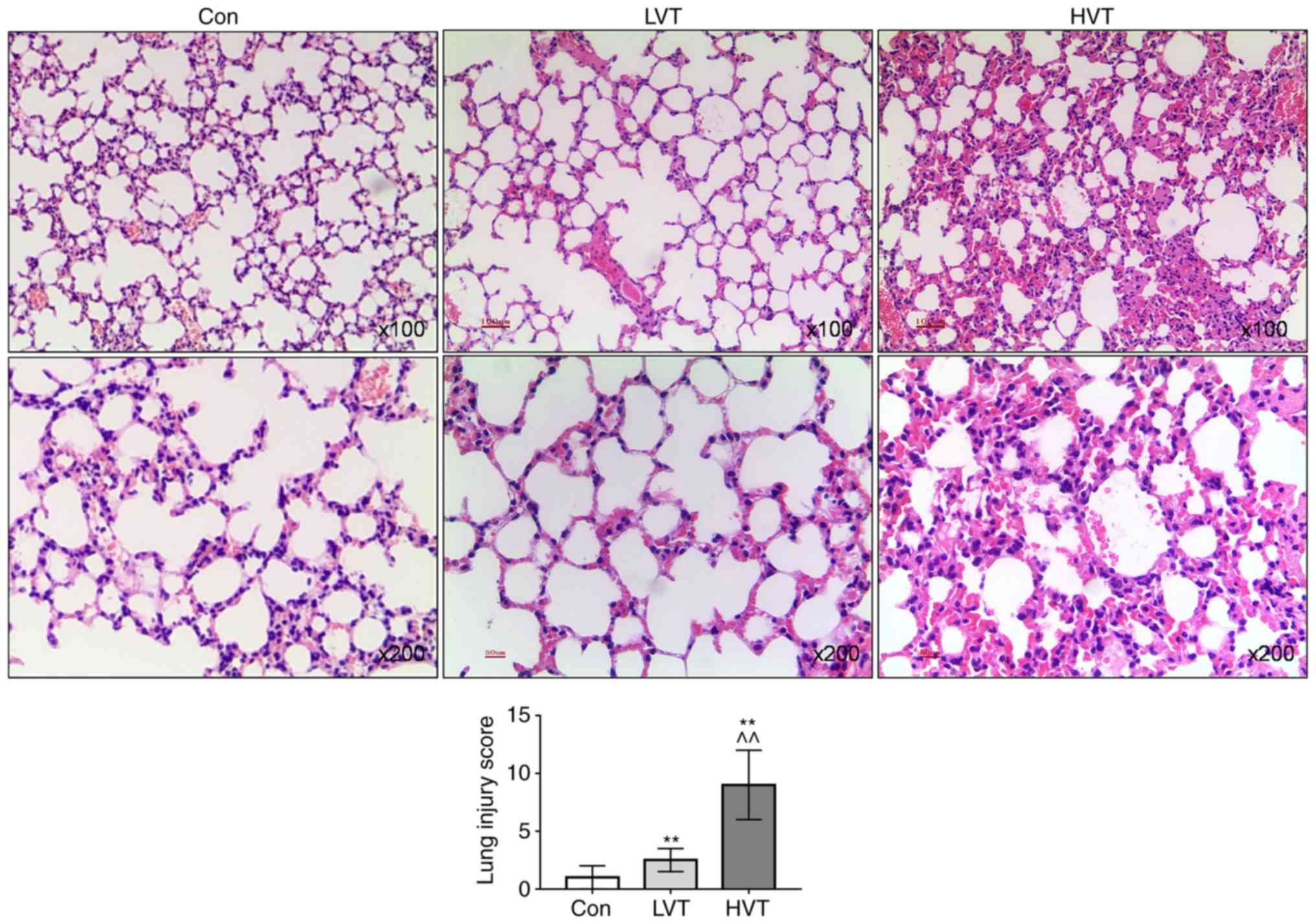
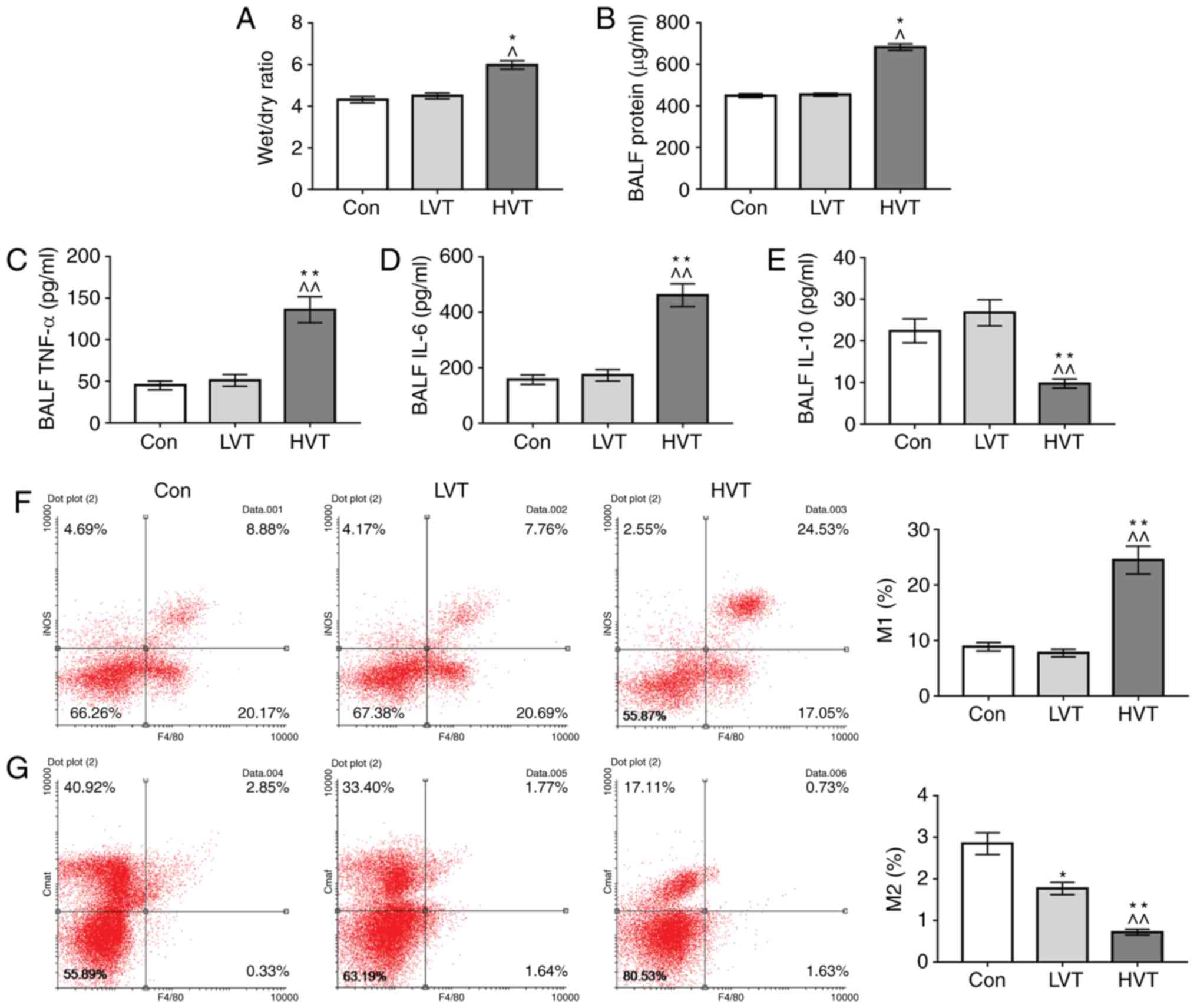
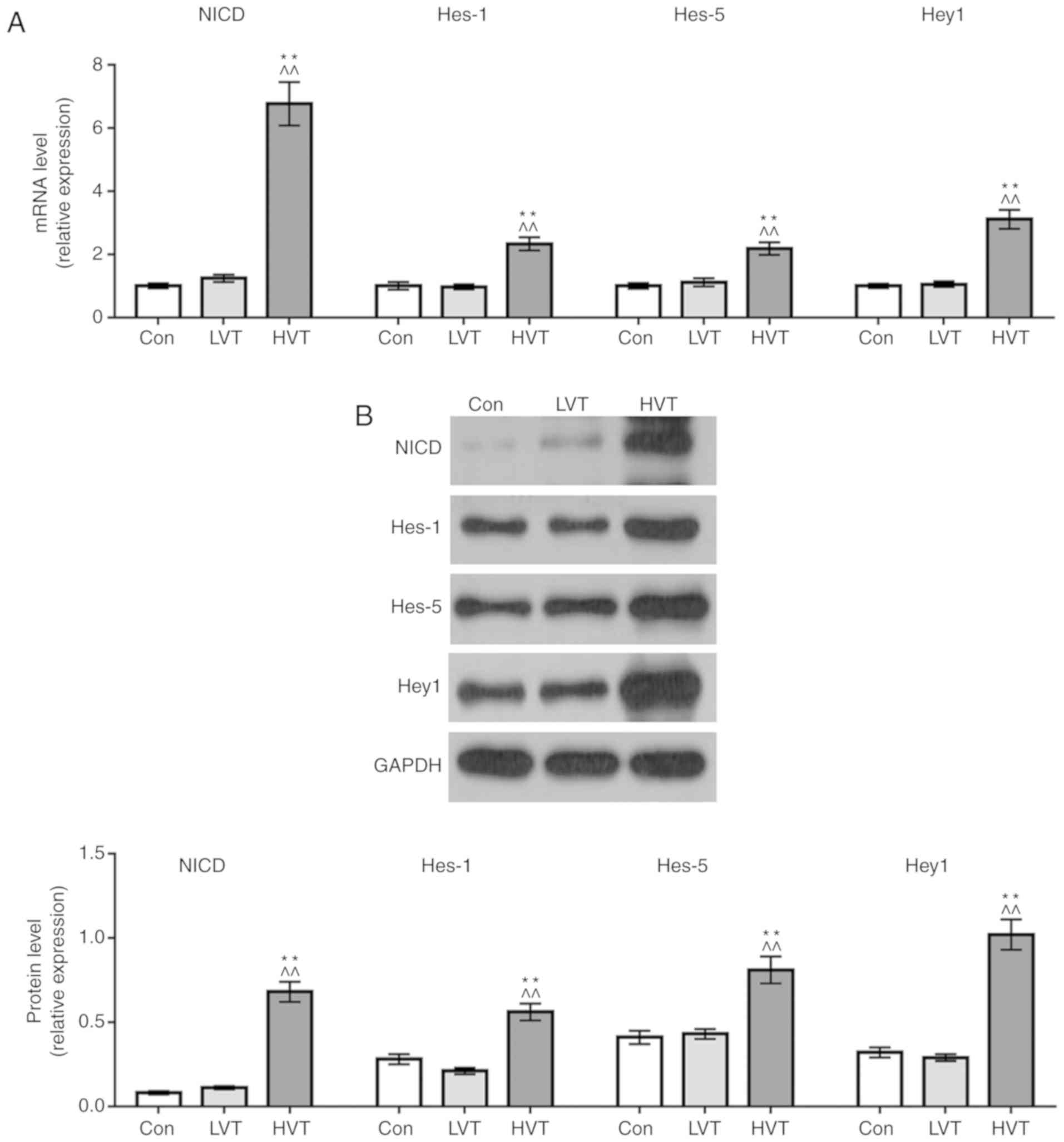
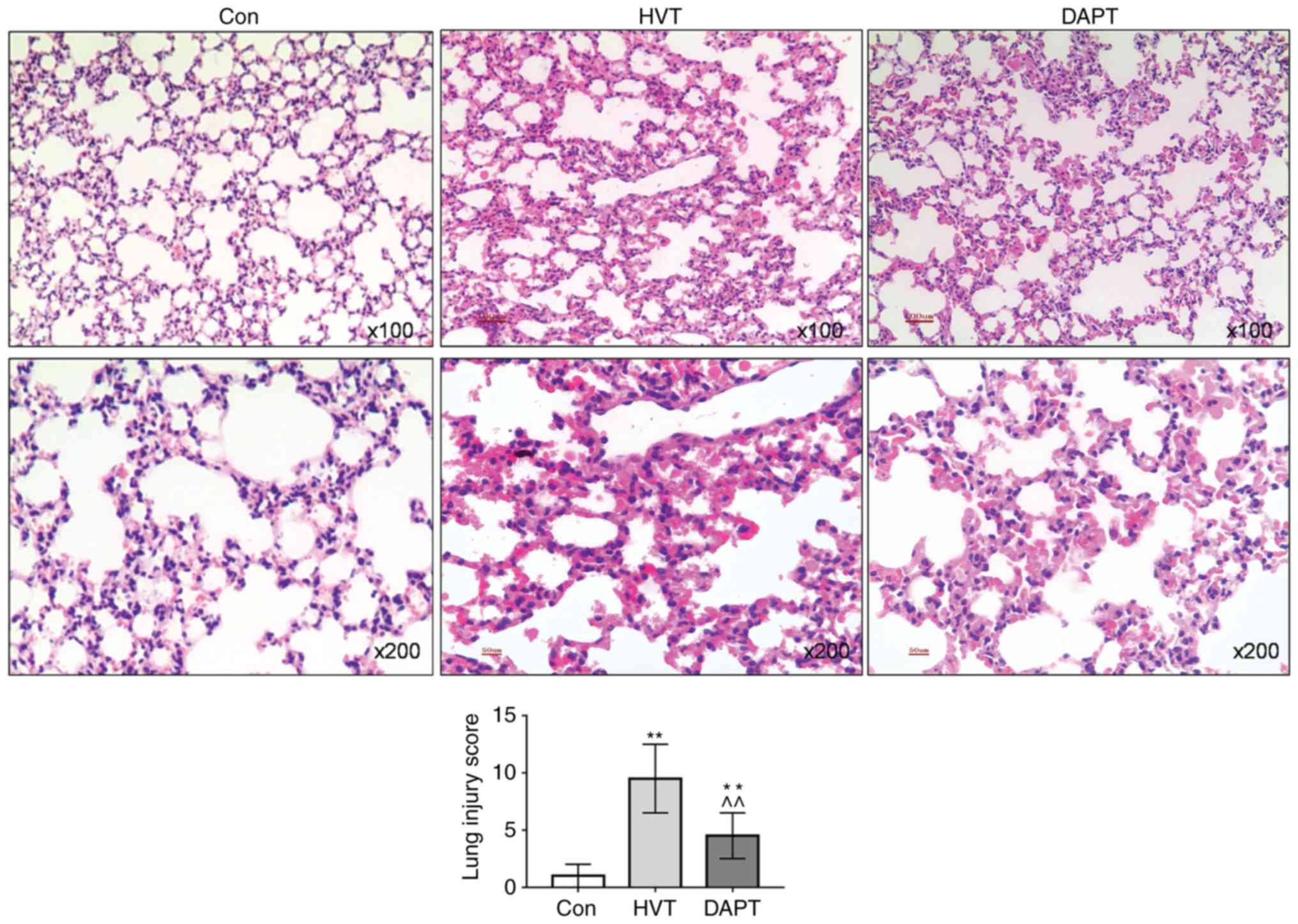
|
1
|
Sutherasan Y, Vargas M and Pelosi P:
Protective mechanical ventilation in the non-injured lung: Review
and meta-analysis. Crit Care. 18:2112014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schnell D, Timsit JF, Darmon M, Vesin A,
Goldgran-Toledano D, Dumenil AS, Garrouste-Orgeas M, Adrie C,
Bouadma L, Planquette B, et al: Noninvasive mechanical ventilation
