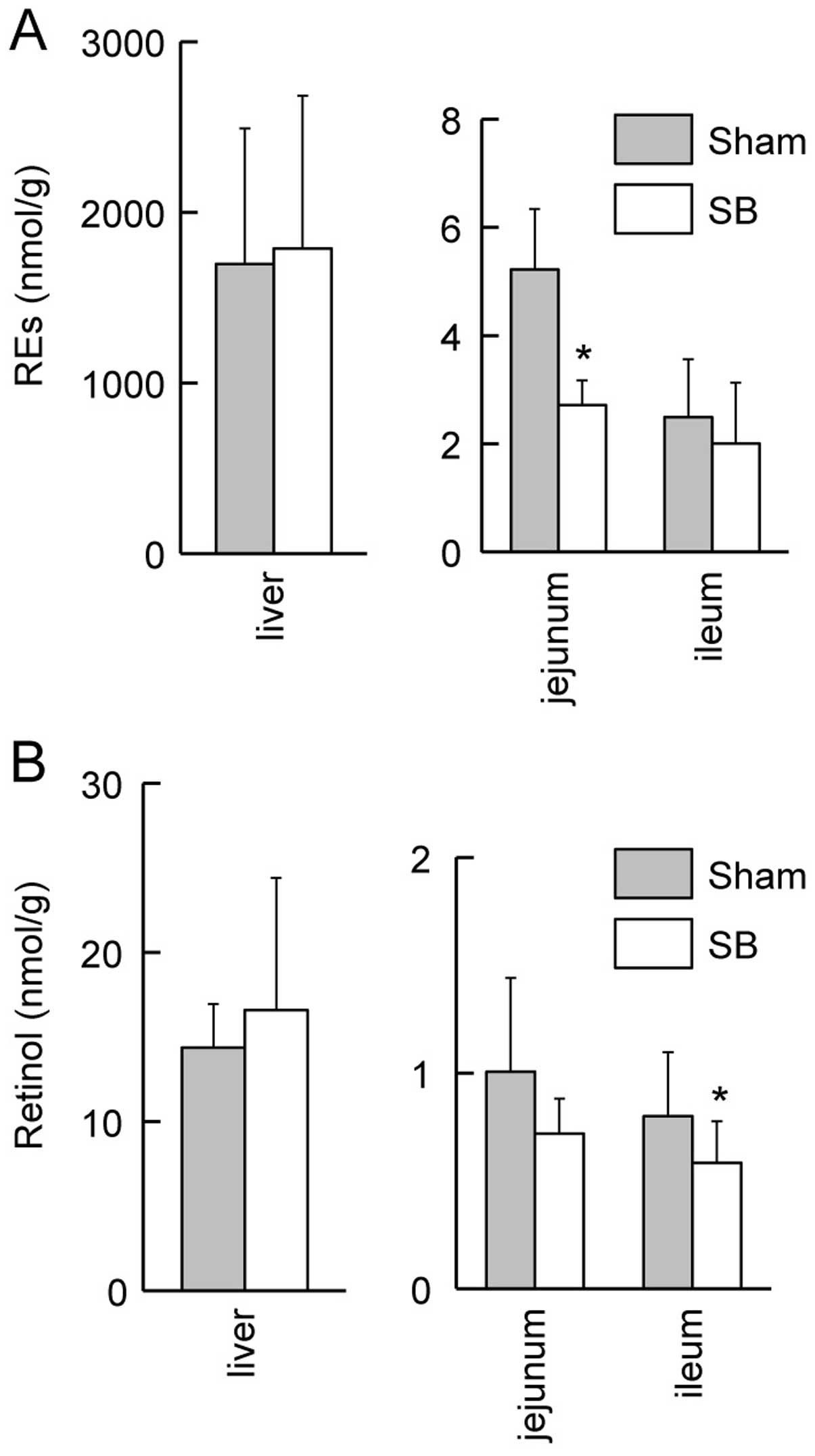
Downregulation of retinol and REs in the
intestines of rats with SB
We then used HPLC to quantify the amounts of retinol
and REs in the livers and the intestines of the rats with SB and
the sham-operated rats. The content of REs in the jejuna of the
rats with SB was lower than that of the sham-operated rats
(Fig. 5A; P<0.05). The retinol
content in the ilea of the rats with SB was lower than that of the
sham-operated rats (Fig. 5B;
P<0.05). There were no significant differences in the contents
of retinol and REs in the livers of the rats with SB and the
sham-operated rats (Fig. 5).