in acute respiratory failure: Trends in use and outcomes. Intensive
Care Med. 40:582–591. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Unroe M, Kahn JM, Carson SS, Govert JA,
Martinu T, Sathy SJ, Clay AS, Chia J, Gray A, Tulsky JA and Cox CE:
One-year trajectories of care and resource utilization for
recipients of prolonged mechanical ventilation: A cohort study. Ann
Intern Med. 153:167–175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Müller-Redetzky HC, Felten M, Hellwig K,
Wienhold SM, Naujoks J, Opitz B, Kershaw O, Gruber AD, Suttorp N
and Witzenrath M: Increasing the inspiratory time and I:E ratio
during mechanical ventilation aggravates ventilator-induced lung
injury in mice. Crit Care. 19:232015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hamlington KL, Smith BJ, Allen GB and
Bates JH: Predicting ventilator-induced lung injury using a lung
injury cost function. J Appl Physiol 1985. 121:106–114. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reddy SP, Hassoun PM and Brower R: Redox
imbalance and ventilator-induced lung injury. Antioxid Redox
Signal. 9:2003–2012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Curley GF, Contreras M, Higgins B, O'Kane
C, McAuley DF, O'Toole D and Laffey JG: Evolution of the
inflammatory and fibroproliferative responses during resolution and
repair after ventilator-induced lung injury in the rat.
Anesthesiology. 115:1022–1032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bertok S, Wilson MR, Morley PJ, de Wildt
R, Bayliffe A and Takata M: Selective inhibition of intra-alveolar
p55 TNF receptor attenuates ventilator-induced lung injury. Thorax.