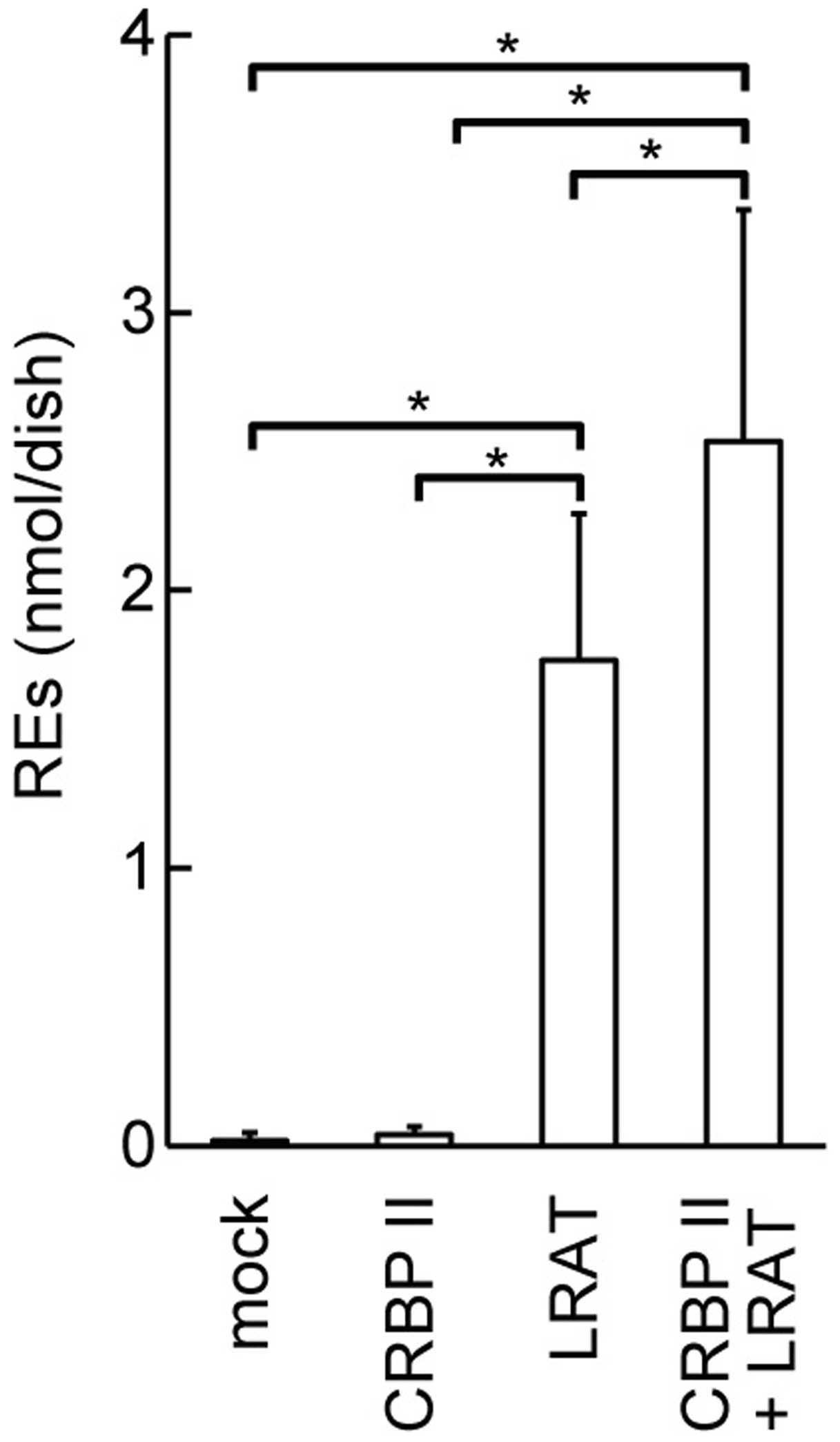
Upregulation of REs in cultured cells
overexpressing CRBP II and LRAT
We wished to determine whether CRBP II affects the
amount of REs produced by LRAT. Thus, we quantified REs in HEK293T
cells overexpressing CRBP II and/or LRAT. The overexpression of
LRAT in the HEK293T cells led to the accumulation of a large amount
of REs in the cells within 10 min of the addition of retinol to the
medium (P<0.05; Fig. 6). CRBP
II overexpression enhanced the accumulation of REs in the
LRAT-transfected HEK293T cells (P<0.05).
Discussion
In this study on SB syndrome, we used a rat model of
SB to demonstrate that the intestinal mRNA expression levels of
Rbp2 were significantly higher in the rats with SB than
those in the sham-operated rats (Fig.
2). The esterification of vitamin A is regulated by the
cooperative actions of LRAT and CRBPs (18,19). In accordance with this, we
demonstrated that the overexpression of CRBP II and LRAT
cooperatively increased the amount of REs in the HEK293T cells
(Fig. 6). However, we observed a
decrease in vitamin A content in the intestines of rats with SB
(Fig. 5). The mRNA expression
levels of Apoa4 in the intestines of rats with SB were
significantly higher than those in the sham-operated animals
(Fig. 2), as was previously
reported by Rubin et al (20). Apolipoprotein A-IV is a component
of chylomicrons (21) in which
REs are transported (22). Thus,
the upregulation of Apoa4 mRNA may lead to the enhanced
transport of vitamin A from the absorptive epithelial cells to the
lymphatic vessels, resulting in the reduction of vitamin A content
in the intestines of rats with SB. In spite of the 75% reduction in
the surface area of the intestine, our quantitative analysis
revealed no significant differences in the contents of retinol or
REs in the livers of rats with SB and the sham-operated rats
(Fig. 5), the major storage organ
for vitamin A. This may be explained by the functional adaptation
of the intestine of rats with SB mediated by the upregulation of
Rbp2 and Apoa4 mRNA levels.
It is generally accepted that LRAT is present in the
endoplasmic reticulum (ER) (23)
and that CRBPs are present in the cytosol (24); our data confirmed these
presumptions (Fig. 4).
Nevertheless, LRAT and CRBPs likely act cooperatively for vitamin A
esterification (25). The
N-terminal portion and/or the C-terminal portion of LRAT are
inserted into the membranes of the ER, exposing remnant hydrophilic
portions to the cytosol (26,27). There, CRBPs probably deliver
vitamin A to the cytosolic portion of LRAT.
In immunohistological staining of the small
intestines, absorptive epithelial cells were positive for both CRBP
II and LRAT (Fig. 3C and D).
Their overlapping presence in the rat intestines provides in
vivo evidence for the cooperative action of these 2 proteins to
increase the absorption of vitamin A. Some fibroblast-like cells in
the lamina propria mucosae also seemed to be positive for both CRBP
II and LRAT. We recently reported that vitamin A was absorbed and
stored in the cells of the lamina propria mucosae of rat intestines
and transmission electron microscopic analysis revealed that these
vitamin A-storing cells in the lamina propria resembled
extrahepatic stellate cells (EHSCs) (28). We speculate that the CRBP II- and
LRAT-positive cells in the lamina propria represent EHSCs. If that
is the case, EHSCs express CRBP II, differing from HSCs that
express CRBP I. It would be intriguing to determine whether the
differential expression of CRBPs in HSCs and EHSCs leads to
functional differences between these cells.