67:244–251. 2012. View Article : Google Scholar :
|
|
9
|
Wilson MR, Wakabayashi K, Bertok S, Oakley
CM, Patel BV, O'Dea KP, Cordy JC, Morley PJ, Bayliffe AI and Takata
M: Inhibition of TNF receptor p55 By a domain antibody attenuates
the initial phase of acid-induced lung injury in mice. Front
Immunol. 8:1282017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Opitz B, van Laak V, Eitel J and Suttorp
N: Innate immune recognition in infectious and noninfectious
diseases of the lung. Am J Respir Crit Care Med. 181:1294–1309.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martinez FO and Gordon S: The M1 and M2
paradigm of macrophage activation: Time for reassessment. F1000
Prime Rep. 6:132014. View
Article : Google Scholar
|
|
12
|
Jablonski KA, Amici SA, Webb LM,
Ruiz-Rosado Jde D, Popovich PG, Partida-Sanchez S and
Guerau-de-Arellano M: Novel markers to delineate murine M1 and M2
macrophages. PLoS One. 10:e01453422015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wynn TA, Chawla A and Pollard JW:
Macrophage biology in development, homeostasis and disease. Nature.
496:445–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jaecklin T, Otulakowski G and Kavanagh BP:
Do soluble mediators cause ventilator-induced lung injury and
multi-organ failure? Intensive Care Med. 36:750–757. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dai H, Pan L, Lin F, Ge W, Li W and He S:
Mechanical ventilation modulates Toll-like receptors 2, 4, and 9 on
alveolar macrophages in a ventilator-induced lung injury model. J
Thorac Dis. 7:616–624. 2015.PubMed/NCBI
|
|
16
|
Kopan R and Ilagan MX: The canonical Notch
signaling pathway: Unfolding the activation mechanism. Cell.
137:216–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Z, Guo L, Liu D, Sun L, Chen H, Deng
Q, Liu Y, Yu M, Ma Y, Guo N and Shi M: Acquisition of resistance to
trastuzumab in gastric cancer cells is associated with activation
of IL-6/STAT3/Jagged-1/Notch positive feedback loop. Oncotarget.
6:5072–5087. 2015.PubMed/NCBI
|
|
18
|
Han H, Gong G, Bai X, Lin YC, Sun J, Wang
W, Zhao Y, Yang L, Wang X, Zhang Z, et al: Inhibition of Notch
signaling protects mouse lung against zymosan-induced injury.
Shock. 40:312–319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsao PN, Wei SC, Huang MT, Lee MC, Chou
HC, Chen CY and Hsieh WS: Lipopolysaccharide-induced Notch
signaling activation through JNK-dependent pathway regulates
inflammatory response. J Biomed Sci. 18:562011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiao Z, Wang W, Hua S, Liu M, Wang H, Wang
X, Chen Y, Xu H and Lu L: Blockade of Notch signaling ameliorates
murine collagen-induced arthritis via suppressing Th1 and Th17 cell
responses. Am J Pathol. 184:1085–1093. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park JS, Kim SH, Kim K, Jin CH, Choi KY,
Jang J, Choi Y, Gwon AR, Baik SH, Yun UJ, et al: Inhibition of
Notch signalling ameliorates experimental inflammatory arthritis.
Ann Rheum Dis. 74:267–274. 2015. View Article : Google Scholar
|
|
22
|
Huang C, Pan L, Lin F, Dai H and Fu R:
Monoclonal antibody against Toll-like receptor 4 attenuates
ventilator-induced lung injury in rats by inhibiting MyD88- and
NF-κB-dependent signaling. Int J Mol Med. 39:693–700. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Whitehead TC, Zhang H, Mullen B and
Slutsky AS: Effect of mechanical ventilation on cytokine response
to intratracheal lipopolysaccharide. Anesthesiology. 101:52–58.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Silva PL, Negrini D and Rocco PR:
Mechanisms of ventilator-induced lung injury in healthy lungs. Best
Pract Res Clin Anaesthesiol. 29:301–313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cressoni M, Gotti M, Chiurazzi C, Massari
D, Algieri I, Amini M, Cammaroto A, Brioni M, Montaruli C, Nikolla
K, et al: Mechanical power and development of Ventilator-induced
lung injury. Anesthesiology. 124:1100–1108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Borges JB, Costa EL, Suarez-Sipmann F,
Widström C, Larsson A, Amato M and Hedenstierna G: Early
inflammation mainly affects normally and poorly aerated lung in
experimental ventilator-induced lung injury*. Crit Care Med.