SB syndrome is linked to the defective absorption of
many types of nutrients, including carbohydrates, fats, amino acids
and vitamins. The resultant malnutrition may lead to diseases in
various organs. For example, it has been reported that arginine
becomes an essential amino acid after massive bowel resection,
because citrulline, a precursor of arginine, is synthesized in the
small intestine (29). We
previously reported that 90% resection of the rat small intestine
caused an insufficiency of citrulline, leading to focal
tubulointerstitial fibrosis in the kidney (30). In addition, liver fibrosis has
been shown to occur after massive small bowel resection in the
neonate piglet SB model (31).
The increased esterification and transport of retinol in the
absorptive epithelial cells of the intestines of rats with SB,
which is suggested in this study, may contribute to prevent such
fibrosis, as the suppressive effects of vitamin A on various types
of fibrosis are known (32).
In conclusion, our results suggest that the
esterification and transport of vitamin A are enhanced in the
absorptive epithelial cells of the intestines of rats with SB
through the upregulation of Rbp2 and Apoa4 mRNA
expression.
Acknowledgments
This study was supported by the JSPS KAKENHI grant
nos. 23592623, 25504001 and 23590228.
References
|
1
|
Duro D, Kamin D and Duggan C: Overview of
pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr.
47(Suppl 1): S33–S36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wall EA: An overview of short bowel
syndrome management: adherence, adaptation, and practical
recommendations. J Acad Nutr Diet. 113:1200–1208. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shaw D, Gohil K and Basson MD: Intestinal
mucosal atrophy and adaptation. World J Gastroenterol.
18:6357–6375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang L, Tang Y, Rubin DC and Levin MS:
Chronically administered retinoic acid has trophic effects in the
rat small intestine and promotes adaptation in a resection model of
short bowel syndrome. Am J Physiol Gastrointest Liver Physiol.
292:G1559–G1569. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harrison EH: Mechanisms of digestion and
absorption of dietary vitamin A. Annu Rev Nutr. 25:87–103. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blomhoff R and Blomhoff HK: Overview of
retinoid metabolism and function. J Neurobiol. 66:606–630. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wake K: ‘Sternzellen’ in the liver:
perisinusoidal cells with special reference to storage of vitamin
A. Am J Anat. 132:429–462. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Senoo H: Structure and function of hepatic
stellate cells. Med Electron Microsc. 37:3–15. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Newcomer ME: Retinoid-binding proteins:
structural determinants important for function. FASEB J. 9:229–239.
1995.PubMed/NCBI
|
|
10
|
Flower DR, North AC and Sansom CE: The
lipocalin protein family: structural and sequence overview. Biochim
Biophys Acta. 1482:9–24. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sherman DR, Lloyd RS and Chytil F: Rat
cellular retinol-binding protein: cDNA sequence and rapid
retinol-dependent accumulation of mRNA. Proc Natl Acad Sci USA.
84:3209–3213. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Colantuoni V, Cortese R, Nilsson M,
Lundvall J, Bavik CO, Eriksson U, Peterson PA and Sundelin J:
Cloning and sequencing of a full length cDNA corresponding to human
cellular retinol-binding protein. Biochem Biophys Res Commun.
130:431–439. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li E, Demmer LA, Sweetser DA, Ong DE and
Gordon JI: Rat cellular retinol-binding protein II: use of a cloned
cDNA to define its primary structure, tissue-specific expression,
and developmental regulation. Proc Natl Acad Sci USA. 83:5779–5783.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
E X, Zhang L, Lu J, Tso P, Blaner WS,
Levin MS and Li E: Increased neonatal mortality in mice lacking
cellular retinol-binding protein II. J Biol Chem. 277:36617–36623.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takase S, Goda T and Shinohara H: Adaptive
changes of intestinal cellular retinol-binding protein, type II
following jejunum-bypass operation in the rat. Biochim Biophys
Acta. 1156:223–231. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dodson BD, Wang JL, Swietlicki EA, Rubin
DC and Levin MS: Analysis of cloned cDNAs differentially expressed
in adapting remnant small intestine after partial resection. Am J
Physiol. 271:G347–G356. 1996.PubMed/NCBI
|
|
17
|
Wang JL, Swartz-Basile DA, Rubin DC and
Levin MS: Retinoic acid stimulates early cellular proliferation in
the adapting remnant rat small intestine after partial resection. J
Nutr. 127:1297–1303. 1997.PubMed/NCBI
|
|
18
|
Ong DE, Kakkad B and MacDonald PN:
Acyl-CoA-independent esterification of retinol bound to cellular
retinol-binding protein (type II) by microsomes from rat small
intestine. J Biol Chem. 262:2729–2736. 1987.PubMed/NCBI
|
|
19
|
Yost RW, Harrison EH and Ross AC:
Esterification by rat liver microsomes of retinol bound to cellular
retinol-binding protein. J Biol Chem. 263:18693–18701.
1988.PubMed/NCBI
|
|
20
|
Rubin DC, Swietlicki EA, Wang JL, Dodson
BD and Levin MS: Enterocytic gene expression in intestinal
adaptation: evidence for a specific cellular response. Am J
Physiol. 270:G143–G152. 1996.PubMed/NCBI
|
|
21
|
Green PH, Glickman RM, Riley JW and Quinet
E: Human apolipoprotein A-IV Intestinal origin and distribution in
plasma. J Clin Invest. 65:911–919. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goodman DW, Huang HS and Shiratori T:
Tissue distribution and metabolism of newly absorbed vitamin A in
the rat. J Lipid Res. 6:390–396. 1965.PubMed/NCBI
|
|
23
|
Ruiz A, Winston A, Lim YH, Gilbert BA,
Rando RR and Bok D: Molecular and biochemical characterization of
lecithin retinol acyltransferase. J Biol Chem. 274:3834–3841. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ong DE and Chytil F: Cellular
retinol-binding protein from rat liver Purification and
characterization. J Biol Chem. 253:828–832. 1978.PubMed/NCBI
|
|
25
|
Napoli JL: Interactions of retinoid
binding proteins and enzymes in retinoid metabolism. Biochim
Biophys Acta. 1440:139–162. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bok D, Ruiz A, Yaron O, Jahng WJ, Ray A,
Xue L and Rando RR: Purification and characterization of a
transmembrane domain-deleted form of lecithin retinol
acyltransferase. Biochemistry. 42:6090–6098. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moise AR, Golczak M, Imanishi Y and
Palczewski K: Topology and membrane association of lecithin:retinol
acyltransferase. J Biol Chem. 282:2081–2090. 2007. View Article : Google Scholar
|
|
28
|
Senoo H, Mezaki Y, Morii M, Hebiguchi T,
Miura M and Imai K: Uptake and storage of vitamin A as lipid
droplets in the cytoplasm of cells in the lamina propria mucosae of
the rat intestine. Cell Biol Int. 37:1171–1180. 2013.PubMed/NCBI
|
|
29
|
Wakabayashi Y, Yamada E, Yoshida T and
Takahashi H: Arginine becomes an essential amino acid after massive
resection of rat small intestine. J Biol Chem. 269:32667–32671.
1994.PubMed/NCBI
|
|
30
|
Hebiguchi T, Kato T, Yoshino H, Mizuno M,
Wakui H, Komatsuda A and Imai H: Extremely short small bowel
induces focal tubulointerstitial fibrosis. J Pediatr Gastroenterol
Nutr. 32:586–592. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Taguchi S, Masumoto K, Yamanouchi T and
Suita S: Decrease in hepatic circulation induces hepatic fibrosis
in a neonatal piglet model with short bowel syndrome. J Pediatr
Surg. 40:1592–1597. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Senoo H and Wake K: Suppression of
experimental hepatic fibrosis by administration of vitamin A. Lab
Invest. 52:182–194. 1985.PubMed/NCBI
|