42:e279–e287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu YH and Guo NL: USP14 inhibitor IU1
prevents ventilator-induced lung injury in rats. Cell Mol Biol
(Noisy-le-grand). 60:50–54. 2014.
|
|
29
|
Malaviya R, Sunil VR, Venosa A, Verissimo
VL, Cervelli JA, Vayas KN, Hall L, Laskin JD and Laskin DL:
Attenuation of nitrogen mustard-induced pulmonary injury and
fibrosis by anti-tumor necrosis factor-α antibody. Toxicol Sci.
148:71–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Halbertsma FJ, Vaneker M, Scheffer GJ and
van der Hoeven JG: Cytokines and biotrauma in ventilator-induced
lung injury: A critical review of the literature. Neth J Med.
63:382–392. 2005.PubMed/NCBI
|
|
31
|
Ko YA, Yang MC, Huang HT, Hsu CM and Chen
LW: NF-κB activation in myeloid cells mediates ventilator-induced
lung injury. Respir Res. 14:692013. View Article : Google Scholar
|
|
32
|
Huang C, Pan L, Lin F, Qian W and Li W:
Alveolar macrophage TLR4/MyD88 signaling pathway contributes to
ventilator-induced lung injury in rats. Xi Bao Yu Fen Zi Mian Yi
Xue Za Zhi. 31:182–185. 1892015.In Chinese.
|
|
33
|
Morimoto M, Nishinakamura R, Saga Y and
Kopan R: Different assemblies of Notch receptors coordinate the
distribution of the major bronchial Clara, ciliated and
neuroendocrine cells. Development. 139:4365–4373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nagase H and Nakayama K:
γ-Secretase-regulated signaling typified by Notch signaling in the
immune system. Curr Stem Cell Res Ther. 8:341–356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Katoh M and Katoh M: Integrative genomic
analyses on HES/HEY family: Notch-independent HES1, HES3
transcription in undifferentiated ES cells, and Notch-dependent
HES1, HES5, HEY1, HEY2, HEYL transcription in fetal tissues, adult
tissues, or cancer. Int J Oncol. 31:461–466. 2007.PubMed/NCBI
|
|
36
|
Ma Y, Bian J and Zhang F: Inhibition of
perillyl alcohol on cell invasion and migration depends on the
Notch signaling pathway in hepatoma cells. Mol Cell Biochem.
411:307–315. 2016. View Article : Google Scholar
|
|
37
|
Liu X, Cong N, Cheng X, Ma R, Wang J,
Huang YB, Zhao M, Wang XW, Chi FL and Ren DD: The role of the Notch
signal pathway in mucosal cell metaplasia in mouse acute otitis
media. Sci Rep. 7:45882017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang YC, He F, Feng F, Liu XW, Dong GY,
Qin HY, Hu XB, Zheng MH, Liang L, Feng L, et al: Notch signaling
determines the M1 versus M2 polarization of macrophages in
antitumor immune responses. Cancer Res. 70:4840–4849. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun W, Zhang H, Wang H, Chiu YG, Wang M,
Ritchlin CT, Kiernan A, Boyce BF and Xing L: Targeting
notch-activated M1 macrophages attenuates joint tissue damage in a
mouse model of inflammatory arthritis. J Bone Miner Res.
32:1469–1480. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang F, Zhao JL, Wang L, Gao CC, Liang
SQ, An DJ, Bai J, Chen Y, Han H and Qin HY: miR-148a-3p mediates
Notch signaling to promote the differentiation and M1 activation of
macrophages. Front Immunol. 8:13272017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wongchana W and Palaga T: Direct
regulation of interleukin-6 expression by Notch signaling in
macrophages. Cell Mol Immunol. 9:155–162. 2012. View Article : Google Scholar
|
|
42
|
Zhang H, Hilton MJ, Anolik JH, Welle SL,
Zhao C, Yao Z, Li X, Wang Z, Boyce BF and Xing L: NOTCH inhibits
osteoblast formation in inflammatory arthritis via noncanonical
NF-κB. J Clin Invest. 124:3200–3214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ortiz-Masia D, Cosin-Roger J, Calatayud S,
Hernández C, Alós R, Hinojosa J, Esplugues JV and Barrachina MD: M1
macrophages activate Notch signalling in epithelial cells:
Relevance in crohn's disease. J Crohns Colitis. 10:582–592. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhao JL, Huang F, He F, Gao CC, Liang SQ,
Ma PF, Dong GY, Han H and Qin HY: Forced activation of Notch in
macrophages represses tumor growth by upregulating miR-125a and
disabling tumor-associated macrophages. Cancer Res. 76:1403–1415.
2016. View Article : Google Scholar : PubMed/NCBI
|